Abstract
Background
Diuron is an environmental component listed as a likely human carcinogen. Several other studies report that diuron can be oncogenic for bladder, urothelial, skin, and mammary cells. No study mentions the putative effect of diuron on the glioma occurrence.
Objectives
We here wanted to investigate the effects of diuron exposure on the glioma occurrence while wishing to incriminate a putative implication of DNA methylation modulation in this process.
Methods
In in vivo model of glioma, diuron exposure was firstly compared or combined with oncogenic overexpressions already known to promote gliomagenesis. ELISA quantifying the 5-methylcytosine level on DNA was performed to examine the global DNA methylation level. Quantitative real-time polymerase chain reaction and proximity ligation in situ assay were performed to identify the molecular causes of the diuron-induced changes of DNA methylation. The signatures diuron-induced changes of DNA methylation were analyzed in a cohort of 23 GBM patients.
Results
Diuron exposure is not sufficient to promote glioma, such as the oncogenic overexpression of Akt or Ras. However, the combination of diuron exposure and Akt overexpression promotes glioma. We observed that the diuron/Akt-induced glioma is characterized by three phenotypic signatures characterizing cancer cells: a global DNA hypomethylation, a loss of sensitivity to cell death induction, and a gain of signals of immune escape. Our data associated these phenotypes with three aberrant DNA methylation signatures: the LLT1, PD-L1, and Bcl-w hypomethylations. Strikingly, we observed that these three concomitant hypomethylations were only observed in GBM patients having a potential exposure to diuron via their professional activity.
Conclusions
As single player, diuron is not an oncogenic of glioma, but it can participate to the glioma formation in association with other events (also devoid of oncogenic property as single player) such as Akt overexpression.
Keywords: DNA methylation, Diuron, Gliomagenesis, Apoptosis, PD-L1
Background
The European Cancer Observatory (ECO) estimates the number of brain tumor at 30715 in Europe (http://eco.iarc.fr) and the Central Brain Tumor Registry of the United States (CBTRUS) estimates at 26070 the number of brain primary malignant tumor in 2017 (http://www.cbtrus.org). In 2016, the World health Organization (WHO) classification of central nervous system (CNS) tumors was revised to integrate molecular signatures to histological parameters in order to enhance the robustness of the classification of CNS tumors.
During the last decades, different molecular oncogenic events have been described to induce primary tumor brain formation. Thus, oncogenic overexpression and induction of global DNA hypomethylation are described to promote the gliomagenesis [1–4]. In addition, several risk factors have been described as potential contributors/inducers of glioma risk. These environmental risk factors include allergies, exposure to ionizing and non-ionizing (cellular phones) radiations, exposure to chemicals, solvents, and pesticides [5–7].
A study performed in southwestern of France reports that a high level of occupational exposure to pesticides might be associated with an excess risk of brain tumors [8]. Meta-analysis of brain cancer and farming indicated that pesticides exposure commonly experienced by farmers may contribute to the increased risk of brain cancer [9]. A study performed in Nebraska significantly associated some specific agricultural pesticide exposures and the risk of glioma among male farmers [10]. However, these studies need to be counterbalanced by study reporting the absence of association between pesticide exposure and risk of glioma [7].
Diuron is a herbicide and an antifoulant listed as a likely human carcinogen by US Environmental Protection Agency (USEPA) in 1997. Due to this dual and extensive utilization, diuron contamination is frequently observed [11]. Diuron and its metabolites appear as the third pesticide detected in surface water in Italy with a concentration of 3.104 ng/L [12]. Diuron is detected in Great Barrier Reef lagoon at a concentration exceeding the Australian and New Zealand guideline trigger value of 0.2 μg/L [13]. The CEREMA study reports that diuron is detected in air with a peak at 0.31 ng/m3. This study also indicated that diuron is detected in approximately 30% of groundwater samples with a maximum concentration of 0.279 μg/L. The CEREMA study also reports that diuron was detected in 70% of samples with a maximum concentration of 0.864 μg/L and the mean concentration of 0.041 μg/L in European river waters. Diuron is also detected in food and vegetables. European Food Safety Authority mentions an acceptable daily intake (ADI) of 0.007 mg/kg bw/day and an acute reference dose (ARfD) of 0.016 mg/kg bw/day. In a document edited in 2011, EFSA reports that the highest chronic exposure represented 1.8% of the ADI (German child) and the highest acute exposure amounted to 6.1% of the ARfD (apple). Thus, in addition to professionals using diuron in their activity, diuron exposure is multiple for human.
Despite its classification as a likely human carcinogen, little is known about the combination effect of oncogenic overexpression and the pesticide exposure on the glioma occurrence. More generally, little is known about the diuron-induced tumor molecular mechanism even if diuron has been already reported to promote the bladder [14, 15], urothelial [16], skin [17, 18], and mammary [15–19] carcinogenesis.
In this article, we asked the question to determine whether the diuron exposure of glial cells could promote glioma formation and if that is the case, we will analyze the putative involvement of DNA methylation modifications in the diuron-induced glioma.
Here, we show that the unique diuron exposure is not oncogenic for glial cells. However, the combination of diuron exposure and Akt overexpression promote glioma. In terms of molecular mechanisms, our data indicate that the diuron/Akt-induced glioma is characterized by active and passive processes of DNA demethylation that promote an epigenetic reprogramming of certain apoptosis and immune system actors. Thus, this supports the escape of cell death and immune system.
Methods
Cell culture and diuron treatment
Ntv-a cell line used was obtained from Holland [1] team and corresponds to newborn tv-a transgenic mice brain cells infected with viruses containing RCAS vectors with LacZ, Ras, and/or Akt. These cells are cultivated with DMEM with 10% fetal calf serum, 1% glutamine, 1% penicillin/streptomycin, and maintained at 37 °C in a humidified atmosphere containing 5% CO2-air. Cells were treated with 100 μM of diuron dissolved in DMSO each 2 days during 14 days, control cells received DMSO only.
Tumorigenicity assay
After diuron exposure, Ntv-a cells were harvested by trypsinization, washed, and resuspended in saline buffer. Cell suspensions were injected s.c. (subcutaneous) as 2.106 cells in 0.05 ml of PBS with equal volume of matrigel matrix (Becton Dickinson, France) in the flank of seven groups of five 7/8-week-old nude NMRI-nu female mice (Janvier, France). Tumor volumes based on caliper measurements were calculated by the modified ellipsoidal formula (tumor volume = 1/2 (length × width2)).
The experimental procedures using animals were in accordance with the guidelines of Institutional Animal Care and the French National Committee of Ethics. In addition, all experiments were conducted according to the Regulations for Animal Experimentation at the “Plate-forme Animalerie” of “Institut de Recherche en Santé de l’Université de Nantes (IRS-UN)” and approved by the French National Committee of Ethics (Agreement number: B44278).
Measure of global DNA methylation
DNA was extracted by using the QiaAmp DNA mini Kit (Qiagen, France). Next, global DNA methylation was estimated by quantifying the presence of 5-methylcytosine using 5-mC DNA ELISA kit (Zymo Research, France) according to the manufacturer’s instructions.
RT-qPCR analysis
RNA extract is performed using RNeasy Mini QIAcube Kit and QIAcube (Qiagen, France). RT-qPCRs are performed using QuantiTect Reverse Transcription Kit, Rotor-Gene SYBR Green PCR Kit, QuantiTect Primer Assays, and Rotor-Gene Q as real-time thermocycler (Qiagen, France). Reference gene RPLP0 was used, with the 2-∆∆Ct relative quantification method.
Proximity ligation in situ assay
Cells were cultured for 24 h on cover slip. Cells were then fixed with 4% paraformaldehyde in PBS pH 7.4 for 15 min at room temperature. Permeabilization is performed with PBS containing 0.5% Triton X-100 for 20 min at room temperature. Blocking, staining, hybridization, ligation, amplification, and detection steps were realized according to manufacturer’s instructions (Olink Bioscience, Sweden). All incubations were performed in a humidity chamber. Amplification and detection steps were performed in dark room. Fluorescence was visualized by using the Axiovert 200 M microscopy system (Zeiss, Le Pecq, France) with ApoTome module (X63 and numerial aperture 1.4). Preparations were mounted by using ProLong® Gold antifade reagent with DAPI (Life Technologies, France). Pictures acquisition was realized in structured illumination microscopy. After decovolving (3.5 Huygens Essential software (SVI)), 3D view was obtained by using Amira.4.1.1 program. Finally, images were analyzed by using the freeware “BlobFinder” available for download from www.cb.uu.se/~amin/BlobFinder. Thus, we obtained either number of signals per nuclei since nuclei can be automatically identified. DNMT1 and UHRF1 were detected with anti-DNMT1 (Santa Cruz, sc10221, France) and anti-UHRF1 (Santa Cruz, sc98817, France).
Measure of genes methylation by quantitative methylation-sensitive restriction enzyme digestion
Quantitative methylation-sensitive restriction enzyme digestion (qMSRE) combines the use of methylation-sensitive restriction enzyme and real-time PCR.
