Short abstract
Background
Endoscopic repair of cerebrospinal fluid (CSF) fistulas is a fundamental practice in anterior skull base surgery due to high success rates and low morbidity profile. However, spontaneous CSF (sCSF) leaks have the highest recurrence rate compared to other etiologies. The most effective management is undetermined due to variations in graft materials and limited evidence.
Objective
We present the largest study of a standardized endoscopic repair technique for sCSF leaks.
Methods
Single-institution retrospective review of patients who underwent endoscopic sCSF leak repair between October 2011 and January 2018. All patients underwent repair using a temporary lumbar drain, intrathecal fluorescein, and multilayer reconstruction using bilayered fascia lata autograft and vascularized nasoseptal flap.
Results
Twenty patients (100% female, mean age: 53.2 years) with 25 separate sCSF leak sites were included. Obesity was present in 15 of 20 patients (mean body mass index [BMI] = 35.3). No patients had previous sinus surgery. Locations of skull base defects included: cribriform plate (44%), ethmoid (32%), lateral sphenoid (12%), and planum sphenoidale (12%). The mean follow-up was 22.8 months and 92% of the leak sites (23/25) were successfully repaired primarily. There were no neurological complications or cases of meningitis. Two patients (mean BMI = 52) with persistent postoperative CSF leaks and elevated intracranial pressure were successfully managed with ventriculoperitoneal shunt placement. BMI was associated with likelihood of repair failure (P = .003).
Conclusions
At our institution, endoscopic repair of sCSF leaks using a composite autograft of fascia and a nasoseptal flap demonstrates high success rates. Elevated BMI was a statistically significant risk factor for revision.
Keywords: cerebrospinal fluid leak, cerebrospinal fluid rhinorrhea, cerebrospinal fluid leak repair, spontaneous, vascularized flap repair, anterior skull base
Introduction
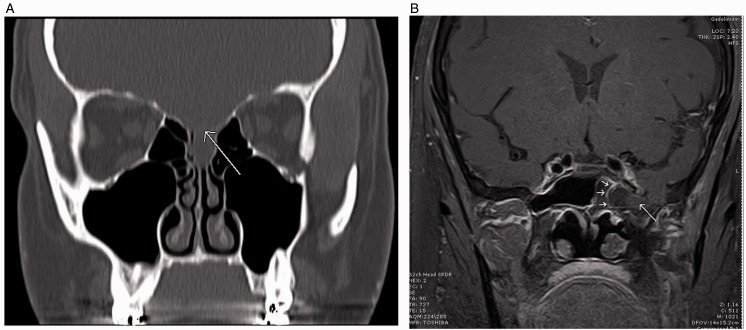
Spontaneous cerebrospinal fluid (sCSF) leaks are associated with sustained increased intracranial pressure (ICP), which may represent a variant of idiopathic (previously known as benign) intracranial hypertension (IIH) or pseudotumor cerebri.1–3 These patients are typically obese (body mass index [BMI] > 30), middle-aged females of childbearing age, who may present with headaches, tinnitus, changes in vision, and pneumonitis.4–8 Common findings on high resolution computed tomography (CT) and magnetic resonance imaging (MRI) may include thinning or attenuation of the skull base, arachnoid pits, multiple defects, encephaloceles, empty sella, and dilated optic nerve sheaths (Figure 1).9–12
Figure 1.
A, Coronal CT sinus w/o contrast. Patient S. M., a 41-year-old woman with thinning of the cribriform plate and fovea ethmoidalis (arrow). Low-attenuation lesion representing the meningoencephalocele. B, Coronal MRI T1-weighted post contrast. Patient N. C., a 62-year-old woman with 8 mm defect in the floor of the left middle cranial fossa with herniating meningoencephalocele into the left sphenoid sinus.
