Abstract
Background.
Although tri-modality therapy is an acceptable standard of care in patients with locally advanced esophageal cancer, data regarding patterns of failure is lacking. We report bi-institutional patterns of failure experience treating patients using tri-modality therapy.
Materials and methods.
We retrospectively reviewed patients who underwent chemoradiation followed by esophagectomy between 2006 and 2011 at two NCI-designated cancer centers. First failure sites were categorized as local, regional nodal, or distant. Statistical analysis was performed using Fisher’s exact test, non-parametric Wilcoxon rank-sum test, and multiple logistic regression. Kaplan-Meier curves were generated for relapse-free survival (RFS) and overall survival.
Results.
A total of 132 patients met the inclusion criteria with a median age of 62 (range 36–80) and median follow-up of 28 months (range 4–128). There were a total of six (4.5%) local, 13 (10%) regional nodal, and 32 (23.5%) distant failures. Local failure was correlated with fewer lymph nodes (LN) assessed (p = 0.01) and close/positive margins (p<0.01). Regional nodal failure was correlated with fewer LN assessed (p<0.01) and larger pretreatment tumor size (p = 0.04). Patients with ≤13 LN evaluated had an inferior locoregional RFS versus patients with >13 LN evaluated (p = 0.003). Distant recurrence was correlated with higher pathologic nodal stage (p<0.001), ulceration (p = 0.017), perineural invasion (p = 0.029), residual disease (p = 0.004), and higher post-treatment PET SUV max (p = 0.049). Patients with a pathologic complete response (OR 0.19, 95% CI 0.05–0.68) were less likely to experience distant recurrence.
Conclusion.
Tumor and treatment factors may predict for failure in patients undergoing tri-modality therapy for locally advanced esophageal cancer. Further data is needed to identify patterns of failure in these patients.
Tri-modality therapy, consisting of neoadjuvant chemoradiation followed by surgery, is the preferred treatment for patients with locally advanced esophageal cancer [1]. Randomized trials have demonstrated a survival benefit with the addition of neoadjuvant chemoradiation versus esophagectomy alone [2,3]. As a result, there has been a significant increase in the utilization of tri-modality therapy across the US with a subsequent improvement in overall survival (OS) in this cohort of patients [4].
Despite significant advances in treatment techniques, the OS for patients with esophageal cancer remains poor with only 20% of patients alive at five years [5]. Identifying means of augmenting therapy remains an important aspect of managing esophageal cancer. One important step in optimizing therapy involves identifying factors which may predict for more aggressive disease biology or an increased risk of failure. Tailoring therapy to address increased risk for these patients may allow for improved locoregional and distant control and a subsequent improvement in disease-free and OS.
Identifying predictors of local, regional, and distant recurrence may uncover opportunities for targeted intensification of therapy in patients with poor prognostic factors. There is limited data regarding patterns and predictors of failure in patients undergoing tri-modality therapy for esophageal cancer. The primary objective of this study was to identify the patterns of failure in patients undergoing tri-modality therapy. The secondary objective of this study was to identify factors predictive for local, distant, and regional recurrence.
Material and methods
Following IRB approval, we identified all patients with locally advanced esophageal cancer (T1N1 or T2–4Nany) undergoing neoadjuvant chemoradiation followed by esophagectomy for esophageal cancer at two NCI-designated cancer centers between 2006 and 2011. Exclusion criteria were surgery or radiation at an outside facility or patients who did not complete tri-modality therapy. Patients with incomplete treatment information were not included in the analysis.
All patients were staged pre- and post-operatively according to the tumor-node-metastasis classification of the American Joint Committee for Cancer Staging seventh edition [6]. Pretreatment clinical staging routinely included computed tomography (CT) scan, esophagogastroduodenoscopy and biopsy, bronchoscopy, endoscopic ultrasound (EUS) and positron emission tomography (PET) scan. Patients with no residual, viable tumor cells in the surgical specimen (ypT0N0M0) were classified as having a pathologic complete response (pCR), all other patients were considered to have residual disease. A close margin was defined as tumor within 3 cm of the specimen edge [7]. Baseline data collected included general patient characteristics (age, gender, diagnosis date, pathology), treatment characteristics (type of chemotherapy, radiation dose, type of surgery), toxicity, and tumor recurrence. Follow-up data was obtained from patient medical records, referring physicians, and telephone interviews.
