Abstract
The majority of cases involving hypercalcemia in the setting of sarcoidosis are explained by the overproduction of calcitriol by activated macrophages. Vitamin D takes part in the regulation of granuloma formation. However, using vitamin D metabolites to assess the activity of the disease is still problematic, and its usefulness is disputable. In some cases, though, a calcium metabolism disorder could be a valuable tool (i.e. as a marker of extrathoracic sarcoidosis). Although sarcoidosis does not cause a decrease in bone mineral density, increased incidence of vertebral deformities is noted. Despite increasing knowledge about calcium homeostasis disorders in patients with sarcoidosis, there is still a need for clear guidelines regarding calcium and vitamin D supplementation in these patients.
Keywords: Sarcoidosis; calcium; vitamin D; hypercalcemia; hypercalciuria; calcitriol; 1,25(OH)2D3; 25(OH)D3
Introduction
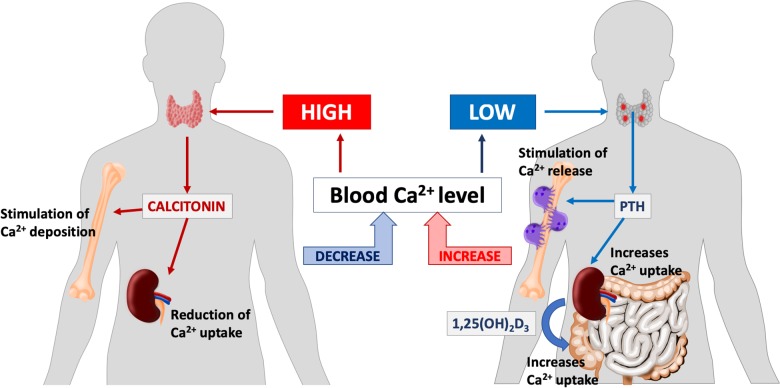
Calcium metabolism in the human body is tightly regulated. Although many of the mechanisms of control are still not fully described, the present state of knowledge shows that they are far more complicated than the already known parathyroid hormone—vitamin D feedback loop (Figure 1). The endocrine activity of osteoblasts and osteoclasts, creating a fibroblast growth factor 23-vitamin D axis, seems to be just as important.1,2
Figure 1.
Selected aspects of human calcium homeostasis.
The majority of hypercalcemia cases in sarcoidosis are explained by the overproduction of 1,25(OH)2D3 (calcitriol) by activated macrophages. Despite quite convincing evidence supporting this hypothesis, some questions have yet to be completely answered. Moreover, some recent studies suggest that vitamin D supplementation may improve not only calcium homeostasis but also the course of sarcoidosis.3 Naturally, many authors are at odds with this opinion and do not recommend cholecalciferol supplementation in patients with sarcoidosis. Still, it is unknown what factors predispose to calcium homeostasis disorders and how its occurrence changes the outcome. Those, among many other doubts, encouraged us to create a summary and analysis of the knowledge connected with this subject.
In the context of sarcoidosis, vitamin D and its turnover are interesting because of its important role in the regulation of the immune system and the process of granulomatous inflammation. It is now known that 1-α-hydroxylase expression is present in many tissues. However, only hydroxylation in kidneys, activated macrophages, and the placenta can influence plasma 1,25(OH)2D3 levels.4 It has been shown that calcitriol inhibits the production of (interferon gamma ) INF-γ, lymphotoxin, interleukin 2 (IL-2), and proliferation of certain T-lymphocyte subpopulations.5–7 A murine model has also proven that calcitriol inhibits IL-6 and tumor necrosis factor alpha (TNF-α) production by lipopolysaccharide (LPS)-stimulated monocytes and macrophages.8 In vitro studies have shown that 1,25(OH)2D3 stimulates monocyte proliferation, differentiation, and transformation into epithelioid cells.9 On the other hand, it inhibits macrophage differentiation into dendritic cells and inhibits dendritic cell maturation, simultaneously stimulating their apoptosis (Figure 2).10 Increased expression of TREM-2 (a receptor playing an important role in cell fusion and granuloma formation) on myeloid cells has been found in pulmonary sarcoidosis and, interestingly, compared to subjects with 25(OH)D3 deficiency (<30 ng/μl), patients with a level between 30 ng/ml and 50 ng/ml had higher total numbers and percentages of TREM2 positive cells in bronchoalveolar lavage fluid.11 Expression of vitamin D receptors has only been found in the alveolar lymphocytes of subjects with sarcoidosis, and not in healthy controls.12,13 Data about polymorphisms of vitamin D receptor in sarcoidosis are inconsistent. According to Niimi et al., Taq1 polymorphisms seem to be irrelevant. Allele B of Bsm1 alleles is more common in sarcoidosis but does not affect localization nor the course of the disease (101 patients and 105 healthy controls from Japanese population).14 One smaller study (n = 35) suggests a higher frequency of allele B in sarcoidosis patients and shows allele B to be present more often in patients with Löfgren syndrome.15 The concentration of vitamin D binding protein is higher in exosomes extracted from bronchoalveolar lavage fluid in patients with sarcoidosis and cerebrospinal fluid from patients with neurosarcoidosis.16,17 No relationship has been found between vitamin D binding protein different alleles and the incidence of sarcoidosis.18
Figure 2.
It is well-known that vitamin D plays a complex and still not fully understood role in regulation of immune system. Part of its actions can be directly connected with formation of granuloma. Some of them are presented here.
Epidemiology
Hypercalcemia occurs in 0.2–4% of the general population. Primary hyperparathyroidism and malignancies are responsible for about 80–90% of all cases.19 Donovan et al. retrospectively analyzed 101 cases of vitamin D3-mediated hypercalcemia and concluded that sarcoidosis was an underlying cause of almost 50% of them. Calcitriol serum concentration above 300 pmol/l was suggestive of another etiology of hypercalcemia.20 Moreover, sarcoidosis is the reason behind about 0.5% of cases of hypercalcemia in patients with a history of malignancies.21 Depending on the studies and population studied, hypercalcemia affects from 7% up to 18% of patients with sarcoidosis.22–25 In a case–control etiologic study of sarcoidosis (ACCESS), a multicenter prospective study with 736 enrolled patients, incidence of sarcoidosis-associated hypercalcemia was 3.7%.26 However, such a low prevalence could be the result of ethnic composition—44% of the study group consisted of African-Americans. It is suggested that hypercalcemia is less common among these individuals.27,28 The biggest up-to-date study found, a single-center retrospective study by Baughman et al. (n = 1606), reports that hypercalcemia appeared in about 6% of sarcoidosis patients.29 Interestingly, the incidence of hypercalcemia in the Japanese population has been progressively rising between 1974 and 2012.30 No similar reports regarding the European population have been found.
