Abstract
Cyclophosphamide (CTX) is a chemotherapeutic agent widely used to treat ovarian, breast, and hematological cancers as well as autoimmune disorders. Such chemotherapy is associated with reproductive failure and premature ovarian insufficiency. The mechanism by which CTX and/or its main metabolite, acrolein, affect female fertility remains unclear, but it is thought to be caused by an overproduction of reactive oxygen species (ROS). Here, we investigated the effect of CTX on metaphase II mouse oocytes obtained from treated animals (120 mg/kg, 24 h of single treatment), and oocytes directly exposed to increasing concentrations of CTX and acrolein (n = 480; 0, 5, 10, 25, 50, and 100 μM) with and without cumulus cells (CCs) for 45 min which correlates to the time of maximum peak plasma concentrations after administration. Oocytes were fixed and subjected to indirect immunofluorescence and were scored based on microtubule spindle structure (MT) and chromosomal alignment (CH). Generation of ROS was evaluated using the Cellular Reactive Oxygen Species Detection Assay Kit. Deterioration of oocyte quality was noted when oocytes were obtained from CTX treated mice along with CTX and acrolein treated oocytes in a dose-dependent manner as shown by an increase in poor scores. Acrolein had an impact at a significantly lower level as compared to CTX, plateau at 10 μM versus 50 μM, respectively. These variation is are associated with the higher amount of ROS generated with acrolein exposure as compared to CTX (p < 0.05). Utilization of antioxidant therapy and acrolein scavengers may mitigate the damaging effects of these compounds and help women undergoing such treatment.
Keywords: Cyclophosphamide, Acrolein, Chemotherapy, Follicle dysfunction, Chromosome alignment, Oocyte quality, Oxidative stress, Meiotic spindle, Infertility
1. Introduction
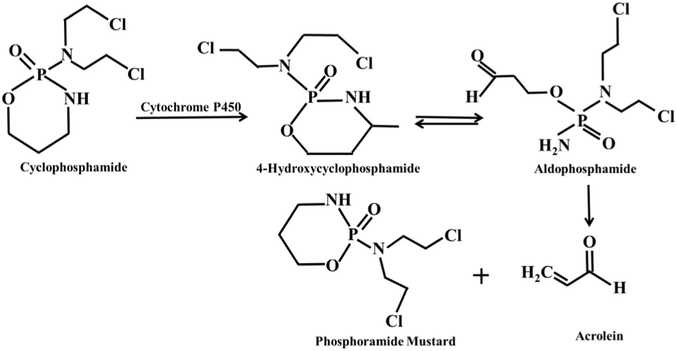
Advances in chemotherapeutics are leading to increased chances of cancer survival, however, the sequela of radiation and chemotherapy in women include premature ovarian failure and subfertility [1]. These patients have limited options to preserve their reproductive function including undergoing in vitro fertilization with egg/embryo freezing and/or undergoing surgery for ovarian tissue cryopreservation. Ovarian failure is associated with the generation of reactive of oxygen species (ROS) damaging the ovarian tissue and leading to follicle depletion [2,3]. Temporary versus permanent infertility depends on the type of chemotherapy, drug dosage, combination of various drugs and importantly the chronological age of women as older women have a lower ovarian reserve to start [2-6]. Cyclophosphamide(CTX), (N,N-bis(2-choloroethyl)tetrahydro-2H-1,3,2-oxazaphosphorin-2-amine 2-oxide), one of the most prevalent chemotherapeutic drugs, is not only used to treat cancers but many autoimmune diseases such as systemic lupus erythematous, rheumatoid arthritis, and minimal change disease [7-11]. Cyclophosphamide is metabolized in the presence of cytochrome P450 to give two stable toxic compounds: acrolein (a highly electrophilic, α,β-unsaturated aldehyde) and phosphoramide mustard through the formation of two unstable transient intermediates, 4-hydroxycyclophosphamide and aldophosphamide (Fig. 1) [12,13].
Fig. 1.
Mechanism of cyclophosphamide breakdown through the cytochrome P450 system to two stable toxic metabolites: Phosphoramide mustard and acrolein through the formation of unstable transient Intermediates: 4-hydroxycyclophosphamide and aldophosphamide.
Acrolein, a reactive aldehyde, is known to be the most toxic metabolite of CTX, through its ability to generate toxic ROS and subsequently effect surrounding tissue [14-16]. ROS have multiple impacts including inhibition of a variety of enzymes, membrane and DNA damage and lipid peroxidation [16-19], which contributes to infertility. Acrolein exposure can also occur through a variety of other sources including cigarette smoke, industrial, and other environmental exposures which are all associated with an enhancement of ROS [20]. Recently we have shown that ROS such as superoxide radical (O2•−), hydrogen peroxide (H2O2), hydroxyl radical (•OH), peroxynitrite (ONOO−), and hypochlorous acid (HOCl) can deteriorate oocyte quality through alterations in the spindle morphology and microtubule arrangement (MT), reflected by disruption of scaffolding proteins and through alterations in chromosomal alignment (CH) [21-23]. Previously, we have shown that oocyte composition is altered in women with inflammatory conditions such as endometriosis, which also produce a higher level of ROS, through increased oocyte, granulosa cell apoptosis, and follicular fluid apoptosis and protein nitration [24]. Cumulus cells (CCs), are cells surrounding the oocytes and are tightly packed in layers forming the cumulus-oocyte complex (COC) [25]. They serve to provide nutrition to the oocytes and operate as a network between the oocyte and its extracellular microenvironment [25-27]. Additionally, the CCs provide protection for the oocytes against ROS, in our previous work they have demonstrated protection against H2O2 and •OH insult at low concentrations, but this protection was noted to be lost at higher concentrations [28]. Phosphoramide mustard works by introducing alkyl radicals into biologically active molecules inducing DNA-DNA crosslinks and eventually cell apoptosis [12]. However previously published studies have shown that it does not generate ROS [29].
Females exposed to CTX and acrolein may experience infertility, ovarian failure, and even premature ovarian insufficiency [30]. However, the link between exposure to CTX and acrolein and infertility is still unclear [31,32]. In the current work, we conducted both in vivo (through direct administration of CTX to mice) and in vitro (incubation of metaphase II mice oocytes with CTX and acrolein) treatments to explore the mechanism through which CTX and its main metabolite, acrolein alter oocyte quality and impact fertility. Our results show that the damaging effects mediated by CTX and acrolein are involve the generation of ROS to different degree. Determining the underlying mechanism of subfertility will help in development of strategies for damage mitigation and future therapeutics to preserve fertility in patients undergoing chemotherapeutic treatment.
