Introduction
The field of renal cancer therapeutics has undergone radical changes in the last decade. The recognition of the vascular endothelial growth factor being the predominant driver of the disease led to the initial advances, heralded by the clinical evaluation of antibody and small molecule tyrosine kinase inhibitors (TKI) of angiogenesis. Agents such as sorafenib, sunitinib, pazopanib, and bevacizumab plus interferon were rapidly evaluated and established Food and Drug Association (FDA) approval in an otherwise orphan indication, with a huge unmet need [1–4]. Everolimus and axitinib were then evaluated in the second-line setting and also approved [5, 6]. Recent advances in the field have led to another wave of novel therapies being evaluated in clinical trials in the post-TKI second- or third-line setting. These therapies have distinct mechanisms of action, patterns of efficacy, and toxicity profiles. Nivolumab, cabozantinib, and the combination of lenvatinib and everolimus [7–10] demonstrated superior clinical efficacy as compared to everolimus alone and have gained FDA approval in the last 12 months.
The focus of this paper is to review the mechanism of action, indications, clinical trial data, and safety information of cabozantinib therapy in advanced renal cancer. We will also discuss the therapeutic use of cabozantinib in the context of numerous other second- to third-line agents that are now available in metastatic RCC. In addition, the data regarding efficacy of cabozantinib in non-clear cell histologies, and the ongoing clinical trials of single agent and cabozantinib-based combination regimens in kidney cancer will be reviewed.
New cases (62,700) of kidney and renal pelvis cancer cases are estimated to be diagnosed in the USA in 2016, which constitutes about 4% of all new cancer cases. It is also estimated to be responsible for 14,240 deaths (about 2% of all cancer deaths estimated in 2016) [11]. Worldwide incidence of kidney cancer in 2012 was estimated around 338,000 new cases [12]. About 70% of all renal cell carcinomas, specifically those which originate from the renal cortex, are clear cell RCC. SEER registry data from 2005 to 2011 show that about 16% of all renal cell carcinoma cases are metastatic at diagnosis, and about 30% of localized RCC will recur after resection [13].
For the last two decades, there has been continuous improvement in treatment of advanced RCC. A disease that was notoriously resistant to cytotoxic chemotherapy and radiation has now succumbed to angiogenesis inhibition and immune checkpoint inhibition. Cytokine therapies with interferon and interleukin were the mainstay of treatment for advanced RCC [14, 15]. Advances in the understanding of the molecular biology underlying RCC have led to the utilization of small molecule vascular endothelial growth factor receptor (VEGFR) TKI as first line of therapy in advanced RCC. Phase III studies have established the therapeutic efficacy of agents like sunitinib and pazopanib as first-line therapy [2, 3, 16]. The efficacy induced by VEGFR TKI in the frontline setting however demonstrated a median progression-free survival of 9–11 months and then created a need for second-line therapeutic options. The first agent to demonstrate efficacy in this setting was the mammalian target of rapamycin (mTOR) inhibitor, everolimus [5]. The placebo-controlled, double-blind crossover design phase III trial showed progression-free survival (PFS) benefit and led to the FDA approval of everolimus [5]. Subsequently, a phase III trial of axitinib, a multikinase VEGFR1, VEGFR2, and VEGFR3 inhibitor, versus sorafenib in cytokine or VEGFR TKI-pretreated patients revealed superior PFS favoring axitinib [6]. Hence, recent evaluation of cabozantinib was conducted on the background of existing FDA-approved therapies of everolimus and axitinib. In the second-line setting, now multiple strategies are available: a multikinase inhibitor cabozantinib [8, 9], programmed death (PD-1) checkpoint inhibition with nivolumab [7], or mTOR inhibition with everolimus in combination with VEGFR and FGFR-inhibiting agent such as lenvatinib [10].
Molecular features of ccRCC
In 1993, the Von Hippel–Lindau (VHL) gene was identified, which was located on the short arm of chromosome 3p25 [17]. Inactivation of the VHL gene was commonly associated with clear cell renal cell carcinoma (ccRCC) and led to increased expression of hypoxia-inducible factors and the expression of multiple genes involved in cancer progression and metastasis, including the vascular endothelial growth factor (VEGF) [18]. VHL inactivation alone was insufficient for ccRCC development, and additional tumor-suppressor genes were later identified on chromosome 3p: BRCA1-associated protein1, SET domain-containing 2, and polybromo 1 [18]. Mutations in these genes have been described in different proportions in ccRCC.
Additional mutations in genes regulating multiple other pathways within ccRCC include MTOR, tuberous sclerosis1, PI3K, and PTEN. Intratumoral mutation heterogeneity abounds in ccRCC, both de novo and treatment-induced mutation changes, which lead to evolution of resistance pathways.