Evidence about the association between a higher risk of elevated serum calcium level in sarcoidosis and age are inconsistent. Namely, in the ACCESS study, patients >40 years old were identified to be more predisposed to this pathology.26 However Brito-Zerón et al., in a smaller study conducted on the Spanish population, reported patients >65 years old in this context.31 On the other hand, hypercalcemia seems to be more common (prevalence rate about 30%) in children. Examined groups are less numerous but reports are coherent.32–34
Association between sex and the incidence of hypercalcemia in sarcoidosis has not been confirmed with some studies showing a higher risk among the male population,22,26,31 whereas others show no statistically significant difference between sexes.23 In the mentioned study, Baughman et al. compared a group of about 100 patients with hypercalcemia to 1500 patients with sarcoidosis without calcium metabolism disturbances and failed to find any differences in sex, age, or ethnicity.29 The higher incidence of hypercalcemia in summer months, due to greater UV exposure, has also been suggested.28,35–38 A significant risk factor for sarcoidosis-associated hypercalcemia (odds ratio 3.6) found in the ACCESS study (over 470 patients analyzed) is the combination of HLA DRB1*1101 allele and exposure to insecticides.39
A more frequent sign of dysregulated calcium homeostasis in sarcoidosis comes in the form of hypercalciuria which may affect between 20%40 and even 40%41 of patients. Also, nephrolithiasis is more common in sarcoidosis than in the general population. This complication will occur in 10–14% of patients in the course of the disease. Asymptomatic stones can be found in 2.7% of subjects at the moment of establishing the diagnosis and in approximately 1% of cases, it can be the first symptom of sarcoidosis.42
The pathophysiology of hypercalcemia in sarcoidosis
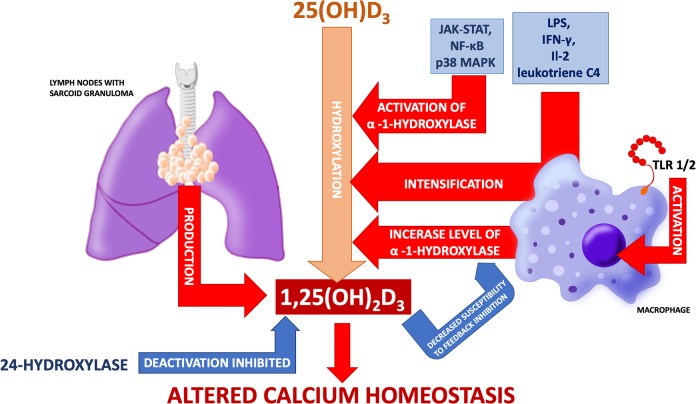
Increased production of 1,25(OH)2D3 is considered to be the main cause of calcium homeostasis disorders in sarcoidosis (Figure 3). Increased calcitriol concentration was observed in a few cases of hypercalcemia in patients with sarcoidosis in the late 1970s.43 The discovery of hypercalcemia in combination with elevated calcitriol levels in patients with sarcoidosis and accompanying kidney failure was the proof of its extrarenal production.44,45 Studies confirmed that homogenate of lymph nodes with sarcoid granuloma produces 1,25(OH)2D3.46 Adams et al. experimentally proved that pulmonary alveolar macrophages from patients with sarcoidosis can hydroxylate 25(OH)D3 to 1,25(OH)2D3 and that the process was augmented in cells derived from patients with hypercalcemia.47,48
Figure 3.
Process of 1-α-hydroxylation of 25(OH)D3 in macrophages is different from the one taking place in kidneys and is not focused on maintaining calcium and phosphate homeostasis but is dependent on immunological processes. The figure shows possible mechanisms of altered vitamin D metabolism and calcium disturbances in sarcoidosis.
Studies in patients with tuberculosis revealed that macrophages activated via toll-like receptor (TLR) 2/1 present increased expression of 1-α-hydroxylase (CYP27B1) and vitamin D receptor.49 In healthy subjects, pulmonary alveolar macrophages synthesize 1,25(OH)2D3 from 25(OH)D3 after activation by IFN-γ and LPS. Meanwhile, in pulmonary alveolar macrophages harvested from patients with sarcoidosis (including those without hypercalcemia), this process occurs without previous activation. However, exposure to LPS, IFN-γ, IL-2, or leukotriene C4 induces intensification of 1-α-hydroxylation of 25(OH)D3.50–53 In turn, Lawrence et al. observed hypercalcemia in three out of four patients, who presented the highest concentrations of soluble receptors for IL-2.54 There has also been a case of sarcoidosis exacerbation with the occurrence of hypercalcemia in the course of treatment with IL-2.55 Interestingly, despite overexpression of CYP27B1 in pulmonary alveolar macrophages of patients with lung cancer (irrespective of histopathological type), no differences in calcium, 25(OH)D3 or 1,25(OH)2D3 levels have been found (in comparison with the control group).56
It is known that JAK-STAT, NF-κB, and p38 MAPK pathways play a role in the activation of 1-α-hydroxylase. After inhibiting any of them, CYP27B1 expression decreases.57 Calcitrol synthesis is tightly regulated in healthy subjects. However, its synthesis in morbid conditions is not so strictly controlled. This has been noted via a couple of different mechanisms. Firstly, 1-α-hydroxylase from macrophages is less susceptible to feedback inhibition by 1,25(OH)2D3 (this resistance is enhanced by IFN-γ). Secondly, 1,25(OH)2D3 deactivation by 24-hydroxylase is inhibited.50,52 Because of this, there may be two effects on circulating calcitriol: firstly, it may exceed reference values. Secondly, and more frequently, it may stay within normal range but be inadequate to circulating serum calcium level. Arguments for “inadequate normal” 1,25(OH)2D3 concentration as the main cause of sarcoidosis-associated hypercalcemia are presented and commented in Table 1.
Table 1.