The endoscopic endonasal approach for repair of CSF rhinorrhea has become a fundamental practice in skull base surgery due to its comparable success rates and low morbidity when compared to open craniotomy techniques.13–17 sCSF leaks, however, are of particular interest due to their high recurrence rates (up to 38%) after surgical repair as compared to <10% for most other etiologies.6,18–22 Despite successful surgical repair, these patients with IIH may have higher incidence of recurrence at the same site or at a distant site if the elevated ICP is not managed by diuretics or CSF diversion.3,23–27
A significant advance in endoscopic closure of CSF leaks was the introduction of the pedicled nasoseptal flap (NSF), first described by Oskar Hirsch in 1952 as an incidental vascularized rotational flap for CSF leak closure.28 It was later reintroduced as the Hadad-Bassagasteguy flap in 2006 for repair of large skull base defects.29 Multiple studies have reported success rates of closure ranging from 90% to 95% of large skull base defects, high-flow CSF leaks, and clival defects with the addition of the NSF.17,29–34 However, this literature analyzed all etiologies of CSF leak or focused on repair of iatrogenic leaks. The most effective surgical technique is undetermined and varies widely because of limited evidence-based guidance. We review the outcomes of our standardized technique in specifically treating sCSF leaks, emphasizing risk factors, secondary management, and postoperative outcomes associated with our institutional paradigm for sCSF leak repair.
Methods
Setting/Participants
The study was approved by the University of Southern California Institutional Review Board. Individual patient consent was not obtained as this was a retrospective review of 20 patients who underwent sCSF leak repair between October 2011 and January 2018. All patients were jointly treated by the senior authors (B. W. and G. Z.) at the private tertiary referral hospital Keck Hospital of University of Southern California (USC) and at the county hospital Los Angeles County + USC (LAC + USC) Medical Center. Electronic medical records were reviewed to obtain the following data: age, gender, BMI, comorbidities, history of sinus or skull base surgery, clinical presentation, site of skull base defect, presence of encephalocele, length of stay, peri- and postoperative morbidity, need for secondary repair or management of CSF leak, and clinical follow-up.
CSF rhinorrhea was confirmed in all cases with preoperative beta-2 transferrin test and a CT and/or MRI to confirm skull base defect location and to exclude all other causes. Leak sites that were separated by a distinct boundary were counted as separate leaks.
Surgical Technique
All patients underwent insertion of a temporary lumbar drain (LD) under general anesthesia, prior to endoscopic endonasal repair. Intrathecal fluorescein (0.1 mL of 10% fluorescein diluted in 10 mL of patient’s CSF) was subsequently injected over 10 minutes for identification of known and concurrent defects. The defect was exposed via endoscopic endonasal dissection of adjacent turbinates and sinuses.
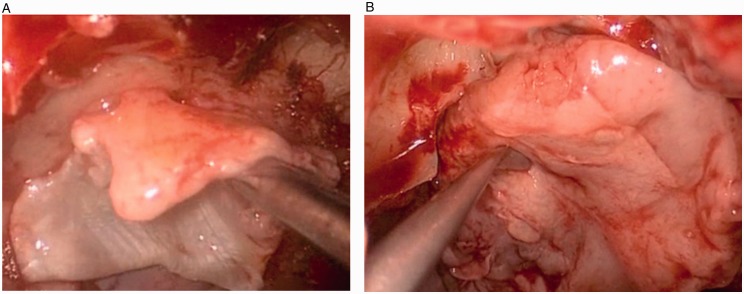
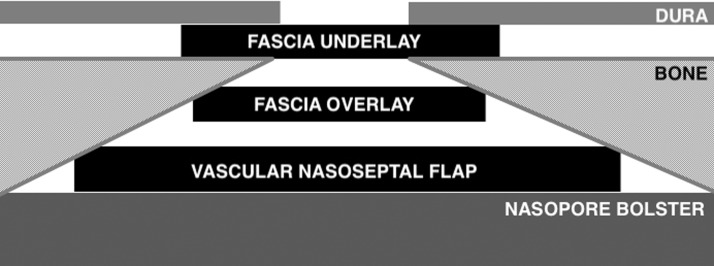
Encephaloceles, if present, were fulgurated using bipolar cautery and reduced in standard fashion. The defect was then repaired in a multilayer fashion using a fascia lata dural underlay, fascia lata dural overlay (Figure 2(A)), and absorbable sheet of oxidized cellulose polymer (Surgicel; Ethicon, Somerville, NJ) in a “cargo net” fashion and a small amount of fibrin sealant (Tisseel; Baxter Healthcare, Deerfield, IL) to reinforce the edges. An overlay of vascularized NSF (Figure 2(B)) was placed; and again, reinforced along the edges with Surgicel and Tisseel. A biodegradable synthetic polyurethane foam (Nasopore; Stryker, Hamilton, Ontario, Canada) provided the final bolster of the reconstruction. An overview of the reconstruction can be visualized in Figure 3. Patients were transferred to the neurointensive care unit for monitoring and lumbar drainage at 5 or 10 mL/h for 48 to 72 hours. LDs were subsequently clamped and removed if no CSF rhinorrhea was evident. Broad spectrum perioperative antibiotics were given while LD was in place.