The radiation treatment technique was at the discretion of the treating radiation oncologist. For radiation treatment planning, patients typically underwent CT simulation with IV and oral contrast, supine in an immobilization cast. Most patients underwent four-dimensional (4D) CT to account for respiratory motion. Radiation treatment was delivered via 3D-conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT). 3D-CRT treatment was typically delivered via a 3–4 beam arrangement at the discretion of the treating radiation oncologist. IMRT was typically delivered using 7–9 step and shoot fields.
The target volume consisted of the primary tumor plus any involved lymph nodes. The gross tumor volume (GTV) consisted of the gross tumor and involved lymph nodes as identified on imaging studies and delineated per the endoscopy report. In general, for patients with gastroesophageal (GE) junction tumors, the celiac axis was electively treated. For proximal esophageal tumors, the supraclavicular lymph nodes were treated. The clinical target volume generally consisted of the GTV plus 4–5 cm longitudinal expansion and 1–2 cm radial expansion. An additional 0.5–1 cm expansion was used for the planning target volume (PTV). Radiation therapy was typically delivered to a total dose of 50.4 Gy in 1.8 Gy fractions.
The chemotherapy regimen used was at the discretion of the treating medical oncologist. Chemotherapy was delivered concurrently and most commonly consisted of either carboplatin/paclitaxel or cisplatin/5-FU. All patients were scheduled to undergo esophagectomy approximately 4–8 weeks after completion of neoadjuvant therapy. The surgery technique was at the discretion of the operating physician and typically employed a trans-hiatal or trans-thoracic approach. Prior to undergoing surgical resection, patients underwent re-staging studies.
Patterns of first failure were characterized in all patients with disease recurrence using available images and/or imaging reports. Local failure was defined as recurrence within the original radiation PTV or at the margin of the treated volume. Regional nodal failure was defined as a recurrence outside of the radiation field within a known regional lymph node draining site depending on the esophageal primary location (i.e. supraclavicular, mediastinal, celiac, para-aortic). Distant failure was defined as all other areas of recurrence. Patterns of recurrence were typically identified by comparing post-treatment imaging including PET and CT scans.
We examined the relationship between patient/tumor characteristics and recurrence outcomes (local, regional nodal, distant) using Fisher’s exact test and Wilcoxon rank-sum tests. We also used multiple logistic regression to simultaneously examine important predictors of distant recurrence, the most common recurrence outcome. For numbers of lymph nodes assessed and tumor size, we identified any optimal cut-points using conditional inference trees [8]. We generated Kaplan-Meier survival curves for relapse-free survival (RFS) and OS, and we generated cumulative incidence curves for the different recurrence types under a competing risks framework [9].
Results
Patient characteristics
A total of 132 patients met the inclusion criteria. The median follow-up was 28 months (range 4–128). The majority of patients had T3/T4 (85%), N0 (66%), and M0 (92%) disease. A total of 110 (83%) patients had adenocarcinoma. The most commonly used chemotherapy regimens were 5-FU based received by 79 (59.8%) or paclitaxel-based received by 31 (23.5%) patients. A total of 19 (14.4%) patients had close or positive margins while 100 (76%) patients had negative margins. Treatment characteristics are further demonstrated in Table I.
Table I.
