Abstract
Pharmacoresistance is a major clinical challenge for approximately 30% of patients with epilepsy. Previous studies indicate nuclear receptors (NRs), efflux transporters and cytochrome P450 enzymes (CYPs) control drug passage across the blood-brain barrier (BBB) in drug resistant epilepsy. Here we 1) evaluate BBB changes, neurovascular nuclear receptors, and drug transporters in lesional/epileptic (EPI) and non-lesional/non-epileptic (NON-EPI) regions of the same brain, 2) examine regional CYP expression and activity, and 3) investigate the association among CYP brain expression, seizure frequency, duration of epilepsy, and antiepileptic drug (AED) combination. We used surgically resected brain specimens from patients with medically intractable epilepsy (n = 22) where the epileptogenic loci were well-characterized by invasive and non-invasive methods; histology confirmed distinction of small NON-EPI regions from EPI tissues. NRs, transporters, CYPs, and tight-junction proteins were assessed by western blots/immunohistochemistry, and CYP metabolic activity was determined and compared. The relationship of CYP expression with seizure frequency, duration of epilepsy and prescribed AEDs was evaluated. Decreased BBB tight-junction proteins accompanied IgG leakage in EPI regions and correlated with upregulated NR and efflux transporter levels. CYP expression and activity significantly increased in EPI compared to NON-EPI tissues. Change in EPI and NON-EPI CYP3A4 expression increased in patients taking AEDs that were CYP substrates, was downregulated when CYP- and non-CYP-substrate AEDs were given together, and correlated with seizure frequency. Our studies suggest focal neurovascular CYP-NR-transporter alterations, as demonstrated by the relationship of seizure frequency and AED combination to brain CYP3A4, might together impact biotransformation machinery of human pharmacoresistant epilepsy.
Keywords: pharmacoresistant epilepsy, blood-brain barrier, antiepileptic drugs, cytochrome P450, multiple drug transporters, nuclear receptors
Introduction
Antiepileptic drugs (AEDs) are widely used in long-term combination therapy regimen or as monotherapy to control recurrent seizures in patients with epilepsy. Multiple factors impact the bioavailability of these medications to the epileptic brain, and poor responsiveness limits drug efficacy in 25-30% of patients with refractory epilepsy [1, 2].
Many AEDs undergo classical pharmacological regulation involving the cytochrome P450 (CYP)-drug-transporter system in the liver and intestines [3-7], where the majority of drug metabolism and biotransformation occurs. However, we and other groups have identified increased expression and function of CYP enzymes along with efflux drug transporters within blood-brain barrier (BBB) endothelial cells in human drug-resistant epilepsy [8-12]. CYP expression predominates at the neurovascular interface and in regions of the epileptic brain showing reactive gliosis [8, 10]. Additionally, we recently demonstrated that the glucocorticoid receptor (GR) seems to be an upstream molecular regulator of pregnane-x receptor (PXR) and CYP-efflux drug transporter in the BBB epileptic brain endothelium [13, 14].
Reports suggest that the expression of CYP-nuclear receptor-drug transporter is variable within tissues in the healthy liver, intestines [15, 16] and brain [17-19]. Nevertheless, it remains unclear whether this variability in CYP-nuclear receptor-efflux transporter system is related to neurovascular changes taking place in the epileptic (EPI) regions compared to non-epileptic (NON-EPI) regions of the human epileptic brain. Further, the influence of such P450-reliant human brain biotransformation machinery on seizure frequency and AED combination or vice-versa, though clinically relevant, remains poorly understood. Thus investigating the functional biotransformation machinery at the neurovascular interface in regulating AEDs may provide a novel perspective on pharmacoresistance and a future target for the treatment of focal epilepsies. In the present study, we used a homogeneous patient cohort with pharmacoresistant epilepsy due primarily to focal cortical dysplasia to 1) evaluate BBB changes, neurovascular nuclear receptors and drug efflux transporters and investigate the associations of these changes in EPI compared to NON-EPI regions of the same brain, 2) determine regional differences in brain CYP expression and activity, and 3) correlate CYP expression in EPI and NON-EPI regions with individual seizure frequency, duration of epilepsy and AED combinations used during therapy.
Materials and Methods
Human Subjects
Brain specimens from human subjects (n = 22) with pharmacoresistant epilepsy were obtained following focal surgical resections according to the principles outlined in the Declaration of Helsinki and the Cleveland Clinic Institutional Review Board-approved protocol (IRB 07-322). Information on seizure frequency, duration of epilepsy, age, gender, the resected brain regions, combination of CYP- and non-CYP-metabolized AEDs prescribed prior to surgery, and experimental use of each specimen is provided in Table 1. Brain tissues from EPI (lesional/epileptic) and NON-EPI (non-lesional/non-epileptic) regions were resected after prior non-invasive (scalp video-EEG monitoring, magnetic resonance imaging/MRI, positron emission tomography/PET) and invasive (stereo-electro encephalography/SEEG) evaluations. A small portion of the NON-EPI area from the excision in each subject was considered as relative/ internal control.
Table 1.
Demographic details
| S. no | Age (yrs) |
Gender | AEDs | AEDs as CYP- substrate |
Seizure Freq. (per week) |
Duration of Epilepsy (yrs) |
Resected tissue region |
Pathology | Exp. use |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23 | F | LCM, LEV, LTG | LCM | 1 | 20.5 | Lateral temporal lobe | HS, focal gliosis | WB; CYP FA |
| 2 | 18 | M | TPM, LTG | TPM | 1 | 14 | Left frontal lobe | FCD, subpial gliosis, mid focal perivascular inflammation. | WB; CYP FA |
| 3 | 37 | M | LCM, LTG | LCM | 1 | 15 | Left temporal lobe | FCD, subpial gliosis, perivascular white matter atrophy. | WB; CYP FA |
| 4 | 8 | M | LCM | LCM | 1 | 6 | Left frontal lobe | FCD, dystrophic calcifications | WB; CYP FA |
| 5 | 65 | M | LEV, CLB, OXC, | OXC, CLB | 7±1 | 64 | Right frontal lobe | Gliosis and perivascular chronic inflammation | WB; CYP FA |
| 6 | 44 | M | ESL, CLB | ESL, CLB | 1 | 37 | Right temporal lobe | Focal gliosis and perivascular atrophy | WB; CYP FA |
| 7 | 3 | F | OXC, LEV, CLB | OXC, CLB | 12±2 | 2 | Left temporal lobe/left frontal lobe; hippocampus | Focal cortical architectural disorganization | WB |
| 8 | 29 | F | LEV, PHT, ZNS, LTG, CLB | CLB, PHT, ZNS | 14±2 | 24 | Left temporal lobe/left frontal lobe | FCD | WB |
| 9 | 13 | F | OXC, LTG, CLB, CLZ, DZP | OXC, CLB, CLZ | 14±2 | 11 | Right frontal lobe | FCD; subpial gliosis | WB |
| 10 | 20 | F | LTG, CLB | CLB | 10±2 | 9 | Left lateral temporal lobe | FCD | WB |
| 11 | 33 | M | OXC, LEV, ZNS | OXC, ZNS | 5±1 | 30 | Left parietal lobe | FCD, subpial gliosis | WB |
| 12 | 14 | M | LTG, ZNS | ZNS | 3±1 | 10 | Right frontal lobe | FCD, nodular heterotopia and focal perivascular white matter atrophy. | WB |
| 13 | 36 | F | OXC, ZNS, GBP | OXC, ZNS | 12±2 | 34 | Mesial left frontal lobe | FCD, subpial gliosis | WB |
| 14 | 2 | F | LCM, VGB | LCM | 2±1 | 1 | Left frontal lobe | FCD | WB |
| 15 | 24 | M | ZNS, CBZ | ZNS, CBZ | 4±1 | 19 | Right occipital lobe | FCD, perivascular white matter atrophy | WB |
| 16 | 1 | M | VGB, LEV | unknown | 0.5 | Right temporal occipital parietal/ right hippocampus | FCD | WB | |
| 17 | 26 | F | LCM, RFM, LEV, PHT, TPM | LCM, PHT, TPM | 7±1 | 13 | Right parieto-occipital lobe | Focal perivascular chronic inflammation and atrophy; Gliosis | IHC |
| 18 | 17 | F | LEV, ZNS | ZNS | 14±2 | 8 | Left lateral temporal lobe | FCD; Gliosis | IHC |
| 19 | 22 | M | LTG, PHT, CLB | CLB, PHT | 1 | 18 | Right frontal lobe | Focal perivascular white matter atrophy; Gliosis | IHC |
| 20 | 22 | M | LTG, OXC | OXC | 1 | 8.5 | Right frontal lobe | FCD; Focal perivascular meningeal chronic inflammation | IHC |
| 21 | 27 | M | LTG, ZNS | ZNS | 1 | 11 | Left frontal lobe | FCD; focal perivascular chronic inflammation and white matter atrophy | IHC |
| 22 | 63 | F | LEV, OXC, TPM, | OXC, TPM | 2±1 | 57 | Left frontal lobe lesion | FCD; cortical architectural disorganization; inflammation; Gliosis | IHC |
Abbreviations: AEDs: antiepileptic drugs; LCM: lacosamide; LEV: levetiracetam; LTG: lamotrigine; OXC: oxcarbazepine; PHT: phenytoin; CLB: clobazam; ESL: eslicarbazepine acetate; VGB: vigabatrin; ZNS: zonisamide; TPM: topiramate; CLZ: clonazepam; DZP: diazepam; CBZ: carbamazepine; RFM: rufinamide; GBP: gabapentin; FCD: focal cortical dysplasia; HS: hippocampal sclerosis; Cau: caucasian; AA: african american; yrs: years; Exp. use: experimental use; WB: western blot; IHC: immunohistochemistry; FA: functional assay.
