Abstract
Background
We aimed to comprehensively explore the associations between serum 25(OH)D deficiency and risk of dementia and Alzheimer’s disease(AD).
Methods
We systematically searched Pubmed, the Cochrane Library, Embase and the reference lists of pertinent review articles for relevant articles published from database inception up until January 2019. Pooled hazard ratios (HRs) and 95% confidence intervals (CIs) were calculated with random effects models using the Stata 12.0 statistical software package.
Results
Twelve prospective cohort studies and four cross-sectional studies were included in this meta-analysis. The pooled HRs of dementia and AD, respectively, were 1.32 (95%CI: 1.16, 1.52) and 1.34 (95%CI: 1.13, 1.60) for vitamin D deficiency (< 20 ng/ml). In the subgroup analyses, the pooled HRs of dementia and AD, respectively, were 1.48 (95%CI: 1.19, 1.85) and 1.51 (95%CI: 1.04, 2.18) for moderate vitamin D deficiency (10–20 ng/ml) and 1.20 (95%CI: 0.99, 1.44) and 1.36 (95%CI: 1.01, 1.84) for severe vitamin D deficiency (< 10 ng/ml).
Conclusion
There are significant associations between vitamin D deficiency and both dementia and AD. There are stronger associations between severe vitamin D deficiency (< 10 ng/ml) and both dementia and AD compared to moderate vitamin D deficiency (10–20 ng/ml).
Keywords: Alzheimer’s disease, Dementia, Vitamin D deficiency, Meta-analysis
Background
Dementia is one of the leading causes of death and disability. Currently, approximately 48 million people around the world are suffering from dementia and this number is expected to increase 2.73 times by 2050 [1]. Almost 10 million people progress to dementia each year; the median age of diagnosis is about 80 years of age [2]. This places an enormous burden on the health system, with AD care costing about $81.8 billion each year worldwide [3]. AD is the most common type of dementia, accounting for 75% of all dementia cases [4]. Dementia is an incurable neurodegenerative disease of unknown cause. As such, many researchers have focused on exploring relevant preventive interventions to delay the development of dementia. Several relevant risk factors have been identified; however, the evidence for these is varied and unconvincing. In addition to other potentially modifiable risk factors for dementia, such as being overweight, smoking, diabetes mellitus, hypertension, hypercholesterolemia, and cardiovascular diseases, a potential prognostic role of vitamin D deficiency has been proposed [5]. Due to the role of vitamin D in neurotrophy, neurotransmission, neuroprotection, and neuroplasticity, it has been suggested that vitamin D deficiency may play a key role in the progression of dementia and AD [5]. Some researchers refer to vitamin D as the “forgotten neurosteroid” [6]. Organisms primarily obtain vitamin D through food intake and skin synthesis. A published clinical study estimated that there are 1 billion people worldwide with vitamin D deficiency due to a lack of exposure to sunlight [7]. In addition, people residing in regions with less sunlight need to consume more foods rich in vitamin D [8]. 7-DHC in the skin is irradiated by ultraviolet rays (solar ultraviolet B radiation of 290–315 nm) and is processed by the liver to produce 25(OH)D3, which is a stable storage and assay form of vitamin D in the body [9]. Kidney-treated 1,25-(OH)2D3 is the only active metabolite of vitamin D in organisms [9]. Enzymes involved in the synthesis and elimination of 1,25-(OH)2D3 are expressed in brain regions such as the thalamus, hippocampus, and basal ganglia, suggesting that vitamin D has both autocrine and paracrine pathways in the central nervous system [10, 11]. In addition, studies have shown that gene polymorphisms of vitamin D are associated with AD susceptibility [12]. To date, several systematic reviews and clinical trials have discussed the relationship between vitamin D deficiency and cognitive decline, but the conclusions of these studies are contradictory. A recent meta-analysis of five cohort studies showed that adequate vitamin D was associated with lower risk of dementia and AD, and the researchers excluded cross-sectional studies, with possible choice bias and publication bias. A recent meta-analysis of five cohort studies showed that adequate vitamin D was associated with lower risk of dementia and AD; the authors of this study excluded cross-sectional studies and those with possible choice bias and publication bias [13]. Another meta-analysis of 18,974 adults reported that severe vitamin D deficiency (< 10 ng/ml) increased the risk of dementia by 54% [14]. However, a recent prospective cohort study of 13,044 participants in the Middle East reported that lower concentrations of vitamin D measured during middle age were not significantly associated with faster cognitive decline during the 20-year follow-up period [15]. Another systematic review also failed to find a significant correlation between cognitive decline and plasma 25-(OH)D concentration [16]. There are several limitations of the existing literature. Previous systematic reviews are now outdated, with the many recently published studies not included in the available meta-analyses, and most published studies had insufficient data for subgroup analyses and exploration of correlations. Further, few previous meta-analyses have examined the associations between moderate vitamin D deficiency (10–20 ng/ml) and both dementia and AD. Further, the inconsistency in the results of meta-analyses may be due to differences in literature search strategies, inclusion criteria, statistical analyses, and adjustments for confounding factors. Vitamin D deficiency is common worldwide among all age subgroups. A cross-sectional analysis using Pearson’s correlations found that low vitamin D levels in the elderly and also in young people (30–60 years old) were correlated with significant declines in cognitive ability [17]. However, there has been no systematic evaluation of whether vitamin D deficiency (< 20 ng/ml) is associated with risk of development of dementia and AD. Such information would be significant for preventing the development of dementia. Therefore, the aim of this study was to estimate the associations between vitamin D deficiency and the risk of developing dementia and AD, respectively.
Methods
Search strategy
A systematic literature search of Pubmed, the Cochrane Library, and Embase was conducted to identify relevant studies published from database inception until 1 January 2019. The literature search strategy was based on the PICO principles [18]. The search included combinations of keywords relevant to “vitamin D” or “25(OH)D”, “Alzheimer’s disease” or “dementia”, and so forth. All terms within each set were combined with a Boolean operator. Boolean operations, also known as logical operations, are mathematical methods that can be applied to a literature search strategy; they are simple terms such as “OR” or “END” that are used to connect key terms. The reference lists of all retrieved articles were also manually searched to identify relevant studies that were missed by the search strategy. Only studies published in English were eligible for inclusion. All considered studies were imported into a reference management software (Endnote X·8·0·0) and duplicate publications were deleted.