MET pathway in RCC
Mutations in MET proto-oncogene which is located on chromosome 7q21 is responsible for both types 1 and 2 hereditary papillary renal cell carcinoma (pRCC). Type 1 usually has a more indolent course, and type 2 presents with worse pathological and clinical features. MET tyrosine kinase receptor is expressed on the surface of epithelial and endothelial cells and is bound by HGF which is an inactive serine protease analog [19]. MET/HGF are involved in tumor growth, invasion, and metastasis. It has also been implicated in resistance to treatment, most importantly to anti-VEGFR therapy [20].
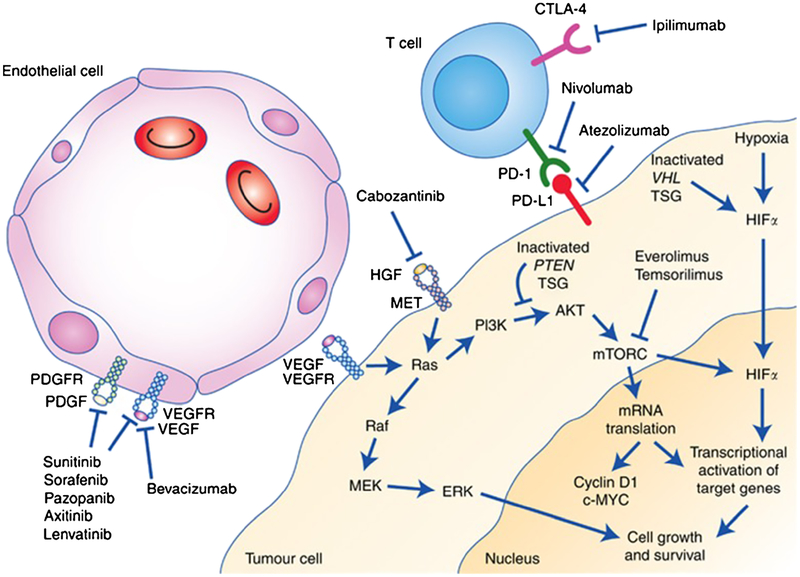
Higher expression of MET is associated with a worse outcome in both clear cell and non-clear cell RCC [21]. In experiments conducted on two ccRCC mice models, one TKI sensitive and the other TKI resistant, combined blockade of VEGF and HGF-MET pathway increased the antitumor effect in both models (Fig. 1) [22, 23].
Fig. 1.
Mechanism of action of treatments for RCC. Reproduced with kind permission from the British Journal of Cancer from Greef B, Eisen T. Medical treatment of renal cancer: new horizons. Br J Cancer. 2016; 115(5):505–16.
Prognostic factors in advanced RCC
Motzer et al. developed a prognostic model for advanced RCC in 1999, which categorized patients into three risk groups based on five prognostic factors [24]. Subsequent work by Heng et al. and Ko et al. established the International Metastatic Renal-Cell Carcinoma Database Consortium Model for prognosis in first- and second-line treatments for metastatic RCC [25, 26]. This again categorized patients into three risk groups. Based on the Heng’s model, the anticipated PFS in the second-line setting for VEGFR TKI-pretreated patients ranged between 3 to 4 months and almost no complete responses were noted. Obviously, there was room for improvement within RCC therapeutics, especially in the pretreated patient population.
Cabozantinib: drug development
Cabozantinib (XL184) is a small molecule with potent inhibitory activity against MET and VEGF receptor 2 (VEGFR2), as well as a number of other receptor tyrosine kinases that have also been implicated in tumor biology, including RET, KIT, AXL, and FLT3 [27] (Fig. 1). The clinical debut of cabozantinib was made by the clinical efficacy noted in medullary thyroid cancer leading to FDA approval in this indication [28]. A phase I study in patients with advanced RCC reported in 2014 showed a promising activity in heavily pretreated patients with a partial response rate of 28% [29]. The primary endpoints of the study were to evaluate the safety and tolerability and antitumor efficacy in subjects with relapsed or refractory RCC. Twenty-five subjects with RCC were enrolled in the study. All the patients had at least one or two lines of prior therapy with systemic agents, and 50% had three or more lines of prior therapy. The majority of patients (88%) had prior anti-VEGF anticancer therapies. Fourteen of the 25 had previously taken sunitinib. The dosage of cabozantinib was 140 mg orally daily, which was determined to be the maximum tolerated dose. Twenty-one of the 25 patients were in the intermediate-risk group per Heng criteria; 3 were in the poor prognosis category and only 1 was in the favorable risk category. The response rate in this study was very promising with 7 (28%) achieving partial responses and 13 patients with stable disease (52%). Only one patient (4%) had disease progression. Median progression-free survival was 12.9 months, and median overall survival was 15.0 months. Four patients had bone metastases and, of these, three out of four revealed a response. Two patients had bone pain and cabozantinib-induced significant symptom relief in both. On the basis of this relatively preliminary, but promising efficacy observed in a drug–drug interaction trial in advanced RCC, the plan for a phase III registration trial was formed.
