Abstract
Background:
Changes in resting state functional connectivity between the insula and dorsal anterior cingulate cortex as well as between the insula and nucleus accumbens have been linked to nicotine withdrawal and/or administration. However, because many of nicotine’s effects in humans appear to depend, at least in part, on the belief that nicotine has been administered, the relative contribution of nicotine’s pharmacological actions to such effects requires clarification.
Aims:
The purpose of this study was to examine the impacts of perceived and actual nicotine administration on neural responses.
Methods:
Twenty-six smokers were randomly assigned to receive either a nicotine inhaler (4 mg deliverable) or a nicotine-free inhaler across two sessions. Inhaler content instructions (told nicotine vs told nicotine-free) differed across sessions. Resting state functional connectivity between sub-regions of the insula and the dorsal anterior cingulate cortex and nucleus accumbens was measured using magnetic resonance imaging before and after inhaler administration.
Results:
Both actual and perceived nicotine administration independently altered resting state functional connectivity between the anterior insula and the dorsal anterior cingulate cortex, with actual administration being associated with decreased resting state functional connectivity, and perceived administration with increased resting state functional connectivity. Actual nicotine administration also contralaterally reduced resting state functional connectivity between the anterior insula and nucleus accumbens, while reductions in resting state functional connectivity between the mid-insula and right nucleus accumbens were observed when nicotine was administered unexpectedly. Changes in resting state functional connectivity associated with actual or perceived nicotine administration were unrelated to changes in subjective withdrawal and craving. Changes in withdrawal and craving were however independently associated with resting state functional connectivity between the nucleus accumbens and insula.
Conclusions:
Our findings highlight the importance of considering non-pharmacological factors when examining drug mechanisms of action.
Keywords: Nicotine, expectancy, insula, anterior cingulate cortex, nucleus accumbens
The insula is believed to be instrumental in mediating the addictive properties of tobacco (Naqvi et al., 2007; Sutherland et al., 2012). Localized insular damage in cigarette smokers is associated with dramatic reductions in smoking behavior (Gaznick et al., 2013), in the expression of abstinence-related withdrawal symptoms (Abdolahi et al., 2015), and in conscious urges to smoke (Naqvi et al., 2007). Although some studies do not differentiate between sub-regions of the insula, there is evidence of sub-regional specificity of function. The posterior insula is believed to be primarily responsible for coding and processing sensory and interoceptive inputs, while the anterior insula (AI) is thought to determine how such inputs impact homeostatic processes (Naqvi et al., 2014; Simmons et al., 2013). With respect to tobacco addiction, the AI has been hypothesized to play a role in transmitting interoceptive signals related to nicotine effects and/or withdrawal to brain regions involved in the appraisal of motivationally relevant stimuli (e.g. nucleus accumbens (NAc)) and in the planning and evaluation of goal directed behavior (e.g. the anterior cingulate cortex (ACC)). These neural processes are thought to give rise to smoking relevant cognitions and behaviors (Naqvi et al., 2014).
Strong resting state co-activation (i.e. resting state functional connectivity (rsFC)) between the bilateral AI and dorsal ACC (dACC) has been associated with enhanced reactivity to smoking salient cues in smokers following 1.5 h of abstinence, suggesting that AI-dACC coupling may cue attention to smoking cues, cue-induced thoughts of smoking, and/or cravings in the absence of full withdrawal symptoms (Janes et al., 2015). Other reports suggest that nicotine administration via lozenges is associated with reduced withdrawal symptoms and reduced co-activation between the right posterior insula and ACC in smokers abstinent for 11.5 h (Cole et al., 2010), that 48-hour abstinence is associated with enhanced rsFC between the right posterior insula and dACC relative to smoking satiation (Fedota et al., 2018), and that acute tobacco smoking attenuates right insula and bilateral ventral striatum co-activation in 24-hour abstinent smokers (Sweitzer et al., 2016). Faulkner and colleagues (2019) recently reported that cigarettes with varying nicotine contents (ranging from 0.027–0.763 mg) were all effective in reducing subjective craving in overnight abstinent smokers, but that only the highest nicotine dose reduced rsFC between the AI and ACC. However, the nicotine-related reductions in rsFC were also found to be positively correlated with the degree of craving reduction from smoking the highest dose cigarette. Therefore, it is not clear from the existing literature to what extent changes in co-activation between the insula and other smoking-relevant neural structures result from nicotine administration, changes in craving or withdrawal, or some combination of these.
Moreover, because nicotine’s acute effects on subjective craving and withdrawal appear to depend, at least in part, on the belief that nicotine has been ingested (Juliano and Brandon 2002; Schlagintweit et al., 2014), the relative contribution of nicotine’s pharmacological actions to such effects requires clarification. Although less abundant than in the thalamus or brainstem, it is well established that nicotinic receptors are located within the insula, ACC, and NAc (e.g. Nashmi and Lester 2006; Picard et al., 2013). However, a recent study reported that nicotine-induced craving reduction and associated reductions in left insula activity was only evident when nicotine was both expected and administered (Gu et al., 2016). In addition, evidence from other drug models suggests that drug-related expectations can elicit drug-like neural responses (de la Fuente-Fernandez et al., 2001; Scott et al., 2008). As a result, the relative impact of nicotine pharmacology and nicotine-related non-pharmacological factors on altered neural connectivity requires clarification.
The purpose of this study was to use magnetic resonance imaging (MRI) to examine the relative impacts of actual and perceived nicotine administration on rsFC between sub-regions of the insula and a priori defined regions of interest (ROIs; dACC and NAc) in three-hour tobacco abstinent smokers. The abstinence duration was selected to allow for recovery of many desensitized nicotinic receptors (Picard et al., 2013; Picciotto et al., 2008), but not to induce major withdrawal symptoms (Brown et al., 2013; Hughes et al., 1994) to enable an examination of acute nicotine effects in the absence of subjective withdrawal relief. Using a mixed within/between-subjects design, each participant was randomly assigned to receive either a nicotine containing or nicotine-free inhaler across two experimental sessions (between-subject factor), but received different instructions about the inhaler’s nicotine content (told nicotine vs told nicotine-free) during each session (within-subject factor). This produced four conditions: (a) told nicotine, administered nicotine, (b) told nicotine, administered nicotine-free, (c) told nicotine-free, administered nicotine, and (d) told nicotine-free, administered nicotine-free, allowing for an assessment of the independent and combined effects of nicotine pharmacology and expectancy (Sutton, 1991). Based on the existing literature, it was hypothesized that acute nicotine administration would lead to reductions in rsFC between the insula and each ROI, and that these effects would primarily involve the AI. It was also expected that these effects would be more pronounced when participants believed that nicotine had been administered.