Inclusion criteria and study selection
The diagnostic criteria for AD referred to the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Diseases and Related Disorders Associations (NINCDS-ADRDA) revised in the United States in 2011. The selection criteria included: (1) human subjects, (2) aged over 18 years, (3) observational (cross-sectional, case-control, longitudinal) or interventional designs with a control group, (4) blood measurement of 25(OH)D, (5) effective neuropsychological tests, (6) reported risk estimates (relative risk or hazard ratios (HRs) and corresponding 95% confidence intervals (CIs) for dementia or AD in relation to each category of serum 25(OH)D, and (7) reported dementia or AD incidence at follow-up. The exclusion criteria included: (1) studies with incomplete data (relative risk or hazard ratios for dementia not reported or study published only as an abstract), (2) studies examining other psychological, metabolic, or neurological conditions, and (3) participants with a diagnosis of dementia or cognitive disorder at baseline. Two independent authors (FL-G, RP-W) screened the titles and abstracts of all retrieved studies against pre-specified criteria. Disputes about inclusion or exclusion of a study were solved by discussion to reach consensus or by involving a third reviewer.
Data extraction and assessment of study quality
Pre-designed, standardized data extraction forms were developed to capture pertinent information from each included study. The collected information included: the first author’s name, year of publication, country, study design, mean age, follow-up duration, gender, adjusted factors, assessment method for serum 25(OH)D, sample size, and reported risk estimates and 95% CIs for dementia and AD across different categories of serum 25(OH)D. If a study did not provide enough information to extract the corresponding data, the authors were contacted to try and obtain the required additional information. Two authors independently extracted the data from each study and compared the data for consistency. Risk of bias was assessed using the Cochrane risk of bias tool and NOS. Any discrepancies were resolved by group discussion to reach consensus.
Data synthesis and statistical analysis
HRs and 95% CIs were considered as measures of effect size for all studies. All statistical analyses were conducted using the Stata 12.0 software package. We followed the recommendations of the Institute of Medicine and divided serum vitamin 25(OH)D into three categories: < 10 ng/ml (severe deficiency), 10–20 ng/ml (moderate deficiency), and ≥ 20 ng/ml (non-deficient or sufficient, as the reference category). Study heterogeneity was assessed by I2 estimations. According to the Cochrane review group criteria, study heterogeneity was divided into three levels: low heterogeneity (I2 < 25%), moderate heterogeneity (I2 25–50%), and high heterogeneity (I2 > 50%). A fixed effects model was used when no statistical heterogeneity was detected; otherwise, a random effects model was used. If there is no heterogeneity among the included studies, the results of the fixed effect model and the random effects model are consistent. Relevant subgroup analyses considered APOE genes and study types. Publication bias was evaluated by Begg’s test. If the funnel plot was visually symmetric or P > 0.05, there was considered to be no publication bias. In addition, we also explored the association between risk of dementia or AD and follow-up time using meta-regression. Correlation coefficients were expressed as β. Sensitivity analysis was also performed; each study was eliminated in turn to examine the stability of the results. Significance was set at a P value less than 0.05.
Results
Study characteristics
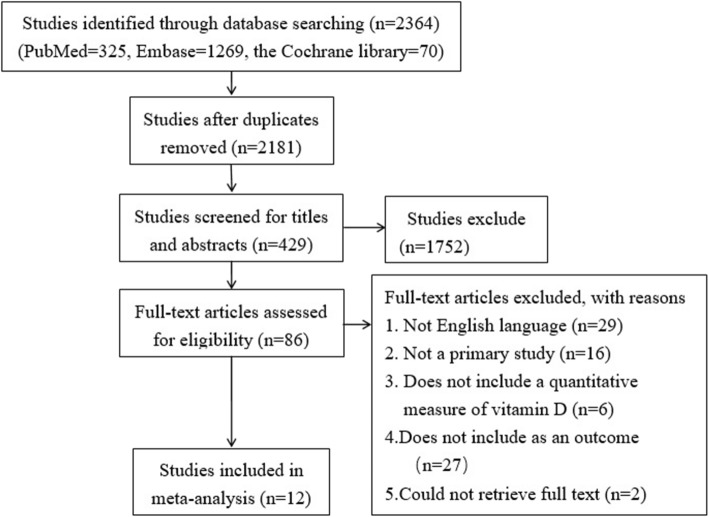
The study screening flow chart is shown in Fig. 1. Overall, the systematic searched identified 2364 potentially relevant studies, of which 183 were duplicate studies and another 1752 were irrelevant and were thus excluded during the initial screening of the title and abstract. The studies included both sexes, except one which included only men [19]. There were eight prospective cohort studies [19–26] and four cross-sectional studies [27–30]. The studies were published between 2010 and 2018 (Table 1 and Table 2). Included studies originated from Denmark, the US, Finland, France, Sweden, Germany, and the Netherlands. Review articles, studies with duplicate data, and studies not published in English were all excluded. Quality assessments indicated that all included studies were of high quality.
Fig. 1.
Flowchart of selection of studies for inclusion in the meta-analysis
Table 1.
