Abstract
Background
Mutant peptides presented by cancer cells are superior vaccine candidates than self peptides. The efficacy of mutant K-Ras, P53 and EGFR (Epidermal Growth Factor Receptor) peptides have been tested as cancer vaccines in pancreatic, colorectal, and lung cancers. The immunogenicity of EGFR Del19 mutations, frequent in Chinese lung adenocarcinoma patients, remains unclear.
Results
We predicted the HLA binding epitopes of Del19 mutations of EGFR in Chinese lung adenocarcinoma patients with NetMHC software. Enzyme-linked immunosorbent assay (ELISA) was performed to detect the EGFR-reactive IgG in lung cancer patients. Del19 mutations may be presented by multiple HLA Class I molecules, with delE746_A750 presented by 37.5% of Chinese population. For HLA Class II molecules, Del19 mutations of EGFR may be presented by multiple HLA-DRB1 molecules, with delE746_A750 presented by 58.1% of Chinese population. Serum reactivity to wild type EGFR protein was significantly higher in patients with Del19 EGFR mutations than those with EGFR L858R point mutation or with EGFR wild type genotype.
Conclusions
These findings suggest that Del19 mutations of EGFR, with an estimated frequency of 40% in Chinese lung adenocarcinoma patients, may serve as unique targets for immunotherapy in Chinese lung cancer patients.
Keywords: Lung cancer, Neo-antigen, INDEL, Immunotherapy, PD1 checkpoint blocking antibody
Background
Vaccine therapy for cancer has gained very limited success, with the example of Provenge (Sipuleucel-T), a dendritic cell based vaccine loaded with a fusion protein, PA2024, composed of recombinant PAP fused to granulocyte–macrophage colony-stimulating factor (GM-CSF), a cytokine that stimulates antigen presenting cells [1, 2]. Sipuleucel-T has extended survival of metastatic prostate cancer patients by median 4.1 months (IMPACT Phase III trial data). Other cancer vaccines based on self-proteins have shown very limited success in improving overall survival, which is not surprising in view of the expression of such self-proteins in healthy tissues and organs. For example, MUC1, a widely studied vaccine candidate, is expressed at similar abundance in healthy tissues as lung cancer according to mRNA array data of 442 lung adenocarcinoma patients deposited in the Cancer Genome Atlas [3, 4].
Mutant vaccines for cancer therapy was proposed decades ago, and have been focus of current immunotherapeutic studies after a series of findings that neoantigens in chronic lymphocytic leukemia, melanoma, and multiple cancer types are functional [5–12]. Neoantigens identified by exon sequencing and predicted by bioinformatics studies could stimulate autologous T cells [13, 14]. Furthermore, dendritic cell (DC) vaccines loaded with predicted neoantigen peptides increased the breadth and diversity of melanoma neoantigen-specific T cells, proving that such neoantigens are functional to stimulate CD8 T cells in vivo [15]. More excitingly, recent clinical data showed that colorectal cancer with a large number of somatic mutations due to mismatch-repair defects are more susceptible to immune checkpoint blockade by anti-PD-1 antibody therapy [16]. Resistance to immune checkpoint blockade therapy by anti-PD1 or anti-PD1/anti-CTLA-4 therapy is associated with loss of neoantigens [17].
Both point mutations and insertion or deletion (INDEL) type of mutations have been found in lung cancer patients. According to the Catalog Of Somatic Mutations In Cancer (COSMIC), 594 types of EGFR mutations have been reported, with 93% of mutations are present in the gene encoding tyrosine kinase domain (exons 18 to 21). East Asian lung cancer patients (40%) showed much higher EGFR mutation frequency than Caucasian patients (20%). Exon 19 deletions constitute 44.8% of EGFR mutations in East Asian patients [18].
In this study, we studied whether the Del19 mutations of EGFR may serve as targets for immunotherapy. We studied the MHC binding capacity of neoantigen peptides by NetMHC [19]. We also measured the serum antibody responses to EGFR protein (wild type) in lung cancer patients with different EGFR mutations.