For qMSRE, 500 ng of purified genomic DNA (QIAamp DNA Mini QIAcube Kit and QIAcube (Qiagen, France)) is subject to digestion with adequate methylation sensitive enzymes. For each assay, these enzymes can be changed, but generally, we used enzymes that can digest unmethylated CG sites but not methylated CG sites. A parallel “mock” reaction containing all reaction components except enzyme (replaced with glycerol) is included for each sample. DNA from the digested or mock reaction is then amplified by real-time PCR with specific primers. Thus, 5 μl of digested or mock solutions were used to perform qPCR using the Rotor-Gene SYBR Green PCR Kit and Rotor-Gene Q as real-time thermocycler (Qiagen, France). DNA was digested by AciI, BspEI, and HpaII (4 h at 37 °C) for the Bcl-w-qMSRE, AciI, and BsrBI (two digestions: 4 h at 37 °C and 4 h at 65 °C) for the LLT1-qMSRE or by Hpa I, HhaI, and AvaI (4 h at 37 °C) for the PD-L1-qMSRE. qPCR was performed with the and the following primers: Bcl-w: CTCTGCTTTTCCTAGGCACGCAA and AGGGCTGTTCAGAGGCCATAGT, LLT1: primers: ACCATCTGGCCTGGATCACA and GGAGAGTTACCCATTTGGCCCATT, and PD-L1: AAATGCAGTGATGGCCCATTTC and GATCCACATAGGTTGCCTTCCTCT. The methylation level for any amplified region can be determined using the following equation Percent Methylation = 100 × 2-ΔCt where ΔCt = the average Ct value from the digested reaction minus the average Ct values from the reference/undigested reaction.
Sequences of the considered promoters were obtained from the Eucaryotic Promoter Database (https://epd.vital-it.ch).
Measure of cell death
Percentages of cell death were evaluated by using a Trypan Blue Stain 0.4%, and the Countess® Automated Cell Counter (ThermoFisher, France). Cell death was induced using temozolomide (25 μM, Torcis, France). Two Gy irradiation was performed by using X-Rad 225cx (Precision X-Ray inc., North Bradford, CT, USA).
siRNA transfection protocol
In a six-well culture plate, 2.105 cells were incubated for 24 h at 37 °C in a CO2 incubator. Then, 60 pmol of siRNA was added and the cells incubated for 7 h at 37 °C in a CO2 incubator. Without removing the siRNA, 1 ml of normal growth medium containing two times the normal serum and antibiotics concentration was added and cells incubated for 24 h. Then, cells were cultured for 48 h in normal culture medium. Thus, analyses were realized 72 h after the siRNA transfection. The following siRNA were used: siRNA-A (control) (sc-37007, Santa Cruz), siRNA-Bcl-w (sc-37294, Santa Cruz) and siRNA-APOBEC3 (sc-60091, Santa Cruz).
Measure of Akt and APOBEC3γ expression
The Akt and APOBEC3γ expression level were estimated by ELISA method and the use of the PathScan® Total Akt1 Sandwich ELISA Kit (Cell Signaling, France) and APOBEC3γ ELISA kit (MyBioSource, USA) according to the manufacturer’s instructions.
Patient samples
Patient samples were collected from GBM patients treated at the “Institut de Cancérologie de l’Ouest” (ICO, http://www.ico-cancer.fr). All patients recruited gave signed, informed consent. All the samples collected and the associated clinical information were registered in the database (N° DC-2018-3321) validated by the French research ministry. Biological resources were stored at the “Centre de Ressources Biologiques-Tumorothèque” (Institut de Cancérologie de l’Ouest, Saint-Herblain, F44800, France).
Statistical analysis
All experiments were done at least in triplicates. Significance of the differences in means ± standard deviations was calculated using Student t test. Significance of correlation between two parameters was calculated using Pearson’s test.
Results
The combination of diuron exposure with Akt overexpression induces glioma, while neither diuron nor Akt alone is sufficient to induce glioma formation
The RCAS/tv-a model has been a very useful and productive tool for studying the gliomagenesis [20]. In this model, PDGF-B overexpression promotes oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes, and the combination of activated Ras and Akt induces high-grade gliomas [1], while neither activated Ras nor Akt alone is sufficient to induce GBM formation [2].
We first have asked the question to know whether the diuron exposure on Ntv-A cells overexpressing LacZ, Ras, or Akt had the ability to promote the gliomagenesis such as the Ras + Akt combination. For this purpose, Ntv-a/LacZ, Ntv-a/Akt, and Ntv-a/Ras cells were exposed to 100 μM diuron each 2 days during 14 days (Fig. 1) to generate Ntv-a/LacZ + diuron, Ntv-a/Akt + diuron, and Ntv-a/Ras + diuron cells. Five independent exposures were performed for each cell types. The diuron exposure dose (100 μM or 23 mg/L) was determined as being (i) a dose devoid of cytotoxicity (Additional file 1: Figure S1), and (ii) a dose inferior to the one seen in human blood (that is 100 mg/L [21]). Tumorigenicity assays were performed via the injection of diuron-exposed cells. Five mice were used for the Ntv-a/LacZ, Ntv-a/Akt, Ntv-a/Ras, and Ntv-a/Ras + Akt cells. Each independent diuron exposure was subcutaneously injected in one mice. As expected, our experiments confirmed that the Ras+ Akt combination acts as oncogenic event for the glioma formation, whereas neither Ras nor Akt alone is sufficient to induce GBM formation (Fig. 1). We next noted that diuron exposure is not sufficient to induce glioma formation, while its combination with Akt promotes the glioma formation in 60% of our experiments (3/5).
Fig. 1.
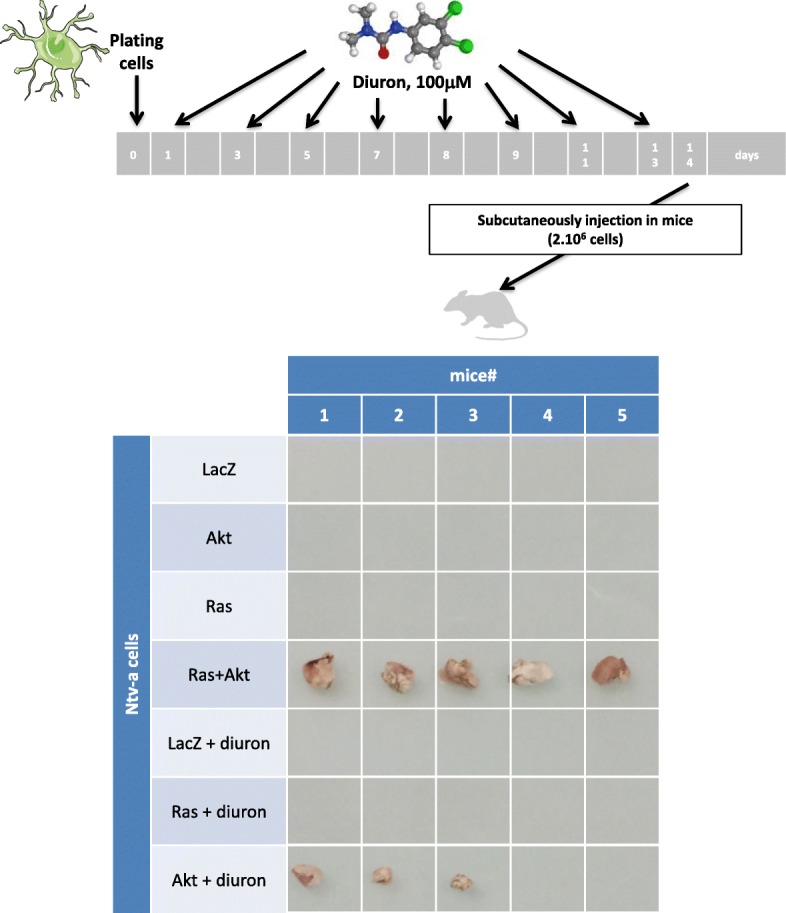
Diuron exposure is not sufficient to induce gliomagenesis, but its combination with Akt overexpression promotes gliomagenesis. Experimental procedures were here summarized. Five mice per condition were xenografted subcutaneously with indicated cells. Sixty days after cells injection, mice were euthanized and tumors were collected. Pictures illustrate the resected tumors
Next, we have compared the tumor-forming potential of Ras + Akt cells and Akt + diuron cells by comparing the tumor growth monitored by caliper measurements. Kinetic of tumor growth and the measure of tumor volume indicated that the volume of tumors induced by the Ras + Akt combination and the tumor growth of these tumors were superior than the one induced by the Akt + diuron combination (Fig. 2a, b).
Fig. 2.