Summary characteristics of studies included in the analysis of vitamin D deficiency and risk of AD
| Author, Publication year& country | Study Type | Sex | Age (Mean) | No.Patients (totle) | Follow-up duration (year) | 25(OH)D (ng/ml) | OR | 95% CI | Quality score | Vitamin D assessment method | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Karakis, 2016, US | Prospective cohort | W/M | 72.4 | 1663 | 9 | < 10 | 0.97 | 0.47–2.00 | 8 | Competitive protein-binding assay and radioimmunoassay | Age, gender, smoking, HTN, DM, prevalent CVD, homocysteine, BMI, and vitamin D supplement use. |
| Afzal, 2014, Denmark | Prospective cohort | W/M | 58.0 | 4087 | 21 | < 10 10–20 | 1.25 1.12 | 0.95–1.64 0.90–1.40 | 9 | Not report | Age, sex, month of blood sample, smoking status, body mass index, leisure time and work-related physical activity, alcohol consumption, income level, education, baseline diabetes mellitus, hypertension, cholesterol, high-density lipoprotein cholesterol, and creatinine |
| Littlejohns 2014, US | Prospective Cohort | W/M | 73.6 | 1547 | 5.6 | < 10 10–20 | 2.22 1.69 | 1.02–4.83 1.06–2.69 | 8 | Liquid chromatography tandemmass spectrometry (LC-MS) | Age, season of vitamin D collection, education, sex, BMI, smoking, alcohol consumption, and depressive symptoms |
| Feart, 2017, France | Prospective cohort | W/M | 73.3 | 916 | 11.4 | < 10 10–20 | 2.21 1.65 | 1.28–3.80 0.98–2.77 | 8 | One-step immunoassay | Gender, education, income, depressive symptomatology, number of drugs per day, apolipoprotein E e4 allele, BMI, practice of physical exercise, DM, history of CVD and stroke, HTN, hypercholesterolemia, hypertriglyceridemia, smoking status, and Mediterranean diet score |
| Buell, 2010, US | Cross-sectional | W/M | 73.5 | 318 | < 20 | 2.65 | 0.99–7.16 | 7 | Radioimmunoassay (DiaSorin, Inc.,Stillwater, MN, USA) on fasting blood sample | Age, race, sex, body mass index, and education, kidney function, multivitamin use, season, diabetes, hypertension, plasma homocysteine, and ApoE allele status | |
| Licher, 2017, Netherlands | Prospective cohort | W/M | 69.2 | 6087 | 13.3 | < 20 | 1.10 | 1.00–1.19 | 9 | Electrochemilumin-escence binding assay | Age, sex, season of blood collection, BMI, SBP, DBP, educational level, smoking, alcohol use, calcium serum levels, ethnicity, eGFR, TC, HDL, history of DM, HF, stroke, MI, depressive symptoms, outdoor activity, and APOE −4 carrier status. |
Table 2.
Summary characteristics of studies included in the analysis of vitamin D deficiency and risk of dementia
| Author& Publication year | Study Type | Sex | Age (Mean) | No.Patients (totle) | Follow-up duration (year) | 25(OH)D (ng/m) | OR | 95% CI | Quality score | Vitamin D assessment method | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Karakis, 2016, US | Prospective cohort | W/M | 72.4 | 1663 | 9 | < 10 | 1.06 | 0.57–1.98 | 8 | Competitive protein-binding assay and radioimmunoassay | Age, gender, smoking, HTN, DM, prevalent CVD, homocysteine, BMI, and vitamin D supplement use. |
| Knekt, 2014, Finland | Prospective cohort | W/M | 56.4 | 5010 | 17 | < 10 | 1.74 | 0.64–3.01 | 8 | Radioimmunoassay | Age, month of blood drawn, education, marital status, physical activity, smoking status, alcohol consumption, BMI, BP, FPG, serum TG, and serum TC. |
| Licher, 2017, Netherlands | Prospective cohort | W/M | 69.2 | 6087 | 13.3 |
< 10 10–20 |
1.22 1.06 | 0.97–1.52 0.90–1.26 | 9 | Electrochemiluminescence binding assay | Age, sex, season of blood collection, BMI, SBP, DBP, educational level, smoking, alcohol use, calcium serum levels, ethnicity, eGFR, TC, HDL, history of DM, HF, stroke, MI, depressive symptoms, outdoor activity, and APOE-4 carrier status. |
| Schneider, 2014, US | Prospective cohort | W/M | 62.0 | 1652 | 16.6 | < 10 10–20 | 1.53 1.22 | 0.84–2.79 0.68–2.19 | 8 | Liquid chromatography-tandem mass spectrometry | Age, sex, education, income, physical activity, smoking, alcohol use, BMI, WC, and vitamin D supplementation. |
| Feart,2017, France | Prospective cohort | W/M | 73.3 | 916 | 11.4 | < 10 ng/ml 10–20 | 2.96 | 1.43–6.11 | 8 | One-step immunoassay | Gender, education, income, |
| ng/ml | 2.29 | 1.14–4.58 | depressive symptomatology, number of drugs per day, apolipoprotein E e4 allele, BMI, practice of physical exercise, DM, history of CVD and stroke, HTN, hypercholesterolemia, hypertriglyceridemia, smoking status, and Mediterranean diet score | ||||||||
| Olsson, 2017, Sweden | Prospective Cohort | M | 71.0 | 1182 | 12 | < 10 10–20 | 1.22 1.06 | 0.97–1.52 0.90–1.26 | 8 | HPLC atmospheric pressure chemical ionization-mass spectrometry | Age, season of blood collection, BMI, education, physical activity, smoking, DM, HTN, hypercholesterolemia, use of vitamin D supplements, and alcohol intake. |
| Littlejohns, 2014,US | Prospective Cohort | W/M | 73.6 | 1615 | 5.6 | < 10 10–20 | 2.25 1.53 | 1.23–4.13 1.06–2.21 | 9 | Liquid chromatography tandemmass spectrometry (LC-MS) | Age, season of vitamin D collection, education, sex, BMI, smoking, alcohol consumption, and depressive symptoms |
| Annweiler, 2011, France | Cross-sectional | W/M | 86.0 | 288 | Not report | < 10 | 2.57 | 1.05–6.27 | 7 | Radioimmunoassay (DiaSorin, Inc.,Stillwat er, MN,USA) on fastingblood sample | Fully adjusted but without detailed information |
| Nagel, 2015, Germany | Cross-sectional | M/W | 75.6 | 1373 | Not report | < 20 | 1.08 | 1.06–2.21 | 8 | ELISA(ImmunodiagnosticSystems Inc., Fountain Hills, AZ,USA) | Adjusted for age, sex, school education, smoking status, season, alcohol consumption, BMI, and history of depression |