The dose level of 60 mg was selected based on a dose de-escalation trial conducted in metastatic prostate cancer that showed comparable efficacy and better tolerability [30]. The cabozantinib tablet (Cabometyx, used in RCC) did not meet prespecified bioequivalence criteria of between 80 and 125% of the Cmax levels achieved by the capsule (Cometriq, used in medullary thyroid cancer) formulations. Cabometyx tablets achieved a Cmax which was about 19% higher, and the upper limit of the 90% confidence interval of the Cmax was 131.5% and hence did not lie within the acceptable range of 80–125%, and bioequivalence was not established [31]. A dose proportional increase in levels was observed with dose escalation ranging from 20 and 40 mg to 60 mg tablets.
Single-dose pharmacokinetics was evaluated in eight patients with mild or moderate liver dysfunction and in ten patients with mild or moderate renal impairment [32]. Comparisons of levels to matched controls with normal function showed an increase of up to 31% in renal impairment cases and 81% in hepatic dysfunction [33]. No clear trends for increased risk of toxicities were noted, but extreme caution and close monitoring for adverse events is advised if cabozantinib is used in patients with mild or moderate renal or liver impairment. In severe hepatic or renal dysfunction, no safety data is available.
A food effect study revealed that a high-fat meal can increase the Cmax by about 40%, and hence, administration in a fasting state (at least 2 h after and 1 h before a meal) is recommended. Proton pump inhibitors did not significantly impact drug levels and hence can be used in conjunction with cabozantinib [34].
Phase III METEOR trial (NCT 018657470)
Study design and eligibility
A pivotal phase III study was conducted in advanced RCC patients, pretreated with VEGFR inhibitors. Patients were required to have a clear cell component; performance status of 0–2; and adequate liver, renal, and bone marrow function. In this open-label, randomized phase III trial, adult patients with advanced or metastatic clear-cell renal cell carcinoma, measurable disease, and previous treatment with one or more VEGFR tyrosine kinase inhibitors were randomized to receive oral 60 mg cabozantinib daily or 10 mg everolimus once a day. Stratification factors consisted of Memorial Sloan Kettering Cancer Center risk group and number of prior therapies with VEGFR tyrosine-kinase inhibitors. The primary endpoint was progression-free survival as assessed by an independent radiology review committee in the first 375 randomly assigned patients with an anticipated hazard ratio of 0.667, and the study was expanded to a total of 658 patients to evaluate the OS endpoint [8, 9]. Secondary endpoints were objective response.
Cabozantinib treatment resulted in improved progression-free survival (hazard ratio (HR) 0·51 [95% CI 0·41–0·62]; p < 0·0001) and objective response (17% (13–22) with cabozantinib vs 3% [2–6] with everolimus; p < 0·0001) per independent radiology review. Median PFS was 7.4 months with cabozantinib as compared to that with everolimus of 3.8 months. The median duration of follow-up for OS and safety at last report was 18·7 months. Median overall survival was 21·4 months (95% CI 18·7–not estimable) with cabozantinib and 16·5 months (14·7–18·8) with everolimus (HR 0·66 [95% CI 0·53–0·83]; p = 0·00026). The overall results as well as subgroup analyses within the METEOR trial are summarized in Table 1.
Table 1.
