Abstract
We investigated the novel combination of dose-dense pemetrexed, gemcitabine and bevacizumab as frontline treatment for advanced non–small cell lung cancer. Thirty-nine patients received pemetrexed (400 mg/m2), gemcitabine (1200 mg/m2), and bevacizumab (10 mg/kg), given every 14 days. Median progression-free survival was 6.1 months (95% confidence interval [CI], 4.2–7.9) and median overall survival was 18.4 months (95% CI, 13.1–29.5). Treatment met the primary endpoint and represents a reasonable therapeutic option.
Introduction:
Platinum-based chemotherapy is standard for untreated, advanced non-small-cell lung cancer (NSCLC). We investigated the activity and tolerability of the novel combination of dose-dense pemetrexed, gemcitabine, and bevacizumab in patients with advanced NSCLC.
Methods:
This multicenter phase II trial evaluated the safety and efficacy of the combination of pemetrexed (400 mg/m2), gemcitabine (1200 mg/m2), and bevacizumab (10 mg/kg), given every 14 days in patients with untreated, advanced NSCLC. The primary endpoint was progression-free survival with secondary endpoints of response rate and overall survival.
Results:
Thirty-nine patients were enrolled. Treatment was well tolerated; the most common grade 3–4 toxicities were neutropenia and fatigue. Of the 38 patients evaluable for tumor response, 1 (3%) had complete response, 15 (39%) had partial response, 12 (31%) had stable disease, and 10 (26%) had progressive disease. Median progression-free survival was 6.1 months (95% confidence interval [CI], 4.2–7.9) and median overall survival was 18.4 months (95% CI, 13.1–29.5). The 1-year overall survival rate was 64% (95% CI, 51%−81%) and the 2-year overall survival rate was 41% (95% CI, 28%−60%).
Conclusions:
Treatment with dose-dense pemetrexed, gemcitabine, and bevacizumab met the primary endpoint with promising efficacy and a manageable safety profile in patients with untreated advanced NSCLC. This regimen represents a reasonable therapeutic option.
Keywords: Adenocarcinoma of the lung, Dose-dense chemotherapy, Frontline chemotherapy, Nonplatinum, VEGF inhibition
Introduction
Platinum-based chemotherapy remains the standard of care for patients with advanced NSCLC; however, no chemotherapy doublet has emerged as superior. Gemcitabine and pemetrexed have significant efficacy in nonsquamous NSCLC and a superior toxicity profile compared with other agents (ie, cisplatin, taxanes). The favorable tolerability allows a “dose-dense” approach that may improve efficacy without intolerable side effects. Although dose-dense regimens have shown clinical benefit in other tumors, such as breast cancer, the benefit in NSCLC remains unproven.1 In vitro models have demonstrated synergy between gemcitabine and pemetrexed, and a phase I study in patients with advanced cancer recommended pemetrexed 500 mg/m2 and gemcitabine 1500 mg/m2 every 2 weeks for further study.2,3
Previous studies have demonstrated safety and clinical benefit when bevacizumab was added to both pemetrexed- and gemcitabine-containing regimens.4,5 In a meta-analysis that included 2194 patients, chemotherapy plus bevacizumab significantly increased both overall survival and progression-free survival compared with chemotherapy alone.6 We conducted a phase II trial of gemcitabine, pemetrexed, and bevacizumab on an every 2 weeks schedule in chemo-naïve patients with advanced NSCLC.
Patients and Methods
Eligibility
Patients with untreated stage IIIB (pleural/pericardial effusion) or IV NSCLC were eligible. Squamous cell was initially allowed if the primary tumor had been removed before the pemetrexed restriction for use in patients with nonsquamous histology. Other requirements included age ≥ 18, Zubrod performance status (PS) 0 to 1, absolute neutrophil count (ANC) ≥ 1500/mm3, platelet count ≥ 100,000/mm3, createnine clearance ≥ 45 mL/min, total bilirubin ≤ 1.5 times the institutional upper limit of normal (ULN) and serum glutamic oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) ≤ 3.0 times ULN. Patients were ineligible if they had uncontrolled hypertension, unstable angina, recent myocardial infarction, or grade ≥ 3 hemorrhage, any history of gross hemoptysis, untreated central nervous system metastases, or required therapeutic anticoagulation. The trial was approved by the local institutional review boards and signed written informed consent was obtained from all patients.
