Abstract
In the present study, we investigated the effect of CDK inhibitors (ribociclib, palbociclib, seliciclib, AZD5438, and dinaciclib) on malignant human glioma cells for cell viability, apoptosis, oxidative stress, and mitochondrial function using various assays. None of the CDK inhibitors induced cell death at a clinically relevant concentration. However, low nanomolar concentrations of dinaciclib showed higher cytotoxic activity against Bcl-xL silenced cells in a time- and concentration-dependent manner. This effect was not seen with other CDK inhibitors. The apoptosis-inducing capability of dinaciclib in Bcl-xL silenced cells was evidenced by cell shrinkage, mitochondrial dysfunction, DNA damage, and increased phosphatidylserine externalization. Dinaciclib was found to disrupt mitochondrial membrane potential, resulting in the release of cytochrome c, AIF, and smac/DIABLO into the cytoplasm. This was accompanied by the downregulation of cyclin-D1, D3, and total Rb. Dinaciclib caused cell cycle arrest in a time- and concentration-dependent manner and with accumulation of cells in the sub-G1 phase. Our results also revealed that dinaciclib, but not ribociclib or palbociclib or seliciclib or AZD5438 induced intrinsic apoptosis via upregulation of the levels of pro-apoptotic proteins (Bax and Bak), resulting in the activation of caspases and cleavage of PARP. We also found an additional mechanism for the dinaciclib-induced augmentation of apoptosis due to abrogation RAD51-cyclin D1 interaction, specifically proteolysis of the DNA repair proteins RAD51 and Ku80. Our results suggest that successfully interfering with Bcl-xL function may restore sensitivity to dinaciclib and could hold the promise for an effective combination therapeutic strategy.
Keywords: Bcl-xL silencing, caspase-dependent cell death, CDK inhibitors, RAD51 and Ku80 cleavage, synergy
1 |. INTRODUCTION
Malignant glioma has a dismal prognosis despite advances in multi-modality therapy. Malignant transformation is characterized by a number of genetic alterations, including inactivation of tumor suppressor genes such as p16, Rb, p53, and PTEN, and amplification of the CDK4 and EGFR genes.1–3 The cyclin-D/CDK4, CDK6/p16INK4a/pRB/E2F pathway, a key regulator of G1 to S phase transition of the cell cycle, is disrupted in the vast majority of human malignant gliomas and leads to dysregulated cell cycle progression. Common genomic alterations include homozygous deletion of CDKN2A/2B (52%), amplification of CDK4 (18%), amplification of CDK6 (1%), and deletion or mutation of RB (12%).1,4,5 Deregulated CDKs induce cell proliferation and chromosomal instability. In normal cells, DNA lesions are recognized and fixed by DNA damage response (DDR) factors and cells will resume normal proliferation. By contrast, when DNA damage is particularly severe, different DNA repair pathways are involved and signal cells to undergo programmed cell death. Malignant cells may evade this process by continuing to proliferate despite DNA damage, leading to accumulation of genomic alterations.
Given the central role of cyclin-D/CDK4/RB pathway in cell cycle control, it has been hypothesized that targeting CDKs may have broad antitumor activity in human malignancies. Although administration of flavopiridol exhibited some clinical activity in hematological malignancies,6 phase II clinical studies showed poor efficacy for solid tumors. Similarly, seliciclib (roscovitine) failed to produce antitumor activity both in preclinical and clinical studies as monotherapy. Because the first-generation pan-CDK inhibitors suffered from a low therapeutic index, second-generation pan-CDK inhibitors were developed; such as ribociclib, palbociclib, AZD5438, and dinaciclib to name a few. Ribociclib (LEE011, Novartis) selectively inhibits CDK4 and CDK6 at a low nanomolar concentration. It inhibits RB phosphorylation and causes cell cycle arrest.7 Ribociclib has shown efficacy in xenograft experimental models of neuroblastoma,7 rhabdomyosarcoma8 and is currently being evaluated in many clinical trials.9 Palbociclib (PD0332991, Pfizer) inhibits both CDK4 and CDK6 kinase activity, prevents RB phosphorylation and induces cell cycle arrest and antitumor activity in glioma.10 Michaud et al11 demonstrated that systemic administration of palbociclib crossed the blood-brain barrier in an orthotopic xenograft model of glioblastoma. AZD5438 (Astra Zeneca, Boston, MA) is an orally bioavailable inhibitor of cyclin ECDK2, cyclin A-CDK2, and cyclin B-CDK1 complexes with a promising clinical efficacy profile.12 However, a post hoc analysis of patients treated with AZD5438 showed a poorer outcome.13 Dinaciclib (SCH 727965/MK-7965; Merck & Co., Kenilworth, NJ), inhibits CDK1, 2, 5, 9, and RB phosphorylation and entered phase 2 and 3 clinical trials. Multiple studies in several cancer types demonstrated modest single-agent clinical activity.14,15 In a recent study, we observed that the effects of ribociclib, palbociclib, AZD5438, and dinaciclib were principally cytostatic rather than cytotoxic in glioma cell lines.16 We also observed that only dinaciclib but not ribociclib, palbociclib, seliciclib, or AZD5438 had significant proapoptotic effects on glioma when combined with ABT737 (Bcl2/Bcl-xL inhibitor), thus providing a rationale for further evaluation.
Through siRNA screening, we were able to identify the critical anti-apoptotic proteins, including Bcl-xL, that when inhibited, greatly promoted apoptotic signaling in glioma cells.16–22 We hypothesized that the functional blockade of Bcl-xL should sensitize these glioma cells to CDK inhibitors by restoring the apoptotic process. Because targeting BCL-2 family members with small molecules is challeng ing,23 and most of the BH3 mimetics bind their targets with poor affinity and induce cell death independently from the mitochondrial apoptotic pathway, in this study, we used lentiviral-based RNA interference to silence Bcl-xL function (genetic inhibition) to examine the impact on efficacy of CDK inhibitors. Our study suggests that low nanomolar concentrations of dinaciclib induced loss of mitochondrial membrane potential and caused a conformational change in proapoptotic molecules Bax and Bak, initiating cytochrome c release and caspase activation that resulted in cell death. We also demonstrated a unique mechanism linking DNA damage response to cell death.
2 |. MATERIALS AND METHODS
2.1 |. Cell lines
The malignant human glioma cell line U87 was obtained from the American Type Culture Collection (Manassas, VA). LNZ308 was provided by Dr. Nicolas de Tribolet (Lausanne, Switzerland). Cell culture conditions of these cell lines were as previously described.16 Cell lines used in this study were authenticated using Short Tandem Repeat (STR) analysis by ATCC cell line authentication service (Manassas, VA). Samples were processed using the ABI Prism® 3500xl Genetic Analyzer and data was analyzed using GeneMapper® ID-X v1.2 software (Applied Biosystems, Foster City, CA). The genetic profiles for the samples were identical to the reported profile.
2.2 |. Reagents and antibodies
Ribociclib, palbociclib, AZD5438, and dinaciclib were from Selleck (Houston, TX). All other chemicals used in this study were from Sigma (St. Louis, MO). The following antibodies were used: β-actin (#4970), Bcl-xL (#2764), phospho-Akt (#4051), total Akt (#9272), cyclin B1 (#4135), cyclin D1 (#2922), cyclin D3 (#2926), CDK4 (#2906), CDK6 (#3136), KU70 (#4104), PTEN (#9559), Phospho-RB (#9307), Bak (#3814), Bax (#2774), Mcl-1 (#4572), Cytochrome c (#4280), smac/DIABLO (#2954), total H2AX (#2595), phospho-H2AX (#2577), HSP70 (#4873), caspase-3 (#9664), caspase-7 (#9494), caspase-8 (#9746), caspase-9 (#9501), caspase-10 (#9752), and PARP (#9546) were from Cell Signaling Technology (Beverly, MA). AIF (sc-5586) was from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-Bax (#556467) and RAD51 (#ab63801) were from BD Pharmingen (San Diego, CA) and Abcam (Cambridge, MA), respectively.
2.3 |. Stable cell line generation
Bcl-xL and non-target control shRNA MISSION Lentiviral transduction particles used in this study were obtained from Sigma. LNZ308 and U87 human glioma cells were seeded in six-well plates and allowed to reach 70–80% confluence and infected according to the manufacturer’s recommendations (Sigma). The day after infection, medium was changed and cells were incubated with complete media containing puromycin (1 μg/mL). After 14 days, cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with Bcl-xL antibody.
2.4 |. Annexin V apoptosis assay
Apoptosis induction in vehicle- or inhibitor-treated cells was assayed by the detection of membrane externalization of phosphatidylserine using an Annexin V assay kit (Molecular Probes, Invitrogen, Carlsbad, CA) as described previously.24 2 × 105 cells were harvested at various intervals after treatment, washed with ice-cold phosphate-buffered saline (PBS), and resuspended in 200 μL of binding buffer. Annexin V-FITC and 1 μg/mL propidium iodide were added and cells were incubated for 15 min in a dark environment. Labeling was analyzed by flow cytometry with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA). Annexin V binds to phosphatidylserine, which trans-locates from the inner leaflet to the outer leaflet of the plasma membrane in apoptotic cells, so cells that are positive for annexin V staining (ie, high Annexin V signal) are undergoing apoptosis. PI staining provides a measure of cell viability and is used to distinguish between cells in early and late apoptosis.