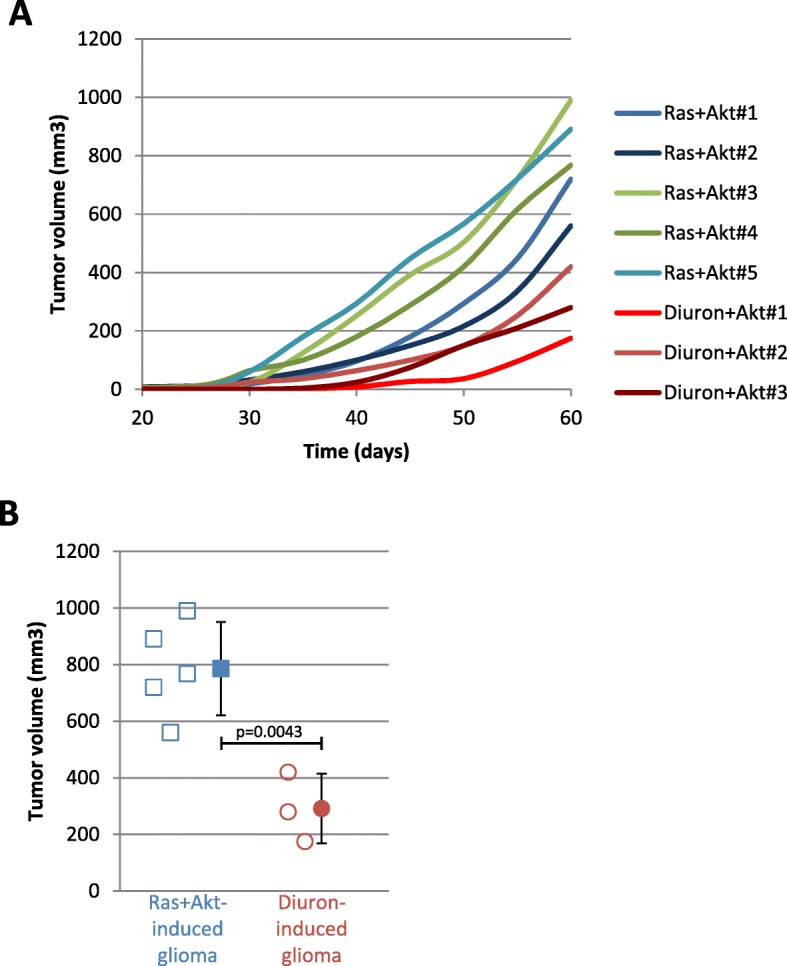
The growth of gliomas induced by the Akt + diuron condition is less aggressive than the one induced by Ras + Akt overexpression. a Curves illustrate, for each mice, the tumor growth of Ras + Akt- and Akt + diuron-induced glioma. b Graph illustrates and compares, 60 days after the cells injection, the volume of all Ras + Akt-induced glioma (n = 5, blue squares) with the volume of all Akt + diuron-induced glioma (n = 3, red circles)
Cells promoting Akt + diuron-induced tumors are globally hypomethylated via active and passive DNA demethylation processes
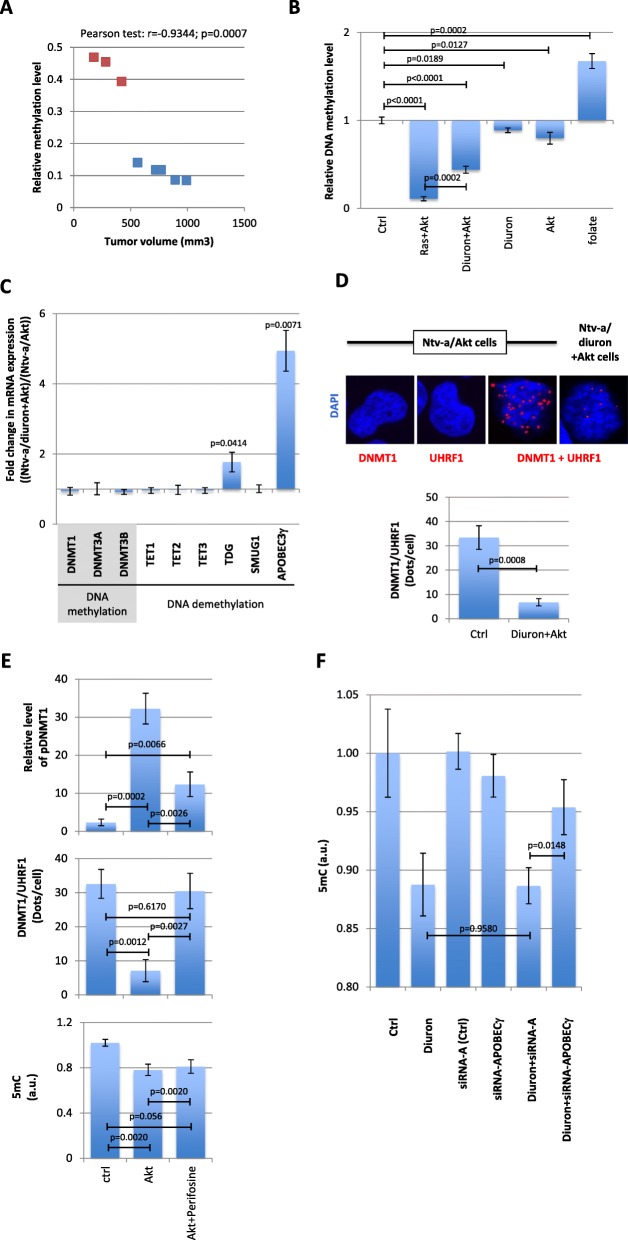
In human glioma, the global level of DNA methylation is associated with the tumor grade and with the aggressiveness of tumors [3, 22, 23]. Our results correlated the relative methylation level of Ras + Akt-induced and Akt + diuron-induced tumors with the tumor volume (Fig. 3a).
Fig. 3.
Global DNA hypomethylation occurs in Akt + diuron-induced glioma. a Correlation between the relative level of DNA methylation and the tumor volume. Blue squares represent the Ras + Akt-induced glioma (n = 5) and red squares represent the Akt + diuron-induced glioma (n = 3). b Graph illustrates the relative DNA methylation level (mean ± SD) seen in Ntv-a/Ras + Akt- and Ntv-a/diuron + Ak cells (n = 3). “Folate” represents the relative DNA methylation level in cells treated with folate, i.e., as a positive control of gain of DNA methylation. “Ctrl” represented the methylation level of Ntv-a/lacZ cells. c Graph represents the fold change expression in mRNA encoding for DNA methylation players (DNMT1, 3A, and 3B) and DNA demethylation players (TET1, 2, and 3, TDG, SMUG1, and APOBEC3γ). d ApoTome view of the DNMT1/UHRF1 dots in indicated cells. Red dots symbolize the DNMT1/UHRF1 interactions. Nucleus/DNA are stained in blue via the use of DAPI. Graph (mean ± SEM) illustrating the number of DNMT1/UHRF1 dots per cells in indicated cells. The number of DNMT1/UHRF1 dots is calculated from the analysis of, at least, 50 nucleus in three independent experimentations. The absence of dots in the presence of the unique use of DNMT1 or UHRF1 antibodies underlines the specificity of signal obtained in presence of the DNMT1 + UHRF1 antibodies. e Graphs illustrate the effect of Akt overexpression and/or inhibition (via the use of Perifosine) on the relative level of phospho-DNMT1 (pDNMT1) (top), the DNMT1/UHRF1 interactions (middle), and on the global level of 5-methylcytosine (5mC, bottom). P-LISA estimates the number of DNMT1/UHRF1 interactions. In cell ELISA performed with anti-pDNMT1S127 was used to analyze the DNMT1 phosphorylation such as previously described [3]. ELISA-5mC (Zymo Research, France) was used to quantify the 5methylcytosine (5mC), i.e., to study the global level of DNA methylation. f Graph illustrates the modification of 5-methylcystosine seen in cells treated with diuron and/or siRNA directed against APOBEC3γ. ELISA-5mC (Zymo Research, France) was used to quantify the 5methylcytosine (5mC), i.e., to study the global level of DNA methylation
To characterize the 5-methylcystosine (5mC) methylation level in cells at the origin of the Akt + diuron-induced glioma (Ntv-a/Akt + diuron cells), we next compared the methylation level of DNA extracted from Ntv-a/LacZ (control, Ctrl), Ntv-a/Ras + Akt, and Ntv-a/Ras + Akt + folate cells. Folate treatment of Ntv-a/Ras + Akt cells (40 μg/ml, 72 h) was here used as a treatment to induced a gain of 5mC. 5mC ELISA reveals that Ntv-a/Akt + diuron cells were hypomethylation in comparison with Ntv-a/LacZ cells (control cells) (Fig. 3b). In addition, we noted that Ntv-a/Ras + Akt cells were more hypomethylated than Ntv-a/Akt + diuron. We also noted that Akt + diuron induced a strong global DNA hypomethylation resulting from synergic effect between the Akt overexpression and the diuron exposure since Akt + diuron DNA demethylation percentage is higher than the theoric addition of diuron effect only and Akt overexpression alone (Fig. 3b).