Potential evidence supporting phenomenon of “inadequate normal” 1,25(OH)2D3 concentration in patients with sarcoidosis.a
| “Inadequate normal” 1,25(OH)2D3 concentration in patients with sarcoidosis | ||
|---|---|---|
| Observation | Comment | References |
| Hypercalcemia accompanied by normal calcitriol level with a decline in its concentration after glucocorticoid treatment | — | Unsal et al.,58 Berlin et al.,59 Shrayyef et al.,60 Falk et al.61 |
| Lack of calcitriol level decrease despite supplementation of calcium in patients with sarcoidosis | In the healthy subjects, the level of calcitriol should decrease after calcium supplementation | Basile et al.62 |
| Increase of 25(OH)D3 as well as 1,25(OH)2D3 after vitamin D2 supplementation | In healthy subjects, supplementation influences only 25(OH)D3 concentration | Stern et al.63 |
aThe concentration of 1,25(OH)2D3 in patients with sarcoidosis, even in those with hypercalcemia, usually stays within normal limits. Still, the overproduction of calcitriol is considered to be the leading cause of sarcoidosis-associated hypercalcemia. This leads to the conclusion that there exists a phenomenon of “inadequate normal” 1,25(OH)2D3 concentration in patients with sarcoidosis. The table shows some evidence supporting this theory.
A positive correlation between 1,25(OH)2D3 level and calcium concentration has been noted in some observational studies (approximately r = 0,55).63–65 However, in one follow-up study with a slightly smaller group (n = 39), this relationship has not been confirmed.66
A few cases of hypercalcemia induced by parathyroid hormone-related protein (PTHrP) have also been described.67–69 The expression of PTHrP was observed in macrophages and giant cells in the majority of patients with granulomatous diseases, sarcoidosis included. Nevertheless, its circulating serum level hardly ever exceeds normal limits.69–72 PTHrP expression is stimulated by factors such as LPS, prostaglandin E (PGE), IL-1, transforming growth factor β (TGF-β), and it most likely acts as an anti-inflammatory agent, which would explain its presence in granuloma cells.70
It is important to remember that sarcoidosis does not exclude other causes of hypercalcemia, beginning with the most common cause: hyperparathyroidism. Such conditions should be excluded during the diagnostic process to enable the introduction of optimal treatment.
The influence of sarcoidosis and related calcium disturbances on skeletal system
The influence of sarcoidosis on the skeletal system is mediated by two main etiologies, both of which are risk factors for osteoporosis. Firstly, it is mediated by calcium homeostasis disorders, including frequent 25(OH)D deficiency. Secondly, by treatment with corticosteroids.
It has been suggested that sarcoidosis itself causes a reduction of bone mineral density (BMD). Heijackmann et al. found that despite higher concentrations of biochemical markers of bone turnover (procollagen type I amino-terminal pro-peptide—PINP and carboxy-terminal cross-linked telopeptide of type I collagen—ICTP), BMD of the hip was normal and did not change in 4-year follow-up.73,74 Also, Bolland et al., in his 2-year follow-up study of 64 patients with sarcoidosis, failed to find a significant change in BMD in regions such as the lumbar spine, total hip, femoral neck, as well as in total body measurements.75 Two large-group case–control studies did not reveal any increase in a total number of bone fractures caused by sarcoidosis alone.76,77 However, higher occurrence of fractures and deformations within vertebrae has been noted, including in patients with normal BMD.73,74,76,78 The effectiveness of Vitamin D3 and calcium supplementation in improving BMD in patients with sarcoidosis remains unconfirmed.75,79 Surprisingly, Saidenberg-Kermanac’h et al., in a cross-sectional study of 142 patients, showed that vitamin D supplementation paradoxically caused BMD reduction and a higher occurrence of bone fractures.78 There is no doubt that the risk of fractures and decreased BMD appear in the course of corticosteroid therapy. Interestingly, population-based studies show that such an effect can be observed only during and up to 3–6 months after the termination of therapy.76,77 The meta-analyses of available randomized controlled trials (in all patients, not specifically with sarcoidosis) confirm moderate effectiveness of vitamin D supplementation on preventing steroid-induced BMD decline with no statistically significant effect on the incidence of fractures. Treatment with bisphosphonates is more effective in improving BMD and seems to be effective in reducing the risk of vertebral fractures. The most effective agent, however, seems to be teriparatide.80–82 This is an important issue because there is currently a discussion about supplementation with vitamin D and calcium and its potential in bringing about hypercalcemia and hypercalciuria.36,62,83,84 Two retrospective studies of patients visiting the outpatient clinic stand in opposition to each other. In a cohort analysis of almost 400 patients (randomly chosen, divided in two equal groups) Sodhi and Aldrich showed that the group of patients, who at least once had vitamin D prescribed, had an almost two times higher risk of developing hypercalcemia in a 2-year follow-up.85 On the other hand, Kamphuis et al. did not observe a higher incidence of hypercalcemia in the group using calcium and vitamin D supplementation. In fact, their study showed a protective effect against hypercalcemia (mean dose 400 UI per day) in the group of 70 patients not treated with corticosteroids (39 with supplementation vs. 31 without supplementation).3
In a randomized trial over 1 year that studied a group of 27 subjects with sarcoidosis taking cholecalciferol supplementation of 50,000 IU/month, there were relevant differences observed in the mean concentration of 25(OH)D3 ranging from suboptimal average <50 nmol/l to average >75 nmol/l. The authors also observed a small, but statistically significant difference in concentrations of 1,25(OH)2D3. One patient from the study group developed hypercalcemia.79 Similarly, in a prospective study, in which a group of 16 African-Americans with average 25(OH)D3 concentration <75 nmol/l were given 50,000 IU/month for 12 weeks, a comparable rise in 25(OH)D3 occurred with no changes in mean concentrations of Ca and Ca2+. Also, no calciuria had been observed. Surprisingly, in the majority of the subjects, 1,25(OH)2D3 concentration decreased by half.86 When supplementation was limited to Ca, increased excretion of calcium in urine was observed, without any effect on calcium and vitamin D metabolites serum concentration.62