2. Materials and methods
2.1. Materials
All the materials used were of the highest grade of purity and did not need further purification. Cyclophosphamide, acrolein, human tubular fluid (HTF) media, anti-α tubulin antibody, fluorescein isothiocyanate (FITC) conjugate anti-goat antibody, 4′,6-diamidino-2-phenylindole (DAPI), 1% BSA (Bovine Serum Albumin), 0.1% M Glycine, and 0.1% Triton X- 100 were obtained from Sigma-Aldrich (St. Louis, MO, USA). Normal Goat Serum (2%) was from Invitrogen (Grand Island, NY) and 0.2% Powder Milk from grocery. Metaphase II oocytes (with and without CC) from a B6C3F1 mouse crossed with a B6D2F1 mouse were obtained commercially (Embryotech Inc.) in cryopreserved straws using ethylene glycol-based slow freeze cryopreservation protocol. The oocyte spindle repolymerizes to normal structure when incubated in media for 60–120 min at 37 °C and 5% CO2 prior to induction of oxidative stress [33]. This mechanism actually has helped support utilization of frozen oocytes for studying spindle damage. The use of frozen-thawed oocytes is well accepted as many studies have been published in the past utilizing frozen thawed oocytes and effects of oxidative stress on spindle morphology.
2.2. Methods
2.2.1. Treatment of mice with cyclophosphamide
The experimental procedures used for live animal care and handling were approved by Institutional Animal Care and Use Committee at Wayne State University (Protocol #A 11-01-15) and Embryotech Laboratories Inc., Haverhill, MA, USA. The methods were carried out in accordance with the approved guidelines. Two separate groups of female B6C3F1 (three weeks of age) mice were obtained (6101F, Envigo, Indianapolis, IN, USA) and superovulated as previously described [34]. Three week old mice are used for superovulation because the number and quality of oocytes are higher from this age group as compared to females past puberty [35]. All mice were housed in groups of three per wire cage and kept under standard laboratory conditions. There were a total of three mice in each group. Group 1 intraperitoneal injection (IP) with CTX (treatment group) and Group 2 injected with phosphate buffer IP (control group). On day 1 – all 6 females were injected with 15IU each of prepared gonadotropin (PMSG) hormone 65hrs prior to oocyte collection. On day 3–24 h prior to collection each mouse in Group 1 received a 120 mg/kg IP of CTX (Sigma Aldrich, St. Louis, MO, USA). Each mouse in Group 2 (control group) received an intraperitoneal injection of prepared phosphate buffer. In addition, 18 h prior to collection groups 1 and 2 were each injected with 15IU of prepared hCG hormone. Day 4 – all females were sacrificed by cervical dislocation, and the oviducts were collected. The oviducts were dissected. Cumulus-oocyte complexes were released by tearing the ampulla of the oviducts. The cumulus cells were removed enzymatically using 85 IU/mL hyaluronidase (Sigma Aldrich, St. Louis, MO, USA) and by mechanical dissociation using a glass pipette. The oocytes were fixed, stained and mounted for grading as described earlier.
2.3. Treatment of metaphase II mouse oocytes with CTX and acrolein
Metaphase II mouse oocytes with and without CC were transferred from straws to phosphate buffer saline (Dulbecco's PBS) and washed to remove excess cryoprotectant for 3–4 min. Oocytes were then transferred to HTF media and incubated at 37 °C and 5% CO2 for 60 min to allow spindle repolymerization and attainment normal oocyte architecture. The oocytes were then screened for the presence of the polar body confirming their metaphase II stage. Two- three oocytes from each group were discarded as they were found to be immature or displayed disrupted zona pellucidas. In the experiment metaphase II oocytes with and without CCs were divided into the following groups: CTX and acrolein treatments.
For CTX treatment, oocytes were divided into four different groups; (group 1, n = 120) oocytes without CCs incubated with increasing concentrations of CTX (0, 5, 10, 25, 50 and 100 μM); (group 2, n = 120) oocytes with CCs incubated with increasing concentrations of CTX (0, 5, 10, 25, 50 and 100 μM). The same treatment protocol was repeated for acrolein treatment, oocytes were divided equally as following: oocytes with (group 1, n = 120) and without (group 2, n = 120) CCs treated with increasing concentrations of acrolein (0, 5, 10, 25, 50 and 100 μM). The treated and untreated oocytes were incubated with the compounds for 45 min to ensure maximum effect.
2.4. Immunofluorescence staining and fluorescence microscopy
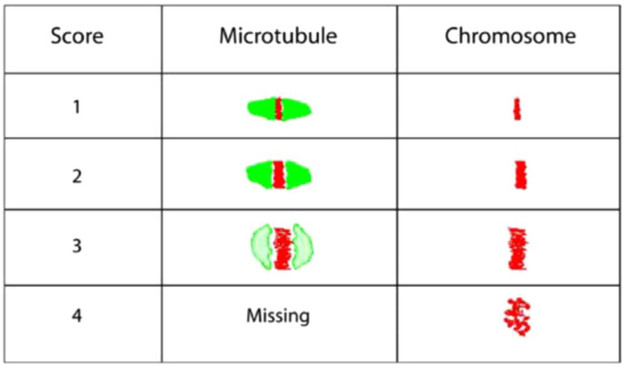
All treated and untreated oocytes were fixed in a solution prepared from 2% formaldehyde and 0.2% Triton X-100 for 30 min at 25 °C [21]. The fixed oocytes were treated with blocking solution (PBS, 0.2% Powdered Milk, 2% Normal Goat Serum, 1% Bovine Serum Albumin (BSA), 0.1 M Glycine and 0.1% Triton X-100) for 30 min then washed with PBS for 3 min [21]. Subsequently, the oocytes were subjected to indirect immunofluorescence staining by incubating in mouse primary anti-α tubulin antibody against the MT for 60 min, and secondary FITC conjugated anti-goat antibody for 30 min. The chromosomes were stained using DAPI, a stain for DNA which binds only to A-T rich regions, and incubated for 1–2 min. Stained oocytes were loaded into an anti-fade agent on slides with two etched rings, and cover slips were affixed using nail varnish. The alterations in the MT and CH were compared with controls and scored by three blinded observers (Fig. 2). Scores of 1–4 were assigned for both MT and CH alterations, with scores 1 and 2 combined for good outcomes meaning microtubules were organized in a barrel-shaped with slightly pointed poles formed by organized microtubules crosswise from pole to pole, and chromosomes were normally arranged in a compact metaphase plate at the equator of the spindle. Scores of 3 and 4 signified poor outcomes and consisted of spindle length reduction, disorganization and/or complete spindle absence, and chromosome dispersion or aberrant condensation appearance (Fig. 2) [33,36]. Images were obtained utilizing both immunofluorescence and confocal microscopy. The alterations in the MTs and CH were compared with controls and scored individually by three blinded observers based on a previously published scoring system [36].