| Buell, 2010, USA | Cross-sectional | M/W | 73.5 | 318 | Not report | < 20 | 2.21 | 1.13–4.32 | 7 | Radioimmunoassay (DiaSorin, Inc., Stillwater, MN,USA) on fasting blood sample | Age, race, sex, body mass index, and education, kidney function, multivitamin use, season, diabetes, hypertension, plasma homocysteine, and ApoE allele status |
| Nourhashemi,2018,French | Cross –sectional | M/W | 76.2 | 1680 | Not report | < 20 | 1.038 | 0.421–2.557 | 9 | a commercially available electro-chemiluminescencecompetitive binding assay | gender, BMI, season of blood collection, educational level, and ApoE ε4 genotype |
Vitamin D status and risk of dementia
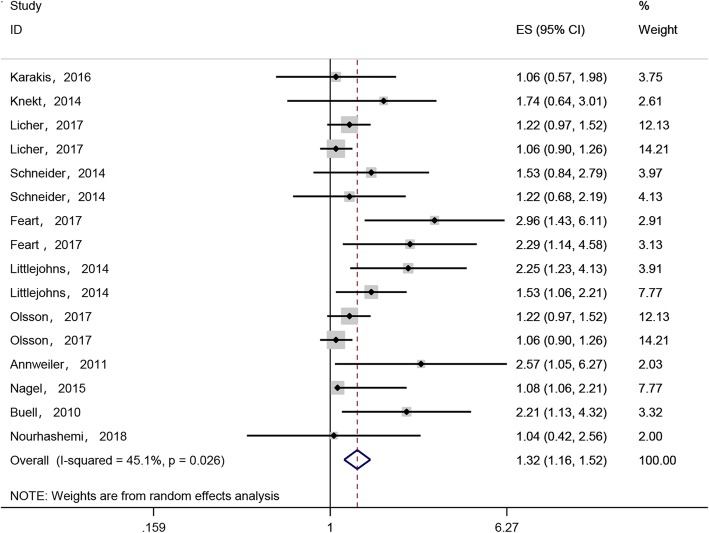
Eleven studies [18–24, 26–29] involving a total of 21,784 participants evaluated the relationship between vitamin D deficiency and dementia. We conducted a meta-analysis (random effects model) to derive a pooled estimate of the association between vitamin D deficiency (< 20 ng/ml) and the risk of dementia. The pooled HR estimate of the risk of dementia for those with vitamin D deficiency (< 20 ng/ml) relative to those with vitamin D levels in the normal range (≥20 ng/ml) was 1.32 (95% CI: 1.16, 1.52, I2 = 45.1%, n = 16, Fig. 2). Severe vitamin D deficiency (< 10 ng/ml) was associated with a high risk of dementia after pooling the results of the subgroup analysis (pooled HR: 1.48, 95%CI: 1.19, 1.85, I 2 = 41.4%, n = 8, Additional file 1: Fig. S4). However, the pooled results for moderate vitamin D deficiency (10–20 ng/ml) showed little association with dementia (pooled HR: 1.20, 95%CI: 0.99, 1.44, I2 = 48.3%, n = 5, Additional file 1: Fig. S4). The I2 value between 25 and 50% indicates evidence of moderate heterogeneity among the studies. In the subgroup analyses, a more significant association between vitamin D deficiency and dementia was observed in the subgroup of studies that considered the serum 25(OH)D APOE gene compared to studies that did not consider the APOE gene (pooled HRs: 1.47, 95%CI: 1.10, 1.98, I2 = 65.9%, n = 6 studies vs. 1.28, 95%CI: 1.10, 1.49, I 2 = 29.1%, n = 10 studies, respectively, Additional file 1: Fig. S6). Similarly, a more significant association was observed in cohort studies compared to cross-sectional studies (pooled HRs: 1.30, 95%CI: 1.13, 1.50, I 2 = 47.5%, n = 12 vs. 1.32, 95%CI: 1.16, 1.52, I2 = 48.4%, n = 4, respectively, Additional file 1: Fig. S8). These subgroup differences appear to be the sources of heterogeneity among the pooled studies. Moreover, meta-regression analysis showed a correlation between dementia risk and follow-up time (P = 0.03; β = 0.90, 95% CI, 0.88 to 1.01). This suggests that follow-up time could account for 90% of the heterogeneity among the studies. When removing each study in turn, the association between vitamin D deficiency and dementia ranged from 1.27 (95%CI: 1.12, 1.43) with the exclusion of the prospective cohort study by Littlejohns et al. [24] to 1.38 (95%CI: 1.19, 1.61) with the exclusion of the cross-sectional study by Annweiler et al. [26] (Additional file 1: Table S3). The sensitivity analysis indicated that omission of any one of the studies did not alter the magnitude of the observed effect, suggesting stability of our findings.
Fig. 2.
HRs of association between dementia and vitamin D deficiency (serum 25(OH)D < 20 ng/ml). The size of each square is proportional to the study’s weight. The estimated pooled HR was 1.32 (95%CI, 1.16 to 1.52) with high statistical significance (P < 0.0001). There was moderate heterogeneity among the studies (I2 = 45.1%)
Vitamin D status and risk of AD
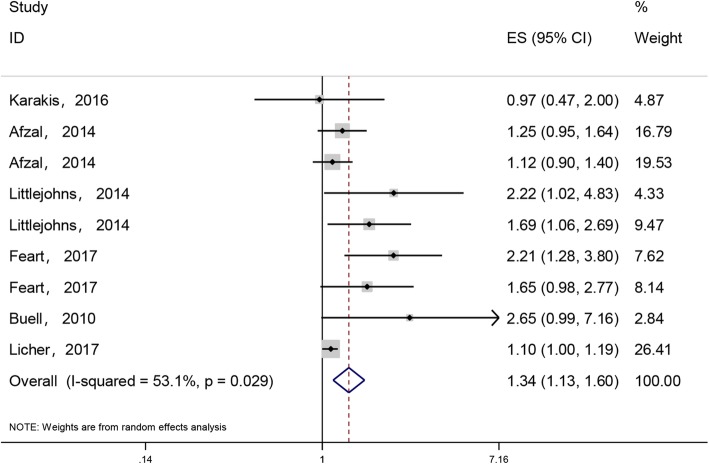
Six studies [19, 21, 23–25, 28] involving a total of 14,618 participants evaluated the relationship between vitamin D deficiency and AD. We performed a meta-analysis (random effects model) to derive a summary estimate of AD risk associated with vitamin D deficiency. The meta-analysis indicated that patients with vitamin D deficiency (< 20 ng/ml) had a higher risk of AD than patients with adequate vitamin D supply (≥20 ng/ml) (pooled HR: 1.34, 95% CI: 1.13, 1.60, I2 = 53.1%, n = 9, Fig. 3). The I2 value was greater than 50%, indicating evidence of heterogeneity among the studies. Severe vitamin D deficiency(< 10 ng/ml) was associated with a high risk of AD compared to moderate vitamin D deficiency (10–20 ng/ml) after pooling the results in the subgroup analysis (pooled HRs: 1.51, 95%CI: 1.04, 2.18, I2 = 47.4%, n = 4 vs. 1.36, 95%CI: 1.01, 1.84, I2 = 39.6%, n = 3, Additional file 1: Fig. S5). The I2 value between 25 and 50% indicates moderate heterogeneity among the studies. In the subgroup analyses, a more significant association between vitamin D deficiency and AD was observed in the subgroup of studies that considered the serum 25(OH)D APOE gene compared to studies that did not consider the APOE gene (pooled HRs: 1.34, 95%CI: 1.13, 1.60, I2 = 72.9%, n = 5 vs. 1.27, 95%CI:1.05, 1.54, I2 = 28.1, n = 4, respectively). Moreover, meta-regression analysis indicated an association between dementia risk and follow-up time (P = 0.02; β = 0.89, 95% CI, 0.87 to 1.06). 11This suggests that follow-up time could account for 89% of the heterogeneity among the studies. In the sensitivity analysis, with removal of each study in turn, the association between vitamin D deficiency and AD ranged from 1.13 (95%CI: 1.10, 1.55) with the exclusion of the cross-sectional study by Bull et al. [28] to 1.46 (95%CI: 1.18, 1.80) with the exclusion of the prospective cohort study by Licher [21] (Additional file 1: Table S4). As a result of the sensitivity analysis, one of the studies[28]was found to be the heterogeneous source of this review after each study was excluded in sequence.