Calcium homeostasis and prognosis
The association between decreased 25(OH)D3 level and a more severe course of disease has been described.12 Kiani et al. compared two groups (n = 40) of patients suffering from sarcoidosis with one group having 25(OH)D3 concentration <50 nmol/l and the other >50 nmol/l. They found that 25(OH)D3 deficiency correlated negatively with lung parenchyma involvement (stages II–IV) and tended to predispose to chronic course of disease in a 2-year follow-up.87 On the other hand, higher mean concentrations of calcitriol were found in patients with active and untreated sarcoidosis.88 Kavathia et al. (in a 59 patient study involving predominantly African-American patients) found a concentration of 1,25(OH)2D3 above 51 pg/ml to be a risk factor for prolonged (>1 year) treatment.89
The correlation between calcitriol concentration and 67 Ga uptake has not been noted,90 but 25(OH)D3 concentration correlated negatively with results of a somatostatin receptor scintigraphy.3
In renal sarcoidosis, a correlation between hypercalcemia and complete response to glucocorticoid treatment has been observed (a retrospective study of 46 patients with biopsy-proven renal sarcoidosis).28 In the observational study performed on 36 Japanese patients, Hamada et al. observed that ionized calcium level above 1.23 mmol/l was a 100% specific indicator for extrapulmonary sarcoidosis.64
Management
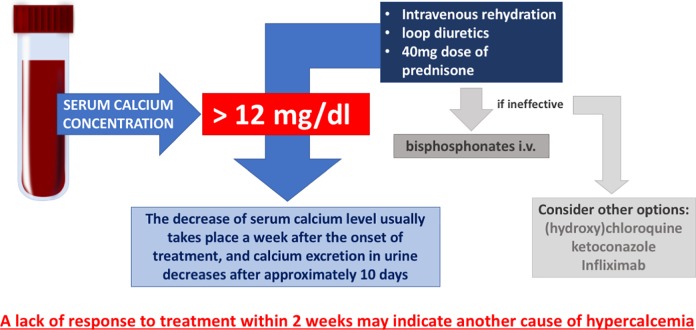
The management of hypercalcemia depends mostly on the level of calcium. Patients with moderate elevations in serum calcium (12.0–14.0 mg/dl) may develop symptoms when levels rise rapidly. This group of symptomatic patients requires immediate intervention. Intensive treatment is usually necessary when serum calcium concentration exceeds 14 mg/dl. Intravenous rehydration and loop diuretics are the treatment of choice. Bisphosphonates should be given intravenously in case other treatment options are ineffective (Figure 4).19
Figure 4.
Proposed scheme of sarcoidosis-associated hypercalcemia treatment.
Corticosteroids are the causal first-line treatment in sarcoidosis-associated hypercalcemia. In vitro studies have confirmed that dexamethasone suppresses calcitriol production in sarcoidosis patients’ pulmonary alveolar macrophages.50 The treatment schedule of an initial 40 mg dose of prednisone followed by a reduction to 20 mg in 1–2 weeks appears to be effective. Further dose reduction should follow within the upcoming weeks. The decrease of serum calcium level usually takes place a week after the onset of treatment, and calcium excretion in urine decreases after approximately 10 days. A lack of response to treatment within 2 weeks may indicate another cause of hypercalcemia.91,92
Our clinical observations suggest that in cases of progressive kidney failure, when dual etiology (hypercalcemic and sarcoidosis of kidneys) could not be excluded, IV methylprednisolone was effective. Other drugs successfully (but rarely) used in the treatment of calcium homeostasis disorders are (hydroxy) chloroquine and ketoconazole.93–96 Both drugs suppress 1-α-hydroxylase and chloroquine additionally stimulates calcitriol deactivation by 24-hydroxylase.50,93 Infliximab has also proven to be an effective option in sarcoidosis-associated hypercalcemia in cases of toxicity or resistance to corticosteroids and other immunosuppressive drugs.97,98 It is worth mentioning that sodium cellulose phosphate and flurbiprofen have been administered in the past for the treatment of hypercalcemia and nephrocalcinosis.99–101
In the case of hypercalciuria, thiazide diuretics are contraindicated due to their potential to induce hypercalcemia. Some authors suggest a low-calcium diet, but studies seem to question this recommendation. Hypercalciuria persisted in 30% of patients despite calcium restriction in their diet. Further, the use of pharmacotherapy to limit calcium absorption did not improve calcium concentration control.102,103 In a 4-year observational study, a lower incidence of nephrolithiasis was observed in patients with higher dietary calcium intake, which may be related to inhibition of oxalates absorption. While renal calculi in patients with sarcoidosis consist mainly of calcium oxalates, calcium restriction in the diet may produce unexpectedly harmful results.104–106
Conclusions
Sarcoidosis-associated hypercalcemia is quite a common problem as it affects about 6% of patients. It is also one of the indications to introduce pharmacotherapy with steroids. Its pathophysiology appears to be quite well explained. Although it seems logical that vitamin D metabolites should be good tools for assessing disease activity, clinically it is not that simple. Increased conversion of 25(OH)D3 to calcitriol suggests that perhaps the ratio of 25(OH)D3 to 1,25(OH)2D3 could be more adequate than absolute values. One study confirms this theory.107
Despite our increasing knowledge about calcium homeostasis disorders in patients with sarcoidosis, there is still a need for clear guidelines regarding calcium and Vitamin D supplementation. Papers concerning this problem are inconclusive. It appears that supplementation increases the risk of hypercalcemia but only in a certain group of patients. Nevertheless, indications for supplementation seem to be limited, as it is probably ineffective in preventing steroid-induced osteoporosis fractures. An improvement in the course of sarcoidosis in patients with corrected vitamin D insufficiency is a tempting perspective, but more evidence is needed.
Management of sarcoidosis-associated hypercalcemia should start with excluding other causes of elevated calcium levels. Intensive hydration coupled with loop diuretics (if needed, especially in the elderly and patients with heart failure) should be introduced to reduce hypercalcemia and prevent its acute complication. Corticosteroids should be introduced to remove the cause of calcium metabolism disturbance. Oral treatment with prednisone (doses of 0.5–1 mg/kg a day) is sufficient. In cases of progressive kidney failure, when dual etiology (hypercalcemic and sarcoidosis of kidneys) could not be excluded, IV methylprednisolone would be used.
The complex action of vitamin D on the immune system coupled with the pathophysiology of granulomatous inflammation seen in sarcoidosis could possibly provide new therapeutic targets in the future.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The costs of this study were defrayed from regular finances of the Department of Pneumology and Allergy of Medical University of Lodz, Poland (503/1-151-03/503-11-001-19-00).