2.5 |. DiOC6 labeling and detection of mitochondrial membrane depolarization
Mitochondrial membrane depolarization was measured as described previously.22 In brief, floating cells were collected, and attached cells were trypsinized and resuspended in PBS. Cells were loaded with 50 nmol/L 3’,3’-dihexyloxacarbo-cyanine iodide (DiOC6, Molecular Probes, Invitrogen) at 37°C for 15 min. The positively charged DiOC6 accumulates in intact mitochondria, whereas mitochondria with depolarized membranes accumulate less DiOC6. Cells were spun at 3000g, and rinsed with PBS twice and resuspended in 1 mL of PBS. Following acquisition of data (CellQuest software (Becton Dickinson, San Jose, CA)), the cell fluorescence information was saved in the Flow Cytometry Standard (.fcs) format. These files were then accessed with the FlowJo analysis software (Tree Star, Inc., Ashland, OR). Through this software, the fluorescence data were plotted as histograms, which were converted into and saved as Scalable Vector Graphics (.svg) files. Using Inkscape (The Inkscape Team), an Open Source vector graphics editor, the data was compiled into two-dimensional histogram overlays for comparative analysis. The loss of mitochondrial membrane potential was quantified in FlowJo by gating any left-shifted populations and subtracting from control, and the percentage of cells with decreased fluorescence was determined.
2.6 |. Determination of reactive oxygen species generation
Reactive oxygen species (ROS) production was monitored with the cell-permeable ROS indicator, 2′7′- dichlorodihydrofluorescein diacetate (H2DCFDA) (Invitrogen) as described by Cossarizza et al.25 After treatment, cells were washed with PBS, incubated with 5 μM H2DCFDA for 30 min, and washed again with PBS. Fluorescence intensity was measured by flow cytometry (Becton Dickinson). This dye (H2DCFDA) is a cell-permeable molecule, which is very sensitive to intracellular redox change.25 The functional role of ROS generation on apoptosis was assessed in additional experiments using the free radical scavenger N-acetyl-l-cysteine (NAC). Cells were pre-incubated with 5 mM NAC for 2 h, followed by co-incubation with inhibitors and assessment of apoptosis or ROS generation as described above.
2.7 |. Cell cycle analysis
The effect of varying concentrations of inhibitors on cell cycle distribution was determined by flow cytometric analysis of the nuclear DNA content as previously described.26 Briefly, cells grown exponentially to 50–60% confluency were exposed to the inhibitors or DMSO for a range of intervals, harvested, washed in ice-cold PBS, and fixed in 70% ethanol. DNA was stained by incubating the cells in PBS containing propidium iodide (50 μg/mL) and RNase A (1 mg/mL) for 60 min at room temperature. Sample was measured and percentages of cells in subG1, G1, S, and G2/M phases were analyzed by using a Becton Dickinson FACScan and Cell Quest software (Becton Dickinson Immunocytometry Systems).
2.8 |. Immunoprecipitation and western blotting analysis
Cells were washed in cold PBS and lysed in buffer containing protease inhibitors (Sigma, Catalog Number P8340) for 15 min on ice. Samples were centrifuged at 12 000g for 15 min, supernatants were isolated, and protein was quantified using Protein Assay Reagent (Pierce Chemical, Rockford, IL). Equal amounts of protein were separated by SDS polyacrylamide gel electrophoresis (PAGE) and electro-transferred onto a nylon membrane (Invitrogen). Nonspecific antibody binding was blocked by incubation of the membranes with 4% bovine serum albumin in Tris-buffered saline (TBS)/Tween 20 (0.1%). The membranes were then probed with appropriate dilutions of primary antibody overnight at 4°C. The antibody-labeled blots were washed three times in TBS/Tween 20 and incubated with a 1:2000 dilution of horseradish peroxidase-conjugated secondary antibody in TBS/Tween 20 at room temperature for 1 h. Proteins were visualized by Western Blot Chemiluminescence Reagent (Cell Signaling). Where indicated, the membranes were reprobed with antibodies against β-actin to ensure equal loading and transfer of proteins. For Bax and Bak immunoprecipitation, cell extracts were prepared by lysing 5 × 106 cells on ice for 30 min in CHAPS lysis buffer (10 mmol/L HEPES (pH 7.4), 150 mmol/L NaCl, 1% CHAPS, protease, phosphatase inhibitors). Lysates were clarified by centrifugation at 15 000g for 10 min at 4°C, and the protein concentrations in the supernatants were determined. Equal amounts of protein extracts were incubated overnight with primary antibody (active Bax, 6A7, Sigma or active Bak, 1Ab). Afterward, Dynabeads Protein G (Invitrogen) was added for 2 h, followed by magnetic separation of the immunoprecipitated fraction; Western blot analysis was carried out as described above.
Scanning densitometry was performed on Western blots using acquisition into Adobe Photoshop (Adobe Systems, Inc., San Jose, CA) followed by image analysis (UN-SCAN-IT gel TM, version 6.1, Silk Scientific, Orem, UT). Values in arbitrary numbers shown in the Western blots represent densitometer quantification of bands normalized to loading control.
2.9 |. Subcellular fractionation
Cells were treated with or without inhibitors and cytosolic proteins were fractionated as described previously.22,27 Briefly, cells were resuspended in a lysis buffer containing 0.025% digitonin, sucrose (250 mM), HEPES (20 mM; pH 7.4), MgCl2 (5 mM), KCl (10 mM), EDTA (1 mM), phenylmethylsulfonyl fluoride (1 mM), 10 μg/mL aprotinin, 10 μg/mL leupeptin. After 10 min incubation at 4°C, cells were centrifuged (2 min at 13 000g), and the supernatant (cytosolic fraction) was removed and frozen at −80°C for subsequent use. Mitochondrial proteins were isolated using Mitochondria Isolation Kit for Mammalian Cells (catalog number, 89874; Thermo Scientific, Rockford, IL) following the manufacturer’s instructions.
2.10 |. Transient transfection
Sequence-specific ON-TARGETplus siRNA for human RAD51 (catalog number J-003530-09-0002 and Ku80 (catalog number J-010491-05-0002), and non-target control siRNA (catalog number D-001830-01-05) sequences were used for this study (Dharmacon, Lafayette, CO). For overexpression studies, pCMV-6 vector (Myc-DDK-tagged, catalog number PS100001), or Myc-DDK tagged RAD51 (catalog number RC218333) or Myc-DDK tagged Ku80 (catalog number RC510649) expression plasmids were obtained from Origene (Rockville, MD). Cells were seeded in six-well plates (for Western blotting and annexin V/PI analysis) and allowed to reach 70–80% confluence. Logarithmically growing glioma cells were transfected as described previously.22 After 48 h post-transfection, medium was changed and cells were incubated with inhibitors for the indicated period. Cell viability (annexin V/propidium iodide binding) or Western blot analysis were carried out as described above.
2.11 |. Fluorescence microscopy
Cells were grown on chamber slides (Nalge Nunc, Naperville, IL) in growth medium, and, after an overnight attachment period, were exposed to selected concentrations of dinaciclib or vehicle (DMSO) for various intervals. Then cells were washed once with PBS and fixed with 3.7% formaldehyde for 30 min. After washing two times in PBS, cells were permeabilized with 0.1% Triton X-100 in PBS for 10 min. Cells were washed with PBS, blocked with 0.5% bovine serum albumin for 1 h and then incubated with primary antibodies overnight at 4°C. Slides were removed from the primary antibodies, washed with PBS and incubated with secondary antibody and Hoechst 33342 (Invitrogen) for 2 h at room temperature. The slides were washed again with PBS, mounted, and examined under a fluorescent microscope (EVOS, Thermo Fisher Scientific, Waltham, MA).
2.12 |. Statistical analysis
Unless otherwise stated, data are expressed as mean ± S.D. The significance of differences between experimental conditions was determined using a two-tailed Student’s t test. Differences were considered significant at P values <0.05.