Treatment
Patients received pemetrexed 400 mg/m2 intravenously over 10 minutes plus gemcitabine 1200 mg/m2 over 30 minutes following pemetrexed on day 1 of a 14-day cycle. Doses were originally 500 mg/m2 and 1500 mg/m2; however, grade 4 neutropenia in the first 2 patients (included in the final analysis) mandated a dose modification for subsequent patients. Bevacizumab 10 mg/kg was given intravenously after the chemotherapy every 2 weeks. Pemetrexed/gemcitabine treatment continued until disease progression or unacceptable toxicity for a maximum of 24 weeks. Bevacizumab was continued as maintenance therapy every 2 weeks until disease progression or unacceptable toxicity. Growth factors were not routinely used but were allowed at the discretion of the treating physician.
Dose Adjustment for Toxicity
Patients who developed grade 4 hematologic toxicity or grade 3/4 nonhematologic toxicity had pemetrexed and gemcitabine held until resolution to grade ≤ 2 or grade ≤ 1, respectively. Pemetrexed and gemcitabine were then restarted at a 25% dose reduction. Bevacizumab dose was not reduced, but was held for grade 3 and discontinued for grade 4 proteinuria, hypertension, hemorrhage, or venous thrombosis. The start of the next cycle could be delayed up to 4 weeks if additional time was required for recovery from treatment-associated toxicity.
Assessment of Response and Toxicity
Patients were considered evaluable for toxicity and response if they received at least 1 dose of pemetrexed, gemcitabine, bevacizumab. Scans were performed every 3 cycles (6 weeks) and response was assessed according to Response Evaluation Criteria In Solid Tumors criteria.7 Treatment was discontinued if a patient required a second dose reduction, developed progressive disease, or had life-threatening, irreversible or unacceptable toxicity. Toxicity was graded according to the National Cancer Institute Common Toxicity Criteria v3.0 (http://ctep.cancer.gov/reporting/ctc.html).
Statistical Analyses
The primary endpoint for the study was progression-free survival (PFS), defined as the time from the first day of treatment until the date of progressive disease, symptomatic deterioration, or death due to any cause. A single-arm and single-stage design was used to test (with 80% power and 1-sided type I error of 0.05) that patients have a median PFS of at least 6 months, in contrast to an assumed historical value of 4 months. The required sample size of 39 patients was determined under the assumption of exponential PFS times, and via the method of Lawless.8
Secondary endpoints were response rate, toxicity, and overall survival (OS). OS was defined as the time from the first day of therapy to the date of death. Point and exact confidence interval estimates of the response rate and point estimates of grade 3 or 4 toxicity were calculated. All time-to-event endpoints (PFS and OS) were estimated using Kaplan-Meier (KM) methods. All the statistical tests are 2 sided at a α = 0.05 and P values were not adjusted for multiple testing. R Survival package was used for KM estimates of time-to-event endpoints and the plot. The SAS system (SAS, Inc, Cary, NC) was used for all other analyses.
Results
Thirty-nine patients were enrolled at 2 sites between September 2006 and August 2010. Patient characteristics are summarized in Table 1. The median number of cycles of pemetrexed/gemcitabine/bevacizumab received was 7 (range, 1–12). Nine patients continued bevacizumab as maintenance therapy for a median of 4 cycles (range, 1–52).
Table 1.