ORCID iD: Adam J Białas  https://orcid.org/0000-0002-3501-167X
https://orcid.org/0000-0002-3501-167X
References
- 1. Blau JE, Collins MT. The PTH-vitamin D-FGF23 axis. Rev Endocr Metab Disord 2015; 16: 165–174. [DOI] [PubMed] [Google Scholar]
- 2. Yokota H, Raposo JF, Chen A, et al. Evaluation of the role of FGF23 in mineral metabolism. Gene Regul Syst Biol 2009; 3: 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamphuis LS, Bonte-Mineur F, van Laar JA, et al. Calcium and vitamin D in sarcoidosis: is supplementation safe? J Bone Miner Res 2014; 29: 2498–2503. [DOI] [PubMed] [Google Scholar]
- 4. Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 2012; 523: 95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rottoli P, Muscettola M, Grasso G, et al. Impaired interferon-gamma production by peripheral blood mononuclear cells and effects of calcitriol in pulmonary sarcoidosis. Sarcoidosis 1993; 10: 108–114. [PubMed] [Google Scholar]
- 6. Muscettola M, Grasso G. Effect of 1,25-dihydroxyvitamin D3 on interferon gamma production in vitro. Immunol Lett 1988; 17: 121–124. [DOI] [PubMed] [Google Scholar]
- 7. Müller K, Bendtzen K. Inhibition of human T lymphocyte proliferation and cytokine production by 1,25-dihydroxyvitamin D3. Differential effects on CD45RA+ and CD45R0+ cells. Autoimmunity 1992; 14: 37–43. [DOI] [PubMed] [Google Scholar]
- 8. Zhang Y, Leung DYM, Richers BN, et al. Vitamin D inhibits monocyte/macrophage pro-inflammatory cytokine production by targeting mitogen-activated protein kinase phosphatase 1. J Immunol Baltim Md 1950 2012; 188: 2127–2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohta M, Okabe T, Ozawa K, et al. In vitro formation of macrophage-epithelioid cells and multinucleated giant cells by 1 alpha,25-dihydroxy vitamin D3 from human circulating monocytes. Ann N Y Acad Sci 1986; 465: 211–220. [DOI] [PubMed] [Google Scholar]
- 10. Penna G, Adorini L. 1α,25-Dihydroxyvitamin d3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol 2000; 164: 2405–2411. [DOI] [PubMed] [Google Scholar]
- 11. Bucova M, Suchankova M, Tibenska E, et al. TREM-2 receptor expression increases with 25(OH)D vitamin serum levels in patients with pulmonary sarcoidosis. Mediators Inflamm 2015. Epub ahead of print 2015 DOI: 10.1155/2015/181986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barna BP, Culver DA, Kanchwala A, et al. Alveolar macrophage cathelicidin deficiency in severe sarcoidosis. J Innate Immun 2012; 4: 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Biyoudi-Vouenze R, Cadranel J, Valeyre D, et al. Expression of 1,25(OH)2D3 receptors on alveolar lymphocytes from patients with pulmonary granulomatous diseases. Am Rev Respir Dis 1991; 143: 1376–1380. [DOI] [PubMed] [Google Scholar]
- 14. Niimi T, Tomita H, Sato S, et al. Vitamin D receptor gene polymorphism in patients with sarcoidosis. Am J Respir Crit Care Med 1999; 160: 1107–1109. [DOI] [PubMed] [Google Scholar]
- 15. Petkovic TR, Pejcic T, Marinkovic M, et al. The role of vitamin D receptor Bsm1 polymorphism in the course of sarcoidosis. Eur Respir J 2017; 50: PA980. [Google Scholar]
- 16. Taibi L, Boursier C, Clodic G, et al. Search for biomarkers of neurosarcoidosis by proteomic analysis of cerebrospinal fluid. Ann Biol Clin (Paris) 2017; 75: 393–402. [DOI] [PubMed] [Google Scholar]
- 17. Martinez-Bravo M-J, Wahlund CJE, Qazi KR, et al. Pulmonary sarcoidosis is associated with exosomal vitamin D-binding protein and inflammatory molecules. J Allergy Clin Immunol 2017; 139: 1186–1194. [DOI] [PubMed] [Google Scholar]
- 18. Milman N, Thymann M, Graudal N, et al. Plasma vitamin D-binding protein (GC) factors, immunoglobulin G heavy chain (GM) allotypes and immunoglobulin kappa light chain (KM1) allotype in patients with sarcoidosis and in healthy control subjects. Sarcoidosis Vasc 2002; 19: 97–100. [PubMed] [Google Scholar]
- 19. Renaghan AD, Rosner MH. Hypercalcemia: etiology and management. Nephrol Dial Transplant 2018; 33: 549–551. [Google Scholar]
- 20. Donovan PJ, Sundac L, Pretorius CJ, et al. Calcitriol-mediated hypercalcemia: causes and course in 101 patients. J Clin Endocrinol Metab 2013; 98: 4023–4029. [DOI] [PubMed] [Google Scholar]
- 21. Soyfoo MS, Brenner K, Paesmans M, et al. Non-malignant causes of hypercalcemia in cancer patients: a frequent and neglected occurrence. Support Care Cancer 2013; 21: 1415–1419. [DOI] [PubMed] [Google Scholar]
- 22. Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 2008; 31: 372–379. [DOI] [PubMed] [Google Scholar]
- 23. Ungprasert P, Crowson CS, Matteson EL. Influence of gender on epidemiology and clinical manifestations of sarcoidosis: a population-based retrospective cohort study 1976-2013. Lung 2017; 195: 87–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. James DG, Neville E, Siltzbach LE. A worldwide review of sarcoidosis. Ann N Y Acad Sci 1976; 278: 321–334. [DOI] [PubMed] [Google Scholar]
- 25. Studdy PR, Bird R, Neville E, et al. Biochemical findings in sarcoidosis. J Clin Pathol 1980; 33: 528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baughman RP, Teirstein AS, Judson MA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med 2001; 164: 1885–1889. [DOI] [PubMed] [Google Scholar]
- 27. Smith C, Feldman C, Reyneke J, et al. Sarcoidosis in Johannesburg – a comparative study of black and white patients. South Afr Med J 1991; 80: 423–427. [PubMed] [Google Scholar]