3 |. RESULTS
3.1 |. Bcl-xL silencing causes an increase in cell death induced by nanomolar concentrations of dinaciclib
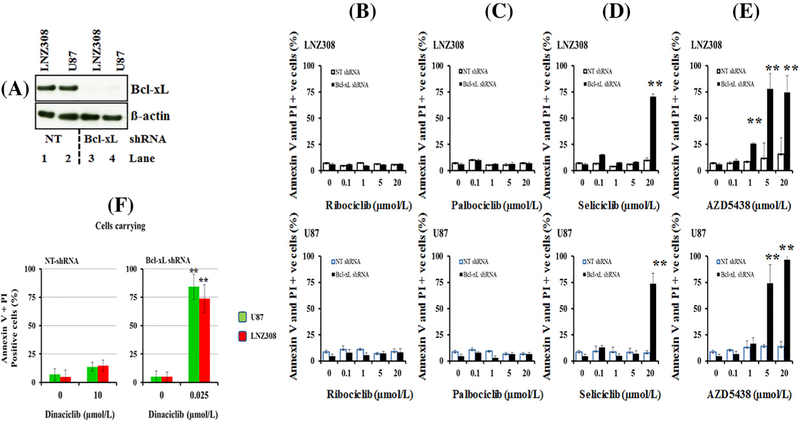
We and others have shown that CDK inhibitors induce cell death by antagonizing the activity of antiapoptotic Bcl-2 family proteins.16,28 In this study, we examined whether Bcl-xL, which is frequently overexpressed in glioma, is associated with resistance to CDK inhibitors. To experimentally address this question, we generated stable cell lines depleted of Bcl-xL or expressing non-target shRNA (Figure 1A). To determine if CDK inhibitors promote apoptosis, non-target control and Bcl-xL-depleted LNZ308 and U87 cells were treated with varying concentrations of ribociclib, palbociclib, seliciclib, AZD5438, and dinaciclib for 24 h. Cell viability was assessed by annexin V/propidium iodide assay. In LNZ308 and U87 cells (non-target shRNA-carrying cell lines), approximately 10% of the cells were double positive for PI and Annexin V after treatment with 20.0 μmol/L ribociclib (Figure 1B) and palbociclib (Figure 1C) for 24 h. This effect was not changed significantly in Bcl-xL silenced cells. However, cell death induced by seliciclib was significantly higher in Bcl-xL silenced cells as compared to non-target shRNA-carrying cells (Figure 1D). While roughly 10% of the non-target shRNA control group of cells were killed with seliciclib (20.0 μmol/L), silencing Bcl-xL significantly increased cell death to 70% (Bcl-xL silenced vs non-target group, P < 0.005). Increasing concentrations ofAZD5438 resulted in a dose-dependent decrease of cell viability in Bcl-xL silenced cells. For example, cells exposed to 5.0 μmol/L AZD5438 enhanced the cell death from 12% to 75% in LNZ308-Bcl-xL silenced cells and 15–65% in U87-Bcl-xL silenced cells compared to respective non-target vector carrying cell lines (Figure 1E). In contrast, unlike seliciclib and AZD5438, we observed a dramatic increase in dinaciclib-induced cell death at a low nanomolar concentration in Bcl-xL silenced cells. As shown in Figure 1F (left panel), 10 μmol/L of dinaciclib produced minimal or no cell death in non-target shRNA-carrying LNZ308 and U87 cells. In contrast, as low as 25 nmol/L of dinaciclib increased the cell death of Bcl-xL silenced cells by approximately 60% and 80% (LNZ308 and U87, respectively; Figure 1F, right panel), suggesting the differential response to CDK inhibitors and the important role of Bcl-xL in protecting cells from dinaciclib-induced cell death.
FIGURE 1.
Bcl-xL silencing causes an increase in cell death to dinaciclib at a nanomolar concentration. (A) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) expressing LNZ308 and U87 stable cell lines were generated as described in the Methods. Cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with Bcl-xL antibody. β-actin served as loading control. (B-F) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT shRNA) expressing LNZ308 (upper panel) or U87 cells (lower panel) were seeded at 60% confluence, allowed to attach overnight, and treated with indicated concentrations of ribociclib (B), palbociclib (C), seliciclib (D), AZD5438 (E), or dinaciclib (F) for 24 h. Control cells received an equivalent amount of DMSO. Apoptosis was analyzed by flow cytometry as described in section 2. The results represent the mean of three independent experiments (** P < 0.001, non-target vs Bcl-xL silenced cells)
3.2 |. Low concentrations of dinaciclib induce mitochondrial dysfunction in Bcl-xL silenced cells
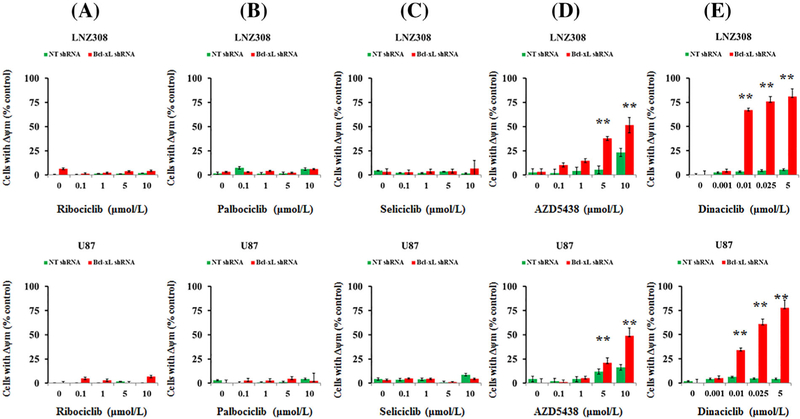
Apoptosis may be initiated by signaling at the plasma membrane or by intracellular pathways that lead to changes in mitochondria.29 Because Bcl-xL is predominantly located in the mitochondria and regulates mitochondrial energetics by stabilizing the membrane potential,30 we hypothesize that CDK inhibitors may cause mitochondrial dysfunction. Both non-target shRNA and Bcl-xL shRNA-transduced cells were treated with CDK inhibitors at the indicated concentrations, and mitochondrial membrane potential loss (Δψm) was analyzed by flow cytometry using DiOC6 dye. Ribociclib (Figure 2A), palbociclib (Figure 2B), seliciclib (Figure 2C), AZD5438 (Figure 2D), and dinaciclib (Figure 2E) caused a minimal or no change of mitochondrial membrane potential in cells stably expressing non-target shRNA vector. This effect was not changed by ribociclib (Figure 2A), palbociclib (Figure 2B), and seliciclib (Figure 2C) in Bcl-xL silenced cells. However, AZD5438 (Figure 2D) and dinaciclib (Figure 2E) caused a significant change in the reduction of mitochondrial membrane potentialin a concentration depend entmanner (ie, appearance of a population to the left suggesting the loss of mitochondrial membrane potential, Δψm; Supplementary Figure S1) in Bcl-xL silenced cells. Quantitative analysis of multiple experiments revealed that as low as 25 nmol/L of dinaciclib resulted in >60% loss of mitochondrial membrane potential in Bcl-xL silenced LNZ308 and U87 cells (Figure 2E).
FIGURE 2.
Low concentrations of dinaciclib induce mitochondrial dysfunction in Bcl-xL silenced cells. Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) expressing cells (LNZ308, upper panel; U87, lower panel) were treated with the indicated concentrations of ribociclib (A), palbociclib (B), seliciclib (C), AZD5438 (D), and dinaciclib (E) for 24 h. DMSO served as vehicle control (0). The integrity of the mitochondrial membranes of the cells was examined by DiOC6 staining and flow cytometry as described in Section 2. Histogram obtained from three independent experiments are shown here (** P < 0.001, non-target vs Bcl-xL silenced cells)
3.3 |. Dinaciclib induces Bax and Bak conformational changes, and the release of caspase activators in BclxL silenced cells
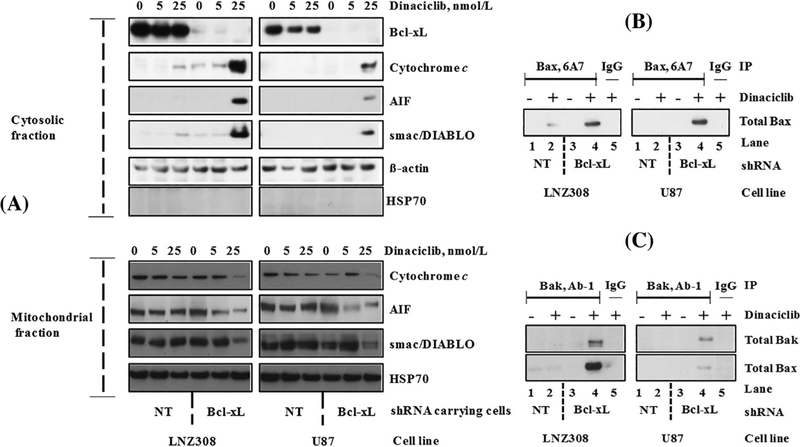
Most of the apoptotic signals that converge on mitochondria trigger the release of caspase activators (such as cytochrome c), changes in electron transport, and loss of mitochondrial transmembrane potential.31 Because cytochrome c release from mitochondria is an early and pivotal event in the apoptosis of many cell types,32 cytosolic and mitochondrial fractions were analyzed by immuno-blotting for cytochrome c, smac/DIABLO, and apoptosis-inducing factor (AIF). As shown in Figure 3A, treatment with dinaciclib strongly increased the release of cytochrome c, AIF and smac/DIABLO into the cytosol (Figure 3A, upper panel) of Bcl-xL depleted cells compared to non-target shRNA-transduced cells. Under the same conditions, the amount of cytochrome c, AIF, and smac/DIABLO in the mitochondrial fraction showed a corresponding decrease (Figure 3A, lower panel).
FIGURE 3.