Summary of the METEOR trial results with subgroup analyses
| Subgroups (no. of pts) (cabozantinib/everolimus) | Overall survival: median HR (95%CI) | Progression-free survival: median HR (95%CI) |
|---|---|---|
| Cabozantinib (330) | 21.4 months (18.4–NR) | 7.4 months (5.6–9.1) |
| Everolimus (328) | 16.5 months(14.7–18.8) | 3.8 months (3.7–5.1) |
| HR = 0·66 [0·53–0·83] | HR = 0·58 [0·45–0·75] | |
| MSKCC risk groups: | ||
| Favorable (150/150) | 0.66 (0.46–0.96) | 0.51 (0.38–0.69) |
| Intermediate (139/135) | 0.67 (0.48–0.94) | 0.47 (0.35–0.65) |
| Poor (41/43) | 0.65 (0.39–1.07) | 0.70 (0.42–1.16) |
| IMDC risk groups: | ||
| Favorable (66/62) | 0.70 (0.34–1.41) | 0.47 (0.30–0.76) |
| Intermediate (210/214) | 0.65 (0.49–0.85) | 0.48 (0.37–0.62) |
| Poor (54/52) | 0.74 (0.48–1.15) | 0.67 (0.48–1.04) |
| Prior nephrectomy: | ||
| No (47/49) | 0.75(0.44–1.27) | 0.51(0.30–0.86) |
| Yes(283/279) | 0.66(0.52–0.84) | 0.51(0.41–0.64) |
| Bone mets | ||
| No (253/263) | 0.71(0.55–0.91) | 0.57(045–0.71) |
| Yes (77/65) | 0.54(0.34–0.84) | 0.33(0.21–0.51) |
| Number of previous VEGFR TKI: | ||
| 1 (235/229) | 0.65(0.50–0.85) | 0.52(0.41–0.66) |
| 2 or more (95/99) | 0.73(0.48–1.10) | 0.51(0.35–0.74) |
| Duration of first VEGFR: | ||
| 6 months or less (88/102) | 0.69(0.47–1.01) | 0.62(0.44–0.89) |
| >6 months (242/224) | 0.69(0.52–0.90) | 0.48(0.38–0.62) |
Predominant adverse events reflected the anti-VEGF activity of cabozantinib. The most common grade 3 or 4 adverse events were hypertension 15%, diarrhea 13%, fatigue 11%, hand-foot syndrome 8%, anemia 6%, hyperglycemia 1%, and hypomagnesemia 5%. Serious adverse events grade 3 or worse occurred in 130 (39%) patients in the cabozantinib group and in 129 (40%) in the everolimus group. One treatment-related death occurred in the cabozantinib group of unclear etiology and two deaths were noted in the everolimus group (one Aspergillus infection and one pneumonia aspiration).
Other significant side effects include dysphonia, stomatitis, headache, dizziness, rash, elevated transaminases, increased serum creatinine, and hypothyroidism.
In addition to supportive care and symptom control, dose reduction is an effective way to manage toxicities. The dose levels of cabozantinib are 40 mg daily and 20 mg daily. It is important to note that about 60% of the patients in the METEOR study required dose reductions secondary to side effects.
Second-line therapy in advanced RCC: how does cabozantinib fit? (Table 2)
Table 2.
Randomized trials in advanced renal cancer pretreated with VEGFR TKI therapy
| Arms (Ref) | No of pts | MSKCC risk group Fav/Int/poor | ORR | Med PFS (months) | Med OS (months) |
|---|---|---|---|---|---|
| Everolimus placebo (5) | 272 | 29%/56%/15% | 1% | 4.9 | 14.8 |
| 138 | 28%/57%/15% | 0% | 1.9 | 14.4 | |
| Axitinib sorafenib (6) | 361 | 28%/37%/35% | 19% | 6.7 | 20.1 |
| 362 | 28%/36%/33% | 9% | 4.7 | 19.2 | |
| Nivolumab everolimus (7) | 406 | 35%/49%/16% | 5% | 4.4 | 25 |
| 397 | 36%/49%/15% | 25% | 4.6 | 19.6 | |
| Cabozantinib everolimus (8,9) | 330 | 43%/43%/15% | 17% | 7.4 | 21.4 |
| 328 | 44%/40%/16% | 3% | 3.8 | 16.5 | |
| Lenvatinib + everolimus Lenvatinib everolimus (10) | 51 | 24%/37%/39% | 43% | 14.6 | 25.5 |
| 52 | 21%/35%/44% | 27% | 7.4 | 19.1 | |
| 50 | 24%/38%/38% | 6% | 5.5 | 15.4 |
A plethora of agents are now FDA approved in metastatic renal cancer. Table 2 summarizes the randomized trials of individual therapies compared with controls in second- or third-line metastatic RCC. Previously, axitinib and everolimus had demonstrated PFS improvement as compared to placebo and sorafenib, respectively. Multiple new treatments have now become approved for second-line therapy of advanced RCC. Within all of these therapies, cabozantinib is the only agent showing RR, PFS, and OS benefit as compared to an active comparator. Since all the agents, especially nivolumab and cabozantinib, were developed in parallel in RCC therapeutics, there is no evidence regarding optimal sequencing. Given the differences in mechanisms of action, toxicity profile, and response rates and PFS, the choice of either agent in the second-line setting seems reasonable. Clinically, the patients who have had prolonged clinical benefit with initial anti-VEGF therapy and demonstrated tolerability to this therapy are likely to have benefit from cabozantinib if switched in the second-line setting at progression.