Histological and Immunohistochemical staining
The resected EPI and NON-EPI brain tissue was fixed immediately in 10% buffered formalin followed by sucrose and later sliced into 10 μm sections on a cryostat and stored at 4°C. Slices were routinely processed for histological, immunohistochemical, and immunofluorescent staining as performed previously [10, 14], Immunohistochemical staining was performed on contiguous sections (n = 5 each per specimen) obtained from resected brain tissues (Table 1).
Histology.
Histopathology of the resected tissue was evaluated using cresyl violet staining on brain slices (n = 5, in triplicates) to identify dysmorphic neurons, balloon cells, and the architectural organization pattern in EPI compared to NON-EPI regions. Staining was performed on contiguous sections obtained from blocks of resected tissue.
Diaminobenzidine (DAB) staining.
BBB integrity was evaluated using antibodies against serum protein IgG and tight-junction protein claudin-5. In brief, sections from both EPI and NON-EPI regions (in triplicates/subject) were permeabilized in 0.3% Tween, followed by blocking for endogenous peroxidase in 0.3% peroxide in methanol and for non-specific binding in a solution of 5% goat serum and 0.4% Triton-X. The sections were incubated in wells with a primary antibody anti-claudin-5 overnight with shaking at 4°C, followed by a 2-hour incubation with the corresponding biotinylated secondary antibody (Supplemental Table 1). Adjacent sections were then washed with PBS and incubated for 2 hours with a biotinylated anti-human IgG (1:100. Vector Laboratories, Burlingame, CA) prepared in 0.4% Triton-X. Sections were incubated for 1 hour with an avidin/biotin complex (Elite Vectastain ABC kit; PK-6102, Vector Labs, Burlingame, CA, USA). Thereafter, the antibody binding sites were visualized using DAB (with or without nickel solution; peroxidase substrate kit, SK-4100, Vector Labs), and mounted using permount solution. Analysis of DAB-labeled sections was performed by light/phase microscopy and the images obtained adjusted with ImageJ software (National institutes of Health, Bethesda, MD, USA).
Immunofluorescence.
Evaluation of nuclear receptors (CAR, constitutive androstane receptor; GR, PXR), drug efflux transporters (BCRP, breast cancer resistance protein; P-gp) and P450 enzymes (CYP3A4, CYP2D6, CYP2C9, CYP2E1) was done by immunofluorescent staining as described earlier [14]. Co-labeling with FITC-conjugated anti-human IgG and tight-junction proteins (claudin-1, claudin-5, occludin-1) was used to determine BBB integrity. Sections were incubated with primary antibody (Supplemental Table 1a) overnight at 4°C after blocking. This was followed by a 2-hour incubation at room temperature with the corresponding fluorescent secondary antibody (Supplemental Table 1b) or tissue simultaneously evaluated with fluorescein goat polyclonal anti-human IgG (1:200; Vector Labs, Burlingame, CA). Autofluorescence was blocked using Sudan Black B solution and all sections were mounted using Vectashield mounting medium with DAPI (H-1200, Vector Laboratories). Sections were analyzed by fluorescence microscopy and the acquired images obtained adjusted using ImageJ software.
Quantitative Assessment.
To quantify overall relative positive staining, images (n = 10/subject) of both the EPI and NON-EPI regions of each patient were taken randomly at 20x magnification using Leica Application Suite 4.12 software (exposure 1.5 sec, gain 1, gamma 0.93). Background was removed using the “rolling ball background subtraction” plugin for ImageJ with a radius of 50 pixels. The image was split into channels using the "split channels" function, and the mean fluorescent intensity and average area of positive staining was recorded. For individual cell types, an outline was drawn around neurons or astrocytes (n = 20/subject) selected randomly from across a group of 5 images per patient, and the average fluorescent intensity was recorded using the measure function. For brain microcapillaries, a line was drawn manually down the center of similarly-sized microvessels (n = 20/subject) and the fluorescent intensity along the line recorded using the Line Analyzer plugin for ImageJ. The non-parametric paired-sample Wilcoxon test (Origin Pro 9.0 Software) was used to identify significant differences in expression between the EPI and NON-EPI brain regions.
Protein isolation and Western blot analysis
Small portions of the snap frozen resected brain tissue from EPI and NON-EPI regions were homogenized in radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich, USA) with protease inhibitor (Sigma-Aldrich, USA). The tissue suspension was centrifuged at 14000G (Avanti-J25I, Beckman Coulter, USA), thereafter supernatant was collected and concentration of protein was measured by Bradford Method.
P-gp/MDR1 was separated by 8% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and later transferred to PVDF (polyvinylidene fluoride) membranes (EMD Millipore Corp., Billerica, MA, USA) in semi-dry transfer (trans-Biot™ SD, Bio-Rad, USA). All other protein targets were separated by 10% SDS-PAGE. In brief, the membranes were probed overnight with primary antibody (see target proteins listed in Supplemental Table 1a). After incubation with primary antibody the membranes were probed with appropriate secondary antibody (Supplemental Table 1b) as previously described [14]. For the target proteins either the PVDF membranes were incubated in stripping buffer in 50 °C for 30 minutes followed by blocking of the membranes or a fresh gel was simultaneously repeated with the samples. In each case, the protein expression was normalized by β-actin (as loading control) and quantified by ImageJ software.
CYP function and resorufin formation
We determined region-specific (EPI vs NON-EPI) 7-ethoxy-resorufin-O-deethylase (EROD) activity by adding the CYP substrate 7-ethoxy-xyresorufin (Sigma-Aldrich, USA) in phosphate buffer (50 mM NaHPO4 with pH 8.0). 7-Ethoxyresorufin stock solutions of 2 mM in dimethyl sulfoxide were prepared. In a reaction mixture, 20 μ1 of 7-ethoxyresorufin was added to 100 μg of isolated protein and phosphate buffer. The reaction time was kept constant for 3 minutes (incubation) at room temperature (based on the threshold and conversion time-noted in the EPI tissue fractions, Supplemental Fig. 1c) and later compared with the corresponding NON-EPI tissue of the brain. The method was standardized in the tissue homogenates as described earlier [20] and the resorufin concentration curve (Supplemental Fig. 1b) with the reaction time (Supplemental Fig. 1c) were calibrated and standardized. The fluorescent product formed was analyzed at excitation of 530 nm and emission of 590 nm in multimode reader (Synergy HT, BIO-TEK instruments, USA). The concentration of fluorescent product formed was analyzed by resorufin standard (Supplemental Fig. 1b) and final concentration was determined in concentration of resorufin/mg of protein [20].
Statistical analysis
Origin 9.0 software (Origin Lab, Northampton, MA, USA) was used for statistical analysis. Wilcoxon signed rank test was used to compare means of obtained data sets, followed by linear regression (Pearson’s coefficient, r) with one-way analysis of variance (ANOVA) to determine the associations between protein expressions and clinical characteristics. All data is represented as mean ± standard error of the mean (SEM). A p-value of < 0.05 was considered statistically significant for all test.