Patient Characteristics
| Age | |
| Median | 62.0 |
| Range | 41–82 |
| Gender, n (%) | |
| Female | 17 (43.6) |
| Male | 22 (56.4) |
| Performance status, n (%) | |
| 0 | 17 (43.6) |
| 1 | 22 (56.4) |
| Stage, n (%) | |
| IIIB | 5 (12.8) |
| IV | 34 (87.2) |
| Brain metastases, n (%) | |
| No | 35 (89.7) |
| Yes | 4 (10.3) |
| Histology, n (%) | |
| Squamous | 1 (2.6)a |
| Nonsquamous | 38 (97.4) |
| Nonsquamous detail, n (%) | |
| Adenocarcinoma | 32 (82.0) |
| Large cell | 2 (5.1) |
| Bronchioloalveolar | 1 (2.6) |
| NSCLC, NOS | 3 (7.7) |
Abbreviation: NSCLC, NOS = non–small-cell lung cancer, not otherwise specified.
The study allowed squamous cell if the primary tumor had been resected.
Toxicity
All 39 patients were evaluable for toxicity analysis. Disease progression was the main reason for discontinuation of treatment (n = 23, 59%). Thirteen patients (33%) discontinued therapy due to toxicity: grade 4 bowel perforation (n = 1), grade 4 neutropenia (n = 2), grade 3 hypertension (n = 2), persistent grade 1 nausea (n = 1), grade 3 alanine transaminase (ALT) elevation (n = 2), persistent grade 2 neuropathy/ataxia/vertigo (n = 3), persistent grade 2 edema (n = 1), and grade 3 retinal hemorrhage (n = 1). Three patients were removed due to symptom progression with no evidence of disease progression.
Toxicities are listed in Table 2. The most common grade 3–4 toxicities were neutropenia (28%), hyperglycemia from dexamethasone pretreatment (23%), fatigue (18%), and ALT elevation (10%). One patient had grade 4 thrombocytopenia, with no bleeding. Eight patients (21%) required a 25% dose reduction of pemetrexed/gemcitabine due to toxicity during cycle 1. One patient required a second dose reduction after cycle 2.
Table 2.
Treatment-related Toxicity
| Type of Adverse Reaction | Grade 3–4 | |
|---|---|---|
| n | (%) | |
| Hematologic | ||
| Neutropenia | 11 | 28 |
| Leukopenia | 3 | 8 |
| Anemia | 1 | 3 |
| Thrombocytopenia | 1 | 3 |
| Febrile neutropenia | 1 | 3 |
| Nonhematologic | ||
| Elevated alanine transaminase/ aspartate transaminase | 4 | 10 |
| Acute renal insufficiency | 1 | 3 |
| Anorexia | 2 | 5 |
| Thrombosis/embolism | 3 | 8 |
| Dehydration | 1 | 3 |
| Fatigue | 7 | 18 |
| Hyperglycemia | 9 | 23 |
| Hypertension | 2 | 5 |
| Nausea/vomiting | 1 | 3 |
| Bowel perforation | 1 | 3 |
| Dyspnea | 4 | 10 |
| Diverticulitis | 2 | 5 |
| Ataxia | 1 | 3 |
Response and Survival
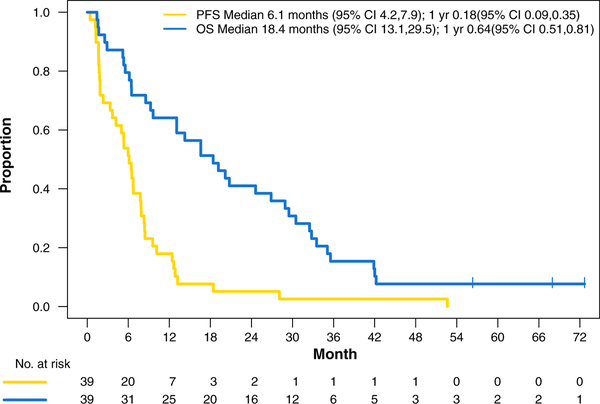
Thirty-eight patients were assessable for response. One patient was removed due to decline in performance status before repeat imaging. The response rate was 42% (95% confidence interval [CI], 0.26–0.59) with 1 (3%) complete response and 15 (39%) partial responses; 12 (31%) patients had stable disease and 10 (26%) had progressive disease, for a disease control rate of 74%. The median PFS was 6.1 months (95% CI, 4.2–7.9) and the median OS was18.4 months (95% CI, 13.1–29.5) (Figure 1). The 1-year OS rate was 64% (95% CI, 51%−81%) and the 2-year OS rate was 41% (95% CI, 28%−60%). Twenty of the 39 patients received second-line therapy. Seven received erlotinib, 3 received docetaxel, and the remaining 10 received other single-agent chemotherapy.