Patient and tumor characteristics.
| N 132 |
Percent 100% |
||
|---|---|---|---|
| Age (years) | Median | 62 | |
| Range | 36–80 | ||
| Sex | Male | 104 | 79% |
| Female | 28 | 21% | |
| cT | T1/T2 | 18 | 14% |
| T3/T4 | 111 | 85% | |
| TX | 2 | 2% | |
| cN | Positive | 92 | 70% |
| Negative | 40 | 30% | |
| cM | M0/M1a | 121 | 92% |
| M1 | 11 | 8% | |
| pT | T0/Tis | 46 | 35% |
| T1/T2 | 49 | 37% | |
| T3/T4 | 37 | 28% | |
| pN | Positive | 45 | 34% |
| Negative | 87 | 66% | |
| Histology | SCC | 110 | 83% |
| Adenocarcinoma | 22 | 17% | |
| Margins | Negative | 109 | 83% |
| Close/Positive | 23 | 17% | |
| LVI | Yes | 9 | 7% |
| No | 121 | 92% | |
| N/A | 2 | 2% | |
| Ulceration | Yes | 7 | 5% |
| No | 109 | 83% | |
| N/A | 16 | 12% | |
| Radiation technique | IMRT | 37 | 28% |
| 3D-CRT | 93 | 70% | |
| Peri-neural invasion | Yes | 8 | 6% |
| No | 121 | 92% | |
| N/A | 3 | 2% | |
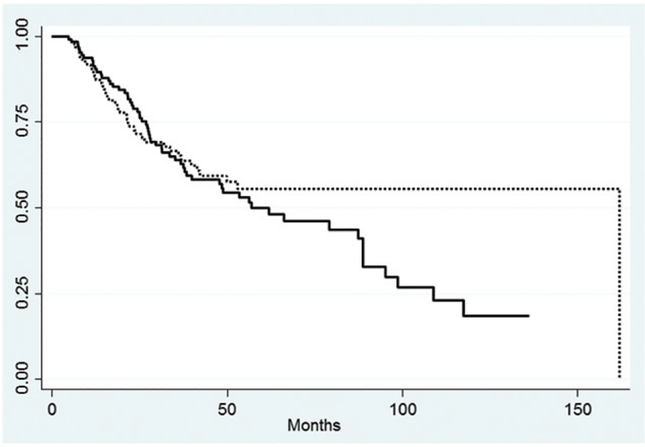
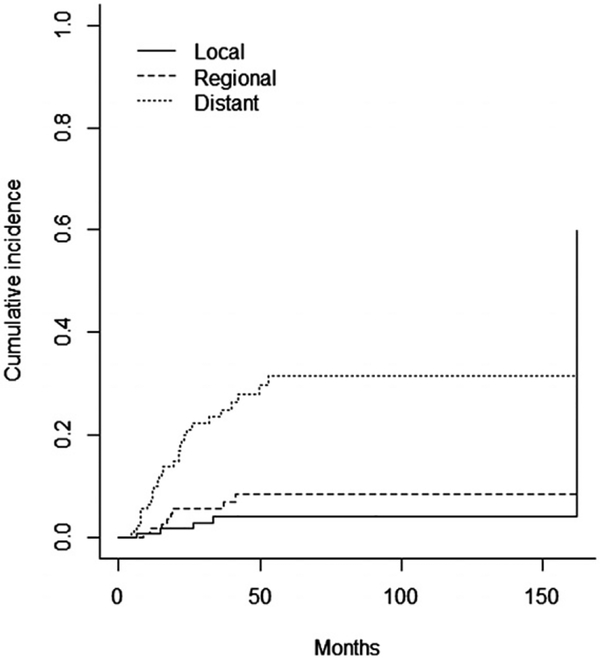
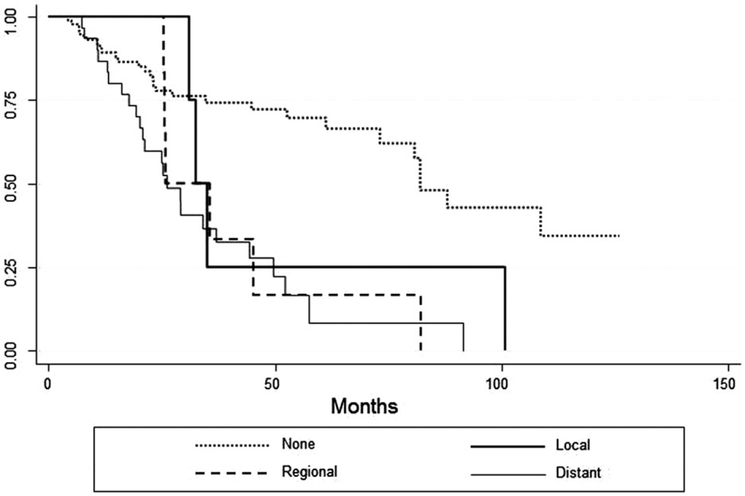
The three- and five-year OS rates were 58% and 48%, respectively (Figure 1A). The three- and five-year RFS rates were 66% and 57%, respectively (Figure 1B). A total of 43 (32.5%) patients experienced disease recurrence, eight patients recurred at multiple sites. Overall, six patients (4.5%) had a local recurrence, 13 (10%) had a regional nodal recurrence, and 32 (23.5%) had a distant failure. Cumulative incidence rates are demonstrated in Figure 2. The type of recurrence was a significant predictor of OS when compared to patients with no recurrence (p<0.0001) (Figure 3). The median OS for patients with a distant recurrence was 26 months, regional recurrence was 26 months, local recurrence was 33 months, and 82.1 months for patients without a recurrence.