Figure 2.
Placement of the (A) fascia overlay and (B) nasoseptal flap.
Figure 3.
Schematic of the multilayer composite repair including a fascia underlay, fascia overlay, Surgicel with Tisseel, nasoseptal flap, Surgicel reinforcement to edges with gelfoam and tisseel, followed by a nasopore bolster.
Statistical Analysis
The primary outcome was surgical success, defined as the closure of skull base defect without recurrence of CSF leak at the same site. Demographic and clinical variables were further examined for associations with recurrence. Fisher exact test and Student’s t test were used accordingly for statistical comparisons to determine whether each variable was associated with leak recurrence with a significant of P < .05.
Results
Fifty charts of patients with NSF repair between October 2011 and January 2018 were reviewed; 20 of whom met the criteria of repair for spontaneous CSF leak. A total of 20 patients with sCSF leaks underwent endoscopic endonasal skull base repair during the study period. All patients were female with a mean age at the time of surgery of 53.2 ± 10.9 years. The mean BMI was 35.3 ± 8.1 kg/m2. Mean follow-up time was 22.8 ± 15.1 months (Table 1). Of note, 1 patient had a history of open repair by another institution of an infratemporal encephalocele. Four of 20 (20%) patients had type II diabetes mellitus, 6 of 20 (30%) were taking acetazolamide, and 7 of 20 (35%) had concomitant chronic rhinosinusitis (CRS). However, no patients had previous sinus surgeries. All patients presented with clear rhinorrhea that was beta-2 transferrin positive. More than half presented with headache, 3 of 20 (15%) presented with meningitis, 4 of 20 (20%) presented with pneumonitis, and 1 of 20 (5%) with seizure. Patients were symptomatic for a mean of 6.6 ± 5.5 months prior to presenting for surgical evaluation.
Table 1.
Patient Demographic and Clinical Data.
| Factor | Patients (n = 20) |
|---|---|
| Women, % | 100 |
| Age, mean (SD, range), years | 53.2 (10.9, 36–76) |
| BMI, mean (SD, range), kg/m2 | 35.3 (8.1, 25–52.5) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 8 (40%) |
| White | 8 (40%) |
| African American | 4 (20%) |
| Past medical history, n (%) | |
| Chronic rhinosinusitis | 7 (35%) |
| Hypertension | 10 (50%) |
| Diabetes | 4 (20%) |
| Seizure disorder | 3 (15%) |
| Neurocysticercosis | 1 (5%) |
| Past surgical history, n (%) | |
| Lateral skull base CSF leak | 1 (5%) |
| Sinus surgery | 0 (0%) |
| Acetazolamide | 6 (30%) |
| Tobacco use | 5 (25%) |
| Time symptomatic, mean (range), months | 6.6 (1–18) |
| Presenting symptoms, n (%) | |
| Clear rhinorrhea | 20 (100%) |
| Headache | 10 (50%) |
| Meningitis | 3 (15%) |
| Pneumonitis | 4 (20%) |
| Seizure | 1 (5%) |
| Empty sella syndrome on MRI | 9 (45%) |
| Postoperative admission, mean (range), days | 7 (4–10) |
| Postoperative use of acetazolamide, n (%) | 5 (25%) |
| Clinic follow-up, mean (range), months | 22.8 (3–66) |
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging; SD, standard deviation.
In total, 25 skull base defects were identified. Four patients presented with 2 separate leaks concurrently, which were repaired simultaneously. The most common location of skull base defect was the cribriform plate (44%), medial ethmoid (32%), lateral sphenoid (12%), and planum sphenoidale (12%). Seventy-two percent of the leak sites had a herniating encephalocele at time of surgery (Table 2).
Table 2.
Skull Base Defect Characteristics.
| Characteristic | Skull Base Defects (n = 25) |
|---|---|
| Location, n (%) | |
| Cribriform | 11 (44%) |
| Ethmoid | 8 (32%) |
| Lateral sphenoid | 3 (12%) |
| Planum sphenoidale | 3 (12%) |
| Encephalocele present, n (%) | 14 (56%) |
All patients had LD placement without complications. All LDs were removed on postoperative days 3 to 4. Six patients had a CSF leak once the LD was clamped. These patients were started on acetazolamide and LD continued for an additional day. Four patients had resolution of their leaks and were monitored postoperatively for papilledema by neuro-ophthalmology. Patients were discharged on average on postoperative day 7 (range: 4–10). There were neither neurological complications or cases of meningitis nor any morbidity associated with the fascia lata donor site.