- 28. Mahévas M, Lescure FX, Boffa J-J, et al. Renal sarcoidosis: clinical, laboratory, and histologic presentation and outcome in 47 patients. Medicine (Baltimore) 2009; 88: 98. [DOI] [PubMed] [Google Scholar]
- 29. Baughman RP, Janovcik J, Ray M, et al. Calcium and vitamin D metabolism in sarcoidosis. Sarcoidosis Vasc 2013; 30: 113–120. [PubMed] [Google Scholar]
- 30. Sawahata M, Sugiyama Y, Nakamura Y, et al. Age-related and historical changes in the clinical characteristics of sarcoidosis in Japan. Respir Med 2015; 109: 272–278. [DOI] [PubMed] [Google Scholar]
- 31. Brito-Zerón P, Sellarés J, Bosch X, et al. Epidemiologic patterns of disease expression in sarcoidosis: age, gender and ethnicity-related differences. Clin Exp Rheumatol 2016; 34: 380–388. [PubMed] [Google Scholar]
- 32. Abernathy RS. Childhood sarcoidosis in Arkansas. South Med J 1985; 78: 435–439. [DOI] [PubMed] [Google Scholar]
- 33. Hoffmann AL, Milman N, Byg KE. Childhood sarcoidosis in Denmark 1979–1994: incidence, clinical features and laboratory results at presentation in 48 children. Acta Paediatr Oslo Nor 1992 2004; 93: 30–36. [PubMed] [Google Scholar]
- 34. Robinson PJ, Olinsky A. Sarcoidosis in children. Aust Paediatr J 1986; 22: 291–293. [DOI] [PubMed] [Google Scholar]
- 35. Smith MJ, Hey GB. Recurring ‘red eyes’ due to seasonal hypercalcaemia. Postgrad Med J 1976; 52: 86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Papapoulos SE, Clemens TL, Fraher LJ, et al. 1, 25-dihydroxycholecalciferol in the pathogenesis of the hypercalcaemia of sarcoidosis. Lancet Lond Engl 1979; 1: 627–630. [DOI] [PubMed] [Google Scholar]
- 37. Cronin CC, Dinneen SF, O’Mahony MS, et al. Precipitation of hypercalcaemia in sarcoidosis by foreign sun holidays: report of four cases. Postgrad Med J 1990; 66: 307–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wiemeyer A, Schwarze EW, Mathias K, et al. Acute kidney failure in a recurrence of sarcoidosis at the height of summer. Dtsch Med Wochenschr 1946 1996; 121: 165–168. [DOI] [PubMed] [Google Scholar]
- 39. Rossman MD, Thompson B, Frederick M, et al. HLA and environmental interactions in sarcoidosis. Sarcoidosis Vasc 2008; 25: 125–132. [PubMed] [Google Scholar]
- 40. Lancina Martín JA, García Freire C, Busto Castañón L, et al. Sarcoidosis and urolithiasis. Arch Esp Urol 1995; 48: 234–239. [PubMed] [Google Scholar]
- 41. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 2007; 357: 2153–2165. [DOI] [PubMed] [Google Scholar]
- 42. Berliner AR, Haas M, Choi MJ. Sarcoidosis: the nephrologist’s perspective. Am J Kidney Dis 2006; 48: 856–870. [DOI] [PubMed] [Google Scholar]
- 43. Bell NH, Stern PH, Pantzer E, et al. Evidence that increased circulating 1 alpha, 25-dihydroxyvitamin D is the probable cause for abnormal calcium metabolism in sarcoidosis. J Clin Invest 1979; 64: 218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barbour GL, Coburn JW, Slatopolsky E, et al. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med 1981; 305: 440–443. [DOI] [PubMed] [Google Scholar]
- 45. Maesaka JK, Batuman V, Pablo NC, et al. Elevated 1,25-dihydroxyvitamin D levels: occurrence with sarcoidosis with end-stage renal disease. Arch Intern Med 1982; 142: 1206–1207. [DOI] [PubMed] [Google Scholar]
- 46. Mason RS, Frankel T, Chan YL, et al. Vitamin D conversion by sarcoid lymph node homogenate. Ann Intern Med 1984; 100: 59–61. [DOI] [PubMed] [Google Scholar]
- 47. Adams JS, Sharma OP, Gacad MA, et al. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest 1983; 72: 1856–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Adams JS, Singer FR, Gacad MA, et al. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. J Clin Endocrinol Metab 1985; 60: 960–966. [DOI] [PubMed] [Google Scholar]
- 49. Adams JS, Chen H, Chun R, et al. Substrate and enzyme trafficking as a means of regulating 1,25-dihydroxyvitamin D synthesis and action: the human innate immune response. J Bone Miner Res 2007; 22(Suppl 2): V20–V24. [DOI] [PubMed] [Google Scholar]
- 50. Reichel H, Koeffler HP, Barbers R, et al. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. J Clin Endocrinol Metab 1987; 65: 1201–1209. [DOI] [PubMed] [Google Scholar]
- 51. Adams JS, Modlin RL, Diz MM, et al. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. J Clin Endocrinol Metab 1989; 69: 457–460. [DOI] [PubMed] [Google Scholar]
- 52. Dusso AS, Kamimura S, Gallieni M. , et al. gamma-Interferon-induced resistance to 1,25-(OH)2D3 in human monocytes and macrophages: a mechanism for the hypercalcemia of various granulomatoses. J Clin Endocrinol Metab 1997; 82: 2222–2232. [DOI] [PubMed] [Google Scholar]
- 53. Adams JS, Gacad MA, Diz MM, et al. A role for endogenous arachidonate metabolites in the regulated expression of the 25-hydroxyvitamin D-1-hydroxylation reaction in cultured alveolar macrophages from patients with sarcoidosis. J Clin Endocrinol Metab 1990; 70: 595–600. [DOI] [PubMed] [Google Scholar]
- 54. Lawrence EC, Berger MB, Brousseau KP, et al. Elevated serum levels of soluble interleukin-2 receptors in active pulmonary sarcoidosis: relative specificity and association with hypercalcemia. Sarcoidosis 1987; 4: 87–93. [PubMed] [Google Scholar]
- 55. Logan T, Bensadoun E. Increased disease activity in a patient with sarcoidosis after high dose interleukin 2 treatment for metastatic renal cancer. Thorax 2005; 60: 610–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yokomura K, Suda T, Sasaki S, et al. Increased expression of the 25-hydroxyvitamin D(3)-1alpha-hydroxylase gene in alveolar macrophages of patients with lung cancer. J Clin Endocrinol Metab 2003; 88: 5704–5709. [DOI] [PubMed] [Google Scholar]