Dinaciclib induces Bax and Bak conformational changes, and the release of caspase activators in Bcl-xL silenced cells. (A) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) carrying LNZ308 and U87 cells were seeded at 60% confluence, allowed to attach overnight, and treated with indicated concentrations of dinaciclib for 24 h. Cytosolic (upper panel) and mitochondrial (lower panel) fractions were prepared, and equal amounts of protein were separated by SDS-PAGE and subjected to Western blotting analysis with the indicated antibodies. Lack of HSP70 in the cytosolic fraction clearly demonstrates that the cytoplasmic cytochrome c, AIF, or smac/DIABLO did not result from the mitochondrial damage during the extraction process. (B) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) expressing LNZ308 or U87 cells were treated with or without dinaciclib (25 nmol/L) for 24 h. An equal amount of protein was immunoprecipitated (IP) with monoclonal anti-Bax (6A7; Sigma-Aldrich) antibody and then immunoblotted with polyclonal anti-Bax antibody (Cell Signaling Technology). Control IgG (Santa Cruz #2025) served as negative control (lane 5). (C) Bcl-xL depleted or non-target shRNA (NT) expressing LNZ308 or U87 cells were treated with or without dinaciclib (25 nmol/L) for 24 h. An equal amount of protein was immunoprecipitated (IP) with monoclonal anti-Bak (Ab-1; EMD Millipore) antibody and then immunoblotted with polyclonal anti-Bak antibody (Cell Signaling Technology) or with polyclonal anti-Bax antibody (Cell Signaling Technology). Dinaciclib induces Bax (B), Bak activation (C, upper panel), and Bax/Bak heterodimer formation (C, lower panel) in Bcl-xL silenced cells (compare lane 2 vs lane 4). Control IgG (Santa Cruz #2025) served as negative control (lane 5). The results of a representative study are shown; three additional experiments produced similar results
Bcl-2 family members Bax and Bak are crucial to the mitochondrial dysfunction-mediated apoptotic cell death pathways33 translocating to mitochondria and undergoing dramatic conformational changes.32 To investigate Bax and Bak involvement, we used Bax (6A7, monoclonal Bax antibody, Sigma) and Bak (1-Ab, monoclonal Bak antibody) antibodies that recognize the active conformations of the respective proteins. Immunoprecipitation followed by Western blot analysis revealed that treatment of cells expressing non-target shRNA (LNZ308 and U87) with dinaciclib induced minimal or no activation of Bax and Bak. However, an increased activation of Bax (Figure 3B) or Bak (Figure 3C) was evident in Bcl-xL silenced stable cell lines. We also observed that depletion of Bcl-xL triggers the formation of Bak/Bax heterodimers after dinaciclib treatment (Figure 3C, bottom panel).
3.4 |. Dinaciclib induces caspase-dependent cell death in Bcl-xL silenced cells
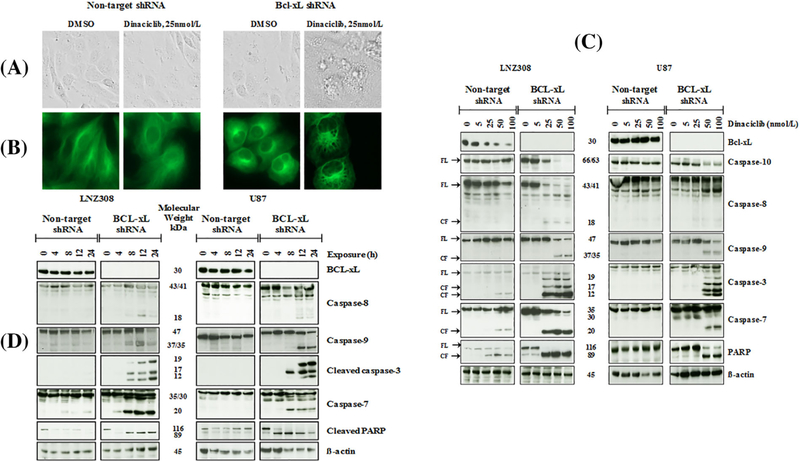
We observed striking morphological (Figure 4A) changes in dinaciclib-treated Bcl-xL silenced cells Figures 4A and B. Bcl-xL silenced cells were smaller than non-target shRNA-carrying cells. Further analysis revealed that low concentrations of dinaciclib led to an extensive formation of vacuoles (Figure 4A, comparing dinaciclib-treated non-target shRNA-carrying cells and dinaciclib-treated Bcl-xL silenced cells). We detected vacuoles within 3 h of treatment (data not shown) and, by 12 h, all cells contained numerous, large vacuoles (Figure 4A). Because loss of mitochondrial membrane potential can trigger a cascade of downstream events to initiate apoptosis, including the activation of caspases resulting in cell death,32 we examined the activation/cleavage of pro-caspase-9 and pro-capsase-8, which activates intrinsic and extrinsic apoptosis pathways, respectively, culminating in the cleavage of executioner caspase-3, caspase-7, and PARP. Silencing of Bcl-xL enhanced dinaciclib-induced caspase and PARP activation in a concentration (Figure 4C) and time (Figure 4D) dependent manner.
FIGURE 4.
Dinaciclib induces caspase-dependent cell death in Bcl-xL silenced cells. Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA expressing LNZ308 cells were grown on chamber slides in growth medium, and, after an overnight attachment period, were exposed to dinaciclib (25 nmol/L) or vehicle (DMSO) for 12 h. Images were taken using phase contrast microscopy (A). Cells were stained with Alexa Flour 488 Phalloidin (1:200, Invitrogen) as described in Section 2 (B). Dinaciclib induces morphological changes such as cell shrinkage, rounding, and membrane blebbing in Bcl-xL silenced cells but not in the non-target vector carrying cells (A). Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA expressing LNZ308 or U87 cells were seeded at 60% confluence, allowed to attach overnight, and exposed to indicated concentrations of dinaciclib for 24 h (C) or with 25 nmol/L dinaciclib for the indicated duration (h, hours) (D). Cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with indicated antibodies. β-actin served as loading control (FL, full length; CF, cleaved fragment). The results of a representative study are shown; three additional experiments produced similar results
3.5 |. Dinaciclib induces ROS generation in Bcl-xL depleted cells
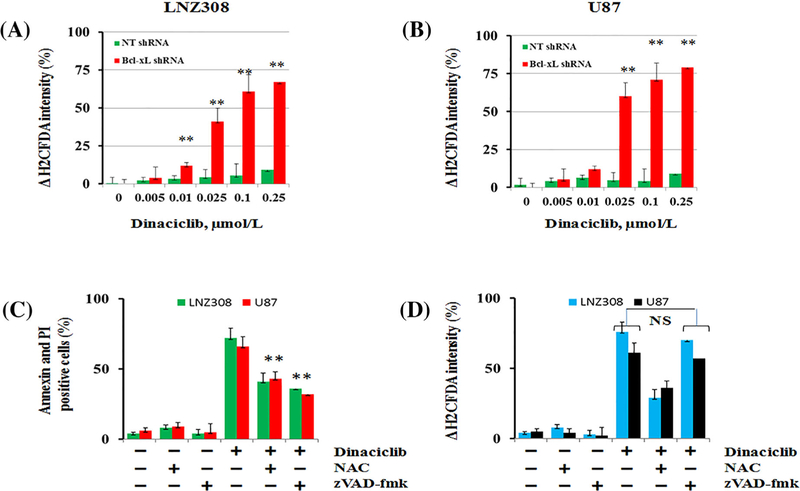
Because intracellular ROS generation following CDK inhibitor treatment can activate several pathways important for the induction of apoptosis,34 experiments were carried out to measure the capacity of dinaciclib to modulate intracellular ROS content. Minimal or no ROS accumulation was evident in non-target shRNA-carrying vector control cells. However, dinaciclib induced ROS generation in a dose-dependent manner in Bcl-xL silenced cells LNZ308 (Figure 5A) and U87 (Figure 5B) as measured by the ROS indicator H2DCFDA. To examine whether the generation of ROS induced by dinaciclib was accompanied by loss of (Δψm) and induction of apoptotic cell death, cells were pretreated with the ROS scavenger N-acetyl-L-cysteine (NAC) or zVAD-fmk (pan caspase inhibitor) before treatment with dinaciclib. NAC or zVAD-fmk alone caused no change in the loss of mitochondrial membrane potential or apoptosis. However, pretreatment with zVAD-fmk protected Bcl-xL silenced cells from dinaciclib-induced apoptosis (Figure 5C) but did not prevent the loss of mitochondrial membrane potential, indicating that zVAD-fmk may act downstream from the mitochondria (Figure 5D). However, pretreatment with NAC significantly protected cells from dinaciclib-induced cell death (Figure 5C) and loss of mitochondrial membrane potential (Figure 5D).
FIGURE 5.
Dinaciclib induces reactive oxygen species (ROS) generation in Bcl-xL silenced cells. Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT shRNA) expressing LNZ308 (A) or U87 (B) cells were exposed to indicated concentration of dinaciclib for 12 h. ROS generation was assessed by H2DCF-DA staining and flow cytometry as described in Section 2. Data are representative of triplicates in three independent experiments (** P < 0.005; non-target vs Bcl-xL silenced cells). (C and D) Bcl-xL depleted (LNZ308 and U87) cells were pretreated with ROS scavenger (NAC, 5 mmol/L) or pan caspase inhibitor, zVAD-fmk (50 μmol/L) for 2 h followed by dinaciclib (25 nmol/L) 24 h. Control cells received equivalent amounts of DMSO. (C) apoptosis (annexin V + PI binding assay) and (D) loss of mitochondrial membrane potential (Δψm, DiOC6 assay) were analyzed by flow cytometry as described in the Section 2. Data are representative of triplicates in three independent experiments. Annexin V and PI apoptosis assay (C) demonstrated that dinaciclib-induced cell death was inhibited by NAC (** P < 0.005) or zVAD-fmk (** P < 0.005). Preincubation of cells with NAC inhibited the loss of mitochondrial membrane potential (D). Preincubation of cells with zVAD-fmk caused no significant change in the dinaciclib-induced loss of mitochondrial membrane potential; NS, not significant).