Two patients had persistent CSF rhinorrhea postoperatively and required ventriculoperitoneal shunt (VPS) during the same admission. They have been without recurrence for 19 and 21 months. The patients with CSF repair failures were 52 and 45 years old and had a BMI 51.5 and 52.5 kg/m2. A t test comparison of recurrence versus no recurrence with regard to BMI was statistically significant to P ≤ .05 (Tables 3 and 4).
Table 3.
Clinical Data by Recurrence.
| Factor | Recurrence (n = 2) | No recurrence (n = 18) | P |
|---|---|---|---|
| Mean age, years | 49.5 | 53.7 | .63 |
| Women, % | 100 | 100 | – |
| Mean BMI, kg/m2 | 52 | 33.6 | .003 |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 1 (50%) | 7 (38%) | 1.0 |
| White | 1 (50%) | 7 (39%) | 1.0 |
| African American | 0 | 4 (22%) | 1.0 |
| Past medical history, n (%) | |||
| Hypertension | 1 (50%) | 9 (50%) | 1.0 |
| Chronic rhinosinusitis | 1 (50%) | 6 (33%) | 1.0 |
| Diabetes | 1 (50%) | 3 (17%) | .37 |
| Seizure disorder | 1 (50%) | 2 (11%) | .27 |
| Neurocysticercosis | 1 (50%) | 0 | 1.0 |
| Past surgical history, n (%) | |||
| Lateral CSF leak repair | 0 | 1 (6%) | 1.0 |
| Sinus surgery | 0 | 0 | – |
| Acetazolamide, n (%) | 1 (50%) | 5 (28%) | .52 |
| Tobacco use | 0 | 5 (28%) | 1.0 |
| Mean symptomatic time, months | 3.5 | 7.2 | .31 |
| Presenting symptoms, n (%) | |||
| Clear rhinorrhea | 2 (100%) | 18 (100%) | 1.0 |
| Headache | 1 (50%) | 8 (44%) | 1.0 |
| Meningitis | 0 | 3 (17%) | 1.0 |
| Pneumonitis | 0 | 4 (22%) | 1.0 |
| Empty sella syndrome on MRI | 2 (100%) | 6 (33%) | .15 |
Abbreviations: BMI, body mass index; CSF, cerebrospinal fluid; MRI, magnetic resonance imaging.
Table 4.
Skull Base Defect Characteristics by Recurrence.
| Characteristic | Recurrence n = 2 | No Recurrence n = 23 | P |
|---|---|---|---|
| Location, n (%) | |||
| Cribriform | 1 (50%) | 10 (43%) | 1.0 |
| Ethmoid | 1 (50%) | 7 (30%) | 1.0 |
| Lateral sphenoid | 0 | 3 (13%) | 1.0 |
| Planum sphenoidale | 0 | 3 (13%) | 1.0 |
| Encephalocele present, n (%) | 2 (100%) | 16 (70%) | 1.0 |
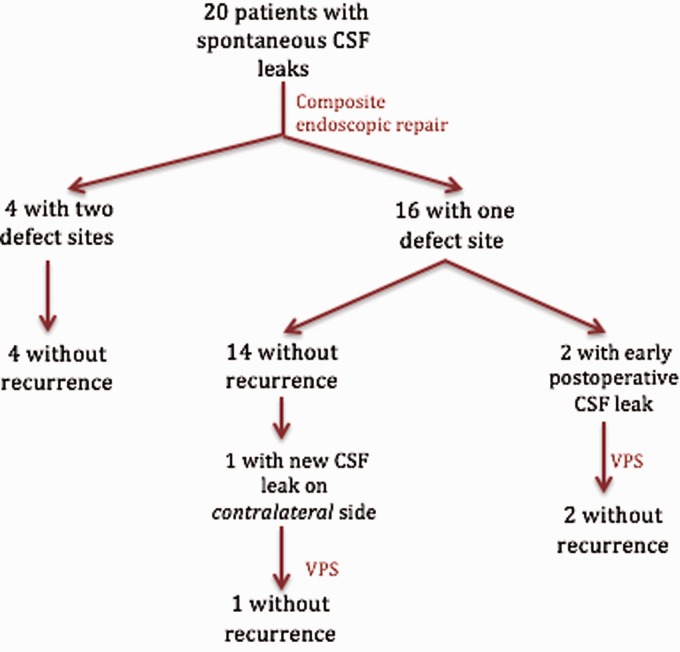
One patient presented with a CSF leak on the contralateral side 37 months after initial endoscopic repair. A VPS was placed and follow-up without recurrence has been 13 months (Figure 4). Of note, this patient had a history of a separate leak repair of a lateral skull base encephalocele at another hospital 11 years prior.