Figure 1.
Kaplan-Meier curves for overall survival and relapse-free survival. Dashed line represents overall survival, solid line represents relapse free survival.
Figure 2.
Cumulative incidence curve of local, regional, and distant recurrence.
Figure 3.
Kaplan-Meier curves for overall survival according to site of first failure.
Local and regional recurrence
On univariate analysis, close/positive margins (p = 0.003) and smaller number of lymph nodes assessed (p = 0.013) were associated with local recurrence. Fewer lymph nodes assessed (p = 0.001) and larger pretreatment tumor size (p = 0.038) were associated with higher rates of regional recurrence. Univariate analysis is further demonstrated in Table II. Due to the limited number of events, multivariable analysis was not performed. Of the 13 patients experiencing regional nodal recurrence, seven patients recurred in the mediastinal lymph nodes, three patients developed a para-aortic recurrence, and three developed a supraclavicular nodal recurrence. Of the patients developing a mediastinal recurrence, six had a GE junction tumor while one had a mid-thoracic tumor. All patients with para-aortic or supraclavicular nodal recurrences had GE junction tumors.
Table II.
Patient and tumor characteristics associated with local, regional, or distant recurrence.
| Local recurrence | Regional recurrence | Distant recurrence | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N | Y | p-Value | N | Y | p-Value | N | Y | p-Value | |
| Age | 63 (36–80) | 60 (42–71) | 0.81 | 62 (36–80) | 66 (40–76) | 0.531 | 63 (36–80) | 61 (37–80) | 0.759 |
| Sex | |||||||||
| Male | 100 | 4 (4%) | 0.61 | 93 | 11 (11%) | 0.74 | 80 | 24 (23%) | 0.806 |
| Female | 26 | 2 (7%) | 26 | 2 (7%) | 21 | 7 (25%) | |||
| cT | |||||||||
| T1 | 2 | 0 (0%) | 0.25 | 1 | 1 (50%) | 0.453 | 2 | 0 (0%) | 1 |
| T2 | 16 | 15 | 1 (6%) | 12 | 4 | ||||
| T3 | 104 | 4 (4%) | 97 | 11 (10%) | 81 | 27 | |||
| T4 | 2 | 1 (33%) | 3 | 0 (0%) | 3 | 0 (0%) | |||
| TX | 2 | 0 (0%) | 2 | 0 (0%) | 2 | 0 (0%) | |||
| cN | |||||||||
| 0 | 37 | 2 (5%) | 0.75 | 35 | 4 (10%) | 1 | 33 | 6 (15%) | 0.144 |
| 1 | 81 | 3 (4%) | 75 | 9 (11%) | 61 | 23 (27%) | |||
| 2 | 7 | 0 (0%) | 7 | 0 (0%) | 6 | 1 (14%) | |||
| 3 | 1 | 0 (0%) | 1 | 0 (0%) | 0 | 1 (100%) | |||
| cM | |||||||||
| M0/M1a | 121 | 5 (4%) | 1.0 | 104 | 10 (9%) | 0.5 | – | – | |
| M1 | 11 | 0 (0%) | 14 | 3 (18%) | – | – | |||
| pT | |||||||||
| T0/Tis | 43 | 3 (7%) | 0.101 | 41 | 5 (11%) | 0.393 | 40 | 6 (13%) | 0.211 |
| T1 | 23 | 0 (0%) | 20 | 3 (13%) | 18 | 5 (22%) | |||