- 57. Stoffels K, Overbergh L, Giulietti A, et al. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res 2006; 21: 37–47. [DOI] [PubMed] [Google Scholar]
- 58. Unsal A, Basturk T, Koc Y, et al. Renal sarcoidosis with normal serum vitamin D and refractory hypercalcemia. Int Urol Nephrol 2013; 45: 1779–1783. [DOI] [PubMed] [Google Scholar]
- 59. Berlin JL, Palamaner Subash Shantha G, Yeager H, et al. Serum vitamin D levels may not reflect tissue-level vitamin D in sarcoidosis. BMJ Case Rep 2014. Epub ahead of print 24 March 2014 DOI: 10.1136/bcr-2014-203759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shrayyef MZ, DePapp Z, Cave WT, et al. Hypercalcemia in two patients with sarcoidosis and Mycobacterium avium intracellulare not mediated by elevated vitamin D metabolites. Am J Med Sci 2011; 342: 336–340. [DOI] [PubMed] [Google Scholar]
- 61. Falk S, Kratzsch J, Paschke R, et al. Hypercalcemia as a result of sarcoidosis with normal serum concentrations of vitamin D. Med Sci Monit Int Med J Exp Clin Res 2007; 13: CS133–CS136. [PubMed] [Google Scholar]
- 62. Basile JN, Liel Y, Shary J, et al. Increased calcium intake does not suppress circulating 1,25-dihydroxyvitamin D in normocalcemic patients with sarcoidosis. J Clin Invest 1993; 91: 1396–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stern PH, De Olazabal J, Bell NH. Evidence for abnormal regulation of circulating 1 alpha,25-dihydroxyvitamin D in patients with sarcoidosis and normal calcium metabolism. J Clin Invest 1980; 66: 852–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hamada K, Nagai S, Tsutsumi T, et al. Ionized calcium and 1,25-dihydroxyvitamin D concentration in serum of patients with sarcoidosis. Eur Respir J 1998; 11: 1015–1020. [DOI] [PubMed] [Google Scholar]
- 65. Adams JS, Gacad MA, Anders A, et al. Biochemical indicators of disordered vitamin D and calcium homeostasis in sarcoidosis. Sarcoidosis 1986; 3: 1–6. [PubMed] [Google Scholar]
- 66. Alberts C, van den Berg H. Calcium metabolism in sarcoidosis. A follow-up study with respect to parathyroid hormone and vitamin D metabolites. Eur J Respir Dis 1986; 68: 186–194. [PubMed] [Google Scholar]
- 67. Krikorian A, Shah S, Wasman J. Parathyroid hormone-related protein: an unusual mechanism for hypercalcemia in sarcoidosis. Endocr Pract 2011; 17: e84–86. [DOI] [PubMed] [Google Scholar]
- 68. van Raalte DH, Goorden SM, Kemper EA, et al. Sarcoidosis-related hypercalcaemia due to production of parathyroid hormone-related peptide. BMJ Case Rep; 2015. Epub ahead of print 9 July 2015 DOI: 10.1136/bcr-2015-210189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Zeimer HJ, Greenaway TM, Slavin J, et al. Parathyroid-hormone-related protein in sarcoidosis. Am J Pathol 1998; 152: 17–21. [PMC free article] [PubMed] [Google Scholar]
- 70. Fierer J, Burton DW, Haghighi P, et al. Hypercalcemia in disseminated coccidioidomycosis: expression of parathyroid hormone-related peptide is characteristic of granulomatous inflammation. Clin Infect Dis 2012; 55: e61–e66. [DOI] [PubMed] [Google Scholar]
- 71. Pandian MR, Morgan CH, Carlton E, et al. Modified immunoradiometric assay of parathyroid hormone-related protein: clinical application in the differential diagnosis of hypercalcemia. Clin Chem 1992; 38: 282–288. [PubMed] [Google Scholar]
- 72. Bucht E, Eklund A, Toss G, et al. Parathyroid hormone-related peptide, measured by a midmolecule radioimmunoassay, in various hypercalcaemic and normocalcaemic conditions. Acta Endocrinol (Copenh) 1992; 127: 294–300. [DOI] [PubMed] [Google Scholar]
- 73. Heijckmann AC, Huijberts MSP, De Vries J, et al. Bone turnover and hip bone mineral density in patients with sarcoidosis. Sarcoidosis Vasc 2007; 24: 51–58. [PubMed] [Google Scholar]
- 74. Heijckmann AC, Drent M, Dumitrescu B, et al. Progressive vertebral deformities despite unchanged bone mineral density in patients with sarcoidosis: a 4-year follow-up study. Osteoporos Int 2008; 19: 839–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Bolland MJ, Wilsher ML, Grey A, et al. Bone density is normal and does not change over 2 years in sarcoidosis. Osteoporos Int 2015; 26: 611–616. [DOI] [PubMed] [Google Scholar]
- 76. Bours S, de Vries F, van den Bergh JPW, et al. Risk of vertebral and non-vertebral fractures in patients with sarcoidosis: a population-based cohort. Osteoporos Int 2016; 27: 1603–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Oshagbemi OA, Driessen JHM, Pieffers A, et al. Use of systemic glucocorticoids and the risk of major osteoporotic fractures in patients with sarcoidosis. Osteoporos Int 2017; 28: 2859–2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Saidenberg-Kermanac’h N, Semerano L, Nunes H, et al. Bone fragility in sarcoidosis and relationships with calcium metabolism disorders: a cross sectional study on 142 patients. Arthritis Res Ther 2014; 16: R78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bolland MJ, Wilsher ML, Grey A, et al. Randomised controlled trial of vitamin D supplementation in sarcoidosis. BMJ Open 2013; 3: e003562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amin S, LaValley MP, Simms RW, et al. The role of vitamin D in corticosteroid-induced osteoporosis: a meta-analytic approach. Arthritis Rheum 1999; 42: 1740–1751. [DOI] [PubMed] [Google Scholar]