Figure 4.
Results of composite endoscopic repair for sCSF leak. CSF, cerebrospinal fluid; VPS, ventriculoperitoneal shunt.
Discussion
The etiology of sCSF leaks remains unknown, but its close relationship with IIH and obesity is well established.1–3 The management of CSF rhinorrhea due to IIH poses a challenge due to its increasing prevalence, unknown etiology, and higher surgical failure rates.6,18–22 Studies have found that conservative management alone (lifestyle changes, diuretics, and LP) was not sufficient for the treatment of sCSF leaks.6,10,35 Endoscopic surgical repair of anterior skull base CSF leaks has increasingly become the gold standard. Success rates range from 33% to 100%, averaging around 90% in endoscopic sCSF leak repair, which is comparable and/or exceeds that of the more morbid open approaches.13–17,36 The most frequent locations of sCSF leaks reported in the literature include the lateral recess of the sphenoid sinus, the cribriform plate, and the ethmoid roof, which is consistent with our findings.5,15,19,22,31,36 Potentially due to small sample size, the only statistically significant risk factor of CSF leak recurrence in this study was increased BMI (P = .003). Although in our study this represents only 2 of 20 cases due to our low N, this is consistent with previous studies linking increased BMI to higher failure rates of primary repair in all etiologies of CSF leaks.37–39
LD in sCSF Leak Repair
A meta-analysis by D’Anza et al. has shown no difference in success rates of repair with or without LD in all etiologies of endonasal repair of skull base CSF leaks.40 The same results were shown in a large retrospective review of middle cranial fossa repair of lateral skull base defects by Nelson et al.41 However, both papers acknowledge a selection bias for patients with high-flow leaks to receive an intraoperative LD. No studies have specifically evaluated the use of LD in sCSF leak. Currently, a majority (68%) of institutions place LDs when repairing anterior sCSF leaks for intraoperative fluorescein use and postoperative short-term ICP monitoring.36 At our institution, an LD is placed in all patients undergoing endoscopic anterior skull base sCSF leak repair.
Long-term CSF Shunting
In this study, 23 of 25 anterior skull base defects were treated successfully. Two patients had persistent CSF rhinorrhea while admitted postoperatively once the LD was clamped. These patients were managed successfully with VPS. Appropriate long-term postoperative ICP management in sCSF leak repair with diuretics or permanent CSF diversion has been shown to improve success rate of primary repair from 81.9% to 92.8%.23–27,42 Secondary repair with permanent CSF diversion in the patients with recurrence proved to be 100% successful unless there were issues with shunt malfunction.22,23,26,27 Similarly, 100% of our patients with permanent CSF diversion (VPS placement) have been recurrence free without shunt issues.
sCSF Leak Repair Grafts
Comparison of endoscopic surgical techniques is difficult due to the wide variety of grafting materials used based on the surgeon preference. Fat, muscle, fascia, bone, cartilage, vascularized flaps, allogenic, and synthetic materials may be used for repair as underlay and/or overlay grafts.14–16,21–23,34,35 Recent evidence-based algorithms have established NSFs as the primary workhorse for larger defects and high-flow leaks.17,29–34 One review showed an overall success rate of 94% for all CSF leaks while free grafts provided an 82% success rate.34 In addition, fascia lata, as opposed to temporalis fascia, offers a very durable, bountifully available material with minimal harvest morbidity that can be harvested by a second team simultaneously.16,18,34,43
Studies looking at endoscopic repair of sCSF leaks do not use a standardized technique across all defect sites with the exception of 3 studies. The largest by Elmorsy and Khafagy42 is a retrospective cohort study of 31 patients with sCSF leaks repaired with septal cartilage or bone overlay followed by a middle turbinate mucosal rotational flap. However, standardization of LD use and addition of abdominal fat graft was not consistent across all defects. Their success rate after first repair was 87.1% (27 of 31 patients) with a mean follow-up time of 32.4 months. Second, Deenadayal et al.44 reported that 7 patients with sCSF leak were repaired with LD placement, fascia lata underlay, and 2 layers of fascia lata overlays, gelfoam. There were no failures after primary surgery with a mean follow-up time of 14.9 months. And finally, Gunaratne and Singh33 reported a case series of 3 patients, without LD placement, who underwent sCSF leak repair with DuraGen (Integra, Plainsboro, NJ), septal cartilage or middle turbinate bone, layered with unilateral or bilateral NSF. No patients had recurrence with a mean follow-up period of 30 months. All other studies looking at sCSF leak repair employed a variety of graft materials and LDs depending on surgeon expert opinion and preference.36
No study has previously examined the success rates of repairing sCSF leaks with a standardized multilayer technique (Figure 2) and LD placement. A bilayered epidural fascia lata as both inlay and overlay is reinforced by a robust vascularized NSF, which achieves a high surgical success rate of 92% in sCSF leaks at our institution. Our study, to the best of our knowledge, is the largest published study of a standardized endoscopic repair technique with NSF (Figure 2) and LD for sCSF leaks of various sizes and locations. This technique also successfully treats patients with multifocal skull base defects and CSF rhinorrhea simultaneously.