Results
Pathological alterations and BBB damage localizes to epileptogenic brain regions
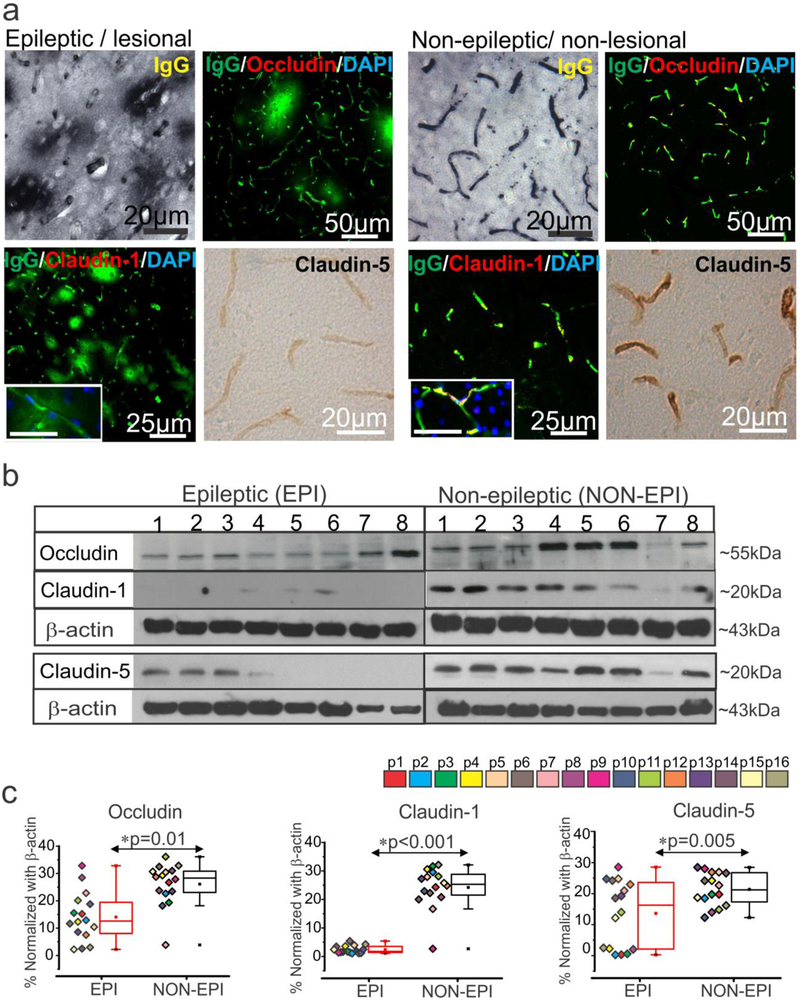
We validated pathology of epileptogenic tissue and found extensive dysmorphic and heterotopic neurons; balloon cells characteristic of focal cortical dysplasia (FCD) type IIb [21-23] were identified in the EPI regions of multiple specimens (Supplemental Fig. 1a) compared to NON-EPI regions. We further identified consistent extravagation of IgGs/immunoglobulins to the brain parenchyma across the vasculature indicating BBB damage in the EPI specimens analyzed (Fig. 1a). This increase in IgG leakage in the EPI area was accompanied by decreased expression of three tight-junction proteins namely, occludin, claudin-1, and claudin-5 in the brain vasculature of the pathological tissue (n = 6). In contrast, the intact BBB in non-pathological tissue was characterized by relatively less IgG leakage and relatively higher expression of the tight-junction proteins analyzed. Western blots further confirmed (n = 16) the decreased expression levels of occludin (*p = 0.01); claudin-1 (*p < 0.001) and claudin-5 (*p = 0.005) within EPI compared to NON-EPI regions (Fig. 1b-c). Together our data indicates that extravagation of IgGs in the brain parenchyma and a significant loss of tight-junction protein expression at the BBB may contribute to pathological alterations in the epileptogenic brain regions.
Fig. 1.
BBB leakage and decrease in tight-junction proteins expression predominates in the epileptogenic region.
(a) Representative images of immunostaining shows increase in IgG extravagation to the brain parenchyma across the brain vasculature and a corresponding decrease in expression of the tight-junction proteins claudin-1, claudin-5, and occludin in EPI/lesional region (n = 6, in triplicates) compared to NON-EPI /non-lesional region. Inset scale bars represents 20 μm. (b, c) Western blot analysis confirms decrease in the level of tight-junction protein expression, occludin (*p = 0.01), claudin-1 (*p < 0.001) and claudin-5 (*p = 0.005) in brain tissue specimens obtained from EPI vs. NON-EPI regions. Representative westerns depicting expression of tight-junction proteins (n = 8 subjects) with β-actin used as loading control (b). Densitometries for individual protein bands (from n = 16 subjects, with color codes p1-p16 depicting each subject) normalized with β-actin (c). Results are expressed as mean ± SEM by non-parametric analysis for paired samples Wilcoxon signed rank test for direct comparison of two population of data.
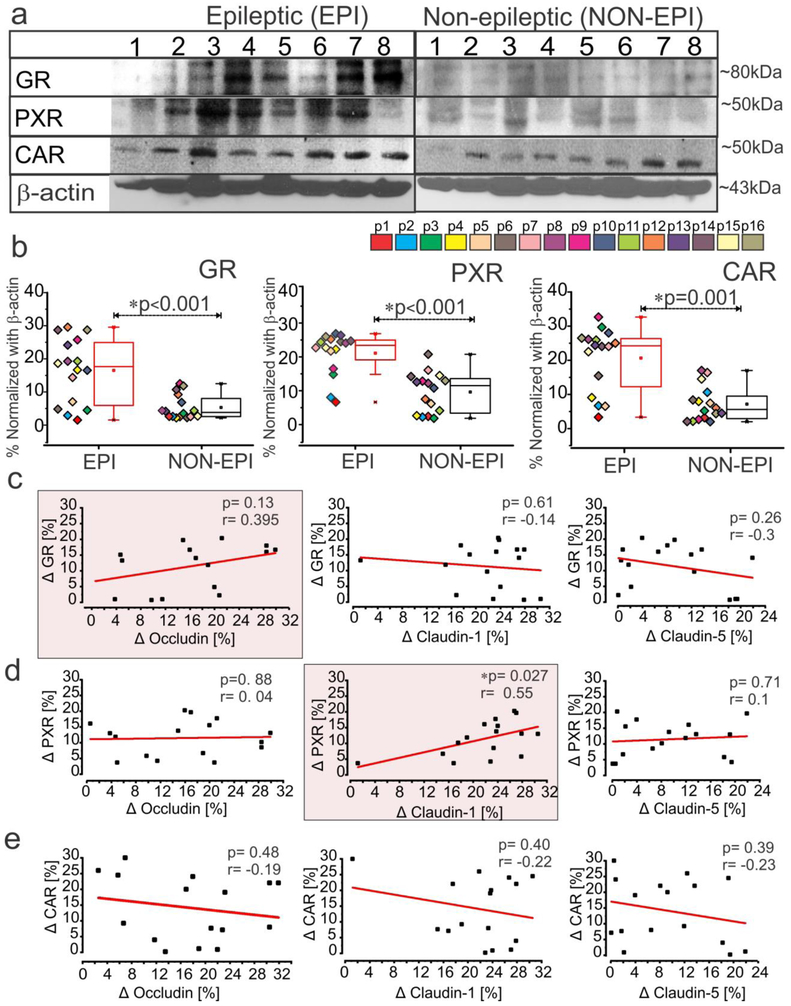
Elevated level of nuclear receptors and drug efflux transporters correlates to tight-junction proteins in epileptic brain regions
We found increased expression of GR (*p < 0.001), PXR (*p < 0.001) and CAR (*p = 0.001) in EPI compared to NON-EPI brain tissue (Fig. 2a-b). Further, the changes in GR, PXR and CAR between EPI and NON-EPI region selectively correlated with expression of tight-junction proteins occludin, claudin-1 and claudin-5 (Fig. 2c-e). Changes in PXR expression significantly correlated with changes in claudin-1 level (*p = 0.027; r = 0.55, n = 16) between the two regions, whereas we only observed a similar relative trend between GR and occludin (p = 0.13; r = 0.395, n = 16). We identified no significant correlations between CAR and tight-junction proteins analyzed in these specimens (Fig. 2c-e).
Fig. 2.
Increased expression of nuclear receptors, GR, PXR and CAR in epileptic region selectively correlates to tight-junction proteins. (a, b) Representative western blots for individual proteins (a) and corresponding densitometric analysis (b) shows a significant increase in GR (*p < 0.001), PXR (*p < 0.001) and CAR expression (*p = 0.001) in EPI regions compared to NON-EPI (n = 16 subjects). Non-parametric analysis for paired samples Wilcoxon signed rank test was performed for direct comparison of two population of data set obtained. Results are expressed as mean ± SEM. (c-e) The association between the EPI to NON-EPI difference in observed expression (Δ) of nuclear receptors GR, PXR and CAR to tight-junction proteins occludin, claudin-1 and claudin-5 is explored within individual subjects (n = 16). Change in GR percentage trended towards a relationship (p = 0.13, r = 0.395) with the change in occludin levels in EPI vs NON-EPI (c); however change in PXR within EPI and NON-EPI regions has a direct correlation to claudin-1 in the same areas (*p = 0.027, r = 0.55), which is statistically significant (d). CAR levels did not correlate with tight-junction proteins (occludin, p = 0.48, r = −0.19; claudin-1, p = 0.40, r = −0.22; claudin-5, p = 0.39, r = −0.23) within the specimens analyzed. The linear correlation (Pearson’s co-efficient, r) for each comparison was determined by one-way ANOVA.
