Main text
Pneumocystis jirovecii pneumonia (PJP) is a severe complication of immunosuppression that is associated with high mortality, depending on the underlying type of immunosuppression [1]. Consequently, the incidence of PJP is higher in non-HIV patients than in HIV patients, because of the increased use of immunosuppressive therapies for widespread indications [2]. So far, there is little evidence for veno-venous extracorporeal membrane oxygenation (ECMO) treatment in cases of PJP-induced severe acute respiratory distress syndrome (ARDS). Particularly, there is no study reporting and comparing the outcome of PJP requiring ECMO therapy in HIV and non-HIV patients.
Therefore, we report retrospective data of a single-centre registry of patients with severe respiratory failure, requiring ECMO support at our centre between January 2009 and April 2019. ECMO support was initiated when lung-protective mechanical ventilation was not able to prevent hypoxemia or hypercapnia, based on the treating medical team’s judgement.
A total of 337 ECMO patients were screened, and 18 patients with PJP were identified (Table 1). Diagnosis of PJP was verified via positive immunofluorescence microscopy in 13 patients (72%). Five patients (28%) displayed high PCR levels (median 67.000 [5.200–250.000] copies/ml) with conclusive symptoms and radiological findings but negative immunofluorescence microscopy. Microbiological testing was performed in bronchoalveolar lavage. In 14 patients (78%), PJP was diagnosed before the initiation of ECMO therapy.
Table 1.
Baseline characteristics and outcome
| All (n = 18) | HIV (n = 6) | non-HIV (n = 12) | p value | |
|---|---|---|---|---|
| Age (years) | 49.7 ± 18.4 | 36.8 ± 9.7 | 56.2 ± 18.6 | 0.032 |
| Sex (male) | 11 (61.1%) | 4 (66.7%) | 7 (58.3%) | 1.0 |
| BMI (kg/m2) | 24.6 ± 3.4 | 23.0 ± 4.2 | 25.5 0 ± 2.6 | 0.149 |
| Underlying pulmonary disease* | 2 (11.1%) | 0 (0%) | 2 (16.7%) | 0.407 |
| Comorbidities | ||||
| Hypertension | 5 (27.8%) | 0 (0%) | 5 (41.27%) | 0.114 |
| Renal insufficiency | 2 (11.1%) | 0 (0%) | 2 (16.7%) | 0.529 |
| Chronic haemodialysis | 1 (5.6%) | 0 (0%) | 1 (8.3%) | 1.0 |
| MV pre-ECMO | ||||
| PEEP (mbar) | 14.9 ± 3.1 | 13.8 ± 2.9 | 15.3 ± 3.2 | 0.489 |
| Plateau pressure (mbar) | 28.5 ± 4.6 | 29.3 ± 4.0 | 28.2 ± 4.9 | 0.571 |
| Driving pressure (mbar) | 13.6 ± 4.2 | 15.5 ± 4.5 | 12.9 ± 4.1 | 0.412 |
| Tidal volume (ml) | 390.7 ± 107.9 | 362.5 ± 104.4 | 400.9 ± 112.3 | 0.571 |
| Minute volume (l/min) | 9.9 ± 3.6 | 10.6 ± 4.3 | 9.6 ± 3.5 | 0.571 |
| Compliance (ml/mbar) | 32.7 ± 15.8 | 23.3 ± 10.4 | 35.5 ± 17.0 | 0.226 |
| FiO2 (%) | 83.8 ± 19.4 | 87.5 ± 19.4 | 81.8 ± 19.4 | 0.660 |
| Horowitz index (mmHg) | 87.6 ± 37.6 | 90.8 ± 40.8 | 85.8 ± 37.6 | 1.0 |
| D(A-a)O2 (mmHg) | 466.4 ± 133.4 | 481.7 ± 132.9 | 458.1 ± 139.4 | 0.884 |
| MV duration before ECMO (days) | 5.4 ± 5.4 | 9.3 ± 6.5 | 3.3 ± 3.3 | 0.048 |
| Acute renal failure | 3 (16.7%) | 0 (0%) | 3 (25.0%) | 0.276 |
| LDHmax (U/l) before ECMO | 734.1 ± 268.2 | 577.2 ± 182.1 | 812.5 ± 275.5 | 0.083 |
| Scores | ||||
| SOFA score | 9.7 ± 3.6 | 8.7 ± 3.4 | 10.3 ± 3.7 | 0.733 |
| APACHE II score | 24.9 ± 8.1 | 25.0 ± 9.0 | 24.9 ± 8.1 | 0.961 |
| RESP score | − 3.3 ± 3.2 | − 2.8 ± 1.9 | − 3.55 ± 3.8 | 1.0 |
| Successful ECMO weaning | 7 (38.9%) | 3 (50%) | 4 (33.3%) | 0.494 |
| Survival† | 4 (22.2%) | 3 (50%) | 1 (8.3%) | 0.045 |
| ICU length of stay (days) | 26.2 ± 20.5 | 33.8 ± 15.4 | 22.4 ± 22.3 | 0.053 |
| ECMO duration (days) | 13.2 ± 8.7 | 13.8 ± 11.0 | 12.9 ± 7.8 | 0.892 |
| MV duration (days) | 20.8 ± 14.8 | 25.2 ± 17.1 | 18.4 ± 13.7 | 0.462 |
| Acute haemodialysis | 6 (33.3%) | 0 (0%) | 6 (50.0%) | 0.054 |
| Prone position while ECMO | 11 (61.1%) | 5 (83.3%) | 6 (50.0%) | 0.588 |
ICU intensive care unit, MV mechanical ventilation
*Underlying pulmonary disease: lung fibrosis (n = 2)
†ICU and hospital survival
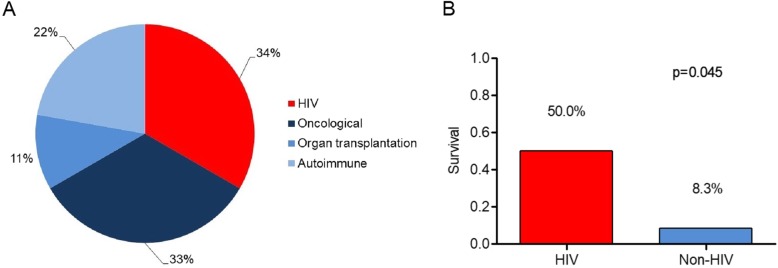
HIV was the cause of immunosuppression in 6 patients, whereas 12 patients had other subtypes of immunosuppression (non-HIV group, Fig. 1a). In all cases, HIV was diagnosed during index hospitalisation. Patients therefore were without previous antiretroviral treatment.