Fig. 3.
HRs of association between AD and vitamin D deficiency (serum 25(OH)D < 20 ng/ml). The size of each square is proportional to the study’s weight. The estimated pooled HR was 1.34 (95%CI, 1.13 to 1.60) with high statistical significance (P < 0.0001). There was high heterogeneity among the studies (I2 = 53.1%)
Publishing bias
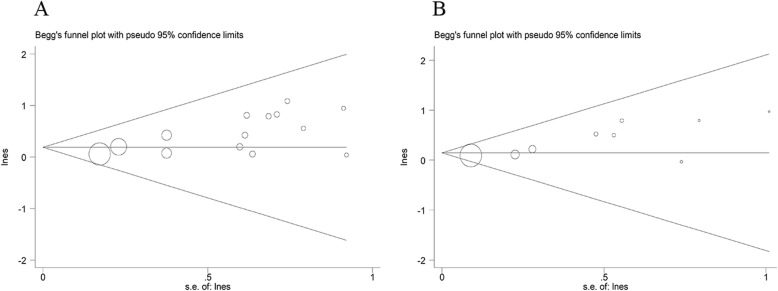
The funnel plot was symmetrical indicating no evidence of publication bias (Fig. 4).
Fig. 4.
Publication bias for studies examining the associations between vitamin D deficiency (serum 25(OH)D < 20 ng/ml) and dementia and AD. The size of each circle is proportional to the study’s weight. The Begg’s tests for the incidence of dementia (A) and AD (B) were roughly symmetrical (P(dementia) = 0.061, P(AD) = 0.076, greater than 0.05)
Discussion
The current meta-analysis provided additional evidence of relationships between vitamin D deficiency and risk of dementia and AD. The present analysis demonstrated positive associations between vitamin D deficiency (< 20 ng/ml) and risk of dementia (by 32%) and AD (by 34%). These results demonstrated that vitamin D deficiency may be a risk factor for dementia or AD. Furthermore, the incidence of AD was higher than dementia when vitamin D was deficient. The subgroup analyses indicated that vitamin D severe deficiency was associated with a greater risk of dementia (by 48%) and AD (by 51%) compared to vitamin D moderate deficiency (by 20 and 36%, respectively), indicating that the risk of dementia and AD was reduced with increased vitamin D. It also suggested that the incidence of AD may be higher than dementia in either mild or moderate vitamin D deficiency. In addition, dementia-related genes and several confounding factors were potential sources of the heterogeneity among the studies. The subgroup of considering the APOE gene was more heterogeneous than the without considering APOE gene. This may reflect different study quality or selectiveness of recruitment. Meta-regression showed that follow-up time may be a source of heterogeneity, which may be related to inconsistencies in the follow-up time of each included study. One study included only male cases and may be a source of potential heterogeneity. A recent review proposed calcium homeostasis as the underlying factor, suggesting that long-term deficiency or inefficient utilization of vitamin D may lead to disruption of calcium homeostasis in neurons, thus causing neuronal aging and neurodegeneration vulnerability [31]. The most common causes of dementia include Lewy body dementia, depression, Alzheimer’s disease, and mild cognitive impairment. Other dementias are less common. AD is the most common neurodegenerative disorder in older people and leads to progressive cognitive impairment. Dementia and AD lead to decreased function and dependence on others for daily life activities; this places a significant financial burden on the health care system. Recent data suggests a possible association between vitamin D deficiency and cognitive dysfunction [32]. Current studies have not fully established the pathophysiological mechanisms underlying the potential effects of vitamin D levels on dementia and AD risk, but several possible mechanisms have been proposed. The potential pathological mechanisms that have been identified to date and that are used to diagnose clinical cognitive dysfunction include Aβ deposition and tau protein tangles in the brain [33]. Animal studies have shown that vitamin D can promote the clearance of Aβ and vitamin D deficiency leads to an increase in Aβ in the brain [34]. In human studies, it has been shown that vitamin D causes an increase in plasma Aβ, especially in the elderly, suggesting a decrease in brain Aβ [35]. In addition, vitamin D modulates the voltage-gated calcium channel targeted by Aβ peptides, indicating that vitamin D can repair neuronal calcium homeostasis altered by Aβ peptides [36]. Finally, vitamin D can prevent glutamate neurotoxicity by upregulating VDR expression and exerting antioxidant effects [12]. AD is a degenerative disease characterized by cognitive and functional decline. Due to their loss of autonomy, it is difficult for patients to receive enough sunlight exposure in order to synthesize a sufficient amount of vitamin D. Similarly, it can be difficult for these patients to eat a sufficient amount of foods rich in vitamin D [37]. This exogenous vitamin D restriction could subsequently lead to low serum vitamin D concentrations in AD patients. Compared with previous reviews, we considered the relationships between different levels of vitamin D and both dementia and AD, and also considered the effects of dementia-related genes and follow-up duration. Compared with previous reviews, our meta-analysis included recently published studies and contained sufficient data for subgroup analysis and exploration of various associations. Thus, this study can provide comprehensive and systematic evidence for clinical adjuvant treatment of dementia and AD. Furthermore, due to small numbers of included studies (< 10) and failure to investigate publication bias, previous reviews may have overestimated the associations between vitamin D deficiency and risk of dementia and AD. Previous meta-analyses have shown that the measures used to assess the relationship between vitamin D and dementia are relatively simple. This may create a bias in the literature as the measures used in the published literature cover less than 30% of the available neuropsychological tests [38]. When all neuropsychological tests were included, despite the increased heterogeneity, we found a significant association between vitamin D deficiency and dementia. In recent years, genes associated with dementia and AD, such as APOE, have received increased research attention. As such, we considered the possible role of genetic factors in this meta-analysis [39].The overall effect size should be interpreted