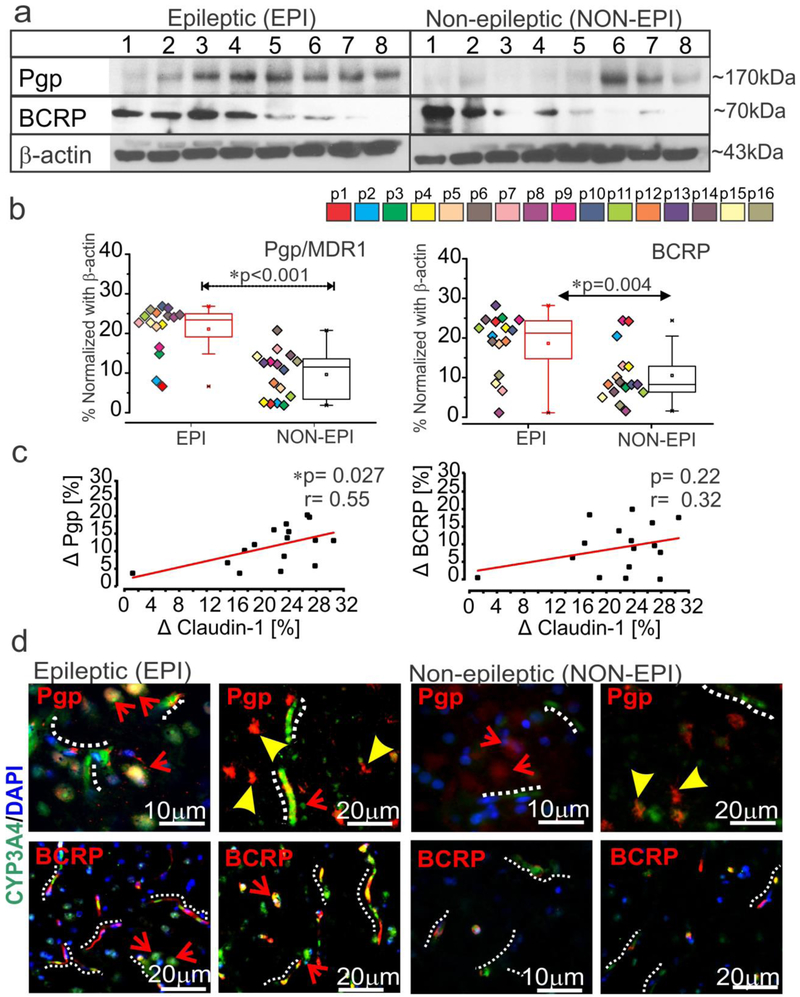
Significant elevation in expression of drug efflux transporter proteins P-gp/MDR1 (*p < 0.001) and BCRP (*p = 0.004) was observed in EPI compared to NON-EPI regions (Fig. 3a-b). The change in claudin-1 levels between EPI and NON-EPI in each specimen significantly correlated (Fig 3c) to a change in P-gp (*p = 0.027 and r = 0.55) and followed a similar trend with BCRP, though the latter failed statistical significance (p = 0.22 and r = 0.32, n = 16). Immunohistochemistry showed consistent neuronal and brain microvascular overexpression of P-gp and BCRP in the EPI regions (n = 6) compared to NON-EPI, which co-localized with CYP3A4 (Fig. 3d). These data indicate that both GR and PXR are sensitive to changes in expression of the tight junction proteins and that efflux transporters (Pgp/MDR1 and BCRP) expression is elevated in EPI regions with lowered claudin-1 levels.
Fig. 3.
Elevated drug efflux transporters co-localize with CYP3A4 and correlate with claudin-1 expression in the epileptic brain. (a-b) Analysis of the brain tissue showing (a) representative western blots (n = 8 subjects) and densitometric quantification normalized with β-actin (b). A significant increase was noted in P-gp (*p < 0.001) and BCRP (*p = 0.004) in EPI vs. NON-EPI region (n = 16 subjects). Non-parametric analysis for paired samples Wilcoxon signed rank test was performed for direct comparison of two population of data set obtained. Results are expressed as mean ± SEM. (c) A positive correlation between the differences between EPI and NON-EPI claudin-1 expression and that of the drug efflux transporter P-gp (*p = 0.027, r = 0.55) was observed within the specimens analyzed (n = 16) by one-way ANOVA. The association between claudin-1 and BCRP (p = 0.22, r = 0.32) failed statistical significance. The percent difference in protein expression between EPI and NON-EPI regions is indicated by Δ. (d) Representative immunostaining of the brain sections show increased CYP3A4 and P-gp/BCRP in the micro-capillaries and neurons of EPI compared to NON-EPI regions (n = 6, in triplicates). Note: micro-capillaries are indicated with dotted white-lines; astrocytes with arrowheads and neurons with arrows. Nuclei are stained with DAPI.
Increased focal neurovascular P450 enzyme expression correlates with increased metabolic transformation and drug efflux transporter protein expression
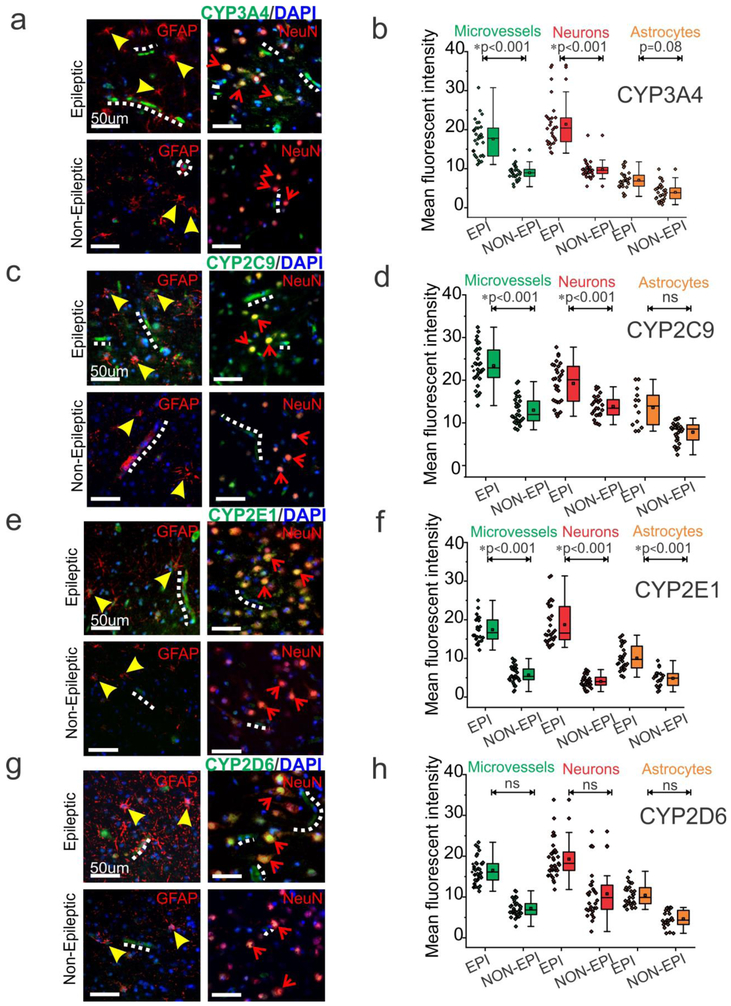
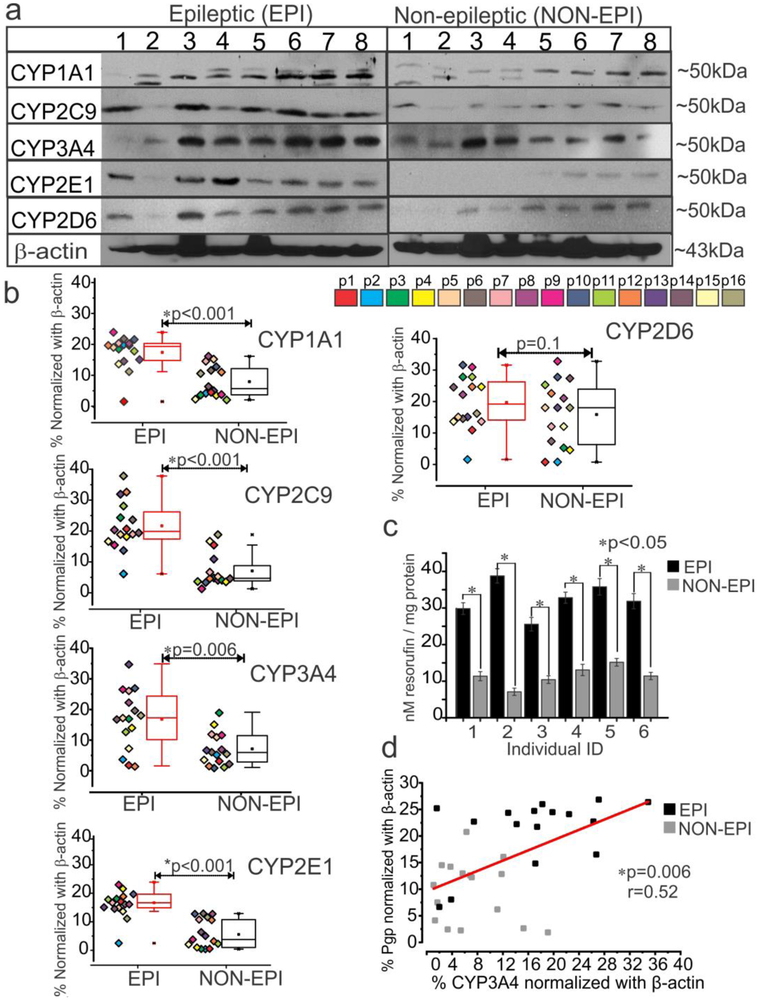
In EPI tissue, CYP3A4 (Fig. 4a-b), CYP2C9 (Fig. 4c-d) CYP2E1 (Fig. 4e-f) and CYP2D6 (Fig. 4g-h) staining is prominent in the microvessels and extensively in the neurons. The presence of these enzymes in astrocytes was relatively sparse across all tissues (n = 6). As compared to EPI tissue, there was a significantly decreased expression of CYP across all cell types in NON-EPI regions: microvessels (*p < 0.001, CYP3A4, CYP2C9, CYP2E1), neurons (*p < 0.001, CYP3A4, CYP2C9, CYP2E1) and astrocytes (*p < 0.001, CYP2E1; p < 0.08, CYP3A4, which narrowly fails significance) (Fig. 4). However, CYP2D6 showed negligible differences within the three cell types in both EPI and NON-EPI regions. A significant increase in overall CYP3A4, CYP2C9 and CYP2E1 was also identified across EPI compared to NON-EPI regions (Supplemental Fig. 1d). Western blot analysis further confirmed the elevated expression of cytochrome P450 enzymes levels of CYP1A1, CYP2C9, CYP3A4, and CYP2E1 (Fig. 5a) in the EPI regions compared to NON-EPI regions of the same individual (n = 16 subjects). For example, the western blots for CYP3A4, CYP2C9 and respective β-actin in all 16 specimens (EPI and NON-EPI) are provided in Supplemental Fig. 2. Within the patient cohort (shown by different color-code. Fig. 5b), the consistent pattern of significant increase in CYP1A1 (*p < 0.001), CYP2C9 (*p < 0.001), CYP3A4 (*p = 0.006) and CYP2E1 (*p < 0.001) expression in EPI brain region is observed compared to NON-EPI counterpart. However, a non-significant difference in CYP2D6 (p = 0.1) by western blot was identified between both regions.