Materials and methods
Participants
Twenty-eight (14 male) smokers were recruited between March 2017–April 2018 from the Halifax, Nova Scotia community. A telephone interview was used to verify selection criteria. Eligible participants were adult daily smokers, with a minimum score of three on the Fagerström Test for Cigarette Dependence (FTCD; Fagerström, 2012). Exclusion criteria were: (a) a current intention to quit smoking, or a plan to quit within 30 days of study participation; (b) a current Diagnotic and Statistical Manual of Mental Disorders -5 (DSM-5; American Psychiatric Association, 2013) diagnosis (excluding tobacco use disorder); (c) a current diagnosis of a serious medical or neurological condition; (d) contraindication for MRI (e.g. implanted ferromagnetic objects, claustrophobia, pregnancy); (e) current use of psychotropic medication; (f) previous participation in a research study that assessed nicotine expectancy effects; and (g) current nicotine replacement therapy use and/or any history of nicotine inhaler use.
The final sample included 26 participants (14 male), as one female participant failed to attend her scheduled MRI sessions and one female participant withdrew shortly after entering the MRI scanner due to claustrophobia symptoms. All participants provided written consent to participate and were compensated CAD$20/hour. The study received ethical approval from the Nova Scotia Health Authority Research Ethics Board.
Inhalers
Commercially purchased ‘Nicorette’ brand inhalers (10 mg of nicotine, 4 mg deliverable; Pharmacia, Mississauga, Ontario, Canada) were used during the nicotine inhaler conditions. Nicotine-free inhalers were identical in appearance but contained inert cellulose filters. Inhalers were flavored with 2 mL of a citrus or mint solution containing thymol, eucalyptol, and menthol (Johnson and Johnson Inc., Markham, Ontario, Canada), such that inhaler flavors always differed between participants’ two experimental conditions.
Withdrawal and craving
Craving and withdrawal symptoms were assessed using the nine-item Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes and Hatsukami, 1986), which includes a "craving" item and eight items that closely correspond to the DSM-5 Tobacco Withdrawal Symptoms (American Psychiatric Association, 2013). Items were rated on a scale ranging from 0 (not present) to 3 (severe). The craving item was analyzed separately. The remaining eight items were averaged to produce an overall "withdrawal" score (Hughes and Hatsukami, 1998).
Procedure
Participants attended one orientation and two experimental sessions scheduled 1–4 weeks apart. Two-hour abstinence from cigarettes and caffeine and >12-hour abstinence from alcohol and other drugs was required before all sessions. During the orientation session, eligibility was verified, participants provided written informed consent, and an expired breath carbon monoxide (CO) sample (Vitalograph, UK) was collected. Given that there is no established CO cut-off for two hours of smoking abstinence, the CO sample served as a bogus pipeline intended to enhance abstinence compliance. Participants were also shown the nicotine inhaler and an information pamphlet describing its effects.
Participants were randomly assigned to receive a nicotine inhaler or nicotine-free inhaler across both experimental study sessions. Male and female participants were assigned to separate blinding lists, each counterbalanced in terms of the order of nicotine content instructions and flavoring conditions. An independent blinder prepared the inhalers. The experimenter provided the nicotine content instructions for the inhalers but remained blind to their actual content.
Experimental sessions began with a one-hour waiting period in order to ensure a minimum period of smoking abstinence prior to imaging. Participants verbally confirmed adherence to the abstinence requirement and provided CO samples before and after the waiting period. After the waiting period, participants rated subjective craving and withdrawal symptoms with the MNWS. They then completed a 25-minute anatomical MRI scan as well as an eight-minute baseline resting state scan. During the scans, participants were instructed to remain still, with their eyes open, and to think of nothing in particular. After these scans, participants exited the scanner, then self-administered their assigned inhaler. The experimenter provided verbal instructions about the nicotine content of the inhaler. In the told nicotine condition participants were given the inhaler in its commercial packaging. In the told nicotine-free condition the inhaler was presented in a clear plastic bag. To standardize inhaler self-administration, participants were instructed, via an audio recording, to self-administer two-second inhaler puffs every 10 s over the course of 20 min for a total of 120 inhalations (Barrett, 2010). Participants then completed a second MNWS assessment, then re-entered the MRI scanner for a second eight-minute resting state scan. This scan started 10 min after inhaler self-administration ended, to correspond with expected peak nicotine plasma levels at 30 min (Schneider et al., 2001). Following the second scan, participants were asked about the nicotine content of the inhaler that they had self-administered. This served as a manipulation check to determine whether participant beliefs regarding drug assignment were consistent with nicotine content instructions. Both experimental sessions followed an identical procedure, except for instructions (told nicotine vs told nicotine-free), corresponding inhaler packaging (commercial packaging vs plastic bag) and inhaler flavoring (mint vs citrus). No additional experimental manipulations were included in either experimental session.
MRI data acquisition
Resting state MRI data was collected using a 3.0 Tesla GE MR750 scanner with a 32-channel radiofrequency head coil. The head coil was positioned with a support to minimize head motion and earplugs were provided to limit any effect of noise.
Pulse sequences and parameters closely matched those used in the Human Connectome Project (Glasser et al., 2013). Specifically, a 3D inversion recovery fast spoiled gradient recalled sequence was used to obtain T1-weighted anatomical 1.0 mm isotropic images with the following parameters: field of view (FOV) 256 mm, 256×256 matrix, 184×1.0 mm sagittal slices, one signal average, repetition time (TR)=4 s, echo time (TE)=1.3 ms, flip angle=9, inversion time=450 ms, bandwidth=62.5 kHz, scan time 7 min 3 s. As well, T2-weighted anatomical 1.0 mm isotropic images were collected using a 3D CUBE T2 Prepped fluid-attenuated inversion recovery sequence with the following parameters: FOV 256 mm, 256×256 matrix, 184×1 mm sagittal slices, TR=5 s, TE=15 ms, bandwidth=62.5 kHz, autocalibrating reconstruction for Cartesian imaging phase factor 1.75, scan time 6 min 6 s.
Resting state functional 3.0 mm isotropic images were collected with a multi-band gradient-echo echo planar imaging sequence obtained from Stanford CNI laboratory (https://cni.stanford.edu/wiki/MUX_EPI) with these parameters: FOV 216 mm, 72×72 matrix, 51×3.0 mm oblique-axial slices, generalized auto-calibrating partially parallel acquisition acceleration factor 2 in-plane, multiplexed acceleration factor 3 slice direction, TR=950 ms, TE=30 ms, 500 time points, scan time 7.9 min. Additionally, EPI reference scans using phase-encode blip direction reversal with identical parameters to the resting state scans were obtained to facilitate field distortion correction (<1 min).