- 81. Allen CS, Yeung JH, Vandermeer B, et al. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst Rev 2016; 10: CD001347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Amiche MA, Albaum JM, Tadrous M, et al. Efficacy of osteoporosis pharmacotherapies in preventing fracture among oral glucocorticoid users: a network meta-analysis. Osteoporos Int J 2016; 27: 1989–1998. [DOI] [PubMed] [Google Scholar]
- 83. Boon ES, Cozijn D, Brombacher PJ. Enhanced production of calcitriol, and hypercalcaemia in a patient with sarcoidosis provoked by daily intake of calciol. Eur J Clin Chem Clin Biochem 1993; 31: 679–681. [PubMed] [Google Scholar]
- 84. Sarathi V, Karethimmaiah H, Goel A. High-dose vitamin D supplementation precipitating hypercalcemic crisis in granulomatous disorders. Indian J Endocrinol Metab 2017; 21: 815–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sodhi A, Aldrich T. Vitamin D supplementation: not so simple in sarcoidosis. Am J Med Sci 2016; 352: 252–257. [DOI] [PubMed] [Google Scholar]
- 86. Capolongo G, Xu LHR, Accardo M, et al. Vitamin-D status and mineral metabolism in two ethnic populations with sarcoidosis. J Investig Med 2016; 64: 1025–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kiani A, Abedini A, Adcock IM, et al. Association between vitamin D deficiencies in sarcoidosis with disease activity, course of disease and stages of lung involvements. J Med Biochem 2018; 37: 103–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Bansal AS, Bruce J, Hogan PG, et al. An assessment of peripheral immunity in patients with sarcoidosis using measurements of serum vitamin D3, cytokines and soluble CD23. Clin Exp Immunol 1997; 110: 92–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kavathia D, Buckley JD, Rao D, et al. Elevated 1,25-dihydroxyvitamin D levels are associated with protracted treatment in sarcoidosis. Respir Med 2010; 104: 564–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Infante JR, Pacheco C, Torres-Avisbal M, et al. Pulmonary activity in sarcoidosis: 67 Ga uptake quantification and plasma determination of 1,25-dihydroxyvitamin D. Rev Esp Med Nucl 2002; 21: 275–280. [DOI] [PubMed] [Google Scholar]
- 91. Sharma OP. Hypercalcemia in granulomatous disorders: a clinical review. Curr Opin Pulm Med 2000; 6: 442–447. [DOI] [PubMed] [Google Scholar]
- 92. Burke RR, Rybicki BA, Rao DS. Calcium and vitamin D in sarcoidosis: how to assess and manage. Semin Respir Crit Care Med 2010; 31: 474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Adams JS, Sharma OP, Diz MM, et al. Ketoconazole decreases the serum 1,25-dihydroxyvitamin D and calcium concentration in sarcoidosis-associated hypercalcemia. J Clin Endocrinol Metab 1990; 70: 1090–1095. [DOI] [PubMed] [Google Scholar]
- 94. Bia MJ, Insogna K. Treatment of sarcoidosis-associated hypercalcemia with ketoconazole. Am J Kidney 1991; 18: 702–705. [DOI] [PubMed] [Google Scholar]
- 95. Barré PE, Gascon-Barré M, Meakins JL, et al. Hydroxychloroquine treatment of hypercalcemia in a patient with sarcoidosis undergoing hemodialysis. Am J Med 1987; 82: 1259–1262. [DOI] [PubMed] [Google Scholar]
- 96. O’Leary TJ, Jones G, Yip A, et al. The effects of chloroquine on serum 1,25-dihydroxyvitamin D and calcium metabolism in sarcoidosis. N Engl J Med 1986; 315: 727–730. [DOI] [PubMed] [Google Scholar]
- 97. Thumfart J, Müller D, Rudolph B, et al. Isolated sarcoid granulomatous interstitial nephritis responding to infliximab therapy. Am J Kidney 2005; 45: 411–414. [DOI] [PubMed] [Google Scholar]
- 98. Huffstutter JG, Huffstutter JE. Hypercalcemia from sarcoidosis successfully treated with infliximab. Sarcoidosis Vasc 2012; 29: 51–52. [PubMed] [Google Scholar]
- 99. Dwarakanathan A, Ryan WG. Hypercalcemia of sarcoidosis treated with cellulose sodium phosphate. Bone Miner 1987; 2: 333–336. [PubMed] [Google Scholar]
- 100. Waron M, Weissgarten J, Gil I, et al. Sarcoid nephrocalcinotic renal failure reversed by sodium cellulose phosphate. Am J Nephrol 1986; 6: 220–223. [DOI] [PubMed] [Google Scholar]
- 101. Littlewood T, Hunter A, Beck P, et al. Treatment of hypercalcaemia in sarcoidosis with flurbiprofen. Br Med J Clin Res Ed 1983; 287: 1762–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Meyrier A, Valeyre D, Bouillon R, et al. Different mechanisms of hypercalciuria in sarcoidosis. Correlations with disease extension and activity. Ann N Y Acad Sci 1986; 465: 575–586. [DOI] [PubMed] [Google Scholar]
- 103. Fuss M, Pepersack T, Gillet C, et al. Calcium and vitamin D metabolism in granulomatous diseases. Clin Rheumatol 1992; 11: 28–36. [DOI] [PubMed] [Google Scholar]
- 104. Rizzato G, Colombo P. Nephrolithiasis as a presenting feature of chronic sarcoidosis: a prospective study. Sarcoidosis Vasc 1996; 13: 167–172. [PubMed] [Google Scholar]
- 105. Lemann J. Composition of the diet and calcium kidney stones. N Engl J Med 1993; 328: 880–882. [DOI] [PubMed] [Google Scholar]
- 106. Thomas WC. Urinary calculi in hypercalcemic states. Endocrinol Metab Clin North Am 1990; 19: 839–849. [PubMed] [Google Scholar]
- 107. Rohmer J, Hadjadj J, Bouzerara A, et al. Serum 1,25(OH)2 vitamin D and 25(OH) vitamin D ratio for the diagnosis of sarcoidosis-related uveitis. Ocul Immunol Inflamm 2018; 5: 1–7. [DOI] [PubMed] [Google Scholar]