In order to determine a molecular cause at the origin of the global DNA hypomethylation seen in Ntv-a/Akt + diuron cells, we first analyzed the expression level of several actors of the DNA methylation and DNA demethylation machineries. RT-qPCR analyses indicated that APOBEC3γ and TDG were overexpressed in Ntv-a/Akt + diuron cells compared to Ntv-a/Akt cells (Fig. 3c).
In addition, we also studied the integrity of the DNMT1/UHRF1/PCNA complex through the integrity of the DNMT1/UHRF1 interaction. This focus is due to the fact that the loss of DNMT1/UHRF1/PCNA integrity promotes global DNA hypomethylation and induces tumorigenesis [3, 4]. Our study reports that the DNMT1/UHRF1 signals decrease in Ntv-a/Akt + diuron cells in comparison with Ntv-a/Akt cells (Fig. 3d). Thus, we conclude that the loss of DNMT1/UHRF1/PCNA and the APOBEC3γ overexpression are the two leading molecular causes of the global DNA hypomethylation seen in cells at the origin of Akt + diuron-induced glioma.
The above data support the hypothesis that Akt induces the global DNA hypomethylation through the disruption of the DNMT1/PCNA/UHRF1 complex via the DNMT1 phosphorylation, such as previously described [3]. To address this point, we analyzed the impact of an Akt inhibitor on the level of pDNMT1, the level of DNMT1/UHRF1 interactions, and 5mC. Our data confirmed that Akt overexpression promotes the DNMT1 phosphorylation, the decrease of DNMT1/UHRF1 interaction, and the decrease of 5mC in DNA studied (Fig. 3e). We also observed that Perifosine, an Akt inhibitor, limited the DNMT1 phosphorylation and restored the DNMT1/UHRF1 interactions but is ineffective to restore the global level of DNA methylation (Fig. 3e).
Concerning the diuron-mediated global DNA hypomethylation, our data suggest a mechanism mainly involving the APOBEC3γ overexpression. To investigate this point, we analyzed the impact of a siRNA-mediated APOBEC3γ downregulation on the global level of 5mC. Our results indicated that siRNA-APOBEC3γ abrogated the diuron-mediated global DNA hypomethylation, while siRNA-A (control) has no effect on the diuron-mediated global DNA hypomethylation (Fig. 3f).
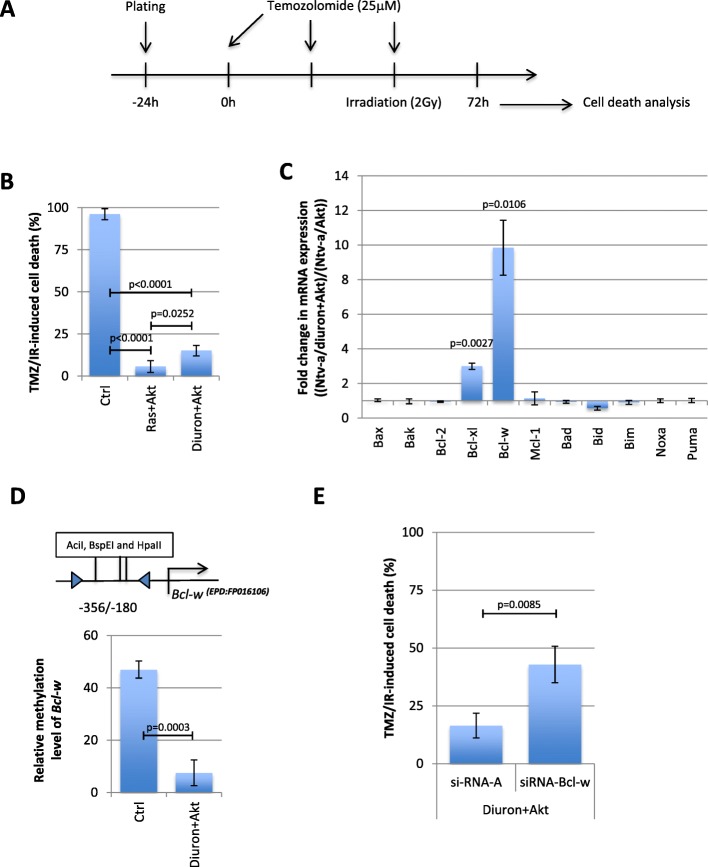
Cells promoting Akt + diuron-induced gliomas are resistant to the temozolomide/irradiation-induced cell death through the Bcl-w overexpression
The standard anti-GBM therapy includes a surgical resection followed by radiation and chemotherapy using temozolomide. Thus, we first studied whether Ntv-a/Akt + diuron cells are sensitive to the temozolomide/radiation therapy. For this purpose, we have calculated the percentage of cell death in Ntv-a/LacZ (Ctrl), Ntv-a/Ras + Akt, and Ntv-a/Akt + diuron. For this purpose, cells were treated with 25 μM of temozolomide (TMZ) for 72 h and irradiated (2 Gy) (Fig. 4a). Thus, we noted that Ntv-a/Ras + Akt and Ntv-a/Akt + diuron cells were strongly resistant to the temozolomide/radiation-induced cell death since this treatment promoted the cell death in a small percentage of cells (5% and 15%, respectively) (Fig. 4b). In order to determine a molecular cause at the origin of this resistance, we analyzed the expression level of 11 apoptotic players. RT-qPCR experiments indicated that mRNA encoding for two anti-apoptotic proteins were overexpressed in Ntv-a/Akt + diuron cells in comparison with Ntv-a/Akt cells: threefold for Bcl-xl and near to tenfold for Bcl-w (Fig. 4c). qMSRE analyzing the methylation level of the Bcl-w promoter indicated that the promoter region of Bcl-w was hypomethylated in Ntv-a/Akt + diuron cells in comparison with Ntv-a/Akt cells (Fig. 4d).
Fig. 4.
The Akt + diuron-induced gliomas are resistant to the temozolomide/irradiation-induced cell death. a Schematic representation of the temozolomide/irradiation-induced cell death. b Graph compares the percentage of temozolomide/irradiation-induced cell death in indicated cells according to Trypan blue method. c Graph represents the fold change expression in mRNA encoding for 11 proteins of the BCL2 family. d qMSRE estimates the relative level of DNA methylation of Bcl-w promoter. e Graph compares the percentage of temozolomide/irradiation-induced cell death in indicated cells according to Trypan blue method
In order to determine whether the diuron-induced Bcl-w overexpression played a crucial role on the temozolomide/radiation resistance, we then measured the percentage of temozolomide/radiation-induced cell death in Akt + diuron cells presenting a Bcl-w downregulation induced through the use of siRNA directed against Bcl-w (Additional file 1: Figure S2). This experiment indicated that siRNA-Bcl-w induced gain of 26% of TMZ/IR-induced cell death (Fig. 4e). This finding confirms that the Bcl-w overexpression plays a role in the TMZ/IR-resistance phenotype seen in cells treated with Akt + diuron.
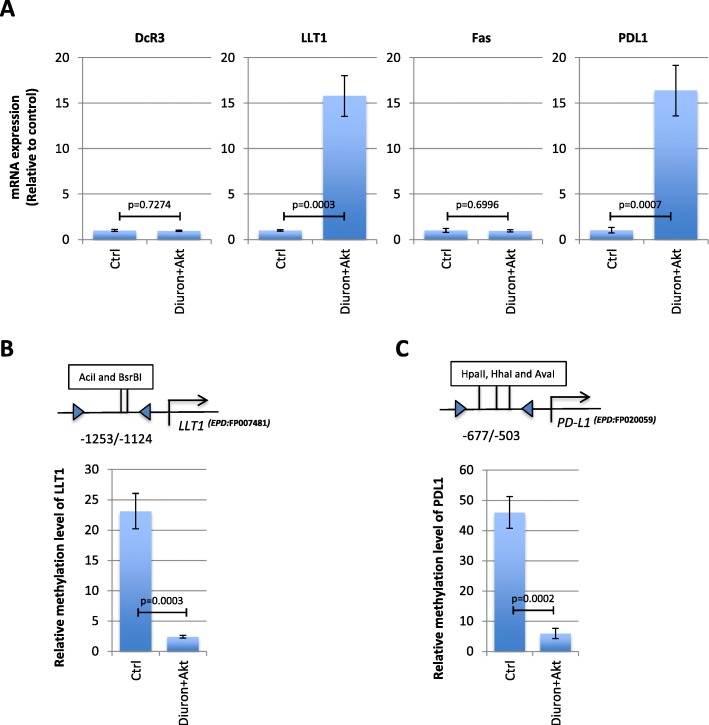
Cells promoting Akt + diuron-induced gliomas are characterized by the DNA hypomethylation-mediated PD-L1 and LLT1 overexpression
Among the various mechanisms of resistance acquired by tumor cells during tumorigenesis, there is the immune system evasion. A pleiotropic number of molecular signatures can be associated to the immune escape of glioma. Here, we have decided to focus our study on four main actors of the immune escape of glioma cells: PD-L1 (since PD-L1 is upregulated in glioma [24]), Fas (a crucial player of the immune system-induced apoptosis [25]), DcR3 (a negative regulator of Fas [26]), and LLT1 (since LLT1 acts as a mediator of immune escape [27]). Our data indicated that Akt + diuron cells overexpressed PD-L1 and LLT1 (Fig. 5a). qMSRE analyses indicated that LLT1 and PD-L1 promoter were hypomethylated in Akt + diuron cells (Fig. 5b, c).