Fig. 1.
a Underlying subtypes of immunosuppression in ECMO patients with PJP. b Survival of PJP with severe respiratory failure and ECMO therapy in HIV vs. non-HIV patients
There were no significant differences between these two groups in relation to sex, comorbidities, ventilator settings, LDH levels or survival prediction scores (SOFA, APACHE II and RESP, Table 1). Patients with HIV were younger than non-HIV patients, and the interval between the start of mechanical ventilation and ECMO therapy was shorter in the non-HIV group.
Overall ECMO weaning rate was 39%, without a significant difference between HIV and non-HIV patients. Overall hospital survival was 22%. Withdrawal of care when further curative treatment was deemed futile was the most common cause of death (nine patients, 64.3%). Survival rate was higher in HIV than in non-HIV patients (50% vs. 8%, p = 0.045, Fig. 1b).
It has been shown previously in a non-ECMO setting that the outcome in HIV-negative PJP patients is worse than in patients with HIV [3], and our data confirm these earlier observations.
There are possible explanations for the better prognosis of HIV in this setting. On average, HIV patients are younger, and immunosuppression in HIV patients is reversible and can be resolved with the initiation of antiretroviral treatment. Moreover, the high mortality of non-HIV patients is associated with the underlying disease itself and a faster and more fulminant progression of the disease with more severe hypoxia and a higher prevalence of shock [4].
One third of our patients in the non-HIV group could be weaned successfully from ECMO support, suggesting that mortality was not only associated with ARDS, but underlying comorbidities may have been predominant. Moreover, there was a trend towards more frequent acute haemodialysis in non-HIV patients, illustrating that these patients had more complications and suffered from multi-organ failure.
In summary, a survival rate of 50% in HIV patients is similar to the average survival of ECMO patients with ARDS of any origin as shown by the CAESAR (63%) or the EOLIA trial (65%) [5, 6]. Therefore, ECMO therapy should not be withheld from patients with HIV-associated PJP.
Acknowledgements
Not applicable.
Abbreviations
- APACHE II
Acute Physiology and Chronic Health Evaluation
- ARDS
Acute respiratory distress syndrome
- HIV
Human immunodeficiency virus
- ICU
Intensive care unit
- MV
Mechanical ventilation
- PJP
Pneumocystis jirovecii pneumonia
- RESP
Respiratory Extracorporeal Membrane Oxygenation Survival Prediction
- SOFA
Sequential Organ Failure Assessment
- VV-ECMO
Veno-venous extracorporeal membrane oxygenation
Authors’ contributions
JR and TW contributed to the conception of the study. JR and TW contributed to the data collection. JR, DLS, SR, DD, CB and TW contributed to the data analysis and interpretation. JR and TW drafted the manuscript. DLS, SR, DD and CB revised the manuscript for important intellectual content. All authors approved the final version of the manuscript.
Funding
None.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The protocol was approved by our institution’s ethical committee (EK-Freiburg 151/14).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Schmidt JJ, Lueck C, Ziesing S, Stoll M, Haller H, Gottlieb J, et al. Clinical course, treatment and outcome of Pneumocystis pneumonia in immunocompromised adults: a retrospective analysis over 17 years. Crit Care. 2018;22:307. doi: 10.1186/s13054-018-2221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roux A, Canet E, Valade S, Gangneux-Robert F, Hamane S, Lafabrie A, et al. Pneumocystis jirovecii pneumonia in patients with or without AIDS. France Emerg Infect Dis. 2014;20:1490–1497. doi: 10.3201/eid2009.131668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monnet X, Vidal-Petiot E, Osman D, Hamzaoui O, Durrbach A, Goujard C, Miceli C, Bouree P, Richard C. Critical care management and outcome of severe Pneumocystis pneumonia in patients with and without HIV infection. Crit Care. 2008;12:R28. doi: 10.1186/cc6806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salzer HJF, Schafer G, Hoenigl M, Gunther G, Hoffmann C, Kalsdorf B, Alanio A, Lange C. Clinical, diagnostic, and treatment disparities between HIV-infected and non-HIV-infected immunocompromised patients with Pneumocystis jirovecii pneumonia. Respiration. 2018;96:52–65. doi: 10.1159/000487713. [DOI] [PubMed] [Google Scholar]
- 5.Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 6.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.