Limitations
Although other studies have shown that small CSF leaks may be repaired with less extensive techniques and grafts (such as free autografts), due to the high rate of recidivism in sCSF leaks, a robust technique with high success rates is justified. Although an NSF may require a prolonged healing period, the morbidity profile is similar to other techniques. Limitations of our study include that it was a retrospective cohort study with a small cohort of 20 patients and limited mean follow-up time of 22.8 months. A prospective study would have allowed for comparisons across different techniques. For future directions, we would consider shedding light on the use of LD and recurrence rates in sCSF leak patients.
Conclusion
We present the largest cohort of sCSF leak repair using a standardized composite repair technique via an endoscopic endonasal approach (Figure 2). Our success rate of 92% is in line with previously reported success rates and very few complications. Higher BMI was a statistically significant associated risk factor for revision.
Authors’ Note
Portions of this work were presented in poster form at the American Rhinology Society Annual Meeting, Chicago, IL, USA, September 8–9, 2017.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval
The study was approved by the USC IRB.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Statement of Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Statement of Informed Consent
There are no human subjects in this article and informed consent is not applicable.
References
- 1.Wise SK, Schlosser RJ. Evaluation of spontaneous nasal cerebrospinal fluid leaks. Curr Opin Otolaryngol Head Neck Surg. 2007; 15:28–34. [DOI] [PubMed] [Google Scholar]
- 2.Schlosser RJ, Wilensky EM, Grady MS, et al. Elevated intracranial pressures in spontaneous cerebrospinal fluid leaks. Am J Rhinol. 2003; 17:191–195. [PubMed] [Google Scholar]
- 3.Schlosser RJ, Woodworth BA, Wilensky EM, et al. Spontaneous cerebrospinal fluid leaks: a variant of benign intracranial hypertension. Ann Otol Rhinol Laryngol. 2006; 115:495–500. [DOI] [PubMed] [Google Scholar]
- 4.Wall M, Corbett JJ. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2014; 83:198–199. [DOI] [PubMed] [Google Scholar]
- 5.Wang EW, Vandergrift WA, Schlosser RJ. Spontaneous CSF leaks. Otolaryngol Clin North Am. 2011; 44:845–856. [DOI] [PubMed] [Google Scholar]
- 6.Stevens SM, Rizk HG, Golnik K, et al. Idiopathic intracranial hypertension: contemporary review and implications for the otolaryngologist. Laryngoscope. 2018; 128:248–256. [DOI] [PubMed] [Google Scholar]
- 7.Seltzer J, Babadjouni A, Wrobel BB, et al. Resolution of chronic aspiration pneumonitis following endoscopic endonasal repair of spontaneous cerebrospinal fluid fistula of the skull base. J Neurol Surg. 2016; 77:e73–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corbett JJ, Savino PJ, Thompson HS, et al. Visual-loss in pseudotumor cerebri- follow-up of 57 patients from 5 to 41 years and a profile of 14 patients with permanent severe visual-loss. Arch Neurol. 1982; 39:461–474. [DOI] [PubMed] [Google Scholar]
- 9.Schlosser RJ, Bolger WE. Significance of empty sella in cerebrospinal fluid leaks. JAMA Otolaryngol Head Neck Surg. 2003; 128:32–38. [DOI] [PubMed] [Google Scholar]
- 10.Lieberman SM, Chen S, Jethanamest D, et al. Spontaneous CSF rhinorrhea: prevalence of multiple simultaneous skull base defects. Am J Rhinol Allergy. 2015; 29:77–81. [DOI] [PubMed] [Google Scholar]