Fig. 2.
Oocyte scoring system based on microtubule and chromosomes. The oocytes are scored from a score of 1–4. A score of 1 and 2 are considered good quality oocytes as the microtubules are well organized, and the chromosome is dense and tightly aligned. As the scores progress to 3 and 4, which are considered poor quality scores you see a ballooning effect of the microtubule and eventual loss and the chromosome starts dispersing away from the midline and scatters.
Confocal microscopy, assessment of microtubules and chromosomal alignment slides were examined with the Axiovert 25 inverted microscope (Zeiss, Thornwood, NY) using DAPI (blue) and FITC (green) fluorescent filters with excitation and emission wavelengths of 358 and 461 nm, and 596 and 613 nm, respectively. Confocal images were obtained utilizing a Zeiss LSM 510 META NLO (Zeiss, Germany) microscope. Oocytes were localized using a 10 × magnification lens and spindle alterations assessed using 40 × oil immersion lens. The MT was stained fluorescent green, which was distinct from the fluorescent blue staining of the chromosomes. Following completion of the experiments each oocyte was closely examined for spindle status by three independent observers blinded to the assigned treatment groups. Observers used comprehensive evaluation of the individual optical sections and the 3-D reconstructed images.
2.5. Detection of ROS generation in oocytes after exposure to CTX and acrolein
The oocytes without CCs (n = 60) were incubated with CTX (untreated and 100 μM), acrolein (untreated and 100 μM) in each group n = 20 for 45 min. For positive oocyte (n = 30) control for ROS signal we used pyocyanin (100 μM) as a known intracellular ROS producer. Generation of ROS was evaluated using the Cellular Reactive Oxygen Species Detection Assay Kit (Abcam, Cambridge, UK) as instructed by the manufacturer. After fixation by 2% formaldehyde for 30 min, nuclei were stained with DAPI. Images of ROS-mediated deep red fluorescence were taken using a Nikon Eclipse 90i epifluorescence microscope. The fluorescence intensity of each oocyte image was measured from quantification of mean pixel intensities using NIS-Element (Nikon, Shinagawa-Ku, Tokyo, Japan). The overall fluorescence unit for CTX and acrolein treatment group of oocytes were calculated relatively to untreated and positive control groups and represented as a normalized relative fluorescent unit (RFU). The Student's t-test was performed on the RFU to test if the difference between control and each compound treatment groups is statistically significant (p < 0.05).
2.6. Statistical analysis
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 22.0. Comparisons of the percentage of oocytes with poor scores (scores 3 and 4) for MT and CH between control and treatment groups were made using One-Way ANOVA with Tukey post hoc testing comparisons between oocytes without and with CCs for each treatment were performed with Student t-test. P < 0.05 was considered significant for all statistical tests.
3. Results
3.1. Intraperitoneal injection of cyclophosphamide in mice deteriorates oocyte quality
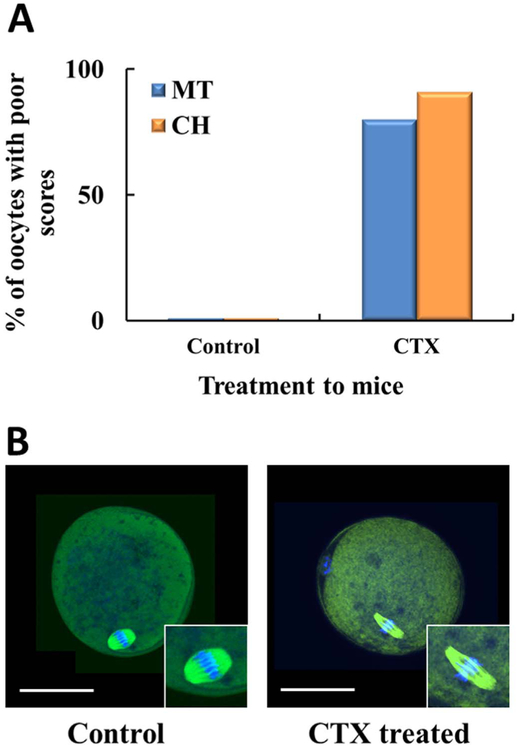
To determine the biological relevance of this study, we first investigated the impact of CTX intraperitoneal injection to female mice and its subsequent effects on oocyte quality. Oocytes obtained from these animals were also scored using the same scoring system mentioned in Fig. 2. Significant deterioration of oocyte quality was observed in oocytes of mice that were injected with CTX as the oocytes that were exposed in vitro. As shown in Fig. 3A, there was dramatic increase in percent of poor scores, MT (80%) and CH (91%), which was substantially different when compared to controls (p < 0.05). Fig. 3B, shows a representative of CTX mouse treated oocyte with spindle elongation and widening of the chromosome. In addition, we have noted that the ovaries in our treatment group were smaller and fibrotic as compared to controls, consistent with previously published results [37,38].
Fig. 3.
Intraperitoneal injection of cyclophosphamide in mice deteriorates oocyte quality. Two separate groups of female mice with a total of three mice in each group. Group 1 cyclophosphamide (treatment group) intraperitoneal injection (IP) and group 2 (control group) with Phosphate Buffer IP. All females from groups 1 & 2 were sacrificed and the oviducts were collected. The oocytes were collected, placed in fixative, primary and secondary antibody, DAPI and scored. A) There was a decline in oocyte quality based on MT and CH in the treatment group. The oocyte score is an average of three scores. B) Confocal microscopy to assess microtubules and spindle of CTX treated mice. Representative images of the control oocyte with normal spindle and chromosomal (CH) alignment as compared to the CTX treated mouse with a thin long spindle and outward movement of the chromosome from the equatorial plate.