Figure 1.
Intent-to-treat Kaplan-Meier Estimates for Progression-free and Overall Survival
Discussion
The combination of pemetrexed, gemcitabine, and bevacizumab given on an every 2 weeks schedule was well tolerated with manageable toxicity in this single-arm, phase II study. The regimen increased median PFS beyond 6 months and the response rate of 42% was encouraging. The median OS of 18.4 months compared favorably with that achieved with other first-line regimens. Three patients remain alive with disease control.
Dose-dense chemotherapy (decreasing the interval between treatment cycles) has been sparsely evaluated in NSCLC. Tumor cell growth is typically characterized by an initially rapid proliferation of cancer cells, then a slowing of the doubling rate as the tumor increases in size.9 Reducing the interval between chemotherapy cycles could theoretically enhance tumor cell kill by inhibiting the rapid growth phase.10 A trial of first-line, dose-dense cisplatin 75 mg/m2 and docetaxel 75 mg/m2 given every 2 weeks for 4 cycles demonstrated a response rate of 53% and median OS of 11 months.11 Unfortunately, significant toxicity, including nausea, dehydration, and neuropathy, limited further development of this regimen. Spigel et al12 randomized 110 elderly patients (age ≥ 70 years) to pemetrexed 500 mg/m2/gemcitabine 1500 mg/m2/bevacizumab 10 mg/kg every 2 weeks or pemetrexed/carboplatin/bevacizumab. The 55 patients in the pemetrexed/gemcitabine/bevacizumab arm received a median of 2.5 cycles of therapy, and 33% required dose reductions, mostly due to fatigue and neutropenia. The response rate was 35% and median time to progression was 4.7 months with a median OS of 7.5 months. Almost 25% of the patients discontinued treatment because of significant toxicities, and the lack of significant survival benefit was likely related to the limited amount of drug received.
Our trial began with “full-dose” gemcitabine (1500 mg/m2) plus pemetrexed (500 mg/m2) every 2 weeks per the phase I trial of this combination. Grade 4 neutropenia forced a dose reduction for the duration of the study. It is possible that the addition of bevacizumab contributed to this unexpected toxicity. This would be consistent with the findings of E4599, which evaluated carboplatin and paclitaxel with or without bevacizumab.13 Five patients in the carboplatin-paclitaxel-bevacizumab group had grade 5 febrile neutropenia. In addition, 17% of the patients in the chemotherapy-alone group had grade 4 neutropenia versus 26% with chemotherapy plus bevacizumab. The reason for increased neutropenia from chemotherapy plus bevacizumab remains unknown.
In conclusion, our trial demonstrated an improvement in PFS with dose-dense gemcitabine/pemetrexed/bevacizumab in patients with nonsquamous NSCLC compared with historical controls. Median OS was also encouraging, but could have been prolonged due to the use of subsequent therapies. This regimen was well tolerated and is a reasonable treatment alternative if a nonplatinum regimen is required for patients with either intolerance or hypersensitivity to platinum therapy.