Results
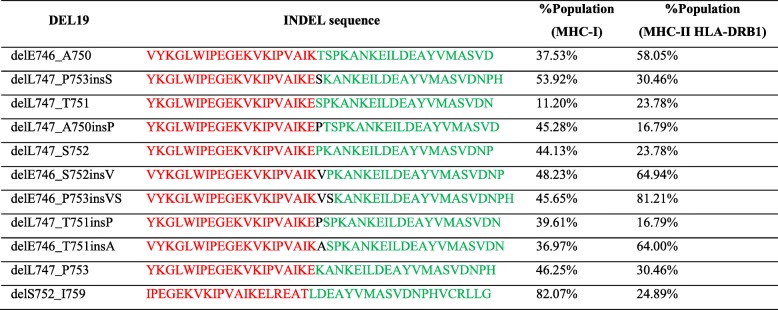
High-affinity candidate T cell epitopes were identified in silico by scanning of the mutant peptides (Table 1). We focused on identified HLA class I gene alleles with high expression levels in humans [20]. 66 neo-peptides were identified for multiple HLA Class I molecules (Additional file 1: Table S1, Additional file 2: Table S2, Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6, Additional file 7: Table S7, Additional file 8: Table S8, Additional file 9: Table S9, Additional file 10: Table S10 and Additional file 11: Table S11). Del19 mutations may be presented by HLA Class I molecules with a frequency ranging from 11.2 to 82.1%, with delE746_A750 presented by 37.5% of Chinese population.
Table 1.
Frequency of EGFR Del19 presentation by Chinese NSCLC patients as predicted by NetMHC4.0
The percentages are the total frequencies of HLA alleles which may present a mutant EGFR. Threshold of binding affinity for potential neoantigens was defined as IC50 < 500 nM. Both MHC I and MHC II were listed. HLA alleles (HLA-A, HLA-B, HLA-C, HLA-DRB1) with population frequency greater than 1% of Chinese population were selected for neoantigen analysis
Since the MHC Class II subtypes are extremely complex due to polymorphisms of both alpha and beta chains, we only studied the binding of EGFR deletions to HLA DRB1 molecules. 378 neo-peptides were identified for HLA Class II HLA DRB1 molecules (Additional file 1: Table S1, Additional file 2: Table S2, Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6, Additional file 7: Table S7, Additional file 8: Table S8, Additional file 9: Table S9, Additional file 10: Table S10 and Additional file 11: Table S11). Del19 mutations of EGFR may be presented by HLA DRB1 molecules with a frequency ranging from 16.7 to 81.2%, with delE746_A750 presented by 58.1% of Chinese population.
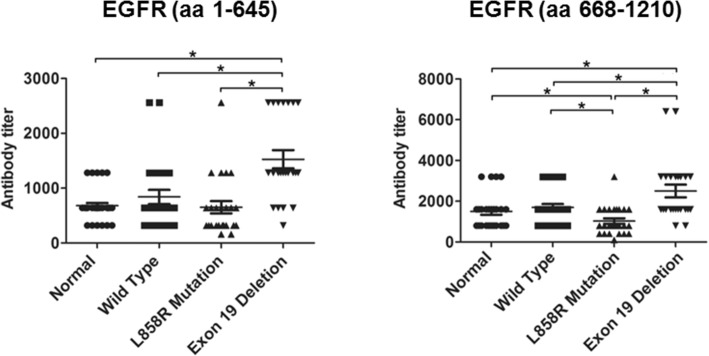
Since the serum cross-reactivity to wild type EGFR protein may reflect the immunogenicity of mutant EGFRs, we compared the antibody responses in lung adenocarcinoma patients with EGFR Del19 as that with EGFR L858R point mutation (Table 2). The results showed that the EGFR Del19 mutations triggered significant higher autoantibody response as compared to EGFR L858R point mutation and wild type genotypes. The medium titer of Del19 patients was about 1 fold higher than that of patient with L858R point mutation (Fig. 1).
Table 2.
Demographics of de-identified patients enrolled in this study
| EGFR | Age-ranges (years) | Pathologic subtype | TNM stage | Smoking |
|---|---|---|---|---|
| L858R mutation | 50–60 | adenocarcinoma | IV | 0 |
| L858R mutation | 50–60 | adenocarcinoma | IV | 300, 30 |
| L858R mutation | 70–80 | adenocarcinoma | IB | 0 |
| L858R mutation | 70–80 | adenocarcinoma | IV | 0 |
| L858R mutation | 70–80 | adenocarcinoma | IV | 1600, 10 |
| L858R mutation | 70–80 | adenocarcinoma | IB | 0 |
| L858R mutation | 70–80 | adenocarcinoma | IV | 450 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 200 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 0 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 0 |
| L858R mutation | 50–60 | adenocarcinoma | IV | 0 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 0 |
| L858R mutation | 50–60 | adenocarcinoma | IV | 0 |
| L858R mutation | 80–90 | adenocarcinoma | IV | 0 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 0 |
| L858R mutation | 70–80 | adenocarcinoma | IV | 0 |
| L858R mutation | 40–50 | adenocarcinoma | IIA | 0 |
| L858R mutation | 50–60 | adenocarcinoma | IV | 0 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 0 |
| L858R mutation | 60–70 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 30–50 | adenocarcinoma | IIIB | 100 |
| Exon 19 deletion | 50–60 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 40–50 | adenocarcinoma | p-IIIB | 0 |
| Exon 19 deletion | 50–60 | adenocarcinoma | IV | 450, 10 |