Fig. 5.
Akt + diuron-induced gliomas are characterized by the DNA hypomethylation-mediated PD-L1 and LLT1 overexpression. a Graphs compare the relative mRNA expression encoding for immune players (DcR3, LLT1, and PD-L1 for immunotolerance, and Fas for immunostimulation) in control (Ctrl) and diuron + Akt cells. b qMSRE estimates the relative level of DNA methylation of LLT1 promoter. c qMSRE estimates the relative level of DNA methylation of PD-L1 promoter
Hypomethylation of Bcl-w and PD-L1 promoters is associated with the APOBEC3γ overexpression, while hypomethylation of LLT1 promoter is associated with the DNMT1/PCNA/UHRF1 disruption
Akt + diuron cells presenting an APOBEC3γ overexpression and a loss of DNMT1/UHRF1/PCNA, we hypothesized that these two causes of DNA hypomethylation could be involved in the hypomethylation of Bcl-w, PD-L1, and LLT1 promoters. To test this hypothesis, APOBEC3γ was overexpressed by transfecting a plasmid coding for this protein (Additional file 1: Figure S3), while the loss of DNMT1/UHRF1/PCNA complex was obtained through the use of a plasmid encoding for a peptide disrupting this complex (named UP, such as previously described [3, 4]). qMSRE analyses indicated that the APOBEC3γ overexpression promoted the Bcl-w and PD-L1 hypomethylation, while the UP-induced loss of DNMT1/UHRF1/PCNA complex induced the LLT1 hypomethylation (Fig. 6).
Fig. 6.
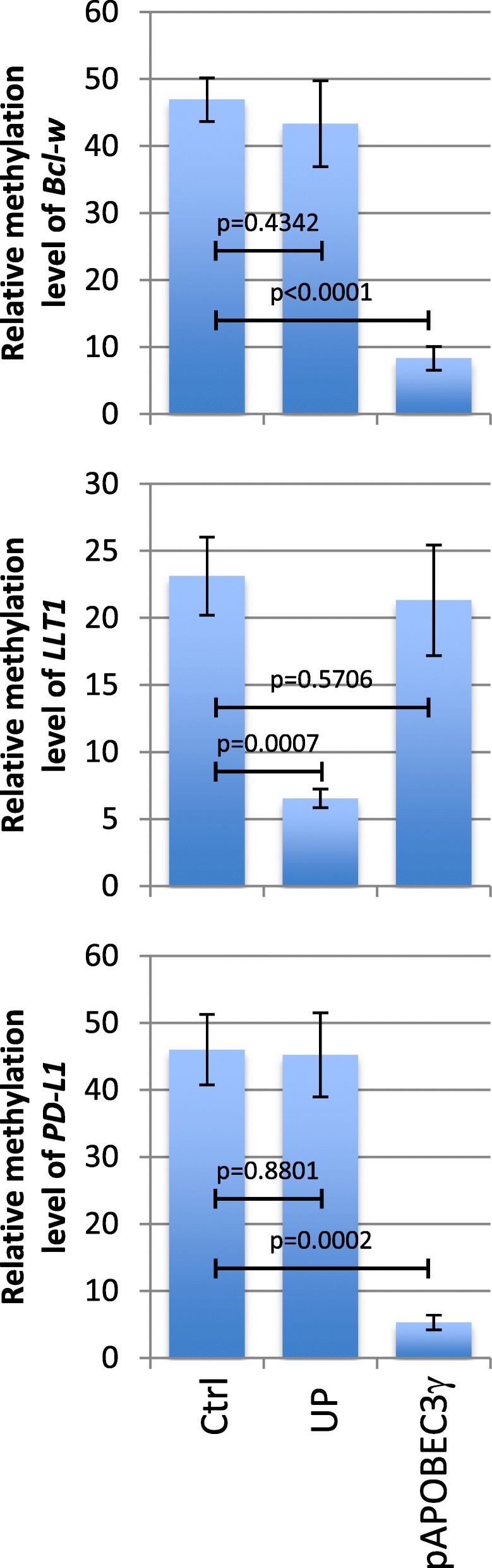
APOBEC3γ promotes the PD-L1 and Bcl-w demethylation, while the DNMT1/PCNA/UHRF1 disruption promotes the LLT1 demethylation. qMSRE (as previously described) compare the relative methylation level of Bcl-w, LLT1, and PD-L1 genes in cells transiently transfected with the pcDNA.3.1 (Ctrl), pcDNA.3.1-UP plasmid, and with pcDNA.3.1-APOBEC3γ plasmids. pcDNA.3.1-UP is a plasmid encoding for a chimeric protein having the ability to selectively disrupt the DNMT1/UHRF1 interaction [3, 4]
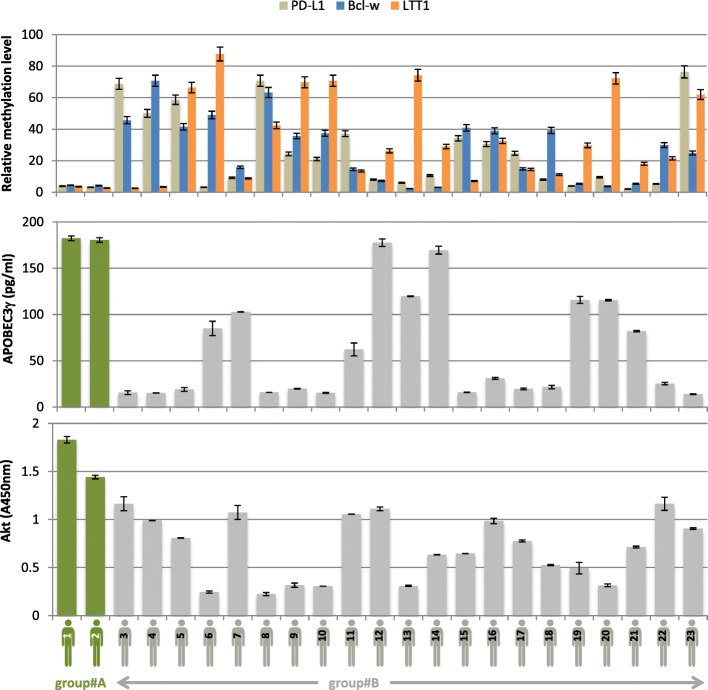
Presence of concomitant LLT1, PD-L1, and Bcl-w hypomethylation in GBM patients
Our data indicate that the gliomagenesis induced by the Akt overexpression and diuron exposure is associated with the hypomethylation of the LLT1, PD-L1, and Bcl-w genes. In other terms, our data indicate that the LLT1, PD-L1, and Bcl-w hypomethylation are associated with the diuron-induced modifications of methylome. Based on this finding, we hypothesized that GBM patients having had a potential exposure to diuron via their professional activity could have tumors characterized by the concomitant LLT1, PD-L1, and Bcl-w hypomethylations. To investigate this idea, a GBM sample cohort of 23 patients was used (Additional file 2: Table S1). Interestingly, two of these patients have had a potential exposure to diuron via their professional activity (group#A), while all other patients have a supposed absence of diuron contact (group#B). qMSRE indicated that the concomitant LLT1, PD-L1, and Bcl-w hypomethylation (defined by a relative methylation level inferior to 5) was only observed in group#A and not in group#B (Fig. 7). We also noted that the two GBM patients included in group#A have high levels of APOBEC3γ and Akt expressions (Fig. 7).
Fig. 7.
Methylation status of PD-L1, Bcl-w, and LLT1 genes in GBM patients putatively exposed to diuron via their professional activity (green, n = 2) or not (gray, n = 21) qMSRE (as previously described) compare the relative methylation level of Bcl-w, LLT1, and PD-L1 genes. APOBEC3γ and AKT expressions were estimated by ELISA method. GBM patients potentially exposed to diuron via their professional activity (farmer, gardener …) are in green (n = 2), and GBM patients potentially unexposed to diuron via their professional activity are in gray (n = 21).
Discussion
During the last decades, several works define the gliomagenesis as a tumor development generated by multistep and/or chromothripsis (a phenomenon characterized by thousands of chromosomal rearrangements) processes that involve various genetic and/or epigenetic alterations. Thus, during tumor initiation step, genetic and/or epigenetic alterations can promote the acquisition of cancer hallmarks such as the apoptosis and immune evasion. In previous articles, we and others have begun to unveil the mechanisms through which DNA hypomethylation or the Akt/Ras overexpression can promote gliomagenesis and the acquisition of apoptosis and immune evasion phenotypes [3, 4, 28, 29]. However, in these papers, DNA hypomethylation was induced by a chimeric peptide having the ability to disrupt the integrity of the main complex responsible for the DNA inheritance, the DNMT1/PCNA/UHRF1 complex.