Both nivolumab and cabozantinib have improved OS and comparable response rates. The two agents have very different toxicity profiles, which can be weighed in decision making. In the era of personalized medicine, the development of clinical and molecular biomarkers to predict benefit with different agents will help with choosing specific therapies.
Cabozantinib in subset populations of RCC
Bone metastases
RCC patients with bone metastasis have a particularly poor prognosis. A retrospective analysis of more than 2000 patients with mRCC from the International Metastatic Renal Cell Carcinoma Database Consortium (IMDC) showed significantly decreased OS of patient with bone metastasis when compared with patients without it (14.9 vs 25.1 months; p < 0.0001) and TTF (5.7 vs 7.6 months; p < 0.0001) [35]. Cabozantinib showed activity on tumor xenografts implanted in bone, in preclinical studies [36]. By inhibiting both VGEFR and MET, it affects osteoclast and osteoblast function which alters the bone microenvironment. A phase II trial in multiple solid tumors showed that 59 of 68 patients with bone metastasis who received cabozantinib had partial or complete bone scan resolution with symptomatic improvement [37]. Similar results were noted on bone scans in metastatic prostate cancer [38]. In patients with bone metastases enrolled in the METEOR trial (77 or 20% randomized to cabozantinib), the HR of progression and death in comparison to everolimus was 0.54 and 0.31, respectively, which demonstrated a relatively favorable outcome for cabozantinib in this population with an otherwise dismal prognosis [8, 9].
Brain metastases
Multiple ongoing and reported phase I and II trials are evaluating the activity of cabozantinib in high-grade gliomas and brain metastases of various tumor types [39]. Cabozantinib crosses the blood–brain barrier and should theoretically have activity in patients with brain metastasis. Although the METEOR study allowed stable and treated brain metastases, only three patients were recruited and minimal evidence is available in RCC.
Nephrectomy
In cases without nephrectomy, another patient population that is underrepresented in clinical trials and has known poor outcomes, the efficacy of cabozantinib was noted in the 47 patients without nephrectomy that were enrolled. The HR for PFS was 0.51 (.0.30–0.86). Although the HR for OS was 0.75 favoring cabozantinib, the confidence interval crossed 1.0 (0.44–1.27). Hence, caution needs to be exercised in drawing conclusions from post hoc subset analyses with sample size limitations.
First line treatment of advanced RCC: CABOSUN trial []
CABOSUN is a randomized, open-label phase II trial that was designed to enroll 150 patients with advanced RCC determined to be intermediate or poor risk by the IMDC criteria. The study was conducted by the Alliance Group. Patients were randomized 1:1 to receive cabozantinib (60 mg once daily) or sunitinib (50 mg once daily, 4 weeks on followed by 2 weeks off). The randomization was stratified by the IMDC risk strata (intermediate or poor risk) and presence of bone metastasis (yes or no). Enrollment was completed in March 2015. The primary endpoint was PFS, defined as time from randomization to disease progression or death, whichever occurs first. Positive PFS results have formed the basis for previous regulatory approvals of treatments in the first-line setting, including sunitinib. Secondary endpoints included overall survival and objective response rate. Eligible patients were required to have locally advanced or metastatic clear-cell RCC, ECOG performance status of 0–2, and had to be intermediate or poor risk as per the IMDC criteria. Prior systemic treatment for RCC was not permitted. With 123 events (disease progression or death), the log-rank statistic has 85% power (with a one-sided type I error rate = 0.12) to detect a hazard ratio of 0.67. Between July 9, 2013 and April 6, 2015, 157 patients were randomized: 79 patients on the cabozantinib arm and 78 patients on the sunitinib arm. The preliminary results announced in a press release by Exelixis on May 23, 2016 revealed superior efficacy of cabozantinib as compared to sunitinib therapy in the front-line setting in clear cell RCC [40••]. The trial met its primary endpoint, demonstrating a statistically significant and clinically meaningful improvement in progression-free survival (PFS) for cabozantinib compared with sunitinib in patients with advanced intermediate- or poor-risk RCC. The safety data in the cabozantinib-treated arm of the study were consistent with those observed in previous studies in patients with advanced RCC. CABOSUN was conducted by the Alliance for Clinical Trials in Oncology as part of Exelixis’ collaboration with the National Cancer Institute’s Cancer Therapy Evaluation Program (NCI-CTEP). The study design resulted in 157 patients enrolled and 80.9% of patients were intermediate risk as per the IMDC criteria while 19.1% were poor risk; 36.3% of patients had bone metastases; 46% of patients had ECOG performance status (PS) 0, 41% had ECOG PS 1, and 13% had ECOG PS 2. All patients were included in the efficacy analyses that followed the intent-to-treat principle. The results of this study were recently reported and showed a statistically significant, improved response rate, PFS, and OS with cabozantinib therapy as compared to sunitinib (HR = 0.69 95% CI 0.48–0.99, p = 0.012). Median PFS was 8.2 months in the cabozantinib arm and 5.6 months in the sunitinib arm [40••]. Response rates were 46 and 18% and median OS were 30 and 21.9 months for cabozantinib and sunitinib, respectively. Patients (157) were enrolled with 19.1% with performance status of 2 and 36.3% had bone metastases. Consistent clinical benefit favoring cabozantinib was noted in either subgroup.