with caution due to the presence of known or unknown confounding factors. The use of a random effects meta-analysis model in this study compensated for the heterogeneity among the studies. It should also be noted that heterogeneity was markedly reduced in our analysis as compared to previous studies; this could reflect the more stringent inclusion criteria. Finally, the significant pooled effect in the current study suggests that these methodological factors were advantageous in our meta-analysis, conferring greater clinical applicability of the findings and greater generalizability of the association between vitamin D deficiency and dementia. When interpreting the current results, the heterogeneity among the included studies should be considered. First, none of the included studies described the methods of collection or duration of preservation of collected serum before 25(OH)D assay. The effects of light, temperature, collection tube properties, and long-term serum storage on assay results are uncertain, particularly with respect to 25(OH)D stability [40]. Second, the methods for determining the concentration of 25(OH)D among the studies were different. The choice of method is crucial since IDEQAS reported differences in results for identical blood samples as a function of method chosen. Therefore, harmonization of techniques seems to be necessary. The most commonly used vitamin D reference assay is the LC-MS assay [41]. However, even inter-laboratory variability in measurements with this technique is unavoidable. Further, since conventional LC-MS analysis may not be able to distinguish molecules of the same mass as 25(OH)D3 and may not be able to distinguish among molecules with similar fragmentation patterns, the concentration of 25(OH)D is often overestimated [41]. In contrast, radioimmunoassay, which is a better analytical method than LC-MS, exhibits good inter-rater reliability and can determine the concentrations of 25(OH)D2 and 25(OH)D3 simultaneously [42]. It appears to be the most economical technology for standardization in future research [43]. Third, none of the included studies tested available vitamin D; however, several studies have shown that available vitamin D may be a more reliable marker of vitamin D status than the total amount of 25(OH)D [44]. Fourth, vitamin D needs to be metabolized by the kidneys to produce active 1,25-(OH)2D3; thus, chronic kidney disease may affect the metabolism of vitamin D. However, only one study considered chronic kidney disease in the multivariate analysis [28]. Fifth, the confounding influence of homocysteine was only adjusted for in a few of the included studies [19, 28]. Excessive homocysteine increases the risk of endothelial dysfunction and increases the cardiovascular risk, which directly contributes to the development of dementia and AD by promoting neurodegenerative changes [45]. Therefore, failure to adjust for this confounding factor may result in biased conclusions. Finally, in the present review, because cross-sectional studies are affected by reverse causality bias, it is not possible to determine whether low levels of vitamin D cause dementia. Instead, dementia may result in reduced intake of vitamin D and reduced outdoor activity with impaired sunlight exposure, both leading to vitamin D deficiency [37]. Several of the included studies improve the reliability of the results by adjusting for two confounding factors, namely, physical activity level and season of sampling [18, 20, 21, 23–25, 27, 28]. In summary, the results of this meta-analysis should be interpreted with caution due to the methodological differences and the confounding factors among the included studies. In the early stages of dementia, for example, mild cognitive impairment is more likely to benefit from therapeutic intervention; thus, more data on the association between mild cognitive impairment and vitamin D would be helpful [46]. Therefore, future prospective studies should examine the association between vitamin D deficiency and the early stages of AD and dementia (e.g., mild cognitive impairment).
Conclusions
The current meta-analysis provided additional evidence of the relationships between vitamin D deficiency (< 20 ng/ml) and risk of dementia and AD in older age people. This study found significant positive associations between vitamin D deficiency (< 20 ng/ml) and risk of dementia and AD. Subgroup analysis indicated that vitamin D moderate deficiency (< 10 ng/ml) was more strongly associated with the risk of dementia and AD compared with severe deficiency (10–20 ng/ml), indicating that the risk of dementia and AD was reduced with increased vitamin D. However, we have no clear evidence of an association between serum 25(OH)D levels > 20 ng/ml and cognitive outcomes. Further longitudinal investigations are needed to assess cognitive outcomes at higher 25(OH)D levels.
Supplementary information
Additional file 1: Appendix 1. Included search strategies, sensitivity analysis tables, and subgroup analyses Appendix 2. Sensitivity analysis supplementary table for systematic reviews. Table S3. Sensitivity analysis of AD associated with vitamin D Deficiency (serum 25(OH)D < 20 ng/ml). Table S4 Sensitivity analysis of AD associated with vitamin D Deficiency (serum 25(OH)D < 20 ng/ml). Appendix 3. Sensitivity analysis supplementary table for systematic reviews. Figure S4. HRs of AD associated with different levels of vitamin D deficiency. Figure S5. HRs of AD associated with different levels of vitamin D deficiency. Figure S6. HRs of dementia that considered the APOE gene of serum 25(OH)D compared to without considering the APOE gene. Figure S7. HRs of AD that considered the APOE gene of serum 25(OH)D compared to without considering the APOE gene. Figure S8. HRs of dementia associated with vitamin D Deficiency that cohort studies compared to cross-sectional studies.
Acknowledgements
None.
Consent to publish
Not applicable.