In the present article, we report that Akt overexpression and diuron exposure promote the glioma formation from neural progenitor cells via the induction of DNA hypomethylation mediated by two distinct pathways: the loss of DNMT1/PCNA/UHRF1 interactions and the APOBEC3γ overexpression. The identification of these two molecular causes of hypomethylation reinforce the idea that DNMT1/PCNA/UHRF1 play a crucial role in the maintenance of DNA methylation level, such as already described in several articles [3, 4, 30, 31]. This last point provides a new evidence for the involvement of APOBEC3γ in DNA demethylation mechanisms [32, 33], despite the discussion surrounding the role of APOBEC3γ on these mechanisms (a discussion mainly associated with the publication of Wijesinghe and Bhagwat [34]).
Our work is the first to report the evidence that the diuron exposure can promote to the gliomagenesis. However, in gliomagenesis, the oncogenic effect is not due to itself since diuron needs to be associated to Akt overexpression to promote the gliomagenesis. The involvement of diuron in carcinogenesis is not new since diuron is already reported for the bladder [14, 15], urothelial [16], skin [17, 18], and mammary [15–19] carcinogenesis. Nevertheless, none of these publications mentioned an impact of diuron on the global level of DNA methylation and on the methylation level of certain gene promoters.
The fact that diuron needs to be associated with another oncogenic hit (Akt overexpression) to promote gliomagenesis reinforces the idea that tumorigenesis can result from the accumulation of several hits such as mentioned in the Knudson hypothesis, also known as the two-hit hypothesis [35, 36] .
To our knowledge, the impact of diuron on methylome is to date only reported in oyster [37–39]. More generally, several publications report the fact that pesticide exposure affects the methylome. Recently, Rusiecki et al. [40] reports that pesticide exposure has been associated with acute and chronic adverse health effects and that DNA methylation may mediate these effects. Besides in this article, the authors report that pesticide exposure could modulate the MGMT methylation. In our study, we noted that the methylation of MGMT methylation was unchanged (Additional file 1: Figure S4). We also observed that the two GBM patients potentially exposed to diuron via their professional activity are “MGMT unmethylated.” Zhang et al. [41] report that diazinon-treated cells exhibited increased DNA methylation levels in certain genes defined as tumor suppressor genes such as RASSF1A and PTEN. A finding supporting the idea that the diazinon-induced hypermethylated could play a pathological role in cancer development [41]. On contrary to this study, our work shows that the Akt + diuron-induced tumors are characterized by both global and local DNA hypomethylations. Our data clearly indicated that the diuron + Akt-induced tumorigenesis is associated with the hypomethylation and the overexpression of genes that responsible for apoptosis (Bcl-w) and immune evasion (PD-L1 and LLT1). Indeed, bcl-w/bcl2l2 (bcl-2-like protein 2) gene encodes an anti-apoptotic protein which expression is regulated by DNA methylation in glioma [42, 43]. In addition, Bcl-w expression contributed to the aggressiveness of glioma [44]. Lectin-like transcript-1 (LLT1) is a newly identified ligand for the inhibitory natural killer (NK) cell receptor CD161 which the expression acts as a mediator of immune escape and contributes to the immunosuppressive properties of glioma cells [27]. PD-L1 is a protein encoded by the CD274 gene, which plays a major role in suppressing the immune response. The high PD-L1 expression is associated with a worse outcome of patients suffering from GBM according to certain study [45], while others studies contradict this finding [46]. Be that as it may on this point, our observation associating the Akt + diuron-induced demethylation/overexpression of Bcl-w, PD-L1,, and LLT1 is consistent with the Akt + diuron-induced gliomagenesis. In other terms, it appears that diuron promotes the reprogramming of expression of certain apoptotic and immune actors. Other pesticides have the same effect. Thus, we recently observed that a pesticides mixture modulates the Mcl1 expression in MSC cells [47].
Finally, we made the dual observing that (1) the gliomagenesis induced by the Akt overexpression and diuron exposure is associated with the hypomethylation of the LLT1, PD-L1, and Bcl-w genes and (2) the concomitant Bcl-w, PD-L1, and LLT1 hypomethylation occurs in 2/2 tumors of patients having had a potential exposure to diuron via their professional activity. We did not observe this situation in 21 tumors of patients devoid of potential exposure to diuron via their professional activity. These observations are troubling and tend to support the idea that diuron exposure can play a role of one oncogenic hit on the road to gliomagenesis.
Conclusion
Since several years, a large number of studies analyzed the pesticide exposure and the carcinogenesis initiation. Our data strongly identify a causal link between diuron exposure and cancer development. However, our data underline that diuron is not oncogenic by itself since diuron promotes gliomagenesis only when its exposure is associated with the Akt overexpression (another non-oncogenic event of gliomagenesis by itself) in our model. Mechanistically, we determined that diuron promotes gliomagenesis through the induction of DNA methylation modification. Thus, a better understanding of the diuron-induced alterations of DNA methylation mechanisms could provide rational biomarkers for detecting diuron-induced glioma and to design efficient therapeutic strategies against these types of tumors. In addition to our finding, several reports mention the idea that the exposure of environmental chemicals can modulate the epigenome to potentialize or directly induce the carcinogenesis [48–50]. Thus, epigenetic reprogramming by environmental chemical could represent a novel mechanism to explain their carcinogen impact. Thus, without being oncogenic alone, exposure to an environmental chemical such as diuron could be the first step on the carcinogenesis road. And this step needs to “be complete” by other(s) step(s) (such as exposure to other(s) environmental chemical(s) or the genes overexpression, mutation, or deletion, etc...) in order to arrive at its term, i.e., the tumor formation (Fig. 8).
Fig. 8.
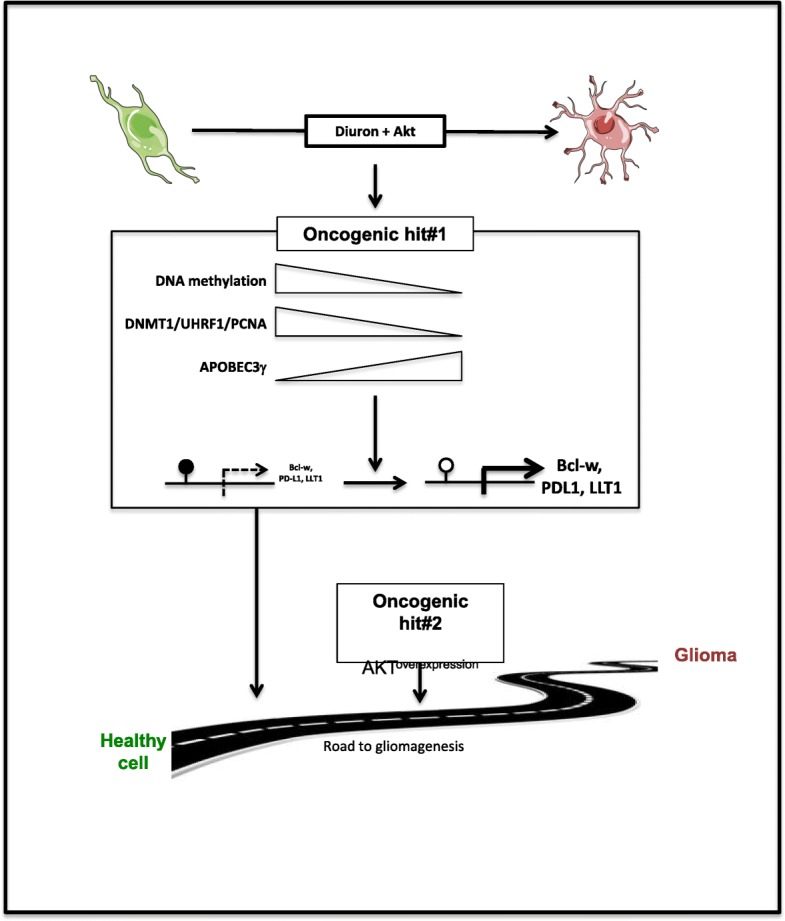
Signaling pathway of the Akt + diuron-induced glioma
Supplementary information
Additional file 1: Figure S1. Cell viability of Ntv-a/lacZ cells exposed to diuron. Different doses of diuron were incubated on Ntv-a/lacZ cells. XTT Cell Viability Kit (Ozyme, France) was used to calculate the percentage of cell viability after 48h of diuron. The values are means±SD from three independent experiments performed in duplicate. Figures S2 and S3. RT-qPCR were done to validated the siRNA downexpression. Down-regulation of Bcl-w and APOBEC3γ were performed via cells transient transfection with siRNA-Bcl-w (Santa-Cruz, sc-37294, France) and siRNA-APOBEC3γ (Santa-Cruz, sc-60091, France). siRNA-A is a control (Santa-Cruz, sc-37007, France). Figure S4. Graphs illustrate the effect of Diuron+Akt on the MGMTmRNA expression (A) and the MGMT methylation level (B). RT-qPCR estimates the MGMTmRNA expression. qMSRE (OneStep qMethyl™ Kit - Zymo Research, France) estimates the MGMT methylation level
Additional file 2: Table S1. Characteristics of BGM patients.