| T2 | 24 | 2 (8%) | 22 | 4 (15%) | 19 | 7 (27%) | |||
| T3 | 36 | 1 (3%) | 36 | 1 (3%) | 24 | 13 (35%) | |||
| T4 | 1 | 1 (50%) | 0 | 0 (0%) | 0 | 0 (0%) | |||
| pN | |||||||||
| 0 | 83 | 4 (5%) | 1.0 | 79 | 8 (9%) | 0.489 | 75 | 12 (14%) | <0.001 |
| 1 | 39 | 2 (5%) | 37 | 4 (10%) | 25 | 16 (39%) | |||
| 2 | 4 | 0 (0%) | 3 | 1 (25%) | 1 | 3 (75%) | |||
| Histology | |||||||||
| SCC | 19 | 3 (14%) | 0.1 | 20 | 2 (9%) | 1 | 17 | 4 (19%) | 0.305 |
| Adenocarcinoma | 107 | 3 (3%) | 99 | 11 (10%) | 84 | 26 (24%) | |||
| Margins | |||||||||
| Negative | 99 | 1 (1%) | 0.003 | 90 | 10 (10%) | 1 | 71 | 29 (29%) | 0.017 |
| Close/Positive | 15 | 4 (21%) | 17 | 2 (11%) | 7 | 2 (22%) | |||
| N/A | 12 | 1 (8%) | 12 | 1 (8%) | 13 | 0 (0%) | |||
| LVI | |||||||||
| Yes | 115 | 6 (5%) | 1.0 | 110 | 11 (9%) | 0.153 | 4 | 5 (56%) | 0.068 |
| No | 2 | 0 (0%) | 8 | 1 (11%) | 95 | 26 (21%) | |||
| N/A | 9 | 0 (0%) | 1 | 1 (50%) | 2 | 0 (0%) | |||
| Ulceration | |||||||||
| Yes | 7 | 0 (0%) | 0.691 | 6 | 1 (14%) | 0.701 | 4 | 3 (43%) | 0.017 |
| No | 104 | 5 (5%) | 98 | 11 (10%) | 81 | 28 (26%) | |||
| N/A | 15 | 1 (6%) | 15 | 1 (6%) | 16 | 0 (0%) | |||
| Radiation technique | |||||||||
| IMRT | 37 | 1 (3%) | 1.0 | 35 | 2 (5%) | 0.411 | 32 | 6 (16%) | 0.256 |
| 3D-CRT | 88 | 4 (4%) | 82 | 11 (12%) | 68 | 24 (26%) | |||
| Perineural invasion | |||||||||
| Yes | 8 | 0 (0%) | 1.0 | 8 | 0 (0%) | 0.294 | 3 | 5 (63%) | 0.029 |
| No | 115 | 6 (5%) | 109 | 12 (10%) | 95 | 26 (21%) | |||
| N/A | 3 | 0 (0%) | 2 | 1 (33%) | 3 | 0 (0%) | |||
| Location | |||||||||
| Lower/GE junction | 117 | 5 (4%) | 0.383 | 110 | 12 (10%) | 1 | 94 | 28 (23%) | 0.699 |
| Middle | 9 | 1 (10%) | 9 | 1 (10%) | 7 | 3 (30%) | |||
| Pathologic response | |||||||||
| pCR | 38 | 2 (5%) | 1.0 | 37 | 3 (8%) | 0.754 | 37 | 3 (8%) | 0.004 |
| Residual disease | 88 | 4 (4%) | 82 | 10 (11%) | 64 | 28 30%) | |||
| Chemotherapy regimen | |||||||||
| 5-FU based | 74 | 5 (6%) | 0.72 | 69 | 10 (13%) | 0.387 | 59 | 20 (25%) | 0.239 |
| Taxol-based | 30 | 1 (3%) | 30 | 1 (3%) | 27 | 4 (13%) | |||
| Other | 22 | 0 (0%) | 20 | 2 (9%) | 15 | 7 (32%) | |||
| Lymph nodes assessed | 6 (4–20) | 15 (2–58) | 0.013 | 16 (2–58) | 7.5 (2–13) | 0.001 | 15 (2–51) | 14 (4–58) | 0.706 |
We attempted to identify cut points for PET tumor size and number of lymph nodes assessed to identify meaningful parameters for clinical practice. For lymph node assessment, patients with ≤13 lymph nodes assessed had an inferior locoregional RFS versus patients with >13 lymph nodes assessed (p = 0.003) (Supplementary Figure 1).