MRI data preprocessing
All preprocessing was conducted using fMRIB Software Library (FSL (Jenkinson et al., 2001); https://fsl.fmrib.ox.ac.uk/fsl) and Freesurfer driven by modified HCP Pipelines v3.4.0 (https://github.com/Washington-University/Pipelines/wiki/v3.4.0-Release-Notes,-Installation,-and-Usage). T1w and T2w anatomical images were skull-stripped using the brain extraction tool, bias-corrected to reduce intensity inhomogeneity with FAST (Zhang et al., 2001) and spatially aligned to the Montreal Neurological Institute (MNI) 152 standard space template using 12-degrees-of-freedom affine registration with the FSL linear registration tool (FLIRT) followed by non-linear image registration with FNIRT. Preprocessing included motion correction with MCFLIRT, field inhomogeneity-induced distortion correction using FSL topup software, brain extraction, spatial smoothing using a Gaussian kernel of 5 mm full width at half maximum, and high-pass temporal filtering at 0.01 Hz to remove low-frequency noise. Boundary-based registration was used to align EPI volumes to their respective high-resolution T1-weighted structural images. Registration matrices were combined with non-linear warp coefficients to register EPI volumes onto MNI152 standard space template (Montreal Neurological Institute, Montreal, Canada).
Analyses
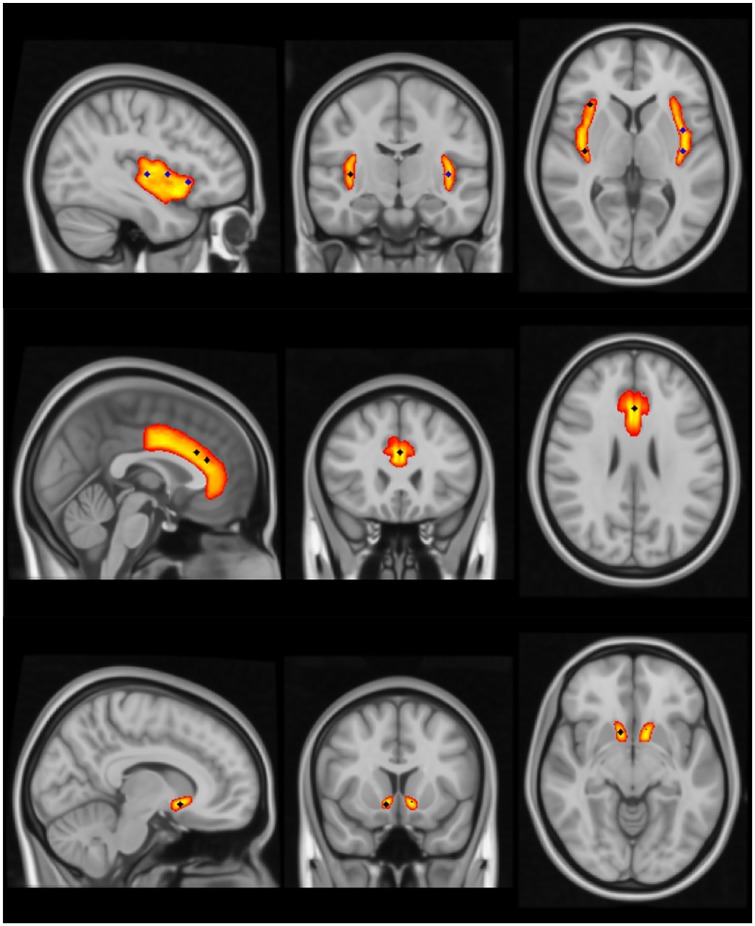
For the rsFC analyses, insula seed voxels were located bilaterally in each of the anterior (right (R): 34, 22, 4; left (L): –38, 18, –2), mid (R: 40, 6, 0; L: –38, 2, 4) and posterior (R: 38, –14, 4; L: –38, –14, 4) sub-regions. Target ROIs were located bilaterally in the NAc (R: 10, 12, –10; L: –10, 14, –8) and centered in the dACC (0, 32, 20 and 0, 24, 26) (Figure 1). The seed and target ROIs were selected a priori based on reports of co-activation of these regions among smokers at rest and evidence that these regions respond to nicotine administration and/or expectancy effects (Cole et al., 2010; Gu et al., 2016; Janes et al., 2015). The insula seed coordinates were based on Gu et al. (2016), with each of the right seeds being modified slightly to improve alignment with the cortical layer in standard MNI152 template space, and to ensure no overlap with cerebrospinal fluid. The coordinates for the dACC targets were replicated directly from those reported in Janes et al. (2015). Since the NAc is a relatively small structure, targets were placed directly at the center of each hemisphere’s defined NAc region (as per the Harvard-Oxford probabilistic atlas in FSL). Each seed (insula) and target (dACC, NAc) location was defined as a 9 mm sphere centered on the coordinates specified above, created using FSLmaths.
Figure 1.
Insula seeds and dorsal anterior cingulate cortex (dACC) and nucleus accumbens (NAc) target regions of interest. Each seed and target location was defined as a 9 mm sphere created using FSLmaths in standard MNI152 template space. Top (left to right): sagittal view of the left posterior (–38, –14, 4), mid (–38, 2, 4), and anterior (–38, 18, –2) insula seeds, coronal view of the right (38, –14, 4) and left posterior insula seeds, and axial view of the right posterior and anterior (34, 22, 4) and left posterior and mid insula seeds. Right mid (40, 6, 0) insula seed location is not visible. Middle: sagittal view of both dACC targets (0, 32, 20 and 0, 24, 26), and coronal and axial views of the more caudal of the dACC targets. Bottom: sagittal, coronal, and axial views of the right NAc (10, 12, –10) target location. Left NAc (–10, 14, –8) target location is also discernable on the coronal and axial NAc views.
First-level (individual run) analyses were performed on spatially normalized data using linear mixed effects modelling implemented in FMRIB’s Improved Linear Model within FSL’s FEAT tool (Woolrich et al., 2001). For each resting state MRI run, multiple first-level analyses were conducted, using the time courses for each seed region of the insula (including anterior, middle, and posterior sub-regions in each of the left and right hemispheres; six seed regions total) as regressors. To control for global patterns in the blood-oxygen-level-dependent signal that could dominate region-specific effects (Fox et al., 2005) we obtained the global time course averaged over the whole brain, as well as time courses averaged over cerebrospinal fluid and white matter separately (each defined and segmented using FSL’s templates). These nuisance time courses were included as additional regressors of no interest in the analysis, as were the six motion correction parameters, to remove any variance associated with these known sources of noise.