Acknowledgments
JB was supported by a fellowship from EpiSAVMEN/Région Pays de la Loire (Programme Dynamique Scientifique 2016). We also thank “En Avant la Vie” (an association supporting the patients suffering from gliomas and their family, and the research on glioma field) for the partial funding of the JB’s fellowship.
Abbreviations
- 5mC
5-Methylcystosine
- P-LISA
Proximity ligation in situ assay
- qMSRE
Quantitative methylation-sensitive restriction enzyme digestion
Authors’ contributions
PFC designed and coordinated the project. JB, AN, GBC, and PFC performed all experiments. JB, GCB, CB, FMV, and PFC interpreted and discussed the data. PFC wrote the first version of the manuscript and all authors reviewed and approved it.
Funding
This work was partially supported by grants from the LIGUE NATIONALE CONTRE LE CANCER, Comité InterRégional Grand Ouest, département de Loire Atlantique, d’Ille et Vilaine, Vendée et Côte d’Armor (Subvention 2015, 2016 and 2017). This work was partially supported by grants from EpiSAVMEN (Dynamique scientifique, Région Pays de la Loire, France).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article and its additional files. All other datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of LaBCT and was performed according to the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Joséphine Briand, Email: josephine.briand@etu.univ-nantes.fr.
Arulraj Nadaradjane, Email: Arulraj.Nadaradjane@univ-nantes.fr.
Gwenola Bougras-Cartron, Email: Gwenola.Cartron@ico.unicancer.fr.
Christophe Olivier, Email: christophe.olivier@univ-nantes.fr.
François M. Vallette, Email: francois.vallette@inserm.fr
Pierre-François Cartron, Phone: 33-25-348-4785, Email: pierre-francois.cartron@inserm.fr.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13148-019-0759-1.
References
- 1.Dai C, Celestino JC, Okada Y, Louis DN, Fuller GN, Holland EC. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001;15:1913–1925. doi: 10.1101/gad.903001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- 3.Hervouet E, Lalier L, Debien E, Cheray M, Geairon A, Rogniaux H, et al. Disruption of Dnmt1/PCNA/UHRF1 interactions promotes tumorigenesis from human and mice glial cells. PLoS One. 2010;5:e11333. doi: 10.1371/journal.pone.0011333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pacaud R, Brocard E, Lalier L, Hervouet E, Vallette FM, Cartron P-F. The DNMT1/PCNA/UHRF1 disruption induces tumorigenesis characterized by similar genetic and epigenetic signatures. Sci Rep. 2014;4:4230. doi: 10.1038/srep04230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H-X, Meng H-Y, Peng X-X, Zong Q, Zhang K, Han G-L. A meta-analysis of association between pesticides exposure and Glioma risk in adults. J Craniofac Surg. 2015;26:e672–e673. doi: 10.1097/SCS.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 6.Ruder AM, Carreón T, Butler MA, Calvert GM, Davis-King KE, Waters MA, et al. Exposure to farm crops, livestock, and farm tasks and risk of glioma: the upper Midwest health study. Am J Epidemiol. 2009;169:1479–1491. doi: 10.1093/aje/kwp075. [DOI] [PubMed] [Google Scholar]
- 7.Samanic CM, De Roos AJ, Stewart PA, Rajaraman P, Waters MA, Inskip PD. Occupational exposure to pesticides and risk of adult brain tumors. Am J Epidemiol. 2008;167:976–985. doi: 10.1093/aje/kwm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Provost D, Cantagrel A, Lebailly P, Jaffré A, Loyant V, Loiseau H, et al. Brain tumours and exposure to pesticides: a case-control study in southwestern France. Occup Environ Med. 2007;64:509–514. doi: 10.1136/oem.2006.028100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khuder SA, Mutgi AB, Schaub EA. Meta-analyses of brain cancer and farming. Am J Ind Med. 1998;34:252–260. doi: 10.1002/(SICI)1097-0274(199809)34:3<252::AID-AJIM7>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Lee WJ, Colt JS, Heineman EF, McComb R, Weisenburger DD, Lijinsky W, et al. Agricultural pesticide use and risk of glioma in Nebraska, United States. Occup Environ Med. 2005;62:786–792. doi: 10.1136/oem.2005.020230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mathys W. Pesticide pollution of groundwater and drinking water by the processes of artificial groundwater enrichment or coastal filtration: underrated sources of contamination. Zentralblatt Hyg Umweltmed Int J Hyg Environ Med. 1994;196:338–359. [PubMed] [Google Scholar]
- 12.Meffe R, de Bustamante I. Emerging organic contaminants in surface water and groundwater: a first overview of the situation in Italy. Sci Total Environ. 2014;481:280–295. doi: 10.1016/j.scitotenv.2014.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Angly FE, Pantos O, Morgan TC, Rich V, Tonin H, Bourne DG, et al. Diuron tolerance and potential degradation by pelagic microbiomes in the great barrier reef lagoon. PeerJ. 2016;4:e1758. doi: 10.7717/peerj.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Rocha MS, Arnold LL, De Oliveira MLCS, Catalano SMI, Cardoso APF, Pontes MGN, et al. Diuron-induced rat urinary bladder carcinogenesis: mode of action and human relevance evaluations using the international Programme on chemical safety framework. Crit Rev Toxicol. 2014;44:393–406. doi: 10.3109/10408444.2013.877870. [DOI] [PubMed] [Google Scholar]
- 15.de Moura NA, Grassi TF, Rodrigues MAM, Barbisan LF. Potential effects of the herbicide diuron on mammary and urinary bladder two-stage carcinogenesis in a female Swiss mouse model. Arch Toxicol. 2010;84:165–173. doi: 10.1007/s00204-009-0477-0. [DOI] [PubMed] [Google Scholar]
- 16.da Rocha MS, Nascimento MG, Cardoso APF, de Lima PLA, Zelandi EA, de Camargo JLV, et al. Cytotoxicity and regenerative proliferation as the mode of action for diuron-induced urothelial carcinogenesis in the rat. Toxicol Sci Off J Soc Toxicol. 2010;113:37–44. doi: 10.1093/toxsci/kfp241. [DOI] [PubMed] [Google Scholar]
- 17.Ferrucio Bianca, Franchi Carla Adriene da Silva, Boldrin Natália Ferreira, Oliveira Maria Luiza Cotrim Sartor de, Camargo João Lauro Viana de. Evaluation of Diuron (3-[3,4-dichlorophenyl]-1,1-dimethyl urea) in a Two-stage Mouse Skin Carcinogenesis Assay. Toxicologic Pathology. 2010;38(5):756–764. doi: 10.1177/0192623310375452. [DOI] [PubMed] [Google Scholar]
- 18.Antony M, Shukla Y, Mehrotra NK. Tumour initiatory activity of a herbicide diuron on mouse skin. Cancer Lett. 1989;48:125–128. doi: 10.1016/0304-3835(89)90048-7. [DOI] [PubMed] [Google Scholar]
- 19.Grassi TF, Rodrigues MAM, de Camargo JLV, Barbisan LF. Evaluation of carcinogenic potential of diuron in a rat mammary two-stage carcinogenesis model. Toxicol Pathol. 2011;39:486–495. doi: 10.1177/0192623310396904. [DOI] [PubMed] [Google Scholar]
- 20.Hambardzumyan D, Amankulor NM, Helmy KY, Becher OJ, Holland EC. Modeling adult Gliomas using RCAS/t-va technology. Transl Oncol. 2009;2:89–95. doi: 10.1593/tlo.09100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verheij ER, van der Greef J, La Vos GF, van der Pol W, Niessen WM. Identification of diuron and four of its metabolites in human postmortem plasma and urine by LC/MS with a moving-belt interface. J Anal Toxicol. 1989;13:8–12. doi: 10.1093/jat/13.1.8. [DOI] [PubMed] [Google Scholar]
- 22.Hervouet E, Debien E, Campion L, Charbord J, Menanteau J, Vallette FM, et al. Folate supplementation limits the aggressiveness of glioma via the remethylation of DNA repeats element and genes governing apoptosis and proliferation. Clin Cancer Res Off J Am Assoc Cancer Res. 2009;15:3519–3529. doi: 10.1158/1078-0432.CCR-08-2062. [DOI] [PubMed] [Google Scholar]
- 23.Zukiel R, Nowak S, Barciszewska A-M, Gawronska I, Keith G, Barciszewska MZ. A simple epigenetic method for the diagnosis and classification of brain tumors. Mol Cancer Res MCR. 2004;2:196–202. [PubMed] [Google Scholar]