Distant recurrence
On univariate analysis, higher pathologic nodal stage (p<0.001), ulceration (p = 0.017), perineural invasion (p = 0.029), residual disease (p = 0.004), and higher post-treatment PET SUV max (p = 0.049) were associated with distant failure. There was a trend towards higher rates of distant recurrence with lymphovascular invasion (p = 0.068). Univariate analysis is further demonstrated in Table II. No post-treatment PET SUV max cut-point could be identified. On multivariate analysis, patients with pCR (OR 0.19, 95% CI 0.05–0.68, p = 0.011) were less likely to experience distant recurrence. Patients achieving a pCR had an improvement in OS (HR 0.25 95% CI 0.06–0.92, p = 0.01) versus patients with residual disease.
Discussion
Tri-modality therapy has resulted in a significant shift in failure patterns for patients with locally advanced esophageal cancer. In the presented series, only 4.5% of patients developed local recurrence versus 23.5% developing distant disease. This is in contrast to RTOG 85–01, which demonstrated that 39% of patients developed local recurrence versus 7% developing distant disease as their first site of recurrence in patients receiving chemoradiation [10]. Tri-modality therapy has resulted in a significant improvement in locoregional outcomes and as a consequence has also greatly improved survival in these patients. Despite significant improvements in outcomes in these patients, outcomes are still poor and further progress is needed.
pCR remains an important predictor of outcomes in patients completing tri-modality therapy. In the presented analysis, patients obtaining a pCR were less likely to experience a distant relapse and had an improved OS versus patients with residual disease. Walsh et al. randomized patients with esophageal cancer to esophagectomy alone versus pre-operative chemoradiation followed by esophagectomy [11]. Of patients receiving pre-operative therapy, 25% had a pCR. These patients had an improved survival versus those with residual disease. Berger et al. demonstrated that patients who achieved a pCR following tri-modality therapy had a median OS of 50 months and a five-year survival rate of 48% versus a median survival of 25 months and a five-year survival of 18% of patients without a pCR [12]. Other studies have demonstrated similar response rates following neoadjuvant chemoradiation [2,13,14]. Identifying targets to increase treatment intensity may allow for higher response rates and improved treatment outcomes.
Multiple covariates were associated with higher rates of distant recurrence suggesting a need for further treatment intensity in this cohort, including higher pathologic nodal stage, ulceration, perineural invasion, residual disease, and higher post-treatment PET SUV max. Multiple other series have also demonstrated poor outcomes in patients demonstrating poor prognostic features [15–17]. Residual uptake on a post-treatment PET scan also has been associated with a poor prognosis due to higher likelihood of residual disease [18]. PET imaging may play a role in risk-adapted therapy in these patients.