Fig. 4.
CYP distribution in the endothelial cells, neurons and astrocytes in epileptic brain. (a, c, e, g) Representative images of resected tissues shows increased expression of CYP in the EPI brain BBB interface and neurons compared to NON-EPI brain tissue (n = 6, in triplicates). (b, d, f, h) Quantification of immunohistochemistry indicating increased CYP isoform expression in EPI compared to NON-EPI tissue. Elevated CYP3A4 levels were found in the microvessels (*p < 0.001), neurons (*p < 0.001), and minimally in astrocytes (p = 0.08). Similarly, CYP2C9 is overexpressed in the microvessels (*p < 0.001) and neurons (*p < 0.001); and CYP2E1 in the microvessels (*p < 0.001), neurons (*p < 0.001), and astrocytes (*p < 0.001). However, CYP2D6 showed non-significant (ns) difference in these cell types among EPI and NON-EPI brain regions. Non-parametric analysis for paired samples Wilcoxon signed rank test was performed for direct comparison of two population of data set obtained. Results are expressed as mean ± SEM. Note: micro-capillaries are indicated with dotted white-lines; astrocytes with arrowheads and neurons with arrows. Nuclei are stained with DAPI.
Fig. 5.
Expression and functional relevance of CYP in the epileptic regions of the brain. (a, b) Representative western blots of (a) CYP isoform expression and densitometric (b) quantification normalized by β-actin. The majority of CYPs (CYP1A1, *p < 0.001; CYP2C9, *p < 0.001; CYP3A4, *p = 0.006 and CYP2E1, *p < 0.001) were overexpressed in EPI regions compared to NON-EPI (n = 16 subjects) counterparts. In contrast, CYP2D6 expression remained unaltered (p = 0.1) in both regions. Results are expressed as mean ± SEM (by Wilcoxon signed rank test). (c) Increased CYP contribution in metabolic conversion of 7-ethoxyresorufin to resorufin was evident in the epileptic brain region (*p < 0.05, n = 6). (d) An apparent direct correlation between levels of P-gp and CYP3A4 expression (*p = 0.006, r = 0.52) was identified in the EPI and NON-EPI regions among the subject cohort. The linear correlation (Pearson’s co-efficient, r) for this comparison was determined by one-way ANOVA.
Functional involvement of CYP in metabolism of exogenous substances was indirectly evaluated by following P450-mediated conversion of 7-ethoxyresorufin to the fluorescent-product resorufin. We found that the EPI brain tissue specimens (n = 6 in triplicates) showed elevated contribution of CYP enzymes to metabolic activity as indicated by increased level (*p < 0.05) of resorufin conversion (Fig. 5c) compared to NON-EPI tissue. We identified a direct correlation between P-gp and CYP3A4 in the specimens analyzed (*p = 0.006, r = 0.52, n = 16 subjects; Fig 5d). This result suggests that both expression and overall metabolic activity of CYP enzymes are high in the EPI brain area, and that a strong nexus exists between levels of P-gp and CYP3A4 in EPI and NON-EPI region.
Association of CYP3A4 with seizure frequency, duration of epilepsy and antiepileptic drug combination
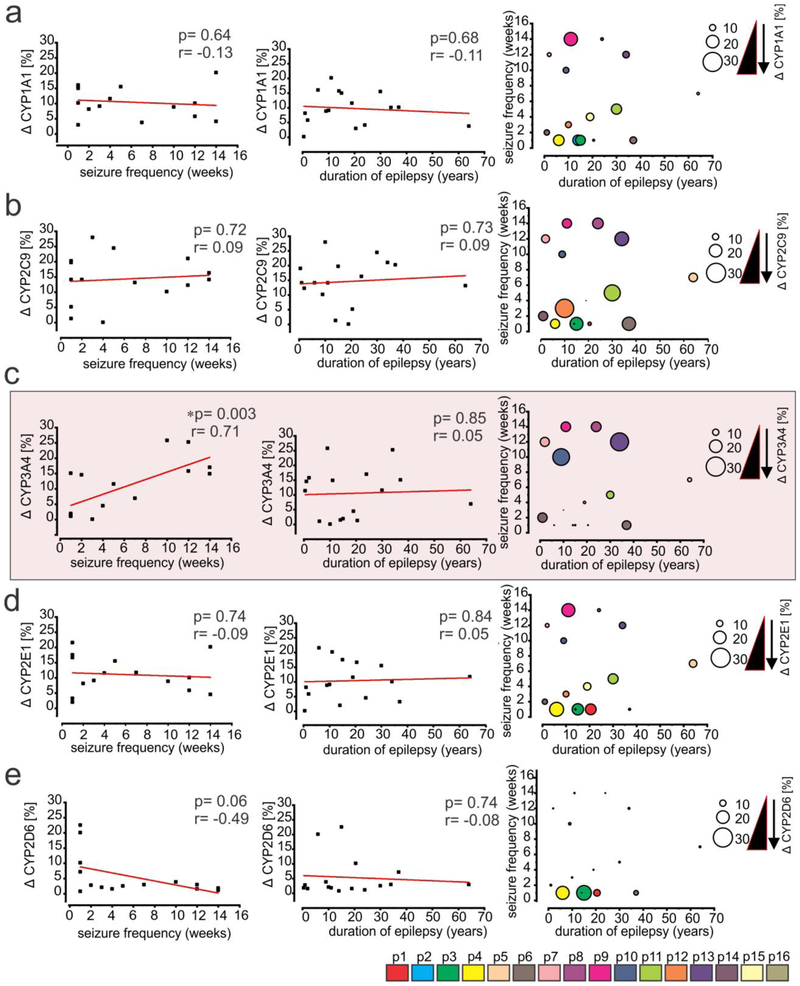
Within this patient cohort, we found a direct correlation (Fig. 6c) between the seizure frequency of the individual subjects (n = 16 subjects) and the percent change in the EPI and NON-EPI CYP3A4 expression in the brain (*p = 0.003, r = 0.71, n = 16 subjects). Such association was not observed between CYP1A1 (p = 0.64, r = −0.13, Fig 6a); CYP2C9 (p = 0.72, r = 0.09, Fig. 6b); CYP2E1 (p = 0.74, r = −0.09, Fig. 6d) or CYP2D6 (p = 0.06, r = −0.49, Fig. 6e) and seizure frequency in this subject cohort. Further, the duration of epilepsy (Fig. 6a-e) showed no significant correlation to percentage change in CYP1A1 (p = 0.68, r = −0.11); CYP2C9 (p = 0.73, r = 0.09); CYP3A4 (p = 0.85, r = 0.05); CYP2E1 (p = 0.84, r = 0.05) or CYP2D6 (p = 0.074, r = −0.08) between EPI and NON-EPI regions in these subjects. The distribution of CYPs-seizure frequency and duration of epilepsy among individual subject (color-coded) is eloquently shown also by a corresponding bubble graph with dot plot (Fig. 6a-e, right).