Contrast of parameter estimate (COPE) values associated with each seed-ROI pairing were analyzed using a marginal linear model approach in the linear mixed model function of SPSS 24 software (SPSS Inc., Chicago, Illinois, USA). For each seed-ROI pairing, the marginal linear effects model structure was the same, with actual drug condition (given nicotine vs nicotine-free; between-subjects) and perceived drug condition (told nicotine vs nicotine-free; within-subjects) entered as fixed factors, and perceived drug condition as a repeated factor. Participants’ corresponding pre-inhaler COPE values from each session were entered as covariates to control for variability in rsFC unrelated to the experimental manipulation and increase statistical power (Pocock et al., 2002). Model simplicity and likelihood ratio tests were used to determine the appropriate covariance structures. Alpha level was set at 0.05, and the Benjamini-Hochberg method (Benjamini and Hochberg, 1995) was used to determine the false detection rate (FDR) for each ROI-insula sub-region combination. To control for type 1 error, FDRs<0.05 were considered to be reliable, while findings where alpha <0.05 but FDRs>0.05 were considered as trends.
Analyses of the CO and MNWS data followed a similar marginal linear model approach, with actual drug condition and perceived drug condition serving as fixed factors, perceived drug condition as a repeated factor, and individual baseline values from each session as covariates. All data used for the production of this manuscript will be made available upon request.
Results
Sample characteristics
Sample characteristics are presented in Table 1. Participants ranged in age from 19–58 years (mean=32.7, standard deviation (SD)=11.5), smoked an average of 13.2 (SD=5.9) cigarettes per day and received a mean score of 5.3 (SD=1.4) on the FTCD. Thirteen participants (seven male) were assigned to each inhaler condition. Participant characteristics in each inhaler condition were compared using t-tests. No significant differences were evident (p values >0.6). Participants reported inhaler contents consistent with instructions in 90.4% (47/52) of the experimental sessions. Sessions where the participant did not believe the nicotine content instructions (n=1; female participant told nicotine, given nicotine-free) or reported being uncertain about the nicotine content (n=4; two participants (one male) told nicotine-free, given nicotine, and two participants (one male) told nicotine, given nicotine-free) were excluded from the analyses. One additional session was excluded (male participant told nicotine, given nicotine-free) due to a breaking of the blind during the participant’s final session. Overall, data from 88.5% (46/52) of the experimental sessions were retained for analyses.
Table 1.
Sample characteristics.
| Entire sample n=26 | Received nicotine n=13 |
Received placebo n=13 |
T-test (p) | |
|---|---|---|---|---|
| Age (mean; SD) | 32.7 (11.5) | 32.8 (12.1) | 32.6 (11.3) | 0.05 (0.96) |
| Cigarettes per day (mean; SD) | 13.2 (5.9) | 13.7 (5.5) | 12.6 (6.4) | 0.48 (0.64) |
| Fagerstrom Test for Cigarette Dependence score (mean; SD) | 5.3 (1.4) | 5.3 (1.6) | 5.2 (1.2) | 0.14 (0.89) |
| Percentage male | 53.8% | 53.8% | 53.8% |
SD: standard deviation.
Breath CO, withdrawal, and craving
On average, breath CO concentrations were 14.8 ppm (SD=8.8) across conditions at the start of the experimental procedures, which is consistent with recent abstinence. No differences between the inhaler and/or instruction conditions were evident (Actual: F(1,41)=0.17, p=0.684; Perceived: F(1,41)=0.89, p=0.350; Actual×Perceived: F(1,41)=0.50, p=0.483).
Post-inhaler withdrawal ratings averaged 0.57 (SD=0.45) out of a maximum of three, which is consistent with low levels of withdrawal. There were no significant differences between the inhaler and/or instruction conditions in levels of withdrawal (Actual: F(1,25.2)=2.35, p=0.138; Perceived: F(1,21.8)=1.00, p=0.328; Actual×Perceived: F(1,21.5)=0.44, p=0.516).
Post-inhaler craving ratings averaged 1.3 (SD=0.9) out of a maximum of three, suggesting that on average participants experienced mild to moderate levels of cigarette craving at the time of the post-inhaler scan. No significant differences between the inhaler and/or instruction conditions were evident (Actual: F(1,41)=3.43, p=0.071; Perceived: F(1,41) 1.11, p=0.298; Actual×Perceived: F(1,41)=0.57, p=0.453).
Resting state functional connectivity
Insula-dACC
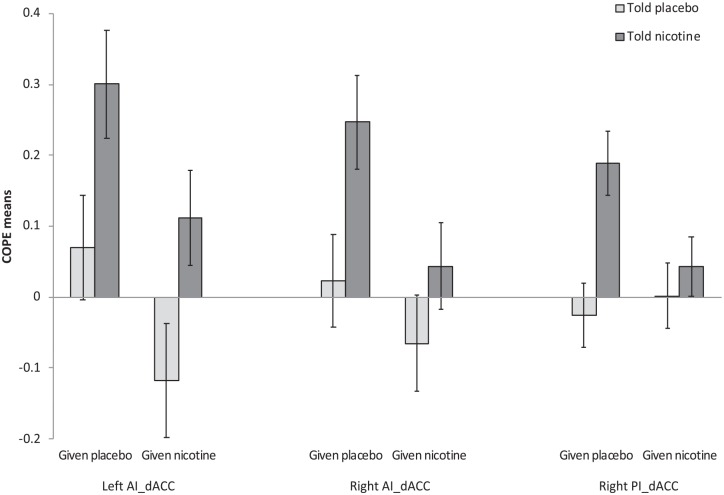
Test statistics involving insula-dACC rsFC are presented in Table 2 and Figure 2. For the more rostral of the dACC ROIs, significant main effects of ‘Actual’ and ‘Perceived’ were found on rsFC with each of the AI seeds (left AI: Actual: F(1,41)=5.92; p=0.019; FDR=0.038; Perceived: F(1,41)=9.32; p=0.004; FDR=0.024; right AI: Actual: F(1,41)=4.92; p=0.032; FDR=0.048; Perceived: F(1,41)=6.37; p=0.016; FDR=0.038) as well as a main effect of ‘Perceived’ for the right posterior seed (F(1,41)=8.14; p=0.007; FDR=0.042). For each ‘Actual’ main effect, receiving nicotine was associated with a significant reduction in rsFC, while for each ‘Perceived’ main effect being told nicotine was associated with increased rsFC. No main effects or interactions were evident for left posterior or either mid-insula seed. No significant effects were evident for the more caudal of the dACC ROIs.
Table 2.