3.2. Cyclophosphamide and acrolein altered the oocyte spindle structure and chromosomal alignment
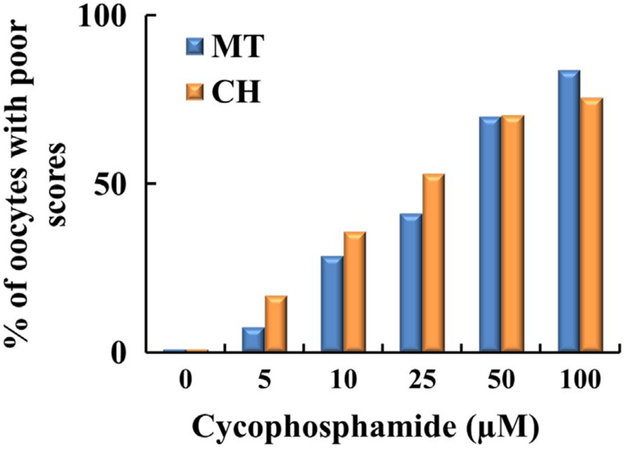
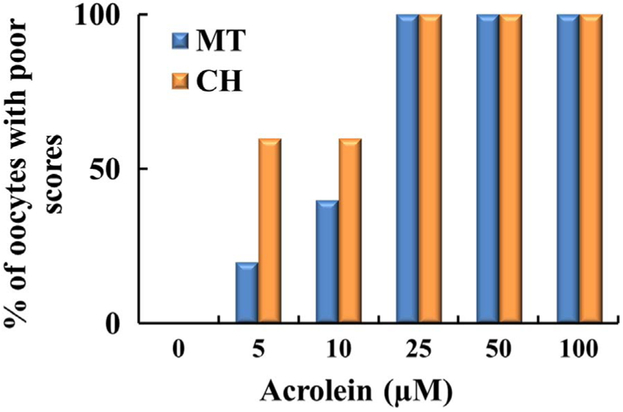
Cyclophosphamide and acrolein differentially deteriorated oocyte quality by disrupting the MT structure and CH. Figs. 4 and 6 show the concentration dependent percentage of poor scores for MT and CH for oocytes without CCs treated with CTX and acrolein, respectively. Cyclophosphamide and acrolein had significantly increased poor scores compared with controls in both MT and CH (p = 0.032 for MT and p = 0.05 for CH) (Fig. 4 and 6). As shown in Fig. 4, incubation of oocytes with 10 μM CTX caused an increase in MT (27%) and CH (35%) poor score, incubation of oocyte with 25 μM CTX cased an increase in MT (40%) and CH (53%) poor score, and 100 μM CTX cased an increase in MT (84%) and CH (76%) poor score. Whereas much less concentration was required to deteriorate oocyte quality when oocytes were exposed to acrolein as noted in Fig. 6. Incubation of oocytes with 5 μM acrolein cased an increase in MT (20%) and CH (61%) poor score, incubation of oocyte with 10 μM acrolein cased an increase in MT (39%) and CH (60%) poor score, whereas the incubation with 25 μM or more of acrolein led to a complete destruction of both MT (100%) and CH (100%). Oocytes with CCs exposed to the CTX show a very similar trend (MT 55% and CH 73%), indicating that CCs did not provide any protection to the oocyte.
Fig. 4.
Plot demonstrating percentage of oocytes with poor scores based on increasing concentration of cyclophosphamide. Oocytes (n = 240) were incubated with increasing concentrations of cyclophosphamide (0–100 μM). As the concentration increased, there was a progressive increase in poor scores based on MT, the blue column and the chromosomes which are represented by the orange column. Each treatment is the average of at least three independent experiments.
Fig. 6.
Plot demonstrating percentage of oocytes with poor scores based on increasing concentration of acrolein. Oocytes (n = 240) were incubated with increasing concentrations of acrolein (0–100 μM). As the concentration is increased of acrolein, there was a progressive decline in oocyte quality based on MT and CH. Each treatment is the average of at least three independent experiments.
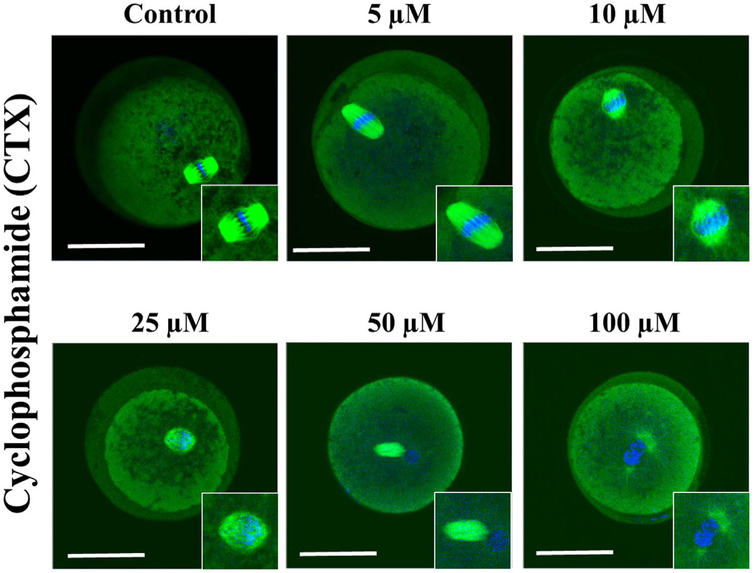
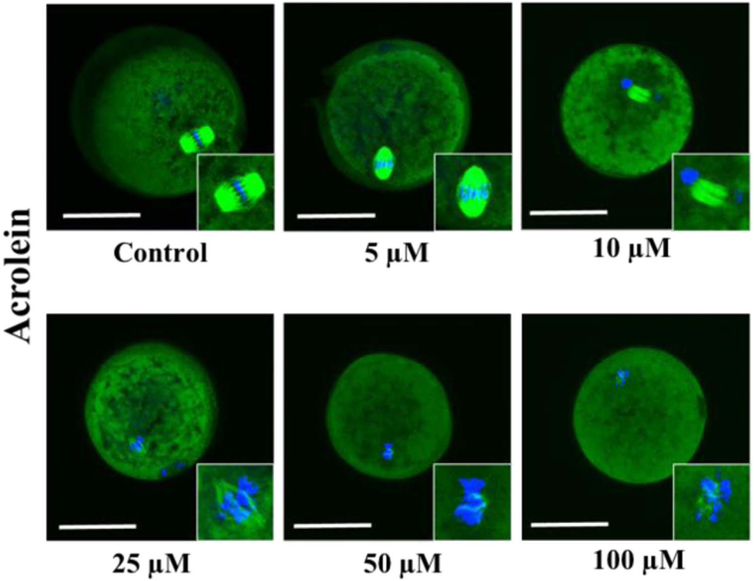
The representative confocal images of spindle morphology and chromosomal alignment exposed to CTX and acrolein compared to controls are shown in Figs. 5 and 7. Untreated oocytes without and with CCs showed a symmetrical well-organized barrel-shaped spindle structure (green) with chromosomes aligned along the spindle equatorial plate (blue) (Fig. 5 and 7). However, a variety of abnormal configurations in MT and CH were noted after exposure to CTX and acrolein. Even with exposure to low concentrations of acrolein, the spindle poles are noted to pivot around the spindle-chromosomal attachments producing a dilated “balloon” shaped spindle and alterations in chromosomal alignment.