Fig. 6.
CYP3A4 expression in the epileptic brain correlates with seizure frequency. (a-e) Differences in CYP isoform expression between EPI and NON-EPI tissues (Δ) were associated with individual subject seizure frequency and duration of epilepsy. A direct correlation between the percent change in CYP3A4 expression (c) in EPI and NON-EPI brain regions (n = 16 subjects) vs. respective seizure frequency was observed in the same subjects (*p < 0.003, r = 0.71). However, non-significant correlations were obtained for (a) CYP1A1 (p = 0.64; r = −0.13); (b) CYP2C9 (p = 0.72; r = 0.09); and (d) CYP2E1 (p = 0.74; r = −0.09). Within the subjects analyzed no association was noticed between CYP isoforms and duration of epilepsy (a) CYP1A1 (p =0.68; r = −0.11); (b) CYP2C9 (p = 0.73; r = 0.09); (c) CYP3A4 (p = 0.85; r = 0.05); (d) CYP2E1 (p = 0.84; r = 0.05); (e) CYP2D6 (p = 0.74; r = −0.08). The bubble graph with dot plot (in the right, a-e) shows the distribution pattern of CYP isoforms together with seizure frequency and duration of epilepsy. The plot suggests that with the same duration of epilepsy, the increase in seizure frequency was exclusively associated with the increased change in CYP3A4 levels between EPI and NON-EPI regions (c; indicated by the different size circle). This trend was absent in case of CYP1A1 (a) and CYP2C9 (b) and somewhat reversed for CYP2E1 (d) and CYP2D6 (e). The linear correlation (Pearson’s co-efficient, r) for each comparison was determined by one-way ANOVA. Asterisks indicate p < 0.05.
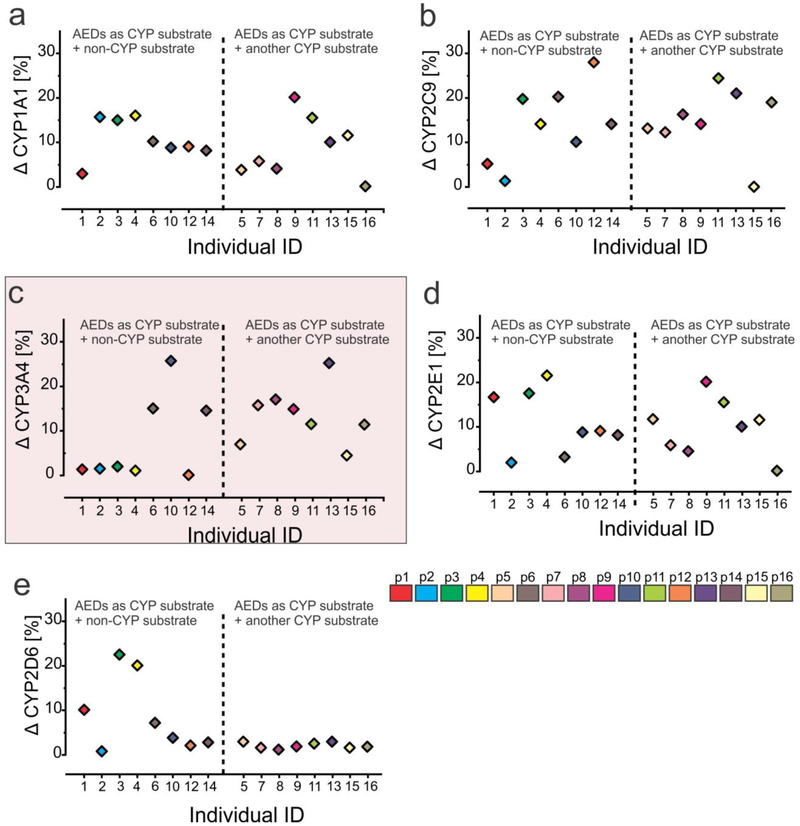
We further found (Fig. 7c) that in 5/8 subjects, there was a negligible difference between EPI and NON-EPI CYP3A4 levels when AEDs that are CYP substrates (e.g., clobazam, lacosamide, etc.) were given alone or in combination with a non-CYP substrate antiepileptic medication (e.g., levetiracetam, lamotrigine, etc.). However, EPI CYP3A4 expression was increased in 7/8 subjects compared to the corresponding NON-EPI regions when multiple AEDs, including two or more CYP substrates, were given in combination. This association was not observed with the expression patterns of CYP1A1, CYP2C9 or CYP2E1, where the regional difference was consistently high irrespective of AED combination. Similarly, CYP2D6 did not show any such pattern as observed in CYP3A4, though the difference in EPI to NON-EPI expression remained consistently low. Thus brain CYP3A4 was the only CYP (among the ones analyzed) whose expression changed with seizure frequency and was also receptive to the influence of AED combination.
Fig. 7.
CYP3A4 expression changes in epileptic brain with antiepileptic drug combination used. (a-b) The percentage change in CYP1A1 (Δ CYP1A1) in EPI vs NON-EPI is increased irrespective of antiepileptic drug (AED) combination used, whether CYP substrate dependent or not. Similar pattern was observed in case of CYP2C9 among the specimens analyzed (n = 16) (b). (c) In the same subjects, a single CYP substrate AED given alone or with another non-CYP substrate resulted in a decreased change of CYP3A4 expression (Δ CYP3A4 [%]; 5/8). However, EPI CYP3A4 expression was increased in 7/8 subjects, compared to the corresponding NON-EPI regions, when multiple AEDs, including two or more CYP substrates, were given in combination, (d) Change of CYP2E1 (Δ CYP2E1 [%]) expression remains consistently high despite drug combination, reflecting a pattern similar to CYP1A1 (a). (e) Consistently low level of CYP2D6 (Δ CYP2D6 [%]) was noticed with negligible association to drug combination in these subjects.
Discussion
The lack of long-lasting pharmacological efficacy in the treatment of intractable epilepsy is a serious clinical concern. Nearly one-third of patients with epilepsy do not respond to available medications and many require surgical intervention to prevent recurrent seizures. While local biotransformation of medication in the brain may limit the effectiveness of drug therapy, it has also been recognized that pathophysiological alterations of the BBB contribute to drug resistance in epilepsy [9, 24, 25]. Therefore, we explicitly examined the neurovascular CYP-nuclear receptor-transporter biotransformation machinery and BBB integrity in EPI and NON-EPI regions of surgically resected brain tissue from patients with medically intractable focal epilepsy. To better understand the significance of therapeutics in the focal epilepsies, we further investigated CYP changes in EPI compared to NON-EPI brain regions and correlated these changes with seizure severity, epilepsy duration and preoperative AED combination.
In the current study, we found a consistent pattern of increased IgG extravagation to the brain parenchyma of EPI regions compared with NON-EPI regions. A simultaneous decrease in tight-junction protein (occludin, claudins) expression was observed in the brain vasculature in EPI regions, suggesting compromised BBB integrity [24, 26-28]. Our results are consistent with previous reports [25, 29] suggesting that the BBB permeability may lead to the accumulation of serum proteins in the brain parenchyma thus contributing to neuronal hyper-excitability. Other reports showed that epileptiform activity can also be induced by direct cortical application of albumin-containing solution and is reduced by blocking cellular uptake of serum proteins near regions of a compromised BBB [29, 30]. The presence of serum proteins in the brain parenchyma could additionally restrict the bioavailability of free protein bound AEDs in epileptogenic regions, [31] thereby leading to significant decrease in drug efficacy.
Cytochrome P450-mediated drug biotransformation is not only limited to the liver, but it has also been identified at the BBB endothelium [9, 12, 32, 33] suggesting CYP and multiple drug efflux transporters operate in conjunction to influence drug resistance in epilepsy [13, 34]. We recently reported that GR in human epileptic brain endothelial cells modulates CYP and MDR1, thereby impacting local drug biotransformation across the BBB [13] and drug bioavailability to the epileptic brain. Additionally, multiple nuclear receptors are overexpressed in the epileptic brain vasculature and in neuronal regions showing reactive gliosis [14]. In the present study, we found that GR, PXR, and CAR, the key nuclear receptors known to regulate CYP and efflux drug transporters in epileptic brain [13, 14, 35, 36] are differently expressed in EPI brain tissue compared to NON-EPI. However, we found an association between GR and PXR expression with changes in levels of tight-junction proteins occludin and claudin-1, respectively. Notably, we observed no direct association between CAR expression and differences between any of the analyzed tight-junction proteins in both regions. The variability in these association is consistent with previous reports, albeit in rodent models, demonstrating that a knockout of nuclear receptors had a selective effect on the expression of tight-junction proteins [37, 38].