Group-level marginal linear model analyses of resting state functional connectivity (rsFC) between the dorsal anterior cingulate cortex (dACC) and insula.
| ROI | Insula seed | Actual drug | Perceived drug | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Main effect | Main effect | |||||||||
| F | p | FDR | F | p | FDR | F | p | FDR | ||
| Rostral dACC | Anterior right | 4.92 | 0.032 | 0.048a | 6.37 | 0.016 | 0.038a | 0.76 | 0.388 | 0.466 |
| Anterior left | 5.92 | 0.019 | 0.038a | 9.32 | 0.004 | 0.024a | 0.01 | 0.993 | 0.993 | |
| Mid right | 2.38 | 0.131 | 0.262 | 0.02 | 0.900 | 0.900 | 1.83 | 0.184 | 0.276 | |
| Mid left | 3.68 | 0.062 | 0.262 | 1.23 | 0.274 | 0.329 | 2.39 | 0.130 | 0.262 | |
| Posterior right | 1.75 | 0.194 | 0.233 | 8.14 | 0.007 | 0.042a | 3.79 | 0.058 | 0.120 | |
| Posterior left | 3.73 | 0.060 | 0.120 | 2.66 | 0.110 | 0.165 | 1.38 | 0.248 | 0.248 | |
| Mid dACC | Anterior right | 0.54 | 0.469 | 0.743 | 0.25 | 0.619 | 0.743 | 0.27 | 0.607 | 0.743 |
| Anterior left | 0.03 | 0.866 | 0.866 | 0.31 | 0.580 | 0.743 | 1.88 | 0.286 | 0.743 | |
| Mid right | 0.20 | 0.660 | 0.880 | 0.10 | 0.753 | 0.880 | 0.76 | 0.389 | 0.880 | |
| Mid left | 0.02 | 0.880 | 0.880 | 0.03 | 0.867 | 0.880 | 0.37 | 0.551 | 0.880 | |
| Posterior right | 1.43 | 0.239 | 0.564 | 3.90 | 0.055 | 0.330 | 1.19 | 0.282 | 0.564 | |
| Posterior left | 0.29 | 0.594 | 0.891 | 0.01 | 0.954 | 0.954 | 0.07 | 0.794 | 0.953 | |
FDR: false detection rate; ROI: region of interest.
FDR<0.05.
Figure 2.
Mean (±standard error of the mean (SEM)) contrast of parameter estimate (COPE) values associated with changes in resting state functional connectivity (rsFC) between insula seeds and the more rostral of the dorsal anterior cingulate cortex (dACC) regions of interest. Significant main effects of Actual drug and Perceived drug were found for each of the anterior insula (AI) seeds as well as a main effect of Perceived drug for the right posterior insula (PI) seed. In each case, receiving nicotine was associated with a significant reduction in rsFC, while being told nicotine was associated with increased rsFC.
Insula-NAc
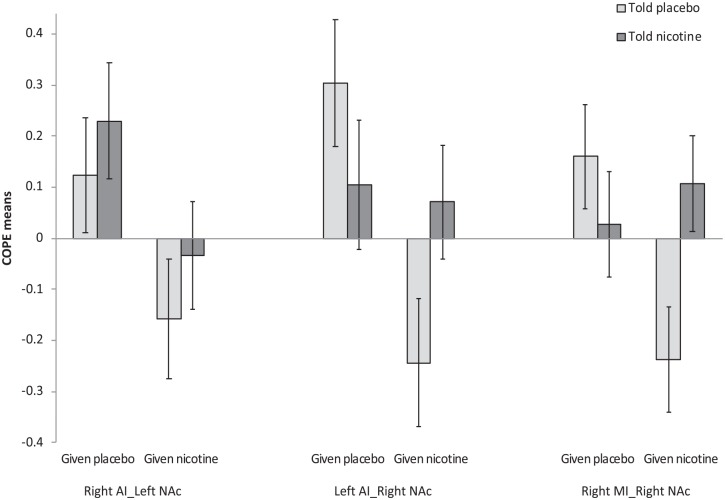
Test statistics involving the insula and NAc are presented in Table 3 and Figure 3. There was evidence of main effects of ‘Actual’ on rsFC between the NAc and contralateral AI seeds. In each case, participants given nicotine displayed reduced rsFC (left AI-right NAc: F(1,20.8)=9.61; p=0.005; FDR=0.03; right AI-left NAc: F(1,41)=5.96; p=0.019; FDR=0.11), although the effect was only present at the trend level for the right AI-left NAc. An Actual×Perceived interaction was also evident at the trend level for rsFC between the right NAc and the right mid insula (F(1,41)=5.68; p=0.022; FDR=0.13). Specifically, there was reduced rsFC when participants were told no nicotine but given nicotine relative to when they were told no nicotine and given no nicotine (F(1,41)=7.566; p=0.009), but no differences were evident between drug conditions when participants were told that they would receive nicotine (F(1,41)=0.33; p=0.571). No other NAc-related main effects or interactions were evident.
Table 3.
Group-level marginal linear model analyses of resting state functional connectivity (rsFC) between the nucleus accumbens (NAc) and insula.
| ROI | Insula seed | Actual drug main effect | Perceived drug main effect | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| F | p | FDR | F | p | FDR | F | p | FDR | ||
| Right NAc | Anterior right | 2.90 | 0.096 | 0.144 | 3.59 | 0.065 | 0.144 | 2.36 | 0.132 | 0.158 |
| Anterior left | 9.61 | 0.005 | 0.030a | 0.15 | 0.702 | 0.702 | 3.09 | 0.092 | 0.144 | |
| Mid right | 2.46 | 0.124 | 0.372 | 1.10 | 0.300 | 0.600 | 5.68 | 0.022 | 0.132 | |
| Mid left | 0.22 | 0.882 | 0.882 | 0.58 | 0.452 | 0.678 | 0.05 | 0.826 | 0.882 | |
| Posterior right | 0.45 | 0.505 | 0.731 | 1.39 | 0.246 | 0.731 | 0.76 | 0.390 | 0.731 | |
| Posterior left | 0.27 | 0.609 | 0.731 | 0.62 | 0.437 | 0.731 | 0.01 | 0.952 | 0.952 | |
| Left NAc | Anterior right | 5.96 | 0.019 | 0.114 | 0.99 | 0.325 | 0.533 | 0.01 | 0.933 | 0.993 |
| Anterior left | 0.87 | 0.355 | 0.533 | 0.52 | 0.474 | 0.569 | 1.17 | 0.287 | 0.553 | |
| Mid right | 0.98 | 0.333 | 0.499 | 3.13 | 0.092 | 0.321 | 2.84 | 0.107 | 0.321 | |
| Mid left | 1.49 | 0.295 | 0.499 | 0.66 | 0.424 | 0.509 | 0.01 | 0.933 | 0.993 | |
| Posterior right | 0.59 | 0.448 | 0.638 | 0.40 | 0.532 | 0.638 | 0.97 | 0.330 | 0.638 | |
| Posterior left | 0.66 | 0.423 | 0.638 | 0.16 | 0.694 | 0.694 | 0.43 | 0.512 | 0.638 | |
FDR: false detection rate; ROI: region of interest.