3.6 |. Dinaciclib downregulates cell cycle regulatory proteins in Bcl-xL depleted cells
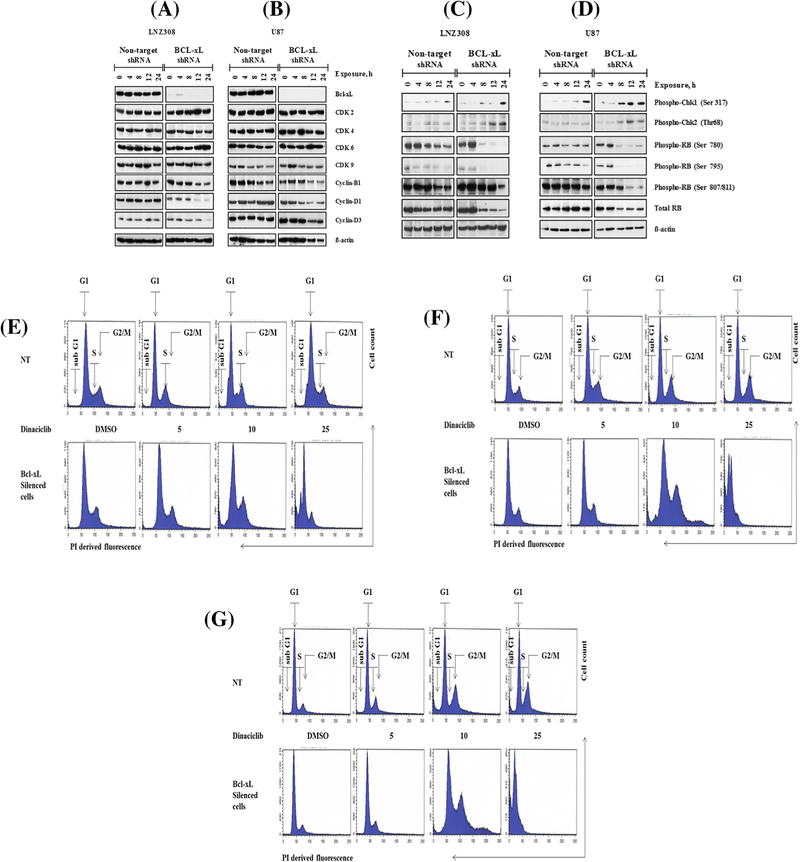
Because dinaciclib demonstrated strong selectivity to inhibit cell proliferation and to induce apoptosis in Bcl-xL silenced cells, we examined the impact of this agent on cell cycle regulatory proteins. As shown in Figures 6A and 6B, dinaciclib did not alter CDK2, CDK4, CDK6, and CDK9, cyclin B1, cyclin D1 and cyclin D3 protein levels in non-target shRNA-carrying LNZ308 (Figure 6A left panel) and U87 (Figure 6B, left panel), respectively. However, a significant reduction of cyclin B1, cyclin D1, and cyclin D3 protein level was seen following exposure to dinaciclib in the Bcl-xL depleted cells (Figure 6A right panel and 6B right panel).
FIGURE 6.
Effect of dinaciclib on the cell cycle profile and the expression levels of cell-cycle regulatory proteins. Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA expressing LNZ308 or U87 cells were seeded, allowed to attach overnight, and exposed to dinaciclib (25 nmol/L) for the indicated durations (h, hour). Cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with indicated antibodies. β-actin served as loading control (A-D). Bcl-xL depleted (Bcl-xL silenced cells) or non-target shRNA expressing (NT) LNZ308 cells were exposed to indicated concentrations of dinaciclib for 24 (E) or 48 (F) or 72 h (G). Control cells received an equivalent amount of DMSO. Cell cycle analysis using propidium iodide (PI) staining was performed as described in the Section 2. Representative cell cycle FACS plots are shown here. Three independent experiments produced similar results
Because Rb is sequentially phosphorylated by the CDK complexes (cyclin D-Cdk4/6 and cyclin E-Cdk2),9 we then examined phosphorylation status of Rb. Dinaciclib suppressed Rb phosphorylation level in a time-dependent manner in both in Bcl-xL depleted or non-target shRNA expressing cells. However, this effect was much more pronounced in Bcl-xL silenced cells than non-target shRNA expressing control cell lines. Treatment with dinaciclib did not affect the total Rb protein levels of non-target shRNA expressing cells. Surprisingly, we observed a marked reduction in dinaciclib-induced total Rb protein levels in Bcl-xL silenced cells (Figures 6C and 6D and Supplementary Figure S2A–D). Densitometric analysis (total Rb versus β-actin ratio) revealed that approximately 50–70% of Rb expression was inhibited in Bcl-xL silenced cells following exposure to dinaciclib. This was not seen in the non-target control shRNA carrying cells (non-target vs Bcl-xL silenced cells, P < 0.005; Supplementary Figure S2D).
Because CDK activity is required for maintaining cell cycle progression,9 we then examined the effect of dinaciclib on the cell cycle profile. Logarithmically growing cells (both Bcl-xL silenced and vector control) were treated with indicated concentrations of dinaciclib for 24, 48, and 72 h and then subjected to flow cytometric analyses. After 24 h, dinaciclib (25 nmol/L) induced an increase in cells in the sub G1 phase (apoptotic phase, >30%), which was only manifested in Bcl-xL silenced cells. This is in agreement with our Annexin V/PI (Figure 1F) and Western blot analysis (Figures 4C and 4D), suggesting that dinaciclib promotes apoptotic (increase in subG1 phase, Figure 6E–G) cell death in Bcl-xL silenced cells. Our data showed that about 25–30% of cells were observed in the G2/M phases in non-target shRNA expressing cells compared to a much lower percentage (~15%) in Bcl-xL-silenced cells. Changes in the cell cycle profile (significant reduction in the population in G1 phase and loss of G2M phase) of Bcl-xL silenced cells reached significance after 48 and 72 h of incubation (Figure 6E–G and Supplementary Figure S3A–C). Furthermore, Chk1 (Ser 317), and Chk 2 (Thr68) phosphorylation was more pronounced in Bcl-xL shRNA-carrying cells than vector carrying cells, suggesting that dinaciclib treatment can interfere with different stages of the cell cycle, and may ultimately lead to cell death.
3.7 |. Dinaciclib treatment promotes RAD51 and Ku80 proteolysis and exacerbates DNA damage response in Bcl-xL silenced cells
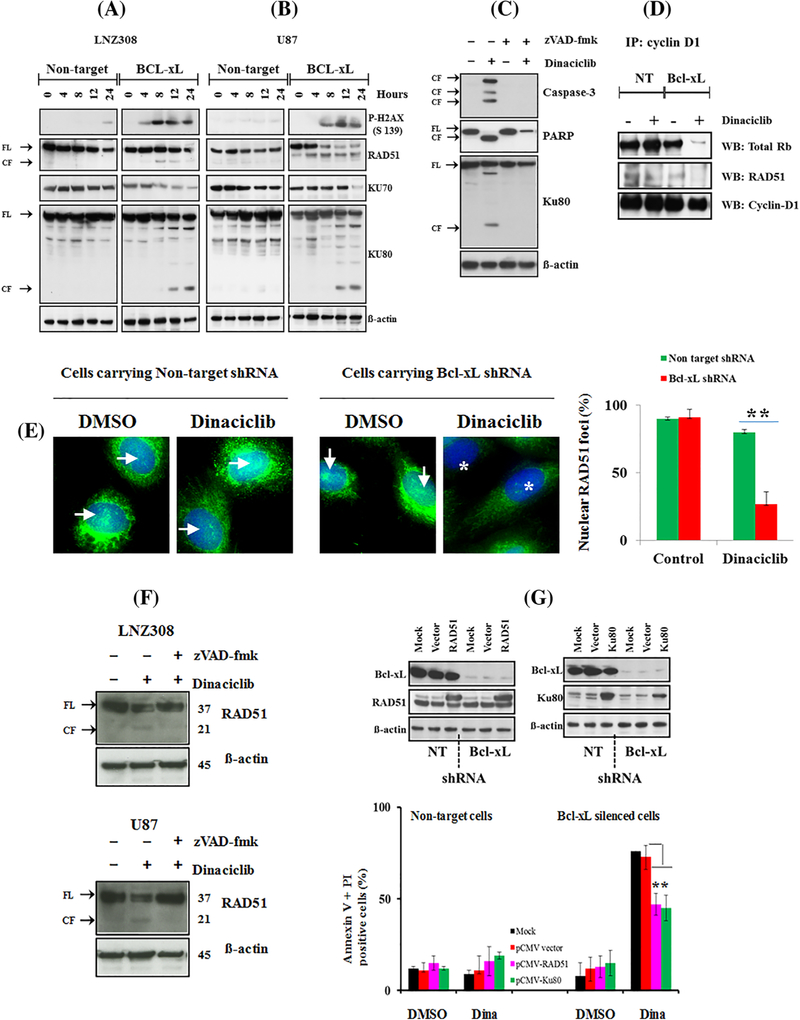
Previous studies have shown that inactivation of Rb (Rb knock-down by siRNA) generates signals similar to that produced in response to double-strand breaks (DSB).35 Because there was a marked reduction in dinaciclib-induced Rb protein (total Rb, Figures 6C and 6D and Supplementary Figure S2D), we employed both immunofluorescence and Western blot analysis to investigate the DNA damage response. First, we examined phosphorylation of γ-H2AX, a sensitive marker for unrepaired double strand breaks36,37 which was shown to correlate with cell death.38 Western blot analysis showed that dinaciclib caused increased levels of γ-H2AX phosphorylation in a time-dependent manner. Phosphorylation of γ-H2AX was more rapidly and robustly increased in Bcl-xL silenced cells than the non-target shRNA control cell lines (Figures 7A and 7B). Similarly, at least 80% of Bcl-xL silenced U87 and LNZ308 cells had γ-H2AX foci compared to only 10% of non-target control cells (Supplementary Figure S4).