Reports on pharmacoresistance indicate elevated expression of efflux transporters in epileptic brain tissue [12, 35, 39]. Similarly, we identified higher expression of both P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP) in the EPI specimens studied compared to their adjacent NON-EPI counterparts. P-gp overexpression was identified in astrocytes, as well as in neurons and microvessels with concurrent expression of CYP3A4. In contrast, we observed higher BCRP expression in microvessels and neurons, co-expressed with CYP3A4. This increased change in efflux transporters from EPI to NON-EPI tissue relates to claudin-1 levels in these subjects. Together, these results suggest that despite compromised BBB integrity, a possible mechanism of pharmacoresistance may be due to hyper-expression of efflux transporter proteins and CYP enzymes in the human focal epileptic brain.
Consistent with our previous findings [8-10, 35], we also found that the major CYPs (e.g. CYP3A4, CYP1A1, CYP2C9 and CYP2E1) responsible for AED metabolism in the liver [3, 4, 40] are overexpressed in EPI brain tissues compared to the NON-EPI regions, with corresponding metabolic activity. Notably, overexpression of CYP3A4, CYP2E1, and CYP2C9 in the EPI regions remained primarily localized to the microvessels and neurons. Expression of CYP3A4 and P-gp also correlated in the EPI and NON-EPI regions in these subjects, consistent with previous reports [8, 10, 12, 19, 39, 41] and further supporting our finding of focal overexpression of co-localized CYP-drug efflux transporter in epileptic regions in the human brain.
Seizure frequency in epilepsy has been previously related to inflammatory mediators in the human epileptic brain and with the loss of dentate gyrus GABAergic neurons in rodent model of temporal lobe epilepsy [42, 43]. However, as clinical characteristics are used to predict surgical outcome, drug choice, and prognosis of epilepsy, we investigated whether a connection existed between some CYP isoforms and clinical information (such as seizure frequency, duration of epilepsy and AEDs used at the time of surgery). The difference in expression of CYP3A4 between the EPI and NON-EPI regions directly correlates to the seizure frequency in the subjects analyzed (*p < 003; r = 0.71, n = 16 subjects). Further, in patients with the same duration of epilepsy, increased seizure frequency was associated exclusively with an increase in the difference of CYP3A4 expression between the EPI and NON-EPI regions in these subjects. This pattern was not observed with other CYP isoforms such as CYP1A1, CYP2C9, CYP2E1 and CYP2D6.
Previous studies showed that the numbers of pre-treatment seizures were greater in pharmacoresistant patients, with those reporting more than 10 seizures prior to initiation of therapy being more than twice as likely to develop refractory epilepsy [44, 45] . In addition, high seizure frequency prior to AED treatment was shown to be a predictor of pharmacoresistant epilepsy in a rat model of temporal lobe epilepsy [44]. Therefore, our results may suggest a mechanistic explanation to these observations through a differential increase in local metabolism of some AEDs in the epileptic focus. AEDs functionally control abnormal neuronal firing by various mechanisms of action including blocking sodium ion channels and glutamate receptor excitation and/or enhancing gamma-aminobutyric acid (GABA)-mediated inhibition [42, 43]. As documented, the metabolism of current AEDs may or may not involve enzymatic CYP induction and certain AEDs are known to modify the expression of CYP enzymes [40, 46]. With this in mind, we investigated the association of two broad categories of AEDs (CYP- or non-CYP substrates) with human brain CYP levels. Based on our results, the percent change in CYP3A4 levels between EPI vs NON-EPI region is increased when a CYP substrate AED is given along with additional medications which are also CYP substrates in combination; conversely, CYP3A4 remains unaltered or decreases when a CYP-mediated AED is given alone or combined with non-CYP substrate AEDs. The differential in situ over-expressions of CYP1A1, CYP2C9, and CYP2E1 and the decreased expression of CYP2D6 in epileptic areas remained independent of the drug combination used at time of surgery. Together, this suggests that the relative change of CYP3A4 level in EPI and NON-EPI brain areas, influenced by AED combination, has an exclusive relationship to individual seizure frequency. During multiple medication selection, AEDs metabolized by complementary pathways (one CYP-mediated AED with an additional non-CYP mediated AED) may decrease CYP3A4 levels in the brain and corresponding seizure frequency. This drug selection might also prevent undesirable side-effects and formation of reactive AED metabolites by CYP enzymes [33, 47, 48], which needs further investigation.
Final remarks and conclusion
Taken together, BBB dysfunction may contribute to drug resistance and epileptogenesis; therefore, alterations of the focal CYP-nuclear receptor-drug efflux transporter biotransformation machinery in the neurovascular epileptogenic area may be a consequence of, or contribute to, further pathophysiological changes. Notably, the in situ expression of CYP3A4 in the epileptic brain focus has potential pharmacological and clinical relevance to human drug resistant epilepsy.
Supplementary Material
Acknowledgements:
This work was supported in part by grants R01NS078307 and R01NS095825 from the National Institute of Neurological Disorders and Stroke/National Institutes of Health and by grants awarded from the Brain and Behavior Research Foundation (formerly NARSAD), the American Heart Association (13SDG13950015), and Alternatives Research & Development Foundation. We would like to acknowledge Saurabh Mishra for initial assistance with western blot.
Footnotes
Disclosure
J.G.M. has conflict of interest with Zimmer Biomet, Cleveland. I.N. serves on the Speaker’ bureau and as a member of ad hoc advisory board for Eisai, Inc. E.P. received speaker’s or consultancy fees from Axovant, Biogen, Eisai, GW Pharma, Sanofi, Takeda, UCB Pharma and Xenon Pharma. None of the other authors has any potential conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Krauss GL, Sperling MR (2011) Treating patients with medically resistant epilepsy. Neurol Clin Pract 1(1):14–23. doi: 10.1212/CPJ.0b013e31823d07d1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwan P (2011) Defining drug-resistant epilepsy. Neurology Asia 16: 67–69. [Google Scholar]
- 3.Zanger UM, Schwab M (2013) Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138(1):103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Johannessen SI, Landmark CJ (2010) Antiepileptic drug interactions - principles and clinical implications. Curr Neuropharmacol 8(3):254–267. doi: 10.2174/157015910792246254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perucca E (2006) Clinically relevant drug interactions with antiepileptic drugs. Br J Clin Pharmacol 61(3):246–255. doi: 10.1111/j.1365-2125.2005.02529.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wallace J, Paauw DS (2015) Appropriate prescribing and important drug interactions in older adults. Med Clin North Am 99(2):295–310. doi: 10.1016/j.mcna.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 7.Zaccara G, Perucca E (2014) Interactions between antiepileptic drugs, and between antiepileptic drugs and other drugs. Epileptic Disord 16(4):409–31. doi: 10.1684/epd.2014.0714 [DOI] [PubMed] [Google Scholar]
- 8.Ghosh C, Gonzalez-Martinez J, Hossain M et al. (2010) Pattern of P450 expression at the human blood-brain barrier: Roles of epileptic condition and laminar flow. Epilepsia 51(8):1408–1417. doi: 10.1111/j.1528-1167.2009.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh C, Hossain M, Solanki J et al. (2016) Pathophysiological implications of neurovascular P450 in brain disorders. Drug Discov Today. 21(10):1609–1619. doi: 10.1016/j.drudis.2016.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghosh C, Marchi N, Desai NK et al. (2011) Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia 52(3):562–571. doi: 10.1111/j.1528-1167.2010.02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lombardo L, Pellitteri R, Balazy M et al. (2008) Induction of nuclear receptors and drug resistance in the brain microvascular endothelial cells treated with antiepileptic drugs. Curr Neurovasc Res 5(2):82–92. doi: 10.2174/156720208784310196 [DOI] [PubMed] [Google Scholar]