- 24.Wang Z, Zhang C, Liu X, Wang Z, Sun L, Li G, et al. Molecular and clinical characterization of PD-L1 expression at transcriptional level via 976 samples of brain glioma. Oncoimmunology. 2016;5:e1196310. doi: 10.1080/2162402X.2016.1196310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gratas C, Tohma Y, Van Meir EG, Klein M, Tenan M, Ishii N, et al. Fas ligand expression in glioblastoma cell lines and primary astrocytic brain tumors. Brain Pathol Zurich Switz. 1997;7:863–869. doi: 10.1111/j.1750-3639.1997.tb00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arakawa Y, Tachibana O, Hasegawa M, Miyamori T, Yamashita J, Hayashi Y. Frequent gene amplification and overexpression of decoy receptor 3 in glioblastoma. Acta Neuropathol (Berl) 2005;109:294–298. doi: 10.1007/s00401-004-0956-6. [DOI] [PubMed] [Google Scholar]
- 27.Roth P, Mittelbronn M, Wick W, Meyermann R, Tatagiba M, Weller M. Malignant glioma cells counteract antitumor immune responses through expression of lectin-like transcript-1. Cancer Res. 2007;67:3540–3544. doi: 10.1158/0008-5472.CAN-06-4783. [DOI] [PubMed] [Google Scholar]
- 28.Cheray M, Pacaud R, Nadaradjane A, Vallette FM, Cartron P-F. Specific inhibition of one DNMT1-including complex influences tumor initiation and progression. Clin Epigenetics. 2013;5:9. doi: 10.1186/1868-7083-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheray M, Nadaradjane A, Bonnet P, Routier S, Vallette FM, Cartron P-F. Specific inhibition of DNMT1/CFP1 reduces cancer phenotypes and enhances chemotherapy effectiveness. Epigenomics. 2014;6:267–275. doi: 10.2217/epi.14.18. [DOI] [PubMed] [Google Scholar]
- 30.Bostick M, Kim JK, Estève P-O, Clark A, Pradhan S, Jacobsen SE. UHRF1 plays a role in maintaining DNA methylation in mammalian cells. Science. 2007;317:1760–1764. doi: 10.1126/science.1147939. [DOI] [PubMed] [Google Scholar]
- 31.Sharif J, Muto M, Takebayashi S, Suetake I, Iwamatsu A, Endo TA, et al. The SRA protein Np95 mediates epigenetic inheritance by recruiting Dnmt1 to methylated DNA. Nature. 2007;450:908–912. doi: 10.1038/nature06397. [DOI] [PubMed] [Google Scholar]
- 32.Teperek-Tkacz M, Pasque V, Gentsch G, Ferguson-Smith AC. Epigenetic reprogramming: is deamination key to active DNA demethylation? Reprod Camb Engl. 2011;142:621–632. doi: 10.1530/REP-11-0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chelico L, Pham P, Calabrese P, Goodman MF. APOBEC3G DNA deaminase acts processively 3′ --> 5′ on single-stranded DNA. Nat Struct Mol Biol. 2006;13:392–399. doi: 10.1038/nsmb1086. [DOI] [PubMed] [Google Scholar]
- 34.Wijesinghe P, Bhagwat AS. Efficient deamination of 5-methylcytosines in DNA by human APOBEC3A, but not by AID or APOBEC3G. Nucleic Acids Res. 2012;40:9206–9217. doi: 10.1093/nar/gks685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knudson AG. Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pedraza-Fariña LG. Mechanisms of oncogenic cooperation in cancer initiation and metastasis. Yale J Biol Med. 2006;79:95–103. [PMC free article] [PubMed] [Google Scholar]
- 37.Bachère E, Barranger A, Bruno R, Rouxel J, Menard D, Piquemal D, et al. Parental diuron-exposure alters offspring transcriptome and fitness in Pacific oyster Crassostrea gigas. Ecotoxicol Environ Saf. 2017;142:51–58. doi: 10.1016/j.ecoenv.2017.03.030. [DOI] [PubMed] [Google Scholar]
- 38.Akcha F, Barranger A, Bachère E, Berthelin CH, Piquemal D, Alonso P, et al. Effects of an environmentally relevant concentration of diuron on oyster genitors during gametogenesis: responses of early molecular and cellular markers and physiological impacts. Environ Sci Pollut Res Int. 2016;23:8008–8020. doi: 10.1007/s11356-015-5969-2. [DOI] [PubMed] [Google Scholar]
- 39.Rondon R, Grunau C, Fallet M, Charlemagne N, Sussarellu R, Chaparro C, Montagnani C, Mitta G, Bachère E, Akcha F, Cosseau C, Rondon R, Grunau C, Fallet M, Charlemagne N, Sussarellu R, Chaparro C. Effects of a parental exposure to diuron on Pacific oyster spat methylome. Environ Epigenet. 2017:1–13. [DOI] [PMC free article] [PubMed]
- 40.Rusiecki JA, Beane Freeman LE, Bonner MR, Alexander M, Chen L, Andreotti G, et al. High pesticide exposure events and DNA methylation among pesticide applicators in the agricultural health study. Environ Mol Mutagen. 2017;58:19–29. doi: 10.1002/em.22067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X, Wallace AD, Du P, Lin S, Baccarelli AA, Jiang H, et al. Genome-wide study of DNA methylation alterations in response to diazinon exposure in vitro. Environ Toxicol Pharmacol. 2012;34:959–968. doi: 10.1016/j.etap.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Debien E, Hervouet E, Gautier F, Juin P, Vallette FM, Cartron P-F. ABT-737 and/or folate reverse the PDGF-induced alterations in the mitochondrial apoptotic pathway in low-grade glioma patients. Clin Epigenetics. 2011;2:369–381. doi: 10.1007/s13148-011-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hervouet E, Vallette FM, Cartron P-F. Impact of the DNA methyltransferases expression on the methylation status of apoptosis-associated genes in glioblastoma multiforme. Cell Death Dis. 2010;1:e8. doi: 10.1038/cddis.2009.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee WS, Kwon J, Yun DH, Lee YN, Woo EY, Park M-J, et al. Specificity protein 1 expression contributes to Bcl-w-induced aggressiveness in glioblastoma multiforme. Mol Cells. 2014;37:17–23. doi: 10.14348/molcells.2014.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cartron P-F, Hervouet E, Debien E, Olivier C, Pouliquen D, Menanteau J, et al. Folate supplementation limits the tumourigenesis in rodent models of gliomagenesis. Eur J Cancer Oxf Engl 1990. 2012;48:2431–2441. doi: 10.1016/j.ejca.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 46.Heiland DH, Haaker G, Delev D, Mercas B, Masalha W, Heynckes S, et al. Comprehensive analysis of PD-L1 expression in glioblastoma multiforme. Oncotarget. 2017; 10.18632/oncotarget.15031. [DOI] [PMC free article] [PubMed]
- 47.Hochane M, Trichet V, Pecqueur C, Avril P, Oliver L, Denis J, et al. Low-dose pesticide mixture induces senescence in normal mesenchymal stem cells (MSC) and promotes tumorigenic phenotype in premalignant MSC. Stem Cells Dayt Ohio. 2017;35:800–811. doi: 10.1002/stem.2539. [DOI] [PubMed] [Google Scholar]
- 48.Prins GS, Ye S-H, Birch L, Zhang X, Cheong A, Lin H, et al. Prostate Cancer risk and DNA methylation signatures in aging rats following developmental BPA exposure: a dose-response analysis. Environ Health Perspect. 2017;125:077007. doi: 10.1289/EHP1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miousse Isabelle, Chang Jianhui, Shao Lijian, Pathak Rupak, Nzabarushimana Étienne, Kutanzi Kristy, Landes Reid, Tackett Alan, Hauer-Jensen Martin, Zhou Daohong, Koturbash Igor. Inter-Strain Differences in LINE-1 DNA Methylation in the Mouse Hematopoietic System in Response to Exposure to Ionizing Radiation. International Journal of Molecular Sciences. 2017;18(7):1430. doi: 10.3390/ijms18071430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chappell G, Pogribny IP, Guyton KZ, Rusyn I. Epigenetic alterations induced by genotoxic occupational and environmental human chemical carcinogens: a systematic literature review. Mutat Res Rev Mutat Res. 2016;768:27–45. doi: 10.1016/j.mrrev.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Cell viability of Ntv-a/lacZ cells exposed to diuron. Different doses of diuron were incubated on Ntv-a/lacZ cells. XTT Cell Viability Kit (Ozyme, France) was used to calculate the percentage of cell viability after 48h of diuron. The values are means±SD from three independent experiments performed in duplicate. Figures S2 and S3. RT-qPCR were done to validated the siRNA downexpression. Down-regulation of Bcl-w and APOBEC3γ were performed via cells transient transfection with siRNA-Bcl-w (Santa-Cruz, sc-37294, France) and siRNA-APOBEC3γ (Santa-Cruz, sc-60091, France). siRNA-A is a control (Santa-Cruz, sc-37007, France). Figure S4. Graphs illustrate the effect of Diuron+Akt on the MGMTmRNA expression (A) and the MGMT methylation level (B). RT-qPCR estimates the MGMTmRNA expression. qMSRE (OneStep qMethyl™ Kit - Zymo Research, France) estimates the MGMT methylation level
Additional file 2: Table S1. Characteristics of BGM patients.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article and its additional files. All other datasets used and analyzed during the current study are available from the corresponding author on reasonable request.