- 11.Shetty PG, Shroff MM, Fatterpekar GM, et al. A retrospective analysis of spontaneous sphenoid sinus fistula: MR and CT findings. Am J Neurorad. 2000; 21:337–342. [PMC free article] [PubMed] [Google Scholar]
- 12.Silver RI, Moonis G, Schlosser RJ, et al. Radiographic signs of elevated intracranial pressure in idiopathic cerebrospinal fluid leaks: a possible presentation of idiopathic intracranial hypertension. Am J Rhinol. 2017; 21:257–261. [DOI] [PubMed] [Google Scholar]
- 13.Mattox DE, Kennedy DW. Endoscopic management of cerebrospinal-fluid leaks and cephaloceles. Laryngoscope. 1990; 100:857–862 [Database] [DOI] [PubMed] [Google Scholar]
- 14.Hegazy HM, Carrau RL, Snyderman CH, et al. Transnasal endoscopic repair of cerebrospinal fluid rhinorrhea: a meta-analysis. Laryngoscope. 2000; 110:1166–1172. [DOI] [PubMed] [Google Scholar]
- 15.Banks CA, Palmer JN, Chiu AG, et al. Endoscopic closure of CSF rhinorrhea: 193 cases over 21 years. JAMA Otolaryngol Head Neck Surg. 2009; 140:826–833. [DOI] [PubMed] [Google Scholar]
- 16.Psaltis AJ, Schlosser RJ, Banks CA, et al. A systematic review of the endoscopic repair of cerebrospinal fluid leaks. JAMA Otolaryngol Head Neck Surg. 2012; 147:196–203. [DOI] [PubMed] [Google Scholar]
- 17.Harvey RJ, Parmar P, Sacks R, et al. Endoscopic skull base reconstruction of large dural defects: a systematic review of published evidence. Laryngoscope. 2012; 122:452–459. [DOI] [PubMed] [Google Scholar]
- 18.Schlosser RJ, Bolger WE. Nasal cerebrospinal fluid leaks: critical review and surgical considerations. Laryngoscope. 2004; 114:255–265. [DOI] [PubMed] [Google Scholar]
- 19.Gassner HG, Ponikau JU, Sherris DA, et al. CSF rhinorrhea: 95 consecutive surgical cases with long term follow-up at the Mayo Clinic. Am J Rhinol. 1999; 13:439–447. [DOI] [PubMed] [Google Scholar]
- 20.Schick B, Ibing R, Brors D, et al. Long-term study of endonasal duraplasty and review of the literature. Ann Otol Rhinol Laryngol. 2001; 110:142–147. [DOI] [PubMed] [Google Scholar]
- 21.Lindstrom DR, Toohill RJ, Loehrl TA, et al. Management of cerebrospinal fluid rhinorrhea: the Medical College of Wisconsin Experience. Laryngoscope. 2004; 114:969–974. [DOI] [PubMed] [Google Scholar]
- 22.Chaaban MR, Illing E, Riley KO, et al. Spontaneous cerebrospinal fluid leak repair: a five-year prospective evaluation. Laryngoscope 2014; 124:70–75. [DOI] [PubMed] [Google Scholar]
- 23.Woodworth BA, Prince A, Chiu AG, et al. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial hypertension. JAMA Otolaryngol Head Neck Surg. 2018; 138:715–720. [DOI] [PubMed] [Google Scholar]
- 24.Reh DD, Gallia GL, Ramanathan M, et al. Perioperative continuous cerebrospinal fluid pressure monitoring in patients with spontaneous cerebrospinal fluid leaks: presentation of a novel technique. Am J Rhinol Allergy. 2010; 24:238–243. [DOI] [PubMed] [Google Scholar]
- 25.Seth R, Rajasekaran K, Luong A, et al. Spontaneous CSF leaks: factors predictive of additional interventions. Laryngoscope. 2010; 120:2141–2146. [DOI] [PubMed] [Google Scholar]
- 26.Abubaker K, Ali Z, Raza K, et al. Idiopathic intracranial hypertension: lumboperitoneal shunts versus ventriculoperitoneal shunts—case series and literature review. Br J Neurosurg. 2011; 25:94–99. [DOI] [PubMed] [Google Scholar]
- 27.Teachey W, Grayson J, Cho DY, et al. Intervention for elevated intracranial pressure improves success rate after repair of spontaneous cerebrospinal fluid leaks. Laryngoscope. 2017; 127:2011–2016. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch O. Successful closure of cerebrospinal fluid rhinorrhea by endonasal surgery. AMA Arch Otolaryngol. 1952; 56:1–12. [DOI] [PubMed] [Google Scholar]