FIGURE 7.
Dinaciclib exacerbates DNA damage response in Bcl-xL silenced cells. Bcl-xL depleted (Bcl-xL) or non-target shRNA expressing LNZ308 (A) or U87 (B) cells were seeded, allowed to attach overnight, and exposed to dinaciclib (25 nmol/L) for the indicated duration (hours). Cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with indicated antibodies (FL, full length; CF, cleaved fragment). β-actin served as loading control (A and B). (C) Bcl-xL depleted LNZ308 cells were pretreated with pan caspase inhibitor, zVAD-fmk (50 μmol/L) for 2 h followed by dinaciclib (25 nmol/L) for 24 h. Control cells received equivalent amount of DMSO. Cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with indicated antibodies. β-actin served as loading control (FL, full length; CF, cleaved fragment). (D) Bcl-xL depleted (Bcl-xL) or non-target shRNA (NT) expressing LNZ308 cells were treated with or without dinaciclib (25 nmol/L) for 24 h. An equal amount of protein was immunoprecipitated with cyclin D1 antibody (IP: cyclin D1) and then immunoblotted with indicated antibodies (WB, Western blot). (E) LNZ308 (NT-shRNA or Bcl-xL shRNA carrying) cells either untreated or treated with dinaciclib (10 nmol/L, for 24 h) were stained with RAD51 antibodies (polyclonal antibody at 1:100 dilutions, catalog number 63 801, Abcam. → indicates the presence of RAD51 staining in the nuclei; * indicates the absence of RAD51 staining in the nuclei). RAD51 foci from at least 200 cells from each treatment were counted. Y-axis represents the percent of cells with nuclear RAD51 staining per cell. Data represent mean ± SD of three independent experiments (** P < 0.005; dinaciclib-treated non-target versus Bcl-xL silenced cells). (F) Bcl-xL depleted LNZ308 (upper panel) and U87 (lower panel) cells were pretreated with pan caspase inhibitor, zVAD-fmk (50 μmol/L) for 2 h followed by dinaciclib (25 nmol/L) for 24 h. Control cells received equivalent amount of DMSO. Cell extracts were separated by SDS-PAGE and subjected to Western blotting analysis with indicated antibodies. β-actin served as loading control (FL, full length; CF, cleaved fragment). (G) Bcl-xL depleted (Bcl-xL shRNA) or non-target shRNA (NT) expressing LNZ308 cells were transfected with mock (no DNA), vector backbone (Myc-DDK vector), RAD51 (Myc-DDK-RAD51) or Ku80 (Myc-DDK-ku80) expression plasmids as described in the section 2. Cell lysates were collected after 48 h post-transfection, and protein was subjected to Western blot analysis using indicated antibodies (G, upper panel). In parallel (G, lower panel), cells were treated with or without dinaciclib (25 nmol/L, Dina) for 24 h. Control cells received equivalent amounts of DMSO. Cell viability (annexin V/propidium iodide assay) was performed as described in the Section 2. Graph representing three independent experiments are shown (**, P < 0.005; empty vector versus RAD51 or Ku80 expression plasmids in dinaciclib-treated Bcl-xL depleted cells)
We then examined Ku70, Ku80, and RAD51, which enable the repair of DNA double strand breaks through the non-homologous end joining pathway.39 As shown in Figures 7A and 7B, treatment with dinaciclib significantly reduced Ku70 protein levels. Interestingly, we observed the appearance of ~18 kDa fragment in the Ku80 blot (cleaved form of Ku80) in a time-dependent manner in Bcl-xL silenced cells but not in non-target shRNA-carrying cells, suggesting that treatment with dinaciclib promotes proteolytic cleavage of Ku80 in Bcl-xL-silenced cells. We also observed that in addition to the full-length RAD51 (molecular weight of 37 kDa), a 21 kDa cleaved fragment appeared after dinaciclib treatment (Figures 7A and 7B). Then to confirm that Ku80 cleavage was prior to caspase activation, we treated cells with zVAD-fmk (pan caspase inhibitor) for 2 h before treatment with dinaciclib and analyzed by Western blotting. As shown in Figure 7C, dinaciclib-induced Ku80 cleavage was blocked by caspase inhibition.
Because RAD51 directly binds cyclin D1 and is recruited to DNA damage sites,40 we hypothesized that Bcl-xL silencing would have a significant impact on the protein-protein interaction following dinaciclib treatment. Immunoprecipitation followed by Western blot analysis revealed that treatment with dinaciclib clearly reduced cyclin D1 and RAD51 interaction in Bcl-xL silenced cells (Figure 7D). Then, we looked at the intracellular distribution of RAD51. Immunofluorescence staining revealed that RAD51 is not only localized in the nuclei but also in the cytoplasm. In vehicle (DMSO) treated cells (both non-target shRNA and Bcl-xL shRNA groups), a large percentage of cells (90%) showed RAD51 protein in the nuclear and cytoplasmic compartments. However, the percentage of focally concentrated RAD51 (nuclear RAD51 staining) significantly decreased after dinaciclib treatment in Bcl-xL silenced cells but not in the vector carrying cells (Figure 7E). Because proteolytic cleavage of RAD51 by caspase 3 results in the loss of RAD51 function leading to DNA damage and apoptosis,41 we examined whether such an event could be caused by dinaciclib in Bcl-xL silenced cells. Pretreating Bcl-xL silenced cells with zVAD-fmk (pan caspase inhibitor) blocks the appearance of 21 kDa RAD51 fragment, suggesting the role for caspase-mediated cleavage of RAD51 (Figure 7F). Finally, to address whether ectopic expression of RAD51 and Ku80 could protect against dinaciclib-induced cell death, cells were transiently transfected with pCMV6 or pCMV6-Myc-DDK-RAD51 or pCMV6-Myc-DDK-Ku80 and treated with or without dinaciclib. Apoptosis was evaluated by annexin V and propidium iodide analysis. In contrast to pCMV6 transfected cells, ectopic expression of RAD51 and Ku80 partially but significantly reduced apoptotic cell death in Bcl-xL silenced cells, suggesting that RAD51 and Ku80 exerts protection against dinaciclib-induced cell death (Figure 7G).
4 |. DISCUSSION
Although the concept of pharmacological inhibition of CDK has scientific rationale, CDK inhibitors have shown poor clinical efficacy as single agents, possibly because of the multiplicity of targets that contribute to cell cycle regulation and cell survival.9,14,42–44 In this report, we showed that glioma cells are resistant to ribociclib, palbociclib, seliciclib, AZD5438, and dinaciclib at concentrations well above the clinically achievable range to induce apoptosis in vitro. However, disruption of Bcl-xL resulted in a marked increase in dinaciclib-induced cell death at low nanomolar concentrations. Importantly, this effect was not seen with other CDK inhibitors. Investigating the molecular mechanisms, we found that silencing Bcl-xL led to severe mitochondrial dysfunction and persistent DNA damage, suggesting a functional role for Bcl-xL which may translate dinaciclib into clinical relevance.
Apoptosis can be initiated by both mitochondrial-dependent (intrinsic) and mitochondrial-independent (extrinsic) pathways.45 Multiple studies suggest that flavopiridol causes outer mitochondrial membrane permeability and mitochondrial dysfunction by depleting Bcl-2 family members, particularly Bcl-xL and Mcl-1.46,47 We observed no drop in the mitochondrial membrane potential after treatment with ribociclib, palbociclib, and seliciclib (both in vector control and Bcl-xL depleted cells). A minimal (~15%) loss of mitochondrial membrane potential at 1.0 μmol/L was observed in AZD5438-treated Bcl-xL silenced cells. However, dinaciclib strongly induced mitochondrial membrane depolarization (>60% loss of mitochondrial membrane potential at 25 nmol/L), in Bcl-xL depleted cells. In contrast, we found no change in the non-target shRNA expressing cells after dinaciclib treatment (~8% loss of mitochondrial membrane potential at 5.0 μmol/L). On the contrary, there was no impairment in mitochondrial function or the activation of caspases after ribociclib, palbociclib, and seliciclib treatments. In this context, Bax/Bak-induced damage of mitochondrial membranes could play an essential role in cell death. Because dinaciclib-induced cell death but not loss of mitochondrial membrane potential could be blocked using the pan-caspase inhibitor zVAD-fmk, it was apparent that mitochondrial dysfunction constituted an upstream event of caspase activation. This observation is in agreement with our recent study involving pharmacological inhibition of Bcl-xL using ABT737 followed by dinaciclib treatment, which synergistically induced apoptosis in malignant human glioma cell lines.16 Numerous reports have linked ROS with mitochondrial dysfunction, DNA damage and apoptosis.48–51 Here, we demonstrated that the ROS scavenger (NAC) repressed not only dinaciclib-induced apoptosis, but also the loss of mitochondrial membrane potential. Although the underlying mechanism by which dinaciclib induces ROS at very low doses in Bcl-xL silenced cells remains unclear, it is possible that as described in other experimental settings,52 the increase in ROS levels produced by dinaciclib could activate the loss of mitochondrial membrane potential, cytochrome c release, and subsequent cell death.