Abbreviations
- 25(OH)D
25-dihydroxyvitamin D
- 7-DHC
7-dehydrocholesterol
- AD
Alzheimer’s disease
- APOE
Apolipoprotein E
- Aβ
beta-amyloid
- BMI
Body mass index
- CI
Confidence interval
- CI
Confidence interval
- ELISA
Enzyme linked immunosorbent assay
- HR
hazard ratio
- IDEQAS
International Vitamin D External Quality Assessment Scheme
- LC-MS
liquid chromatography-tandem mass spectrometry
- NOS
Newcastle-Ottawa Scale
- PICO
Patient intervention comparison outcome
- US
United States
- VDR
vitamin D recepter
Authors’ contributions
BYC conceived and designed the entire study; FLG and RPW analyzed the data; QRL and CG performed literature research and statistical analysis; YZ and TD drafted the paper. YZ supervised the entire study and revised the manuscript before submission. All authors have read and agreed with the final version of this manuscript.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Availability of data and materials
All data analyzed during this study are included in this article.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bingyan Chai, Email: 2279262895@qq.com.
Fulin Gao, Email: gaofulin990@hotmail.com.
Ruipeng Wu, Email: wuwenli488@163.com.
Tong Dong, Email: dongtong03@163.com.
Cheng Gu, Email: gucheng408@163.com.
Qiaoran Lin, Email: 723763848@qq.com.
Yi Zhang, Email: zhangyi9310@21cn.com.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12883-019-1500-6.
References
- 1.Haapasalo A, Hiltunen M. A report from the 8th Kuopio Alzheimer symposium. Neurodegener Dis Manag. 2018;8(5):289–299. doi: 10.2217/nmt-2018-0029. [DOI] [PubMed] [Google Scholar]
- 2.Wolters FJ, Ikram MA. Erratum to: epidemiology of dementia: the burden on society, the challenges for research. Methods Mol Biol. 2018;1750:E3. doi: 10.1007/978-1-4939-7704-8_27. [DOI] [PubMed] [Google Scholar]
- 3.Kelley AS, et al. The burden of health care costs for patients with dementia in the last 5 years of life. Ann Intern Med. 2015;163(10):729–736. doi: 10.7326/M15-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiu C, Kivipelto M, von Strauss E. Epidemiology of Alzheimer's disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci. 2009;11(2):111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anjum I, et al. The role of vitamin D in brain health: a mini literature review. Cureus. 2018;10(7):e2960. doi: 10.7759/cureus.2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prabhakar P, et al. Vitamin D status and vascular dementia due to cerebral small vessel disease in the elderly Asian Indian population. J Neurol Sci. 2015;359(1–2):108–111. doi: 10.1016/j.jns.2015.10.050. [DOI] [PubMed] [Google Scholar]
- 7.Owusu JE, et al. Cognition and vitamin D in older African-American women- physical performance and osteoporosis prevention with vitamin D in older African Americans trial and dementia. J Am Geriatr Soc. 2019;67(1):81–86. doi: 10.1111/jgs.15607. [DOI] [PubMed] [Google Scholar]
- 8.Mithal A, et al. Global vitamin D status and determinants of hypovitaminosis D. Osteoporos Int. 2009;20(11):1807–1820. doi: 10.1007/s00198-009-0954-6. [DOI] [PubMed] [Google Scholar]
- 9.Jones G. Extrarenal vitamin D activation and interactions between vitamin D(2), vitamin D(3), and vitamin D analogs. Annu Rev Nutr. 2013;33:23–44. doi: 10.1146/annurev-nutr-071812-161203. [DOI] [PubMed] [Google Scholar]
- 10.Gomes FP, Shaw PN, Hewavitharana AK. Determination of four sulfated vitamin D compounds in human biological fluids by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1009-1010:80–86. doi: 10.1016/j.jchromb.2015.12.014. [DOI] [PubMed] [Google Scholar]
- 11.Kattner L, Bernardi D. An efficient synthesis of 1alpha,25-dihydroxyvitamin D3 LC-biotin. J Steroid Biochem Mol Biol. 2017;173:89–92. doi: 10.1016/j.jsbmb.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 12.Supriya M, et al. Vitamin D receptor (VDR) gene polymorphism and vascular dementia due to cerebral small vessel disease in an Asian Indian cohort. J Neurol Sci. 2018;391:84–89. doi: 10.1016/j.jns.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 13.Jayedi, A., A. Rashidy-Pour, and S. Shab-Bidar, Vitamin D status and risk of dementia and Alzheimer's disease: A meta-analysis of dose-response. Nutr Neurosci, 2018: p. 1–10. [DOI] [PubMed]
- 14.Sommer I, et al. Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):16. doi: 10.1186/s12877-016-0405-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider ALC, et al. Serum vitamin D concentrations and cognitive change over 20 years: the atherosclerosis risk in communities neurocognitive study. Neuroepidemiology. 2018;51(3–4):131–137. doi: 10.1159/000490912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnard K, Colon-Emeric C. Extraskeletal effects of vitamin D in older adults: cardiovascular disease, mortality, mood, and cognition. Am J Geriatr Pharmacother. 2010;8(1):4–33. doi: 10.1016/j.amjopharm.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Darwish H, et al. Serum 25-hydroxyvitamin D predicts cognitive performance in adults. Neuropsychiatr Dis Treat. 2015;11:2217–2223. doi: 10.2147/NDT.S87014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chene G, Wittkop L, Saves M. Evidence-based medicine: basic principles. Rev Prat. 2017;67(8):903–907. [PubMed] [Google Scholar]
- 19.Olsson E, et al. Vitamin D is not associated with incident dementia or cognitive impairment: an 18-y follow-up study in community-living old men. Am J Clin Nutr. 2017;105(4):936–943. doi: 10.3945/ajcn.116.141531. [DOI] [PubMed] [Google Scholar]
- 20.Karakis I, et al. Association of Serum Vitamin D with the risk of incident dementia and subclinical indices of brain aging: the Framingham heart study. J Alzheimers Dis. 2016;51(2):451–461. doi: 10.3233/JAD-150991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knekt P, et al. Serum 25-hydroxyvitamin d concentration and risk of dementia. Epidemiology. 2014;25(6):799–804. doi: 10.1097/EDE.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 22.Licher S, et al. Vitamin D and the risk of dementia: the Rotterdam study. J Alzheimers Dis. 2017;60(3):989–997. doi: 10.3233/JAD-170407. [DOI] [PubMed] [Google Scholar]