- 29.Hadad G, Bassagasteguy L, Carrau RL, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006; 116:1882–1886. [DOI] [PubMed] [Google Scholar]
- 30.Munich SA, Fenstermaker RA, Fabiano AJ, et al. Cranial base repair with combined vascularized nasal septal flap and autologous tissue graft following expanded endonasal endoscopic neurosurgery. J Neurol Surg A Cent Eur Neurosurg. 2013; 74:101–108. [DOI] [PubMed] [Google Scholar]
- 31.Soudry E, Turner JH, Nayak JV, et al. Endoscopic reconstruction of surgically created skull base defects: a systematic review. JAMA Otolaryngol Head Neck Surg. 2014; 150:730–738. [DOI] [PubMed] [Google Scholar]
- 32.Clavenna MJ, Turner JH, Chandra RK. Pedicled flaps in endoscopic skull base reconstruction: review of current techniques. Curr Opin Otolaryngol Head Neck Surg. 2015; 23:71–77. [DOI] [PubMed] [Google Scholar]
- 33.Gunaratne DA, Singh NP. Endoscopic pedicled nasoseptal flap repair of spontaneous sphenoid sinus cerebrospinal fluid leaks. BMJ Case Rep. 2015; 67:412–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sigler AC, D’Anza B, Lobo BC, et al. Endoscopic skull base reconstruction: an evolution of materials and methods. Otolaryngol Clin North Am. 2017; 50:643–653. [DOI] [PubMed] [Google Scholar]
- 35.Perez MA, Bialer OY, Bruce BB, et al. Primary spontaneous cerebrospinal fluid leaks and idiopathic intracranial hypertension. J Neuroophthalmol. 2013; 33:330–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lobo BC, Baumanis MM, Nelson RF. Surgical repair of spontaneous cerebrospinal fluid (CSF) leaks: a systematic review. Laryngoscope Investig Otolaryngol. 2017; 2:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav YR, Parihar V, Janakiram N, et al. Endoscopic management of cerebrospinal fluid rhinorrhea. Asian J Neurosurg. 2016; 11:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Song X, Wang D, Sun X, et al. Endoscopic repairs of sinonasal cerebrospinal leaks: outcome and prognostic factors. J Craniofac Surg. 2018; 29:182–187. [DOI] [PubMed] [Google Scholar]
- 39.Fraser S, Gardner PA, Koutourousiou M, et al. Risk factors associated with postoperative cerebrospinal fluid leak after endoscopic endonasal skull base surgery. J Neurosurg. 2018; 128:1066–1071. [DOI] [PubMed] [Google Scholar]
- 40.D’Anza B, Tien D, Stokken JK, et al. Role of lumbar drains in contemporary endonasal skull base surgery: meta-analysis and systematic review. Am J Rhinol Allergy. 2016; 30:420–435. [DOI] [PubMed] [Google Scholar]
- 41.Nelson RF, Roche JP, Gantz BJ, et al. Middle cranial fossa (MCF) approach without the use of lumbar drain for the management of spontaneous cerebral spinal fluid (CSF) leaks. Otol Neurotol. 2016; 37:1625–1629. [DOI] [PubMed] [Google Scholar]
- 42.Elmorsy SM, Khafagy YW. Endoscopic management of spontaneous CSF rhinorrhea with septal graft and middle turbinate rotational flap technique: a review of 31 cases. Ear Nose Throat J. 2014; 93:E14–E19. [PubMed] [Google Scholar]
- 43.Zheng WJ, Zhang XJ, Ji T, et al. Neuroendoscopic endonasal management of cerebrospinal fluid rhinorrhea. J Craniofac Surg. 2015; 26:459–463. [DOI] [PubMed] [Google Scholar]
- 44.Deenadayal DS, Vidyasagar D, Naveen Kumar M, et al. Spontaneous CSF rhinorrhea our experience. Indian J Otolaryngol Head Neck Surg. 2013; 65:271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]