FDR<0.05.
Figure 3.
Mean (±standard error of the mean (SEM)) contrast of parameter estimate (COPE) values associated with changes in resting state functional connectivity (rsFC) between insula seeds and the nucleus accumbens (NAc). There were significant main effects of Actual drug on rsFC between the NAc and contralateral anterior insula (AI) seeds. In each case, participants given nicotine displayed reduced rsFC. An Actual×Perceived interaction was also apparent for rsFC between the right NAc and the right mid insula (MI). Specifically, there was reduced rsFC when participants were told no nicotine but given nicotine relative to when they were told no nicotine and given no nicotine, but no differences were evident between drug conditions when participants were told that they would receive nicotine.
To determine the potential impacts of changes in subjective withdrawal and craving on the rsFC findings, post-hoc analyses were conducted considering both of these variables as a covariate. Neither was found to be consistently related to our outcomes and their inclusion in analyses led to only minor changes in the magnitude of the reported effects (i.e. all reported ‘Perceived’, ‘Actual’ and ‘Perceived×Actual’ effects remained statistically significant with the inclusion of the covariates). However, findings also revealed that changes in withdrawal and craving appeared to be independently associated with rsFC between sub-regions of the insula and the NAc. Specifically, change in withdrawal was found to be a significant covariate in analyses examining rsFC between the right AI and left NAc (F(1,40)=5.32; p=0.007), as well as between the right mid-insula and right NAc (F(1,40)=9.78; p=0.003), while change in craving was associated with rsFC between the left posterior insula and left NAc (F(1,40)=8.37; p=0.006). Post-hoc correlational analyses revealed that in each case relative decreases in withdrawal (right AI-Left NAc: r=0.291; p=0.05; right mid-insula-right NAc: r=0.354, p=0.016) or craving (left posterior insula-left NAc: r=0.363, p=0.013) were associated with lower levels of rsFC after inhaler administration.
Discussion
This study aimed to examine the relative impacts of actual and perceived nicotine administration on rsFC between sub-regions of the insula and a priori defined ROIs (dACC and NAc) in smokers following 3-hours of smoking abstinence. Findings suggest that both actual and perceived nicotine administration significantly impact rsFC between the insula and dACC as well as between the insula and NAc. As predicted, nicotine administration reduced rsFC between the AI and sub-regions of the dACC and NAc. These effects were independent from changes in subjective withdrawal and craving, and raise the possibility that previous reports of abstinence related increases in rsFC (e.g. Fedota et al., 2018; Sutherland et al., 2013) may be at least in part attributable to nicotinic effects in the non-abstinent comparison conditions. The nicotine administration parameters used in this study were selected to deliver a relatively low and steady dose of nicotine with peak levels being achieved during the scan. In past research, inhalation procedures that have required 33% fewer inhalations of the same nicotine dose, over the same time period, have resulted in plasma levels of 8.1±2.5 µg/L, similar to levels found 30–40 min following the administration of a single regular strength cigarette (Schneider et al., 2001). It is expected that this would be sufficient to result in a significant desensitization of the α4β2/α6β2 nicotinic receptors located in one or more of the seeds/ROIs (Benowitz 2010; Rollema and Hurst, 2018). Interestingly, previous evidence suggests that acute nicotine administration decreases cerebral blood flow (CBF) in the ACC in both smokers and non-smokers alike (Ghatan, 1998) and that tobacco smoking decreases CBF both globally and in the ACC and the ventral striatum (including the NAc) in deprived smokers (Zubieta et al., 2005). Global reductions in cerebral glucose metabolism have also been observed following acute nicotine administration in smokers and non-smokers with region-specific effects in the ACC and insula (Stapleton et al., 2003).
The dACC displayed significantly increased coupling with the right and left AI when participants believed that they had received nicotine. These effects were independent of nicotine administration, craving and withdrawal. This suggests that the observed changes in rsFC may be related to nicotine-related cognitions, rather than to the detection of interoceptive signals. This would be consistent with previous observations that AI-dACC coupling was positively associated with smoking cue reactivity but not with subjective tonic craving levels in minimally deprived smokers (Janes et al., 2015). However, because the AI and dACC are known to be co-activated in response to a variety of affective and cognitive processes (Menon, 2015), the specificity of these effects to nicotine-related cognitions requires additional verification. Perceived nicotine administration was associated with increased rsFC between the right posterior insula and dACC. In contrast with the AI related results, this effect was not observed bilaterally and there was not a significant corresponding decrease in rsFC associated with actual nicotine administration. Interestingly, previous reports have linked smoking/abstinence-related effects with changes in rsFC between the ACC and right, but not left, posterior insula (Cole et al., 2010; Fedota et al 2018). The present observations raise the possibility that such findings may be mediated by non-pharmacological components of nicotine administration and/or abstinence. One relatively unexpected finding was that dACC effects were restricted to the more rostral of the two areas examined. It is possible that more diffuse alterations in AI-dACC coupling would have been observed in the presence of elevated craving and/or withdrawal.
In contrast to the dACC effects, perceived and actual nicotine administration appeared to interact (see Figure 2) to impact rsFC between the right mid-insula and right NAc. Specifically, nicotine administration resulted in a greater reduction in rsFC between these regions when it was unexpected; however, this finding was only present at the trend level. One potential explanation for these findings is that the belief that nicotine has been administered elicits homeostatic processes that lead to a dampened pharmacological response to nicotine in the NAc. In animal models, the contingent delivery of nicotine results in greater nicotinic receptor upregulation in the ventral tegmental area relative to non-contingent delivery of nicotine (Metaxas et al., 2010). Moreover, there is evidence that at least for some drugs, providing contextual cues can dampen acute drug responses (Siegel et al., 1982). Insofar as a similar process is driving the present findings, one might expect some of nicotine’s neural effects to be relatively enhanced during adequately blinded trials.