These results have the potential to change the entire therapeutic paradigm in advanced RCC where cabozantinib will be used front line and followed by consideration of other VEGFR TKI or immune therapy. However, front line immune therapy combination trials are also expected to report results within the next 12 months. Two large randomized trials include the combination of ipilimumab + nivolumab as compared to sunitinib as the control arm and the combination of bevacizumab and atezolizumab also compared to sunitinib in untreated advanced RCC. Whether in the front-line or second-line setting, the dilemma in systemic therapy decisions will remain between the use of immune therapy or TKI-based therapy. Predictive biomarkers to help guide therapy will be of critical importance. To date, the clinical utility of biomarkers in RCC has been disappointing. The PD-1 expression has not been predictive of nivolumab efficacy. The Met expression has not been predictive of benefit with cabozantinib. Large databases such as IMDC need to be explored to develop clinical biomarkers until molecular biomarkers are validated and reproducible. A clinical trial of cabozantinib and nivolumab, with or without ipilimumab () in all genitourinary tumors, is active at the National Cancer Institute. The dose escalation phase is ongoing and safety and efficacy needs to be determined.
Cabozantinib in non-clear cell RCC
Papillary renal cancer (pRCC) constitutes about 10–15% of RCC and usually harbors a MET mutation. Therefore, MET inhibition is a logical step and cabozantinib with the met inhibition has clear rationale in papillary RCC. Cabozantinib is under investigation in the Southwest Oncology Group (SWOG) 1500 trial, which is a phase II trial comparing efficacy of three distinct MET inhibitors: cabozantinib, crizotinib, and savolitinib, with a control arm of sunitinib in the treatment of metastatic papillary RCC. The study does not select patients based on met mutations; however, testing will be conducted on archival tissue for all the patients enrolled. No formal clinical trial testing has been conducted in other non-clear cell histologies such as sarcomatoid, collecting duct, and translocation RCC, but cabozantinib may be considered for clinical use in the second-line setting.
Conclusions
Cabozantinib revealed efficacy in advanced pretreated RCC, with statistically significant improvements in RR, PFS, and OS. The drug is incorporated in the NCCN treatment guidelines for advanced RCC as a category 1 guideline. In front-line therapy of RCC, cabozantinib has demonstrated preliminary results of improved efficacy as compared to sunitinib control. In papillary RCC, clinical trials are ongoing. In the future, cabozantinib is likely to play a seminal role in cancer therapeutics of RCC.
Opinion statement.
Cabozantinib was approved by the FDA in April 2016 for the treatment of advanced renal cancer, pretreated with at least one prior antiangiogenic therapy. This is the first agent in the therapy of kidney cancer to show a statistically significant improvement in all three endpoints of clinical efficacy, response rate, progression free survival, and overall survival (OS), in a phase III randomized trial. The reporting of METEOR coincided with that of the Checkmate 025 study which randomized similarly eligible patients to receive nivolumab or everolimus 10 mg daily. As the drug development has occurred in parallel for cabozantinib and nivolumab, no evidence exists for decision making regarding optimal sequencing of these agents. A third option of lenvatinib and everolimus was also rapidly approved based on a phase II randomized trial demonstrating promising magnitude of improvement in response, progression-free survival (PFS), and OS. The differences in toxicity profiles, duration and toxicities of prior therapy, presence of brain metastases, concomitant immunosuppressive therapies, or autoimmune conditions are the factors that are taken into account when choosing therapy. The patients who have demonstrated response, prolonged clinical benefit and tolerability, and with anti-VEGF therapy are likely to benefit from continued antiangiogenic activity combined with MET and HGF inhibition with cabozantinib at progression. The patients who have intolerance or poor response to anti-VEGF TKI should be switched to nivolumab as the preferential therapy of choice. Clearly, better predictors are required to aid in guiding therapeutic decisions. The CABOSUN trial will likely shift the entire paradigm. The CABOSUN trial demonstrated superior PFS and response rates favoring cabozantinib as compared to sunitinib in untreated, intermediate, or poor-risk RCC and can be predicted to become the front-line therapy of choice. Immune-based regimens such as the combinations of nivolumab + ipilimumab and bevacizumab + atezolizumab have completed phase III trials, comparing to sunitinib, and results are awaited. In the future, a similar clinical dilemma will be shifted to the front-line therapy and the nuances of trial eligibility, and patient comorbidities will remain important factors. Optimal sequencing and predictive biomarkers are the questions that need to be incorporated in future clinical trials within RCC.