Distant relapse remains the most common site of recurrence in patients completing tri-modality therapy. In the presented data, only six patients (4.5%) developed a local recurrence, 13 (10%) had a regional nodal recurrence, while 32 (23.5%) had a distant failure. The presented data is concordant with the patterns of failure from the CROSS trial where 14% of tri-modality patients developed locoregional recurrence and only 5% of patients developed a local recurrence [19]. In our series, the site of relapse has a significant impact on survival on patients with locally advanced esophageal cancer. Patients with distant or regional relapse had a median OS of 26 months versus 33 months in patients with a local relapse. This likely reflects better salvage treatment available for patients with an isolated recurrence, but may also be dictated by more aggressive disease biology metastasizing earlier. Patients who did not relapse had a median OS of 82.1 months. Further optimizing systemic therapy options remains important to minimize recurrence rates, although randomized data evaluating the impact of adjuvant chemotherapy in this disease are not currently available.
Multiple series have demonstrated the importance of number of lymph nodes assessed in patients undergoing esophagectomy alone [20–22]. As a result, the American Joint Committee on Cancer Staging Manual recommends at least 12 lymph nodes resected during surgery [23]. There is limited data in the literature regarding the impact of the extent of lymphadenectomy on outcomes in patients receiving multi-modality therapy. In the presented series, patients with >13 lymph nodes evaluated had a statistically significant improvement in locoregional RFS versus patients with ≤13 lymph nodes assessed. Our series confirms that a thorough surgical and pathological lymph node evaluation is still warranted.
The goal of pre-operative chemoradiation is to treat both macroscopic and microscopic disease, to improve resectability without a significant increase in morbidity. Identifying the appropriate radiation treatment volume is important in order to adequately treat any microscopic disease. In the presented dataset, patients with GE junction tumors received elective nodal irradiation to the celiac axis and proximal tumors received elective nodal irradiation to the supraclavicular fossa per NCCN guidelines [1]. Per our analysis, locoregional recurrence rates were low with only 13 (10%) of patients experiencing regional nodal failure. In addition, there was no meaningful impact of tumor location on regional nodal failure. It is unlikely that treating additional lymph node regions would have a meaningful clinical impact.
The limitations of our study include those that are inherent to any retrospective series. Patients were not randomly assigned to therapy and as a result there is inherent selection bias in the presented series. In addition, radiation techniques, chemotherapy regimens, and surgical techniques were not standardized for all patients, which may have impacted outcomes. Furthermore, patients were included in this analysis only if they received all planned therapy, and this may potentially limit the generalizability of our findings. Finally, the small sample size and low event rate prevented us from performing a multivariable analysis. For these reasons, it is difficult to draw broad conclusions that may be extrapolated to other patients.
In conclusion, the presented series demonstrates multiple risk factors which portend higher rates of local, regional, and distant recurrence in patients undergoing tri-modality therapy. Margin status, number of lymph nodes assessed, and pretreatment tumor size may modify risk of locoregional recurrence. We identified >13 lymph nodes evaluated as a potential quality metric in this series of patients. Furthermore, our results again demonstrate the strong correlation between pathologic response and treatment outcomes. Despite significant advances and improvement in outcomes in these patients, survival rates remain poor. Optimizing therapy by risk-adapted treatment may be one method of potentially improving outcomes in patients with poor risk factors. Furthermore, identifying patients with good prognostic factors may allow for sparing of treatment intensification in these patients. Further studies are needed to intensify treatment in patients with locally advanced esophageal cancer undergoing multi-modality therapy.