Cyclin D proteins play an important role in the progression through G1 and entry into S phase. Suppression of CDK activity elicits multiple aspects of DNA damage response, including checkpoint control and repair in Bcl-xL silenced cells.53–55 As other investigators have noted,7,56–58 we observed that elevated levels of cyclin B1 and phosphorylation of Rb were greatly attenuated by dinaciclib. As suggested by Lundberg and Weinberg,59 selective inactivation of either Cdk4/6 or Cdk2 by dinaciclib may result in an inability of cyclin D/Cdk4/6 complexes to completely phosphorylate RB. Furthermore, enhanced phosphorylation of histone γH2AX, CHK1, and CHK2 by dinaciclib may be due to the collapse of replication forks and increase in double stranded DNA breaks.60 These findings led us to investigate Rad51, a key component of homologous recombination and DNA damage response.61,62 We observed that substantial amounts of RAD51 exist in both in the cytoplasm and nuclei of untreated control cells. However, dinaciclib treatment significantly decreased levels of nuclear RAD51. Western analysis showed the appearance of 21 kDa RAD51 fragment in Bcl-xL silenced cells. This effect was not seen in dinaciclib-treated vector-carrying control cells. This could be due to proteolysis during the course of apoptosis as described by Huang et al41 where they have shown that RAD51 is cleaved by caspase-3 and other apoptosis-related proteases. This was supported by our finding that pretreating cells with zVAD-fmk blocked dinaciclib-induced RAD51 cleavage. Because RAD51 was identified as a cyclin D1 protein binding partner,40 we also examined this interaction by immunoprecipitation analysis. Our study revealed that thedinaciclib treatmentcaused a reductionofcyclinD1/RAD51 association and could impair the recruitment of RAD51 to damaged DNA (as evidenced by lack of nuclear RAD51 after dinaciclib treatment), thus impeding homologous recombination-mediated DNA repair in Bcl-xL silenced cells. Our results also demonstrated that treatment with dinaciclib resulted in proteolysis of Ku80. This was in agreement with Song et al,63 where they have shown that Kuproteins were downregulated and cleaved by proteases under oxidative stress conditions. Their study also suggests that ROS played an important role in reducing the DNA repair activity by degrading Ku proteins.
Because several studies have implicated p53 in mediating differential responses to CDK inhibitors,64–68 we used p53 wild-type (U87) and p53-deleted (LNZ308) cell lines and clearly demonstrated that the apoptotic response observed in this study was not influenced by p53 functional status. In summary, because a substantial amount of mitochondrial energy is required for cell-cycle progression,69 we conclude that the loss of overall mitochondrial integrity and resultant DNA damage may contribute to the observed cell death in response to dinaciclib in Bcl-xL silenced cells. The concentrations of dinaciclib we evaluated were within the physiologically relevant/clinically achievable (82–184 nmol/L) range.14,43 Thus, successfully interfering with Bcl-xL function may promote sensitivity to dinaciclib.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Alexis Styche for FACS analysis. This work was supported by the Connor’s Cure Foundation Fund, the Translational Brain Tumor Research Fund, and the Scientific Program Fund of the Children’s Hospital of Pittsburgh Foundation (all to Ian F. Pollack).
Funding information
Connor’s Cure Foundation; Translational Brain Tumor Research; Scientific Program Fund of the Children’s Hospital of Pittsburgh Foundation
Abbreviations:
- AIF
apoptosis inducing factor
- BSA
bovine serum albumin
- CDK
cyclin dependent kinase
- DMSO
dimethyl sulfoxide
- DSB
double strand break
- FACS
fluorescence activated cell sorting
- FITC
fluorescein isothiocyanate
- PAGE
polyacrylamide gel electrophoresis
- PARP
poly ADP-ribose polymerase
- PBS
phosphate-buffered saline
- PDGFR
platelet derived growth factor receptor
- PI
propidium iodide
- ROS
reactive oxygen species
- TBS
Tris-buffered saline
Footnotes
CONFLICTS OF INTEREST
No potential conflicts of interest were disclosed.
SUPPORTING INFORMATION
Additional Supporting Information may be found online in the supporting information tab for this article.
REFERENCES
- 1.Bastien JI, McNeill KA Fine HA. Molecular characterizations of glioblastoma, targeted therapy, and clinical results to date. Cancer. 2015;121:502–516. [DOI] [PubMed] [Google Scholar]
- 2.Bleeker FE, Molenaar RJ Leenstra S. Recent advances in the molecular understanding of glioblastoma. J Neurooncol. 2012;108:11–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wen PY, Kesari S Drappatz J. Malignant gliomas: strategies to increase the effectiveness of targeted molecular treatment. Expert Rev Anticancer Ther. 2006;6:733–754. [DOI] [PubMed] [Google Scholar]
- 4.Ohgaki H, Dessen P, Jourde B, et al. Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64:6892–6899. [DOI] [PubMed] [Google Scholar]
- 5.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin TS, Ruppert AS, Johnson AJ, et al. Phase II study of flavopiridol in relapsed chronic lymphocytic leukemia demonstrating high response rates in genetically high-risk disease. J Clin Oncol. 2009;27:6012–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rader J, Russell MR, Hart LS, et al. Dual CDK4/CDK6 inhibition induces cell-cycle arrest and senescence in neuroblastoma. Clin Cancer Res. 2013;19:6173–6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olanich ME, Sun W, Hewitt SM, Abdullaev Z, Pack SD, Barr FG. CDK4 amplification reduces sensitivity to CDK4/6 inhibition in fusion-positive rhabdomyosarcoma. Clin Cancer Res. 2015;21:4947–4959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Otto T Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17:93–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fry DW, Harvey PJ, Keller PR, et al. Specific inhibition of cyclin-dependent kinase 4/6 by PD 0332991 and associated antitumor activity in human tumor xenografts. Mol Cancer Ther. 2004;3:1427–1438. [PubMed] [Google Scholar]
- 11.Michaud K, Solomon DA, Oermann E, et al. Pharmacologic inhibition of cyclin-dependent kinases 4 and 6 arrests the growth of glioblastoma multiforme intracranial xenografts. Cancer Res. 2010;70:3228–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Camidge DR, Smethurst D, Growcott J, et al. A first-in-man phase I tolerability and pharmacokinetic study of the cyclin-dependent kinase-inhibitor AZD5438 in healthy male volunteers. Cancer Chemother Pharmacol. 2007;60:391–398. [DOI] [PubMed] [Google Scholar]
- 13.Boss DS, Schwartz GK, Middleton MR, et al. Safety, tolerability, pharmacokinetics and pharmacodynamics of the oral cyclin-dependent kinase inhibitor AZD5438 when administered at intermittent and continuous dosing schedules in patients with advanced solid tumours. Ann Oncol. 2010; 21:884–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gojo I, Sadowska M, Walker A, et al. Clinical and laboratory studies of the novel cyclin-dependent kinase inhibitor dinaciclib (SCH 727965) in acute leukemias. Cancer Chemother Pharmacol. 2013;72:897–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar SK, LaPlant B, Chng WJ, et al. Dinaciclib, a novel CDK inhibitor, demonstrates encouraging single-agent activity in patients with relapsed multiple myeloma. Blood. 2015; 125:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jane EP, Premkumar DR, Cavaleri JM, Sutera PA, Rajasekar T, Pollack IF. Dinaciclib, a cyclin-dependent kinase inhibitor promotes proteasomal degradation of mcl-1 and enhances ABT-737-mediated cell death in malignant human glioma cell lines. J Pharmacol Exp Ther. 2016; 356:354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kitchens CA, McDonald PR, Shun TY, Pollack IF Lazo JS. Identification of chemosensitivity nodes for vinblastine through small interfering RNA high-throughput screens. J Pharmacol Exp Ther. 2011;339:851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thaker NG, Zhang F, McDonald PR, Shun TY, Lazo JS, Pollack IF. Functional genomic analysis of glioblastoma multiforme through short interfering RNA screening: a paradigm for therapeutic development. Neurosurg Focus. 2010;28:E4. [DOI] [PubMed] [Google Scholar]
- 19.Thaker NG Pollack IF. Molecularly targeted therapies for malignant glioma: rationale for combinatorial strategies. Expert Rev Neurother. 2009;9:1815–1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thaker NG, Zhang F, McDonald PR, et al. Identification of survival genes in human glioblastoma cells by small interfering RNA screening. Mol Pharmacol. 2009;76:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thaker NG, McDonald PR, Zhang F, et al. Designing, optimizing, and implementing high-throughput siRNA genomic screening with glioma cells for the discovery of survival genes and novel drug targets. J Neurosci Methods. 2010; 185:204–212. [DOI] [PubMed] [Google Scholar]
- 22.Jane EP, Premkumar DR, Sutera PA, Cavaleri JM Pollack IF. Survivin inhibitor YM155 induces mitochondrial dysfunction, autophagy, DNA damage and apoptosis in Bcl-xL silenced glioma cell lines. Mol Carcinog. 2017;56:1251–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juin P, Geneste O, Gautier F, Depil S Campone M. Decoding and unlocking the BCL-2 dependency of cancer cells. Nat Rev Cancer. 2013;13:455–465. [DOI] [PubMed] [Google Scholar]