Fig. 5.
Confocal microscopy to assess microtubules and spindle in the presence of increasing concentration of cyclophosphamide. Starting with the control oocyte with normal spindle and chromosomal (CH) alignment, then as the concentration increased progressively altered spindle and CH alignment with the maximum deterioration of oocyte quality noted at 100 μM. Scale bars: 50 μm. Results depict observations made after three experiments. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article).
Fig. 7.
Confocal microscopy to assess microtubules and spindle in the presence of increasing concentration of acrolein. Starting with the control oocyte with normal spindle and chromosomal (CH) alignment, then as the concentration increased progressively altered spindle and CH alignment with significant deterioration noted at even 10 μM of acrolein. Scale bars: 50 μm. Results depict observations made after three experiments. (For interpretation of the references to color in this figure, the reader is referred to the web version of this article).
3.3. CTX and its metabolites increased ROS generation in metaphase II mouse oocyte
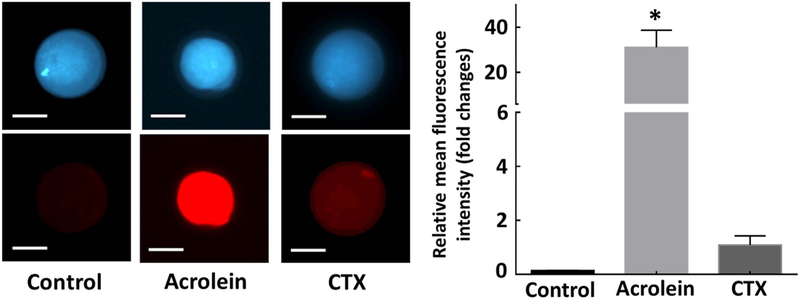
To further understand the mechanism of action behind CTX and acrolein induced damage to oocyte MT and CH, we evaluated ROS generation (Fig. 8). Treatment with CTX and acrolein led to increase in ROS generation in the oocyte as compared to controls as indicated by increased ROS-mediated deep red fluorescence (Fig. 8, left panel). Treated oocytes demonstrated fluorescence in the oocyte indicating that the ROS generation was higher in treated oocytes as compared to controls (relative mean fluorescence intensity, 0.0 for control, 31.0 for acrolein and 1.0 for CTX, p < 0.05). Although CTX generated ROS, it was not as significant as compared with oocytes exposed to acrolein (Fig. 8, right panel).
Fig. 8.
Verification of generation of damage to the oocytes was mediated through the production of reactive oxygen species. Oocytes without CCs (n = 60) were exposed to cyclophosphamide and acrolein for 45-min incubation to determine intracellular ROS generation. The fluorescence intensity was estimated as relative fluorescence unit (RFU) and plotted as a bar graph to present the fold changes in ROS production upon treatment relative to untreated control oocytes. Error bars indicate ± relative standard error of mean (SEM). * p < 0.05 vs. controls. Inset presents representative images of control and oocytes exposed to each compound for 45 min. Scale bars: 50 μm. Images shown are from a typical experiment performed at least three times.
4. Discussion
Our results showed that CTX and its most toxic and stable metabolite, acrolein, deteriorate metaphase-II mouse oocyte quality as judged by alterations in the MT and CH complex. Most importantly, these results are biologically significant as the same adverse effect was noted when oocytes were obtained from CTX treated mice as compared to controls. The damage appears to be through mechanisms that involve ROS overproduction regardless of the presence or absence of CC. ROS production mediated by CTX and acrolein exposure could be the major cause of adverse reproductive outcomes in females treated with CTX. This study clarifies the pathophysiology of CTX use and its implications on potential subfertility and premature menopause.
Chemotherapy mediated generation of ROS deteriorates oocyte quality through dismantling of the microtubule structure and chromosomal alignment through a disruption of the spindle force balance mediated by the derangement of the spindle scaffold proteins such α-tublin. At low concentrations, CTX and acrolein exposure caused shortening and ballooning of the spindle along the pole-to-pole axis leading to widening of the chromosomal plate and eventual complete derangement of both the MT and CH as the concentrations increased. These results were found to be generalizable as our previous studies produced similar spindle morphologic patterns [23]. Furthermore, it has been reported that superoxide and hydroxyl radical are the major ROS mediated by CTX exposure [39]. In addition, the ovaries are smaller and fibrotic in the treatment group, consistent with previously published results which are likely mediated with ROS overproduction [37,38]. We noted similar behavior in mice that were injected with CTX; the oocytes had the same deterioration in spindle and chromosome morphology when oocytes treated with various ROS.