Neither actual nor perceived nicotine administration was found to significantly alter subjective craving or withdrawal in the present study. Participants reported relatively low levels of craving and withdrawal prior to inhaler administration and it is possible that floor effects contributed to these null findings. There was, however, evidence that within session changes in withdrawal and craving were positively associated with rsFC between the NAc and various sub-regions of insula. Interestingly, Faulkner and colleagues (2019) recently reported that non-nicotine aspects of smoking reduced both craving and rsFC between the NAc and the orbitofrontal cortex in smokers deprived for 12 h, but nicotine administration itself did not significantly impact rsFC between these regions. Taken together with the present results, such findings raise the possibility that the NAc coupling with both the insula and orbitofrontal cortex may be involved in modulating smoking-related motivational processes such as subjective craving and withdrawal even in the absence of direct nicotinic effects. However, it is important to note that both the present study and Faulkner et al. (2019)’s involved the administration of relatively low doses of nicotine, and it is possible that nicotine-specific craving/withdrawal relief would have been observed with higher doses.
Our findings should be interpreted in light of the following considerations. First, participants were only required to abstain from smoking for a minimum of three hours prior to experimental procedures, and although there is evidence that early withdrawal symptoms can begin to emerge within three hours of abstinence in dependent smokers (Brown et al., 2013), such symptoms typically do not peak until 24–72 h of abstinence (Hughes et al., 1994). Additional research is required to determine how perceived and actual nicotine administration impact NAc-related rsFC in the presence of significantly elevated craving and withdrawal. Second, to ensure that the nicotine inhalers would be well tolerated, and that participants’ smoking status would remain stable, the protocol required that participants be daily smokers with no immediate intentions to quit. However, heaviness of smoking and quit intentions have been shown to impact nicotine and expectancy-related responses (Darredeau et al., 2013; Schlagintweit et al., 2017). Thus, it is possible that some of the reported findings may not generalize to all smokers. Next, although reliable nicotine administration and expectancy effects were evident, our sample size was relatively modest, with 26 participants each completing two experimental sessions involving identical inhaler contents but different nicotine content instructions. Because nicotine content instructions served as a within-subject factor, we had greater power to detect perceived than actual drug effects. To maximize statistical power, each participant completed two scans (pre and post inhaler administration) during each session, and participants’ pre-inhaler rsFC COPE values were included as covariates in the analyses (Pocock et al., 2002). However, it remains possible that additional main effects or interactions would have been observed with a larger sample size, or through the use of a full factorial design. Further, the present study was underpowered to examine sex differences, which are important to consider when evaluating drug and placebo responses. There is evidence to suggest that men may be more sensitive to the reinforcing effects of nicotine relative to women (Cosgrove et al., 2014; Faulkner et al., 2018; Perkins et al., 1999; Perkins and Karelitz 2015) but that women may be more sensitive to expectancy-related effects relative to men (Darredeau et al., 2013; Perkins et al., 2006). It is possible that sex differences also exist in rsFC associated with actual and perceived nicotine administration, and that a larger sample size would generate adequate statistical power to evaluate such differences. Finally, the seed-based approach to analyzing the MRI data allowed for targeting connectivity with specific seed-to-target ROIs. Although our seeds/ROIs were derived from the literature, it is likely that the exact coordinates selected did not correspond with the specific areas most impacted by our manipulations. Moreover, our analytic approach did not allow for the evaluation of inter-network correlations, and there is growing evidence that acute nicotine and/or abstinence can impact insula rsFC with a larger set of salience and default mode networks (e.g. Fedota et al., 2018). The extent to which such network-wide systems are impacted by the belief that nicotine had been administered remains undetermined.
In conclusion, our findings demonstrate that both perceived and actual nicotine administration can impact rsFC between the insula and the dACC and NAc independently from subjective withdrawal. Because perceived nicotine administration can alter both the magnitude and nature of nicotine-related neural responses, it is important that studies adequately assess and control for participants’ perceptions about their assigned drug conditions in order to make valid inferences about neuropharmacological actions.
Acknowledgments
The following author contributions were made: conception of study: SB, HS; acquisition of funding: SB, KG, HS; study design: SB, CD, KG, RP, HS; participant screening and recruitment: RP; acquisition of data: CH, RP; data analyses: SB, CD, CH, AN, RP, HS; manuscript preparation and approval: SB, CD, KG, CH, AN, RP, HS.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by funding from the Natural Sciences and Engineering Research Council of Canada (S Barrett), the Nova Scotia Health Authority (S Barrett and K Good) and the Nova Scotia Health Research Fund (S Barrett, K Good and H Schlagintweit).
ORCID iD: Sean P Barrett  https://orcid.org/0000-0002-9495-8265
https://orcid.org/0000-0002-9495-8265
References
- Abdolahi A, Williams GC, Benesch CG, et al. (2015) Damage to the insula leads to decreased nicotine withdrawal during abstinence. Addiction 110: 1994–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Publishing. [Google Scholar]
- Barrett SP. (2010) The effects of nicotine, denicotinized tobacco, and nicotine-containing tobacco on cigarette craving, withdrawal, and self-administration in male and female smokers. Behav Pharmacol 21: 144–152. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol 57: 287–300. [Google Scholar]
- Benowitz NL. (2010) Nicotine addiction. N Engl J Med 362: 2295–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Hajek P, McRobbie H, et al. (2013) Cigarette craving and withdrawal symptoms during temporary abstinence and the effect of nicotine gum. Psychopharmacology 229: 209–218. [DOI] [PubMed] [Google Scholar]
- Cole DM, Beckmann CF, Long CJ, et al. (2010) Nicotine replacement in abstinent smokers improves cognitive withdrawal symptoms with modulation of resting brain network dynamics. NeuroImage 52: 590–599. [DOI] [PubMed] [Google Scholar]
- Cosgrove KP, Wang S, Kim SJ, et al. (2014) Sex differences in the Brain’s dopamine signature of cigarette smoking. J Neurosci 34: 16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darredeau C, Stewart SH, Barrett SP. (2013) The effects of nicotine content information on subjective and behavioural responses to nicotine-containing and denicotinized cigarettes. Behav Pharmacol 24: 291–297. [DOI] [PubMed] [Google Scholar]
- de la Fuente-Fernández R, Ruth TJ, Sossi V, et al. (2001) Expectation and dopamine release: Mechanism of the placebo effect in Parkinson’s disease. Science 293: 1164–1166. [DOI] [PubMed] [Google Scholar]
- Fagerström KO. (2012) Determinants of tobacco use and renaming the FTND to the Fagerstrom test for cigarette dependence. Nic Tob Res 14: 75–78. [DOI] [PubMed] [Google Scholar]