- 24.Jane EP, Premkumar DR, Morales A, Foster KA Pollack IF. Inhibition of phosphatidylinositol 3-kinase/AKT signaling by NVP-BKM120 promotes ABT-737-induced toxicity in a caspase-dependent manner through mitochondrial dysfunction and DNA damage response in established and primary cultured glioblastoma cells. J Pharmacol Exp Ther. 2014;350:22–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cossarizza A, Ferraresi R, Troiano L, et al. Simultaneous analysis of reactive oxygen species and reduced glutathione content in living cells by polychromatic flow cytometry. Nat Protoc. 2009;4: 1790–1797. [DOI] [PubMed] [Google Scholar]
- 26.Jane EP, Premkumar DR Pollack IF. AG490 influences UCN-01-induced cytotoxicity in glioma cells in a p53-dependent fashion, correlating with effects on BAX cleavage and BAD phosphorylation. Cancer Lett. 2007;257:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Premkumar DR, Jane EP, Agostino NR, Didomenico JD Pollack IF. Bortezomib-induced sensitization of malignant human glioma cells to vorinostat-induced apoptosis depends on reactive oxygen species production, mitochondrial dysfunction, Noxa upregulation, Mcl-1 cleavage, and DNA damage. Mol Carcinog, 2012;52:118–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen S, Dai Y, Pei XY, et al. CDK inhibitors upregulate BH3-only proteins to sensitize human myeloma cells to BH3 mimetic therapies. Cancer Res. 2012;72:4225–4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christofferson DE Yuan J. Necroptosis as an alternative form of programmed cell death. Curr Opin Cell Biol. 2010;22:263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen YB, Aon MA, Hsu YT, et al. Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J Cell Biol. 2011;195:263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green DR Reed JC. Mitochondria and apoptosis. Science. 1998; 281:1309–1312. [DOI] [PubMed] [Google Scholar]
- 32.Youle RJ Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. [DOI] [PubMed] [Google Scholar]
- 33.Wei MC, Zong WX, Cheng EH, Lindsten T,. et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001; 292:727–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oh JH, Park EJ, Park JW, Lee J, Lee SH, Kwon TK. A novel cyclin-dependent kinase inhibitor down-regulates tumor necrosis factor-alpha (TNF-alpha)-induced expression of cell adhesion molecules by inhibition of NF-kappaB activation in human pulmonary epithelial cells. Int Immunopharmacol. 2010;10:572–579. [DOI] [PubMed] [Google Scholar]
- 35.Pickering MT Kowalik TF. Rb inactivation leads to E2F1-mediated DNA double-strand break accumulation. Oncogene. 2006;25:746–755. [DOI] [PubMed] [Google Scholar]
- 36.Taneja N, Davis M, Choy JS, et al. Histone H2AX phosphorylation as a predictor of radiosensitivity and target for radiotherapy. J Biol Chem. 2004;279:2273–2280. [DOI] [PubMed] [Google Scholar]
- 37.Kao GD, Jiang Z, Fernandes AM, Gupta AK Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem 2007; 282:21206–21212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banath JP Olive PL. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003;63:4347–4350. [PubMed] [Google Scholar]
- 39.Pierce AJ, Stark JM, Araujo FD, Moynahan ME, Berwick M, Jasin M. Double-strand breaks and tumorigenesis. Trends Cell Biol. 2001;11:S52–59. [DOI] [PubMed] [Google Scholar]
- 40.Jirawatnotai S, Hu Y, Michowski W, et al. A function for cyclin D1 in DNA repair uncovered by protein interactome analyses in human cancers. Nature. 2011;474:230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, Nakada S, Ishiko T, et al. Role for caspase-mediated cleavage of Rad51 in induction of apoptosis by DNA damage. Mol Cell Biol. 1999;19:2986–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Flaherty KT, Lorusso PM, Demichele A, et al. Phase I, dose-escalation trial of the oral cyclin-dependent kinase 4/6 inhibitor PD 0332991, administered using a 21-day schedule in patients with advanced cancer. Clin Cancer Res. 2012; 18:568–576. [DOI] [PubMed] [Google Scholar]
- 43.Mita MM, Joy AA, Mita A, et al. Randomized phase II trial of the cyclin-dependent kinase inhibitor dinaciclib (MK-7965) versus capecitabine in patients with advanced breast cancer. Clin Breast Cancer. 2014;14:169–176. [DOI] [PubMed] [Google Scholar]
- 44.Camidge DR, Pemberton M, Growcott J, et al. A phase I pharmacodynamic study of the effects of the cyclin-dependent kinase-inhibitor AZD5438 on cell cycle markers within the buccal mucosa, plucked scalp hairs and peripheral blood mononucleocytes of healthy male volunteers. Cancer Chemother Pharmacol. 2007;60:479–488. [DOI] [PubMed] [Google Scholar]
- 45.Roos WP, Thomas AD Kaina B. DNA damage and the balance between survival and death in cancer biology. Nat Rev Cancer. 2016;16:20–33. [DOI] [PubMed] [Google Scholar]
- 46.Kroemer G, Galluzzi L Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. [DOI] [PubMed] [Google Scholar]
- 47.Hussain SR, Lucas DM, Johnson AJ, et al. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 49.Storz P. Reactive oxygen species in tumor progression. Front Biosci. 2005;10:1881–1896. [DOI] [PubMed] [Google Scholar]
- 50.Bergers G Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. [DOI] [PubMed] [Google Scholar]
- 51.Moody CS Hassan HM. Mutagenicity of oxygen free radicals. Proc Natl Acad Sci U S A. 1982;79:2855–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cai J Jones DP. Superoxide in apoptosis. Mitochondrial generation triggered by cytochrome c loss. J Biol Chem. 1998;273:11401–11404. [DOI] [PubMed] [Google Scholar]
- 53.Parsels LA, Tanska DM, Parsels JD, et al. Dissociation of gemcitabine chemosensitization by CHK1 inhibition from cell cycle checkpoint abrogation and aberrant mitotic entry. Cell Cycle. 2016;15:730–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collins I Garrett MD. Targeting the cell division cycle in cancer: CDK and cell cycle checkpoint kinase inhibitors. Curr Opin Pharmacol. 2005;5:366–373. [DOI] [PubMed] [Google Scholar]
- 55.Guzi TJ, Paruch K, Dwyer MP, et al. Targeting the replication checkpoint using SCH 900776, a potent and functionally selective CHK1 inhibitor identified via high content screening. Mol Cancer Ther. 2011; 10:591–602. [DOI] [PubMed] [Google Scholar]
- 56.Bose P, Simmons GL Grant S. Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin Investig Drugs. 2013;22:723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barton KL, Misuraca K, Cordero F, et al. PD-0332991, a CDK4/6 inhibitor, significantly prolongs survival in a genetically engineered mouse model of brainstem glioma. PLoS ONE. 2013;8:e77639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Logan JE, Mostofizadeh N, Desai AJ, et al. PD-0332991, a potent and selective inhibitor of cyclin-dependent kinase 4/6, demonstrates inhibition of proliferation in renal cell carcinoma at nanomolar concentrations and molecular markers predict for sensitivity. Anticancer Res. 2013; 33:2997–3004. [PubMed] [Google Scholar]
- 59.Lundberg AS Weinberg RA. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin-cdk complexes. Mol Cell Biol. 1998;18:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou BB Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433–439. [DOI] [PubMed] [Google Scholar]
- 61.Sak A, Stueben G, Groneberg M, Bocker W Stuschke M. Targeting of Rad51-dependent homologous recombination: implications for the radiation sensitivity of human lung cancer cell lines. Br J Cancer. 2005;92:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kiyohara E, Tamai K, Katayama I Kaneda Y. The combination of chemotherapy with HVJ-E containing Rad51 siRNA elicited diverse anti-tumor effects and synergistically suppressed melanoma. Gene Ther. 2012;19:734–741. [DOI] [PubMed] [Google Scholar]
- 63.Song JY, Lim JW, Kim H, Morio T Kim KH. Oxidative stress induces nuclear loss of DNA repair proteins Ku70 and Ku80 and apoptosis in pancreatic acinar AR42J cells. J Biol Chem. 2003;278:36676–36687. [DOI] [PubMed] [Google Scholar]
- 64.Huskey NE, Guo T, Evason KJ, et al. CDK1 inhibition targets the p53-NOXA-MCL1 axis, selectively kills embryonic stem cells, and prevents teratoma formation. Stem Cell Rep. 2015;4:374–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cheok CF, Dey A Lane DP. Cyclin-dependent kinase inhibitors sensitize tumor cells to nutlin-induced apoptosis: a potent drug combination. Mol Cancer Res. 2007;5:1133–1145. [DOI] [PubMed] [Google Scholar]
- 66.Coley HM, Shotton CF, Kokkinos MI Thomas H. The effects of the CDK inhibitor seliciclib alone or in combination with cisplatin in human uterine sarcoma cell lines. Gynecol Oncol. 2007;105:462–469. [DOI] [PubMed] [Google Scholar]
- 67.Hahntow IN, Schneller F, Oelsner M, et al. Cyclin-dependent kinase inhibitor Roscovitine induces apoptosis in chronic lymphocytic leukemia cells. Leukemia. 2004;18:747–755. [DOI] [PubMed] [Google Scholar]
- 68.Fu W, Ma L, Chu B, et al. The cyclin-dependent kinase inhibitor SCH 727965 (dinacliclib) induces the apoptosis of osteosarcoma cells. Mol Cancer Ther. 2011;10:1018–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le HV, Minn AJ, Massague J. Cyclin-dependent kinase inhibitors uncouple cell cycle progression from mitochondrial apoptotic functions in DNA-damaged cancer cells. J Biol Chem. 2005; 280:32018–32025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.