- 12.Dauchy S, Dutheil F, Weaver RJ et al. (2008) ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood-brain barrier. J Neurochem 107(6):1518–1528. doi: 10.1111/j.1471-4159.2008.05720.x. [DOI] [PubMed] [Google Scholar]
- 13.Ghosh C, Hossain M, Mishra S et al. (2018) Modulation of glucocorticoid receptor in human epileptic endothelial cells impacts drug biotransformation in an in vitro blood-brain barrier model. Epilepsia. 59(11):2049–2060. doi: 10.1111/epi.14567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghosh C, Hossain M, Solanki J et al. (2017) Overexpression of pregnane X and glucocorticoid receptors and the regulation of cytochrome P450 in human epileptic brain endothelial cells. Epilepsia 58(4):576–585. doi: 10.1111/epi.13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urquhart BL, Tirona RG, Kim RB (2007) Nuclear receptors and the regulation of drug-metabolizing enzymes and drug transporters: implications for interindividual variability in response to drugs. J Clin Pharmacol 47(5):566–578. doi: 10.1177/0091270007299930 [DOI] [PubMed] [Google Scholar]
- 16.Pascussi JM, Gerbal-Chaloin S, Duret C et al. (2008) The tangle of nuclear receptors that controls xenobiotic metabolism and transport: Crosstalk and consequences. Annu Rev Pharmacol Toxicol 48: 1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349 [DOI] [PubMed] [Google Scholar]
- 17.McFadyen MCE, Melvin WT, Murray GI (1998) Regional distribution of individual forms of cytochrome P450 mRNA in normal adult human brain. Biochem Pharmacol 55(6):825–830. doi: 10.1146/annurev.pharmtox.47.120505.105349 [DOI] [PubMed] [Google Scholar]
- 18.Miksys S, Rao Y, Sellers EM et al. (2000) Regional and cellular distribution of CYP2D subfamily members in rat brain. Xenobiotica 30(6):547–564. doi: 10.1080/004982500406390 [DOI] [PubMed] [Google Scholar]
- 19.Miksys SL, Tyndale RF (2002) Drug-metabolizing cytochrome P450s in the brain. J Psychiatry Neurosci 27(6):406–415. [PMC free article] [PubMed] [Google Scholar]
- 20.Zamaratskaia G, Zlabek V (2009) EROD and MROD as Markers of Cytochrome P450 1A Activities in Hepatic Microsomes from Entire and Castrated Male Pigs. Sensors (Basel) 9(3):2134–2147. doi: 10.3390/s90302134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kabat J, Krol P (2012) Focal cortical dysplasia - review. Pol J Radiol 77(2):35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Najm IM, Sarnat HB, Blumcke I (2018) Review: The international consensus classification of Focal Cortical Dysplasia - a critical update 2018. Neuropathol Appl Neurobiol 44(1):18–31. doi: 10.1111/nan.12462 [DOI] [PubMed] [Google Scholar]
- 23.Najm IM, Tassi L, Sarnat HB et al. (2014) Epilepsies associated with focal cortical dysplasias (FCDs). Acta Neuropathol 128(1):5–19. doi: 10.1007/s00401-014-1304-0 [DOI] [PubMed] [Google Scholar]
- 24.Liu JY, Thom M, Catarino CB et al. (2012) Neuropathology of the blood-brain barrier and pharmaco-resistance in human epilepsy. Brain 135(Pt 10):3115–3133. doi: 10.1093/brain/aws147 [DOI] [PubMed] [Google Scholar]
- 25.van Vliet EA, Aronica E, Gorter JA (2014) Role of Blood-Brain Barrier in Temporal Lobe Epilepsy and Pharmacoresistance. Neuroscience 277:455–473. doi: 10.1016/j.neuroscience [DOI] [PubMed] [Google Scholar]
- 26.Ishihara H, Kubota H, Lindberg RL et al. (2008) Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J Neuropathol Exp Neurol 67(5):435–448. doi: 10.1097/NEN.0b013e31816fd622 [DOI] [PubMed] [Google Scholar]
- 27.Librizzi L, de Cutis M, Janigro D et al. (2018) Cerebrovascular heterogeneity and neuronal excitability. Neurosci Lett 667:75–83. doi: 10.1016/j.neulet.2017.01.013 [DOI] [PubMed] [Google Scholar]
- 28.Wolburg H, Wolburg-Buchholz K, Kraus J et al. (2003) Localization of claudin-3 in tight junctions of the blood-brain barrier is selectively lost during experimental autoimmune encephalomyelitis and human glioblastoma multiforme. Acta Neuropathol 105(6):586–592. doi: 10.1007/s00401-003-0688-z [DOI] [PubMed] [Google Scholar]
- 29.Seiffert E, Dreier JP, Ivens S et al. (2004) Lasting blood-brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci 24(36):7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivens S, Kaufer D, Flores LP et al. (2007) TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130(Pt 2):535–547. doi: 10.1093/brain/aw1317 [DOI] [PubMed] [Google Scholar]
- 31.Marchi N, Betto G, Fazio V et al. (2009) Blood-brain barrier damage and brain penetration of antiepileptic drugs: role of serum proteins and brain edema. Epilepsia 50(4):664–77. doi: 10.1111/j.1528-1167.2008.01989.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ghersi-Egea JF, Leininger-Muller B, Cecchelli R et al. (1995) Blood-brain interfaces: relevance to cerebral drug metabolism. Toxicol. Lett 82-83:645–653. [DOI] [PubMed] [Google Scholar]
- 33.Ghosh C, Marchi N, Hossain M et al. (2012) A pro-convulsive carbamazepine metabolite: quinolinic acid in drug resistant epileptic human brain. Neurobiol. Dis 46(3):692–700. doi: S0969-9961(12)00081-2 [pii]; 10.1016/j.nbd.2012.03.010 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh C, Puvenna V, Gonzalez-Martinez J et al. (2011) Blood-Brain Barrier P450 Enzymes and Multidrug Transporters in Drug Resistance: A Synergistic Role in Neurological Diseases. Curr Drug Metab 12(8):742–749. doi : 10.2174/138920011798357051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bauer B, Hartz AM, Fricker G et al. (2004) Pregnane X receptor up-regulation of P-glycoprotein expression and transport function at the blood-brain barrier. Mol Pharmacol 66(3):413–419. doi: 10.1124/mol.66.3. [DOI] [PubMed] [Google Scholar]
- 36.Hartz AM, Pekcec A, Soldner EL et al. (2017) P-gp Protein Expression and Transport Activity in Rodent Seizure Models and Human Epilepsy. Mol Pharm 14(4):999–1011. doi: 10.1021/acs.molpharmaceut.6b00770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boussadia B, Gangarossa G, Mselli-Lakhal L et al. (2016) Lack of CAR impacts neuronal function and cerebrovascular integrity in vivo. Exp Neurol 283:39–48. doi: 10.1016/j.expneurol.2016.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boussadia B, Lakhal L, Payrastre L et al. (2018) Pregnane X Receptor Deletion Modifies Recognition Memory and Electroencephalographic Activity. Neuroscience 370:130–138. doi: 10.1016/j.neuroscience.2017.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dauchy S, Miller F, Couraud PO et al. (2009) Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem. Pharmacol 77(5):897–909. doi: 10.1016/j.bcp.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 40.Brodie MJ, Mintzer S, Pack AM et al. (2013) Enzyme induction with antiepileptic drugs: cause for concern? Epilepsia 54(1):11–27. doi: 10.1111/j.1528-1167.2012.03671.x [DOI] [PubMed] [Google Scholar]
- 41.Loscher W, Potschka H (2002) Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther 301(1):7–14. doi: 10.1124/jpet.301.1.7 [DOI] [PubMed] [Google Scholar]
- 42.Buckmaster PS, Abrams E, Wen X (2017) Seizure frequency correlates with loss of dentate gyrus GABAergic neurons in a mouse model of temporal lobe epilepsy. J Comp Neurol 525(11):2592–2610. doi: 10.1002/cne.24226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pernhorst K, Herms S, Hoffmann P et al. (2013) TLR4, ATF-3 and IL8 inflammation mediator expression correlates with seizure frequency in human epileptic brain tissue. Seizure 22(8):675–678. doi: 10.1016/j.seizure.2013.04.023 [DOI] [PubMed] [Google Scholar]
- 44.Loscher W, Brandt C (2010) High seizure frequency prior to antiepileptic treatment is a predictor of pharmacoresistant epilepsy in a rat model of temporal lobe epilepsy. Epilepsia 51(1):89–97. doi: 10.1111/j.1528-1167.2009.02183.x [DOI] [PubMed] [Google Scholar]
- 45.Hitiris N, Mohanraj R, Norrie J et al. (2007) Predictors of pharmacoresistant epilepsy. Epilepsy Res 75(2-3):192–196. doi: 10.1016/j.eplepsyres.2007.06.003 [DOI] [PubMed] [Google Scholar]
- 46.Meyer RP, Gehlhaus M, Knoth R et al. (2007) Expression and function of cytochrome p450 in brain drug metabolism. Curr Drug Metab 8(4):297–306. doi : 10.2174/138920007780655478 [DOI] [PubMed] [Google Scholar]
- 47.Ghosh C, Hossain M, Spriggs A et al. (2015) Sertraline-induced potentiation of the CYP3A4-dependent neurotoxicity of carbamazepine: An in vitro study. Epilepsia 56(3):439–449. doi: 10.1111/epi.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spina E, Pisani F, Perucca E (1996) Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet 31(3):198–214. doi: 10.2165/00003088-199631030-00004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.