- 23.Schneider AL, et al. Vitamin D and cognitive function and dementia risk in a biracial cohort: the ARIC brain MRI study. Eur J Neurol. 2014;21(9):1211–1218. doi: 10.1111/ene.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feart C, et al. Associations of lower vitamin D concentrations with cognitive decline and long-term risk of dementia and Alzheimer's disease in older adults. Alzheimers Dement. 2017;13(11):1207–1216. doi: 10.1016/j.jalz.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Littlejohns TJ, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83(10):920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzal S, Bojesen SE, Nordestgaard BG. Reduced 25-hydroxyvitamin D and risk of Alzheimer's disease and vascular dementia. Alzheimers Dement. 2014;10(3):296–302. doi: 10.1016/j.jalz.2013.05.1765. [DOI] [PubMed] [Google Scholar]
- 27.Nagel G, et al. Serum vitamin D concentrations and cognitive function in a population-based study among older adults in South Germany. J Alzheimers Dis. 2015;45(4):1119–1126. doi: 10.3233/JAD-143219. [DOI] [PubMed] [Google Scholar]
- 28.Annweiler C, et al. 25-hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;75(1):95. doi: 10.1212/WNL.0b013e3181e00ddb. [DOI] [PubMed] [Google Scholar]
- 29.Buell JS, et al. 25-Hydroxyvitamin D, dementia, and cerebrovascular pathology in elders receiving home services. Neurology. 2010;74(1):18–26. doi: 10.1212/WNL.0b013e3181beecb7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nourhashemi F, et al. Cross-sectional associations of plasma vitamin D with cerebral beta-amyloid in older adults at risk of dementia. Alzheimers Res Ther. 2018;10(1):43. doi: 10.1186/s13195-018-0371-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gezen-Ak Duygu, Dursun Erdinç. Molecular basis of vitamin D action in neurodegeneration: the story of a team perspective. Hormones. 2018;18(1):17–21. doi: 10.1007/s42000-018-0087-4. [DOI] [PubMed] [Google Scholar]
- 32.Balion C, et al. Vitamin D, cognition, and dementia: a systematic review and meta-analysis. Neurology. 2012;79(13):1397–1405. doi: 10.1212/WNL.0b013e31826c197f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veerhuis R, Nielsen HM, Tenner AJ. Complement in the brain. Mol Immunol. 2011;48(14):1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Durk MR, et al. 1alpha,25-Dihydroxyvitamin D3 reduces cerebral amyloid-beta accumulation and improves cognition in mouse models of Alzheimer's disease. J Neurosci. 2014;34(21):7091–7101. doi: 10.1523/JNEUROSCI.2711-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller BJ, Whisner CM, Johnston CS. Vitamin D supplementation appears to increase plasma Abeta40 in vitamin D insufficient older adults: a pilot randomized controlled trial. J Alzheimers Dis. 2016;52(3):843–847. doi: 10.3233/JAD-150901. [DOI] [PubMed] [Google Scholar]
- 36.Gunn AP, et al. Amyloid-beta peptide Abeta3pE-42 induces lipid peroxidation, membrane Permeabilization, and calcium influx in neurons. J Biol Chem. 2016;291(12):6134–6145. doi: 10.1074/jbc.M115.655183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Onojighofia Tobore T. On the Etiopathogenesis and pathophysiology of Alzheimer's disease: a comprehensive theoretical review. J Alzheimers Dis. 2019. [DOI] [PubMed]
- 38.Aghajafari F, et al. Quality assessment of systematic reviews of vitamin D, cognition and dementia. BJPsych Open. 2018;4(4):238–249. doi: 10.1192/bjo.2018.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oliveira ACR, et al. BsmI polymorphism in the vitamin D receptor gene is associated with 25-hydroxy vitamin D levels in individuals with cognitive decline. Arq Neuropsiquiatr. 2018;76(11):760–766. doi: 10.1590/0004-282x20180116. [DOI] [PubMed] [Google Scholar]
- 40.Goring H. Vitamin D in nature: a product of synthesis and/or degradation of cell membrane components. Biochemistry (Mosc) 2018;83(11):1350–1357. doi: 10.1134/S0006297918110056. [DOI] [PubMed] [Google Scholar]
- 41.Wan D, et al. A new sensitive LC/MS/MS analysis of vitamin D metabolites using a click derivatization reagent, 2-nitrosopyridine. J Lipid Res. 2017;58(4):798–808. doi: 10.1194/jlr.D073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fabregat-Cabello N, et al. A fast and simple method for simultaneous measurements of 25(OH)D, 24,25(OH)2D and the vitamin D metabolite ratio (VMR) in serum samples by LC-MS/MS. Clin Chim Acta. 2017;473:116–123. doi: 10.1016/j.cca.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 43.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu S, et al. The high prevalence of hypovitaminosis D in China: a multicenter vitamin D status survey. Medicine (Baltimore) 2015;94(8):e585. doi: 10.1097/MD.0000000000000585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, et al. Associations between Alzheimer's disease and blood homocysteine, vitamin B12, and folate: a case-control study. Curr Alzheimer Res. 2015;12(1):88–94. doi: 10.2174/1567205012666141218144035. [DOI] [PubMed] [Google Scholar]
- 46.Al-Amin M, et al. Vitamin D deficiency is associated with reduced hippocampal volume and disrupted structural connectivity in patients with mild cognitive impairment. Hum Brain Mapp. 2019;40(2):394–406. doi: 10.1002/hbm.24380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Appendix 1. Included search strategies, sensitivity analysis tables, and subgroup analyses Appendix 2. Sensitivity analysis supplementary table for systematic reviews. Table S3. Sensitivity analysis of AD associated with vitamin D Deficiency (serum 25(OH)D < 20 ng/ml). Table S4 Sensitivity analysis of AD associated with vitamin D Deficiency (serum 25(OH)D < 20 ng/ml). Appendix 3. Sensitivity analysis supplementary table for systematic reviews. Figure S4. HRs of AD associated with different levels of vitamin D deficiency. Figure S5. HRs of AD associated with different levels of vitamin D deficiency. Figure S6. HRs of dementia that considered the APOE gene of serum 25(OH)D compared to without considering the APOE gene. Figure S7. HRs of AD that considered the APOE gene of serum 25(OH)D compared to without considering the APOE gene. Figure S8. HRs of dementia associated with vitamin D Deficiency that cohort studies compared to cross-sectional studies.
Data Availability Statement
All data analyzed during this study are included in this article.