- Faulkner P, Ghahremani DG, Tyndale RF, et al. (2019) Neural basis of smoking-induced relief of craving and negative affect: Contribution of nicotine. Addict Biol 24: 1087–1095 . [DOI] [PubMed] [Google Scholar]
- Faulkner P, Petersen N, Ghahremani DG, et al. (2018) Sex differences in tobacco withdrawal and responses to smoking reduced-nicotine cigarettes in young smokers. Psychopharmacology 235: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedota JR, Ding X, Matous AL, et al. (2018) Nicotine abstinence influences the calculation of salience in discrete insular circuits. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, et al. (2005) The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A 102: 9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaznick N, Tranel D, McNutt A, et al. (2013) Basal ganglia plus insula damage yields stronger disruption of smoking addiction than basal ganglia damage alone. Nic Tob Res 16: 445–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghatan PH, Ingvar M, Eriksson L, et al. (1998) Cerebral effects of nicotine during cognition in smokers and non-smokers. Psychopharmacology 136: 179–189. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, et al. (2013) The minimal preprocessing pipelines for the human connectome project. Neuroimage 80: 105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Lohrenz T, Salas R, et al. (2016) Belief about nicotine modulates subjective craving and insula activity in deprived smokers. Front Psychiatry 7: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami DK. (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43: 289–294. [DOI] [PubMed] [Google Scholar]
- Hughes J, Hatsukami DK. (1998) Errors in using tobacco withdrawal scale. Tob Control 7: 92–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR, Higgins ST, Bickel WK. (1994) Nicotine withdrawal versus other drug withdrawal syndromes: Similarities and dissimilarities. Addiction 89: 1461–1470. [DOI] [PubMed] [Google Scholar]
- Janes AC, Farmer S, Peechatka AL, et al. (2015) Insula-dorsal anterior cingulate cortex coupling is associated with enhanced brain reactivity to smoking cues. Neuropsychopharmacology 40: 1561–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, et al. (2001) FSL. Neuroimage 62: 782–790. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Brandon TH. (2002) Effects of nicotine dose, instructional set, and outcome expectancies on the subjective effects of smoking in the presence of a stressor. J Abnorm Psychol 111: 88–97. [DOI] [PubMed] [Google Scholar]
- Menon V. (2015) Salience network. In: AW Toga. (ed.) Brain Mapping: An Encyclopedic Reference. Academic Press: Elsevier, vol; 2, pp. 597–611. [Google Scholar]
- Metaxas A, Bailey A, Barbano M, et al. (2010) Differential region-specific regulation of alpha 4 beta 2nAChRs by self-administered and non-contingent nicotine in C57BL/6J mice. Addict Biol 15: 464–479. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Gaznick N, Tranel D, et al. (2014) The insula: A critical neural substrate for craving and drug seeking under conflict and risk. Ann N Y Acad Sci 1316: 53–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi NH, Rudrauf D, Damasio H, et al. (2007) Damage to the insula disrupts addiction to cigarette smoking. Science 315: 531–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashmi R, Lester HA. (2006) CNS localization of neuronal nicotinic receptors. J Mol Neurosci 30: 181–184. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Donny E, Caggiula AR. (1999) Sex differences in nicotine effects and self-administration: Review of human and animal evidence. Nic Tob Res 1: 301–315. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Doyle T, Ciccocioppo M, et al. (2006) Sex differences in the influence of nicotine dose instructions on the reinforcing and self-reported rewarding effects of smoking. Psychopharmacology 2006; 184: 600–607. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL. (2015) Sex differences in acute relief of abstinence-induced withdrawal and negative affect due to nicotine content in cigarettes. Nic Tob Res 2015; 17: 443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Sadaghiani S, Leroy C, et al. (2013) High density of nicotinic receptors in the cingulo-insular network. NeuroImage 79: 42–51. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, et al. (2008) It is not “either/or”: Activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Prog Neurobiol 84: 329–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pocock SJ, Assmann SE, Enos LE, et al. (2002) Subgroup analysis, covariate adjustment and baseline comparisons in clinical trial reporting: Current practice and problems. Statist Med 21: 2917–2930. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hurst R. (2018) The contribution of agonist and antagonist activities of α4β2 nAChR ligands to smoking cessation efficacy: A quantitative analysis of literature data. Psychopharmacology 235: 2479–2505. [DOI] [PubMed] [Google Scholar]
- Schlagintweit HE, Campbell NK, Barrett SP. (2017) Quit intentions moderate subjective and physiological responses to acute nicotine replacement therapy administration in dependent smokers. Nic Tob Res 19: 922–929. [DOI] [PubMed] [Google Scholar]
- Schlagintweit HE, Good KP, Barrett SP. (2014) The impact of nicotine lozenges and stimulus expectancies on cigarette craving. J Psychopharmacol 28: 773–779. [DOI] [PubMed] [Google Scholar]
- Schneider NG, Olmstead RE, Franzon MA, et al. (2001) The nicotine inhaler: Clinical pharmacokinetics and comparison with other nicotine treatments. Clin Pharmacokinet 40: 661–684. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, et al. (2008) Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Arch Gen Psychiatry 65: 220–231. [DOI] [PubMed] [Google Scholar]
- Siegel S, Hinson RE, Krank MS, et al. (1982) Heroin “overdose” death: Contribution of drug-associated environmental cues. Science 216: 436–437. [DOI] [PubMed] [Google Scholar]
- Simmons WK, Rapuano KM, Kallman SJ, et al. (2013) Category-specific integration of homeostatic signals in caudal but not rostral human insula. Nat Neurosci 16: 1551–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton JM, Gilson SF, Wong DF, et al. (2003) Intravenous nicotine reduces cerebral glucose metabolism: A preliminary study. Neuropsychopharmacology 28: 765–772. [DOI] [PubMed] [Google Scholar]
- Sutherland MT, Carroll AJ, Salmeron BJ, et al. (2013) Down-regulation of amygdala and insula functional circuits by varenicline and nicotine in abstinent cigarette smokers. Biol Psychiatry 74: 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MT, McHugh MJ, Pariyadath V, et al. (2012) Resting state functional connectivity in addiction: Lessons learned and a road ahead. Neuroimage 62: 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton SR. (1991) Great expectations: Some suggestions for applying the balanced placebo design to nicotine and smoking. Br J Addict 86: 659–662. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Geier CF, Addicott MA, et al. (2016) Smoking abstinence-induced changes in resting state functional connectivity with ventral striatum predict lapse during a quit attempt. Neuropsychopharmacology 41: 2521–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Ripley BD, Brady JM, et al. (2001) Temporal autocorrelation in univariate linear modelling of FMRI data. NeuroImage 14: 1370–1386. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. (2001) Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm (2001). IEEE Trans Med Image 20: 45–57. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Heitzeg MM, Xu Y, et al. (2005) Regional cerebral blood flow responses to smoking in tobacco smokers after overnight abstinence. Am J Psychiatry 162: 567–577. [DOI] [PubMed] [Google Scholar]