Clinical Practice Points
Platinum-based chemotherapy is the standard frontline treatment for patients with metastatic non–small-cell lung cancer (NSCLC) without actionable driver mutations. Although cisplatin or carboplatin are commonly used, patient intolerance, renal toxicity, peripheral neuropathy, and allergic reactions may limit use of these agents. There are limited data on the use of nonplatinum regimens plus bevacizumab for advanced NSCLC. This study used a combination of gemcitabine, pemetrexed, and bevacizumab for patients with untreated, metastatic NSCLC. The regimen was well tolerated with a manageable safety profile and no new safety signals were identified with the combination of these agents. The trial demonstrated an overall response rate of 42%, a median progression-free survival of 6.1 months and a median overall survival of 18.4 months. These results compare very favorably with standard frontline, platinum-based regimens. Therefore, pemetrexed, gemcitabine, and bevacizumab could be considered as an initial treatment strategy for patients with metastatic nonsquamous NSCLC unable to receive platinum compounds.
Acknowledgments
Eli Lilly and Company and Genentech, Inc. provided funding/drugs.
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Presented in part at the 45th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, June 2009.
References
- 1.Citron ML, Berry DA, Cirrincione C, et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003; 21:1431. [DOI] [PubMed] [Google Scholar]
- 2.Adjei AA. Gemcitabine and pemetrexed disodium combinations in vitro and in vivo. Lung Cancer 2001; 34(suppl 4):S103–5. [DOI] [PubMed] [Google Scholar]
- 3.Adjei AA, Erlichman C, Sloan JA, et al. Phase I and pharmacologic study of sequences of gemcitabine and the multitargeted antifolate agent in patients with advanced solid tumors. J Clin Oncol 2000; 18:1748–57. [DOI] [PubMed] [Google Scholar]
- 4.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009; 27:1227–34. [DOI] [PubMed] [Google Scholar]
- 5.Patel JD, Socinski MA, Garon EB, et al. PointBreak: a randomized phase III study of pemetrexed plus carboplatin and bevacizumab followed by maintenance pemetrexed and bevacizumab versus paclitaxel plus carboplatin and bevacizumab followed by maintenance bevacizumab in patients with stage IIIB or IV nonsquamous non-small-cell lung cancer. J Clin Oncol 2013; 31: 4349–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soria JC, Mauguen A, Reck M, et al. Systematic review and meta-analysis of randomised, phase II/III trials adding bevacizumab to platinum-based chemotherapy as first-line treatment in patients with advanced non-small-cell lung cancer. Ann Oncol 2013; 24:20–30. [DOI] [PubMed] [Google Scholar]
- 7.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst 2000; 92:205–16. [DOI] [PubMed] [Google Scholar]
- 8.Lawless J Statistical Models and Methods for Lifetime Data. New York, NY: Wiley and Sons; 1982:108. [Google Scholar]
- 9.Norton L A Gompertzian model of human breast cancer growth. Cancer Res 1988; 48:7067–71. [PubMed] [Google Scholar]
- 10.Norton L, Simon R. Tumor size, sensitivity to therapy, and design of treatment schedules. Cancer Treat Rep 1977; 61:1307–17. [PubMed] [Google Scholar]
- 11.Miller AA, Wang XF, Gu L, et al. Phase II randomized study of dose-dense docetaxel and cisplatin every 2 weeks with pegfilgrastim and darbepoetin alfa with and without chemo-protector BNP7787 in patients with advanced non-small cell lung cancer (CALGB 30303). J Thorac Oncol 2008; 3:1159–65. [DOI] [PubMed] [Google Scholar]
- 12.Spigel DR, Hainsworth JD, Shipley DL, et al. A randomized phase II trial of pemetrexed/gemcitabine/bevacizumab or pemetrexed/carboplatin/bevacizumab in the first-line treatment of elderly patients with advanced non-small cell lung cancer. J Thorac Oncol 2012; 7:196–202. [DOI] [PubMed] [Google Scholar]
- 13.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355:2542–50. [DOI] [PubMed] [Google Scholar]