Abbreviations
- CTLA4
Cytotoxic T-lymphocyte-associated antigen 4
- HGF
Hepatocyte growth factor
- PDGF
Platelet-derived growth factor
- PDGFR
Platelet-derived growth factor receptor
- PD-1
Programmed cell death 1
- PDL-1
Programmed cell death ligand 1
- VEGF
Vascular endothelial growth factor
- VEGFR
Vascular endothelial growth factor receptor
Footnotes
Conflict of Interest
Ahmed Abdelaziz declares that he has no conflict of interest. Ulka Vaishampayan has received research support through grants, honoraria, and compensation from Exelixis, Pfizer, Novartis, and Bristol-Myers Squibb for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as:
•• Of major importance
- 1.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356(2):125–34. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–24. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, Hawkins RE, Wagstaff J, Salman P, Mardiak J, Barrios CH, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49(6):1287–96. [DOI] [PubMed] [Google Scholar]
- 4.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Archer L, Atkins JN, et al. Phase III trial of bevacizumab plus interferon alfa versus interferon alfa monotherapy in patients with metastatic renal cell carcinoma: final results of CALGB 90206. J Clin Oncol. 2010;28(13):2137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372(9637):449–56. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–9. [DOI] [PubMed] [Google Scholar]
- 7.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.••.Choueiri TK, Escudier B, Powles T, Mainwaring PN, Rini BI, Donskov F, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]; The trial that established the efficacy of cabozantinib
- 9.••.Choueiri TK, Escudier B, Powles T, Tannir NM, Mainwaring PN, Rini BI, et al. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): final results from a randomised, open-label, phase 3 trial. Lancet Oncol. 2016;17(7):917–27. [DOI] [PubMed] [Google Scholar]; The trial results established the efficacy of cabozantinib for overall survival
- 10.Motzer RJ, Hutson TE, Glen H, Michaelson MD, Molina A, Eisen T, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: a randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16(15):1473–82. [DOI] [PubMed] [Google Scholar]
- 11.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 12.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86. [DOI] [PubMed] [Google Scholar]
- 13.SEER Stat Fact Sheets: Kidney and renal pelvis. http://seer.cancer.gov/statfacts/html/kidrp.html.
- 14.Negrier S, Escudier B, Lasset C, Douillard JY, Savary J, Chevreau C, Ravaud A, Mercatello A, Peny J, Mousseau M, Philip T. Recombinant human interleukin-2, recombinant human interferon alfa-2a, or both in metastatic renal-cell carcinoma. N Engl J Med. 1998;338(18):1272–8. [DOI] [PubMed] [Google Scholar]
- 15.Motzer RJ, Bander NH, Nanus DM. Renal-cell carcinoma. N Engl J Med. 1996;335(12):865–75. [DOI] [PubMed] [Google Scholar]
- 16.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–31. [DOI] [PubMed] [Google Scholar]
- 17.Latif F, Tory K, Gnarra J, Yao M, Duh FM, Orcutt ML, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–20. [DOI] [PubMed] [Google Scholar]
- 18.Gossage L, Eisen T, Maher ER. VHL, the story of a tumour suppressor gene. Nat Rev Cancer. 2015;15(1):55–64. [DOI] [PubMed] [Google Scholar]
- 19.Garajová I, Giovannetti E, Biasco G, Peters GJ. C-met as a target for personalized therapy. Transl Oncogenomics. 2015;7(Suppl 1):13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shojaei F, Lee JH, Simmons BH, Wong A, Esparza CO, Plumlee PA, et al. HGF/c-met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 2010;70(24):10090–100. [DOI] [PubMed] [Google Scholar]
- 21.Gibney GT, Aziz SA, Camp RL, Conrad P, Schwartz BE, Chen CR, et al. C-met is a prognostic marker and potential therapeutic target in clear cell renal cell carcinoma. Ann Oncol. 2013;24(2):343–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ciamporcero E, Miles KM, Adelaiye R, Ramakrishnan S, Shen L, Ku S, et al. Combination strategy targeting VEGF and HGF/c-met in human renal cell carcinoma models. Mol Cancer Ther. 2015;14(1):101–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Greef B, Eisen T. Medical treatment of renal cancer: new horizons. Br J Cancer. 2016;115(5):505–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Motzer RJ, Mazumdar M, Bacik J, Berg W, Amsterdam A, Ferrara J. Survival and prognostic stratification of 670 patients with advanced renal cell carcinoma. J Clin Oncol. 1999;17(8):2530–40. [DOI] [PubMed] [Google Scholar]