| Exon 19 deletion | 50–60 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 300 |
| Exon 19 deletion | 60–70 | non-small cell lung cancer | IV | 140 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 800 |
| Exon 19 deletion | 70–80 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 80–90 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 50–60 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 70–80 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 800, 1 |
| Exon 19 deletion | 70–80 | adenocarcinoma | IV | 1800 |
| Exon 19 deletion | 60–70 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 60–70 | adenocarcinoma | cT4N3Mx | 0 |
| Exon 19 deletion | 40–50 | adenocarcinoma | IV | 0 |
| Exon 19 deletion | 40–50 | adenocarcinoma | IV | 200 |
| Wild type | 60–70 | adenocarcinoma | IV | 600 |
| Wild type | 60–70 | adenocarcinoma | IV | 2400 |
| Wild type | 50–60 | mediastinal malignant tumor | ? | 600 |
| Wild type | 60–70 | non-small cell lung cancer | IIIB | 0 |
| Wild type | 50–60 | adenocarcinoma | IV | 600 |
| Wild type | 60–70 | small cell lung cancer | IIIB | 1000 |
| Wild type | 50–60 | non-small cell lung cancer | IIIA | 0 |
| Wild type | 60–70 | adenocarcinoma | IV | 800 |
| Wild type | 60–70 | adenocarcinoma | p-IIA | 800, 7 |
| Wild type | 60–70 | small cell lung cancer | IV | 800 |
| Wild type | 60–70 | squamous cell carcinoma | cT4N2Mx | 0 |
| Wild type | 60–70 | small cell lung cancer | IV | 0 |
| Wild type | 70–80 | adenocarcinoma | IIIB | 0 |
| Wild type | 60–70 | non-small cell lung cancer | IIIB | 1600 |
| Wild type | 60–70 | non-small cell lung cancer | cT3N3Mx | 1200 |
| Wild type | 60–70 | adenocarcinoma | IIB | 1600, 2 |
| Wild type | 60–70 | neuroendocrine carcinoma | IV | 800 |
| Wild type | 70–80 | squamous cell carcinoma | IIIA | 1200 |
| Wild type | 60–70 | adenocarcinoma | IV | 1200 |
| Wild type | 50–60 | non-small cell lung cancer | IV | 0 |
*(cigarettes/year), Years after quitting
Fig. 1.
Antibody response to mutant EGFR in lung adenocarcinoma patients. Anti-EGFR antibody titer in serum from lung adenocarcinoma cancer patients was measured by ELISA. Antibody titer was compared among patients with EGFR Exon 19 deletion, EGFR L858 point mutation, EGF wild type lung adenocarcinoma, and healthy individuals. Data were representative of 3 independent experiments. * means p < 0.05
Discussion
Immunotherapy targeting PD1 and PDL1 molecules have been approved to treat non-small cell lung carcinoma [21]. Molecular alterations in oncogenes such as EGFR and ALK alterations are associated with a reduced benefit as compared to wild-type patients [22, 23]. EGFR TKI combination with immunotherapy was found to be associated with a high incidence of interstitial lung disease. However, Immunotherapy by PD1 blockade combined with VEGF blockade plus chemotherapy significantly improved progression-free survival (PFS) and overall survival (OS) among patients with metastatic nonsquamous NSCLC with EGFR or ALK genetic alteration status (medium 9.7 months versus 6.1 months), suggesting that immunotherapy is a major area of research for therapy in EGFR-TKI resistant patients [24], especially in East Asian NSCLC patients.
Dong et al. recently analyzed the immunoglical profiles of Chinese NSCLC patients with EGFR mutations [25]. They reported that patients with EGFR mutation showed a lack of T-cell infiltration and shrinking proportion of PD-L1+/CD8+ TIL, suggesting an uninflamed tumor microenvironment. The other finding is that patients with EGFR mutations showed a significantly decreased mutation burden. Thus the induction of tumor-specific T cells is critical for treating Chinese NSCLC patients. Our analysis on Del19 mutations of EGFR, as well as EGFR point mutations, suggest that they may serve as candidate neoantigen peptide vaccines to induce both CD4 and CD8 T cells, a prerequisite for NSCLC patients to benefit from PD1 blockade drugs.