Based on our previous work these alterations in MT and CH are irreversible and associated with poor embryo developmental potential thus subfertility [21,23,40]. ROS generation mediated by acrolein exposure could be from direct mitochondrial dysfunction, increased intracellular calcium, which in turn activates xanthine oxidase this then stimulates an overproduction of ROS and glutathione depletion [41]. The amount of ROS produced is clearly able to overwhelm all antioxidant defenses of the cumulus oocyte complex. We have previously shown that when CCs failed to protect oocyte quality against ROS, the mechanism appeared to be mainly through the loss of CC number or viability [28]. In marked contrast, CTX exposure results in a mild increase in ROS generation in oocytes, which also overwhelms the antioxidant defenses of the oocyte. Previous investigations have shown that CTX mediates ROS generation through multiple pathways including the activation of an intracellular signaling pathway important in regulating the cell cycle (e.g., PI3K/Akt/mTOR pathway), the NADPH complex (NADPH/NADP +), and the mitochondrial electron respiratory chain [31,42,43]. In addition, receptor tyrosine kinase-mediated activation of PI3K results in the recruitment of p47phox, a cytosolic protein, to the plasma membrane and consequent production of ROS. PI3K can regulate the phosphorylation of the NADPH oxidase and the subsequent activation of NADPH oxidase complex, which can increase ROS production as well [42]. The aforementioned three pathways combined can lead to classical apoptosis signaling pathways to be hyper-activated and induce primordial follicle loss and ultimately damage oocytes leading to infertility or premature ovarian failure [31].
Acrolein is generated by cytochrome P450 isozymes including CYP 3A4, 2B6, 2C9, and 2C19 [44] which are absent in the oocytes. Acrolein enters the ovarian tissue directly from the bloodstream by transfer across the blood follicular barrier. In addition to its production as a metabolite of CTX, acrolein can also be generated endogenously through lipid peroxidation, found in dietary sources such as alcohol and fried foods, industrial waste, cigarette smoke, and even automobile exhaust [45]. Indeed, acrolein has been noted to exert more toxic effects versus the parent compound CTX, as it required lower concentrations to deteriorate oocyte quality in addition to generating significantly more ROS. It has been noted that the limitation of CTX use is due to the toxic effects of acrolein [46]. Importantly, in both cases the maximum effect of the aforementioned compounds required significantly lower concentrations to alter oocyte quality as compared to the plasma levels noted during treatment of any neoplastic disease or autoimmune disorder. Our current results support the fact that CTX can lead to infertility through direct damage to oocytes. Acrolein is highly reactive and is recognized as the initiator of lipid peroxidation [47]. The cytotoxicity of acrolein [48] is often what limits CTX use in clinical practice. Furthermore, CTX induced oxidative stress through the generation of free radicals leading to biochemical and physiological disturbances in animal models have been attributed to acrolein [49].
In general, CTX treatment is associated with an imbalance of oxidant and antioxidants machinery, which leads to the accumulation of ROS such as O2•−, H2O2, singlet oxygen, and •OH. It has also been shown that the disruptions in the redox balance through CTX may also occur through higher release of NO during inflammatory conditions, and when coupled with O2•− at near diffusion rate generating ONOO−, a more toxic oxidizing agent [50-52]. Recently, we have shown that insufficient amount of NO accelerates oocyte aging whereas ONOO− deteriorates oocyte quality through the alterations in the microtubule morphology and chromosomal alignment. In addition to a recent study which further shows that acrolein can potentiate oocyte aging through the same mechanism [23,53-55].
Oxidative stress affects oocyte integrity by disruption of the spindle structure, premature primordial follicle activation, antral follicle destruction, impaired oocyte fusibility, deficit of mitochondria-derived ATP, and induction of postovulatory aging [56-59]. ROS overproduction can mediate mitochondrial dysfunction and decrease antioxidant machinery which both can contribute to more ROS generation leading to eventual apoptosis [59]. This conclusion is consistent with recent studies in which animal model uptake of chemotherapy displays increased generation of ROS and caspase 3 in ovarian follicles in a dose-dependent manner [31]. As of late, it has been shown that ROS may affect oocyte quality as evidenced by hypergranulation of cytoplasm, absence of perivitelline space, and abnormal spindle structure [33]. Our results show that treated oocytes displayed increased ROS production that mediated the above mentioned damage. Overproduction of ROS mediated by acrolein exposure leads to lipid peroxidation, oocyte aging and eventually apoptosis [31]. This notion is consistent with our previous published studies which revealed that ROS accelerates oocyte aging [40,53,60].
In the past females treated with CTX had limited options to preserve fertility, i.e. in vitro fertilization or ovarian tissue cryopreservation. Understanding the mechanism behind CTX mediated infertility, through ROS mediated follicular dysfunction, opens the door to potential therapeutic options. Acrolein's long-lived potential to propagate oxidative stress provides one possible explanation for the ineffectiveness of ROS scavengers in clinical trials, therefore represents a novel and potentially more effective target for attenuating oxidative stress [61,62]. Supplementation with a combination of acrolein scavengers and antioxidants could represent as a potential mechanism of preventing oocyte deterioration not only in chemotherapy treatment but also in exposure to cigarette or industrial smoke where you have acrolein exposure. Administration of ROS or acrolein scavengers, together with CTX therapy could protect ovarian function, but inadvertently it may decrease CTX effectiveness for the primary disease [63]. Due to acrolein stability that allows the overwhelming generation of ROS, future investigations are needed to study a combination of acrolein scavengers and antioxidants to preserve fertility and ovarian function of reproductive age women.