Supplementary Material
Acknowledgments
This publication was supported by grant number P30 CA006927 from the National Cancer Institute, NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health. This publication was also supported in part by a grant from Varian Medical Systems, Inc. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Varian Medical Systems, Inc.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Ajani JA, D’Amico TA, Almhanna K, Bentrem DJ, Besh S, Chao J, et al. Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Canc Netw. 2015;13:194–227. [DOI] [PubMed] [Google Scholar]
- 2.Tepper J, Krasna MJ, Niedzwiecki D, Hollis D, Reed CE, Goldberg R, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Hagen P, Hulshof MC, van Lanschot JJ, Steyerberg EW, van Berge Henegouwen MI, Wijnhoven BP, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. New England J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 4.Speicher PJ, Wang X, Englum BR, Ganapathi AM, Yerokun B, Hartwig MG, et al. Induction chemoradiation therapy prior to esophagectomy is associated with superior long-term survival for esophageal cancer. Dis Esophagus September 12 2014. DOI: 10.1111/dote.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: Cancer J Clin. 2015;65:5–29. [DOI] [PubMed] [Google Scholar]
- 6.Edge SB, American Joint Committee on Cancer, American Cancer Society. AJCC Cancer Staging Handbook: From the AJCC Cancer Staging Manual. 7th ed. New York: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 7.Tsutsui S, Kuwano H, Watanabe M, Kitamura M, Sugimachi K. Resection margin for squamous cell carcinoma of the esophagus. Ann Surg. 1995;222:193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hothorn T, Hornik K, Zeileis A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J Comput Graph Stat 15:651–74. [Google Scholar]
- 9.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–54. [Google Scholar]
- 10.Herskovic A, Martz K, al-Sarraf M, Leichman L, Brindle J, Vaitkevicius V, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. New England J Med. 1992;326:1593–8. [DOI] [PubMed] [Google Scholar]
- 11.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. New England J Med. 1996;335:462–7. [DOI] [PubMed] [Google Scholar]
- 12.Berger AC, Farma J, Scott WJ, Freedman G, Weiner L, Cheng JD, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol 2005;23:4330–7. [DOI] [PubMed] [Google Scholar]
- 13.Apinop C, Puttisak P, Preecha N. A prospective study of combined therapy in esophageal cancer. Hepato-gastroenterology. 1994;41:391–3. [PubMed] [Google Scholar]
- 14.Urba SG, Orringer MB, Turrisi A, Iannettoni M, Forastiere A, Strawderman M. Randomized trial of preoperative chemoradiation versus surgery alone in patients with locoregional esophageal carcinoma. J Clin Oncol. 2001;19:305–13. [DOI] [PubMed] [Google Scholar]
- 15.Li H, Zhang Q, Xu L, Chen Y, Wei Y, Zhou G. Factors predictive of prognosis after esophagectomy for squamous cell cancer. J Thorac Cardiovasc Surg. 2009;137:55–59. [DOI] [PubMed] [Google Scholar]
- 16.Chen JW, Xie JD, Ling YH, Li P, Yan SM, Xi SY, et al. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eloubeidi MA, Desmond R, Arguedas MR, Reed CE, Wilcox CM. Prognostic factors for the survival of patients with esophageal carcinoma in the U.S.: the importance of tumor length and lymph node status. Cancer. 2002;95:1434–43. [DOI] [PubMed] [Google Scholar]
- 18.Swisher SG, Erasmus J, Maish M, Correa AM, Macapinlac H, Ajani JA, et al. 2-Fluoro-2-deoxy-D-glucose positron emission tomography imaging is predictive of pathologic response and survival after preoperative chemoradiation in patients with esophageal carcinoma. Cancer. 2004;101:1776–85. [DOI] [PubMed] [Google Scholar]
- 19.Oppedijk V, van der Gaast A, van Lanschot JJ, van Hagen P, van Os R, van Rij CM, et al. Patterns of recurrence after surgery alone versus preoperative chemo-radiotherapy and surgery in the CROSS trials. J Clin Oncol 2014;32:385–391. [DOI] [PubMed] [Google Scholar]
- 20.Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP. Effect of the number of lymph nodes sampled on postoperative survival of lymph node-negative esophageal cancer. Cancer. 2008;112:1239–46. [DOI] [PubMed] [Google Scholar]
- 21.Rizk NP, Ishwaran H, Rice TW, Chen LQ, Schipper PH, Kesler KA, et al. Optimum lymphadenectomy for esophageal cancer. Ann Surg. 2010;251:46–50. [DOI] [PubMed] [Google Scholar]
- 22.Kang CH, Kim YT, Jeon SH, Sung SW, Kim JH. Lymphadenectomy extent is closely related to long-term survival in esophageal cancer. Eur J Cardiothorac Surg 2007;31:154–160. [DOI] [PubMed] [Google Scholar]
- 23.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.