- 25.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ko JJ, Xie W, Kroeger N, Lee JL, Rini BI, Knox JJ, et al. The international metastatic renal cell carcinoma database consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol. 2015;16(3):293–300. [DOI] [PubMed] [Google Scholar]
- 27.Yakes FM, Chen J, Tan J, Yamaguchi K, Shi Y, Yu P, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308. [DOI] [PubMed] [Google Scholar]
- 28.Kurzrock R, Sherman SI, Ball DW, Forastiere AA, Cohen RB, Mehra R, et al. Activity of XL184 (cabozantinib), an oral tyrosine kinase inhibitor, in patients with medullary thyroid cancer. J Clin Oncol. 2011;29(19):2660–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choueiri TK, Pal SK, McDermott DF, Morrissey S, Ferguson KC, Holland J, et al. A phase I study of cabozantinib (XL184) in patients with renal cell cancer. Ann Oncol. 2014;25(8):1603–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vaishampayan U. Cabozantinib as a novel therapy for renal cell carcinoma. Curr Oncol Rep. 2013;15(2):76–82. [DOI] [PubMed] [Google Scholar]
- 31.Lee RJ, Saylor PJ, Michaelson MD, Rothenberg SM, Smas ME, Miyamoto DT, et al. A dose-ranging study of cabozantinib in men with castration-resistant prostate cancer and bone metastases. Clin Cancer Res. 2013;19(11):3088–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nguyen L, Benrimoh N, Xie Y, Offman E, Lacy S. Pharmacokinetics of cabozantinib tablet and capsule formulations in healthy adults. Anti-Cancer Drugs. 2016;27(7):669–78. [DOI] [PubMed] [Google Scholar]
- 33.Nguyen L, Holland J, Ramies D, Mamelok R, Benrimoh N, Ciric S, et al. Effect of renal and hepatic impairment on the pharmacokinetics of cabozantinib. J Clin Pharmacol. 2016;56(9):1130–40. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen L, Holland J, Mamelok R, Laberge MK, Grenier J, Swearingen D, et al. Evaluation of the effect of food and gastric pH on the single-dose pharmacokinetics of cabozantinib in healthy adult subjects. J Clin Pharmacol. 2015;55(11):1293–302. [DOI] [PubMed] [Google Scholar]
- 35.McKay RR, Kroeger N, Xie W, Lee JL, Knox JJ, Bjarnason GA, et al. Impact of bone and liver metastases on patients with renal cell carcinoma treated with targeted therapy. Eur Urol. 2014;65(3):577–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen HM, Ruppender N, Zhang X, Brown LG, Gross TS, Morrissey C, Gulati R, Vessella RL, Schimmoller F, Aftab DT, Corey E. Cabozantinib inhibits growth of androgen-sensitive and castration-resistant prostate cancer and affects bone remodeling. PLoS One. 2013;8(10):e78881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gordon MS, Vogelzang NJ, Schoffski P, Daud A, Spira AI, O’keeffe BA, et al. Activity of cabozantinib (XL184) in soft tissue and bone: results of a phase II randomized discontinuation trial (RDT) in patients (pts) with advanced solid tumors. Abstract, ASCO Annual Meeting Proceedings 2011. May 20 (Vol. 29, No. 15_suppl, p. 3010). [Google Scholar]
- 38.Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, et al. Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol. 2013;31(4):412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schiff D, Desjardins A, Cloughesy T, Mikkelsen T, Glantz M, Chamberlain MC, et al. Phase 1 dose escalation trial of the safety and pharmacokinetics of cabozantinib concurrent with temozolomide and radiotherapy or temozolomide after radiotherapy in newly diagnosed patients with high-grade gliomas. Cancer. 2016;122(4):582–7. [DOI] [PubMed] [Google Scholar]
- 40.••.Choueiri T, Halabi S, Sanford BL, et al. Cabozantinib Versus Sunitinib As Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J Clin Oncol. 2017; 35:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]; The publication reporting that the CABOSUN trial revealed efficacy favoring cabozantinib and met the primary endpoint.