References
- [1].Guida M, et al. , Reproductive issues in patients undergoing hematopoietic stem cell transplantation: an update, J. Ovarian Res 9 (1) (2016) 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Morgan S, et al. , How do chemotherapeutic agents damage the ovary? Hum. Reprod. Update 18 (5) (2012) 525–535. [DOI] [PubMed] [Google Scholar]
- [3].Blumenfeld Z, Chemotherapy and fertility, Best. Pract. Res. Clin. Obstet. Gynaecol 26 (3) (2012) 379–390. [DOI] [PubMed] [Google Scholar]
- [4].Garrido-Colino C, et al. , Current situation on fertility preservation in cancer patients in Spain: level of knowledge, information, and professional involvement, An. Pediatr (2016). [DOI] [PubMed] [Google Scholar]
- [5].Nieman CL, et al. , Cancer survivors and infertility: a review of a new problem and novel answers, J. Support Oncol 4 (4) (2006) 171–178. [PubMed] [Google Scholar]
- [6].Thrall MM, et al. , Trends in treatment of advanced epithelial ovarian cancer in the Medicare population, Gynecol. Oncol 122 (1) (2011) 100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Kallenberg CG, Pro: cyclophosphamide in lupus nephritis, Nephrol. Dial. Transplant 31 (7) (2016) 1047–1052. [DOI] [PubMed] [Google Scholar]
- [8].Yasuda S, et al. , Prognosis and progress in immunotherapies for organ involvements in systemic autoimmune diseases, Nihon Rinsho Meneki Gakkai Kaishi 39 (1) (2016) 8–17. [DOI] [PubMed] [Google Scholar]
- [9].Kim J, Chan JJ, Cyclophosphamide in dermatology, Australas. J. Dermatol (2016). [DOI] [PubMed] [Google Scholar]
- [10].Aouba A, et al. , Complete remission of Schnitzler syndrome and Waldenstrom macroglobulinemia under rituximab-cyclophosphamide-dexamethasone, Dermatology 230 (1) (2015) 18–22. [DOI] [PubMed] [Google Scholar]
- [11].Townes AS, Sowa JM, Shulman LE, Controlled trial of cyclophosphamide in rheumatoid arthritis, Arthritis Rheum. 19 (3) (1976) 563–573. [DOI] [PubMed] [Google Scholar]
- [12].Zhang J, et al. , Metabolism and transport of oxazaphosphorines and the clinical implications, Drug Metab. Rev 37 (4) (2005) 611–703. [DOI] [PubMed] [Google Scholar]
- [13].Boddy AV, Yule SM, Metabolism and pharmacokinetics of oxazaphosphorines, Clin. Pharmacokinet 38 (4) (2000) 291–304. [DOI] [PubMed] [Google Scholar]
- [14].Qin WS, Deng YH, Cui FC, Sulforaphane protects against acrolein-induced oxidative stress and inflammatory responses: modulation of Nrf-2 and COX-2 expression, Arch. Med. Sci 12 (4) (2016) 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kenche H, et al. , Adverse outcomes associated with cigarette smoke radicals related to damage to protein-disulfide isomerase, J. Biol. Chem 291 (9) (2016) 4763–4778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sun Y, et al. , Enhancement of the acrolein-induced production of reactive oxygen species and lung injury by GADD34, Oxid. Med. Cell Longev 2015 (2015) 170309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Esterbauer H, Schaur RJ, Zollner H, Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes, Free Radic. Biol. Med 11 (1) (1991) 81–128. [DOI] [PubMed] [Google Scholar]
- [18].Hoff HF, O'Neil J, Structural and functional changes in LDL after modification with both 4-hydroxynonenal and malondialdehyde, J. Lipid Res 34 (7) (1993) 1209–1217. [PubMed] [Google Scholar]
- [19].Yang MH, Schaich KM, Factors affecting DNA damage caused by lipid hydroperoxides and aldehydes, Free Radic. Biol. Med 20 (2) (1996) 225–236. [DOI] [PubMed] [Google Scholar]
- [20].Horiyama S, et al. , Intracellular metabolism of alpha,beta-unsaturated carbonyl compounds, acrolein, crotonaldehyde and methyl vinyl ketone, active toxicants in cigarette smoke: participation of glutathione conjugation ability and aldehydeketone sensitive reductase activity, Chem. Pharm. Bull 64 (6) (2016) 585–593. [DOI] [PubMed] [Google Scholar]
- [21].Shaeib F, et al. , The impact of myeloperoxidase and activated macrophages on metaphase II mouse oocyte quality, PLoS One 11 (3) (2016) e0151160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Banerjee J, et al. , Melatonin prevents hypochlorous acid-induced alterations in microtubule and chromosomal structure in metaphase-II mouse oocytes, J. Pineal Res 53 (2) (2012) 122–128. [DOI] [PubMed] [Google Scholar]
- [23].Khan SN, et al. , Peroxynitrite deteriorates oocyte quality through disassembly of microtubule organizing centers, Free Radic. Biol. Med 91 (2016) 275–280. [DOI] [PubMed] [Google Scholar]
- [24].Nassif J, et al. , The role of NADPH-derived reactive oxygen species production in the pathogenesis of endometriosis: a novel mechanistic approach, J. Biol. Regul. Homeost. Agents 30 (1) (2016) 31–40. [PubMed] [Google Scholar]
- [25].Huang Z, Wells D, The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome, Mol. Hum. Reprod 16 (10) (2010) 715–725. [DOI] [PubMed] [Google Scholar]
- [26].Wang Q, et al. , An intercellular pathway for glucose transport into mouse oocytes, Am. J. Physiol. Endocrinol. Metab 302 (12) (2012) E1511–E1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hashimoto S, et al. , Effects of cumulus cell density during in vitro maturation of the developmental competence of bovine oocytes, Theriogenology 49 (8) (1998) 1451–1463. [DOI] [PubMed] [Google Scholar]
- [28].Shaeib F, et al. , The defensive role of cumulus cells against reactive oxygen species insult in metaphase II mouse oocytes, Reprod. Sci 23 (4) (2016) 498–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Patel JM, Block ER, Hood CI, Biochemical indices of cyclophosphamide-induced lung toxicity, Toxicol. Appl. Pharmacol 76 (1) (1984) 128–138. [DOI] [PubMed] [Google Scholar]
- [30].Detti L, et al. , Serum markers of ovarian reserve and ovarian histology in adult mice treated with cyclophosphamide in pre-pubertal age, J. Assist. Reprod. Genet 30 (11) (2013) 1421–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Chen XY, et al. , Follicle loss and apoptosis in cyclophosphamide-treated mice: what's the matter? Int. J. Mol. Sci 17 (6) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Song D, et al. , Human umbilical cord mesenchymal stem cells therapy in cyclophosphamide-induced premature ovarian failure rat model, Biomed. Res. Int 2016 (2016) 2517514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Banerjee J, et al. , IL-6 and mouse oocyte spindle, PLoS One 7 (4) (2012) e35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Goud PT, et al. , Nitric oxide extends the oocyte temporal window for optimal fertilization, Free Radic. Biol. Med 45 (4) (2008) 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hoogenkamp H, Lewing P, Superovulation in mice in relation to their age, Vet. Q 4 (1) (1982) 47–48. [DOI] [PubMed] [Google Scholar]
- [36].Choi WJ, et al. , Oxidative stress and tumor necrosis factor-alpha-induced alterations in metaphase II mouse oocyte spindle structure, Fertil. Steril 88 (4 Suppl) (2007) S1220–S1231. [DOI] [PubMed] [Google Scholar]
- [37].Jiang Y, et al. , Accelerated ovarian aging in mice by treatment of busulfan and cyclophosphamide, J. Zhejiang Univ. Sci. B 14 (4) (2013) 318–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Miller JJ 3rd, Williams GF, Leissring JC, Multiple late complications of therapy with cyclophosphamide, including ovarian destruction, Am. J. Med 50 (4) (1971) 530–535. [DOI] [PubMed] [Google Scholar]
- [39].Korkmaz A, Topal T, Oter S, Pathophysiological aspects of cyclophosphamide and ifosfamide induced hemorrhagic cystitis; implication of reactive oxygen and nitrogen species as well as PARP activation, Cell Biol. Toxicol 23 (5) (2007) 303–312. [DOI] [PubMed] [Google Scholar]
- [40].Khan SN, et al. , Diffused intra-oocyte hydrogen peroxide activates myeloperoxidase and deteriorates oocyte quality, PLoS One 10 (7) (2015) e0132388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].O'Toole TE, et al. , Acrolein activates matrix metalloproteinases by increasing reactive oxygen species in macrophages, Toxicol. Appl. Pharmacol 236 (2) (2009) 194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Silva A, et al. , Intracellular reactive oxygen species are essential for PI3K/Akt/mTOR-dependent IL-7-mediated viability of T-cell acute lymphoblastic leukemia cells, Leukemia 25 (6) (2011) 960–967. [DOI] [PubMed] [Google Scholar]
- [43].Park KR, et al. , Beta-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation, Cancer Lett. 312 (2) (2011) 178–188. [DOI] [PubMed] [Google Scholar]
- [44].Rodriguez-Antona C, Ingelman-Sundberg M, Cytochrome P450 pharmacogenetics and cancer, Oncogene 25 (11) (2006) 1679–1691. [DOI] [PubMed] [Google Scholar]
- [45].Moghe A, et al. , Molecular mechanisms of acrolein toxicity: relevance to human disease, Toxicol. Sci 143 (2) (2015) 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sheweita SA, El-Hosseiny LS, Nashashibi MA, Protective effects of essential oils as natural antioxidants against hepatotoxicity induced by cyclophosphamide in mice, PLoS One 11 (11) (2016) e0165667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Gianaris A, et al. , Unilateral microinjection of acrolein into thoracic spinal cord produces acute and chronic injury and functional deficits, Neuroscience 326 (2016) 84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kehrer JP, Biswal SS, The molecular effects of acrolein, Toxicol. Sci. 57 (1) (2000) 6–15. [DOI] [PubMed] [Google Scholar]
- [49].Wahlang B, et al. , Role of cytochrome P450 monooxygenase in carcinogen and chemotherapeutic drug metabolism, Adv. Pharmacol 74 (2015) 1–33. [DOI] [PubMed] [Google Scholar]
- [50].Chen Y, et al. , Collateral damage in cancer chemotherapy: oxidative stress in nontargeted tissues, Mol. Interv 7 (3) (2007) 147–156. [DOI] [PubMed] [Google Scholar]
- [51].Stankiewicz A, Skrzydlewska E, Makiela M, Effects of amifostine on liver oxidative stress caused by cyclophosphamide administration to rats, Drug Metabol. Drug Interact 19 (2) (2002) 67–82. [DOI] [PubMed] [Google Scholar]
- [52].Oyagbemi AA, et al. , Cyclophosphamide-induced hepatotoxicity in wistar rats: the modulatory role of gallic acid as a hepatoprotective and chemopreventive phytochemical, Int. J. Prev. Med 7 (2016) 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Goud PT, et al. , Direct real-time measurement of intra-oocyte nitric oxide concentration in vivo, PLoS One 9 (6) (2014) e98720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Banerjee J, et al. , Peroxynitrite affects the cumulus cell defense of metaphase II mouse oocytes leading to disruption of the spindle structure in vitro, Fertil. Steril 100 (2) (2013) 578–584. [DOI] [PubMed] [Google Scholar]
- [55].Lord T, Martin JH, Aitken RJ, Accumulation of electrophilic aldehydes during postovulatory aging of mouse oocytes causes reduced fertility, oxidative stress, and apoptosis, Biol. Reprod 92 (2) (2015) 33. [DOI] [PubMed] [Google Scholar]
- [56].Suzuki T, et al. , Superoxide dismutase in normal cycling human ovaries: immunohistochemical localization and characterization, Fertil. Steril 72 (4) (1999) 720–726. [DOI] [PubMed] [Google Scholar]
- [57].Karuputhula NB, et al. , Oxidative status in granulosa cells of infertile women undergoing IVF, Syst. Biol. Reprod. Med 59 (2) (2013) 91–98. [DOI] [PubMed] [Google Scholar]
- [58].Dumesic DA, et al. , Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health, Fertil. Steril 103 (2) (2015) 303–316. [DOI] [PubMed] [Google Scholar]
- [59].Goud PT, et al. , Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis, Fertil. Steril 102 (1) (2014) 151–159. [DOI] [PubMed] [Google Scholar]
- [60].Goud AP, et al. , Reactive oxygen species and oocyte aging: role of superoxide, hydrogen peroxide, and hypochlorous acid, Free Radic. Biol. Med 44 (7) (2008) 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Hamann K, Shi R, Acrolein scavenging: a potential novel mechanism of attenuating oxidative stress following spinal cord injury, J. Neurochem 111 (6) (2009) 1348–1356. [DOI] [PubMed] [Google Scholar]
- [62].Muzio G, et al. , Liver cancer is induced by a subnecrogenic dose of DENA when associated with fasting/refeeding: role of glutathione-transferase and lipid peroxidation, Free Radic. Biol. Med 26 (9–10) (1999) 1314–1320. [DOI] [PubMed] [Google Scholar]
- [63].Detti L, et al. , Goserelin fosters bone elongation but does not prevent ovarian damage in cyclophosphamide-treated prepubertal mice, Fertil. Steril 101 (4) (2014) 1157–1164. [DOI] [PubMed] [Google Scholar]