In this study, we found that Del19 mutations triggered significant higher antibody responses as compared to EGFR L858 point mtations in Chinese lung adenocarcinoma patients, suggesting that Del19 mutations of EGFR are expressed at protein level and immunogenic. The presence of autoantibodies toward wild type EGFR was also reported by Azuma et al. in Japanese NSCLC patients treated by EGFR TKI [26]. Together with our results from Chinese NSCLC patients, these data suggest that Del19 mutations of EGFR may serve as excellent candidate targets for immunotherapy as T cell epitopes. Indeed, another type of Del mutation of EGFR, EGFR type III variant (EGFRvIII), which has a deletion in its extracellular domain, has been tested as a vaccine candidate, and triggered both potent antibody responses and T cell responses against tumor cells bearing EGFRvIII mutation [27]. Interestingly, EGFR L858R point mutation did not trigger higher antibody response as compared to EGFR wild type adenocarcinoma patients, nor healthy group. Whether Del19 and L858R mutations may trigger T cell responses remain to be studied. Using X –ray crystallographic analysis, studies have shown that protein conformations of Del19 and L858R are different in both continuous state of kinase activation and conformation upon disruption of dimerization [28]. Other studies also showed Del19 and L858R are different bio-medically such as ability of triggering cell proliferation [29]. Similarly, analysis of clinical data from two large randomized phase III clinical trials of LUX-lung 3 and LUX-lung 6 also demonstrated greater benefit from 2nd generation EGFR-TKI afatinib than standard chemotherapy in patients with EGFR del19 as compared to patients with L858R, indicating potential bio-medical difference between EGFR del19 and L858R [30]. Although the current clinical practice guideline classifies lung adenocarcinoma patients with EGFR del19 and L858R in the same group, our data along with above evidence indicates that these two subtypes may be different not only in biology, but also in immunogenicity. Further studies to understand the difference among these two most common EGFR alteration subtypes are warranted. Although PD1 blockade combined with VEGF blockade and chemotherapy significantly improved PFS and OS among patients with lung adenocarcinomas with EGFR mutations [24, 31], whether or not one subtype benefits more than the other remains un-known. To select the patients who will most likely benefit and avoid the toxicities in patients who are unlikely benefit will be critical.
K-Ras, TP53, and EGFR mutants are well known vaccine candidates which are currently in clinical trials [32–36]. In addition, neo-antigens of passenger mutations are also attractive targets for individualized precision therapy when loaded to dendritic cells or other vaccine formulations. However, it might be technically difficult to determine whether the predicted neo-antigen mutations are presented by MHC Class I molecules. ELISPOT assay by synthetic candidate peptide epitopes may give some clues, but the antigenic epitopes may still show negative results due to loss of neo-antigen, mutations of HLA class I presentation pathway or immune suppression in tumor micro environment. Custom synthesis of pure mutant peptides are required for every patient to perform ELISPOT assay, which is both time consuming and expensive. We are developing ELISPOT assays by 444 neo-peptides identified in Additional file 1: Table S1, Additional file 2: Table S2, Additional file 3: Table S3, Additional file 4: Table S4, Additional file 5: Table S5, Additional file 6: Table S6, Additional file 7: Table S7, Additional file 8: Table S8, Additional file 9: Table S9, Additional file 10: Table S10 and Additional file 11: Table S11, which may be used to predict the function of T cells which destruct tumor cells in cancer patients bearing Del19 mutations of EGFR.
Both CD4 and CD8 epitopes are considered as important for effective anti-tumor immunity. Long peptide vaccines which contain both T cell epitopes for HLA Class I molecules and HLA Class II molecules have shown promise in eliciting both CD4 and CD8 T cell immunity against tumor cells expressing viral or tumor antigens. For example, the long peptide containing E6 and E7 proteins of Human Papilloma virus can be presented by both HLA Class I and II pathways, and showed efficacy in eliciting both CD4 and CD8 T cell responses which reduced tumor burden in cervical cancer patients [37, 38]. The immunogenicity of 40-mer long peptides containing the 11 Del19 mutations of EGFR as a component of cancer vaccine (Table 1) is currently being studied.
During this study, Turajlic et al. reported that neoantigens derived from indel mutations were nine times enriched for mutant specific binding, as compared with non-synonymous SNV derived neoantigens [39]. In the renal clear cell carcinoma cohort, indel mutation was associated with upregulation of antigen presentation genes and T-cell activation as measured by CD8-positive expression. Indel count was significantly associated with checkpoint inhibitor response across three separate melanoma cohorts. The immune responses in patients bearing Del19 mutations of EGFR treated by checkpoint inhibitor remain to be studied, as well as EGFR L858R mutation (Additional file 12).
Conclusions
Our results showed that serum reactivity to wild type EGFR protein was significantly higher in patients with Del19 EGFR mutations than those with EGFR L858R point mutation or with EGFR wild type genotype. These findings suggest that Del19 mutations of EGFR, with an estimated frequency of 40% in Chinese lung adenocarcinoma patients, may serve as unique targets for immunotherapy in Chinese lung cancer patients.
Methods
Neo-peptides prediction
29-mer polypeptides centered on mutated residues were scanned to identify candidate peptides binding to HLA class I or II, i.e., peptide sequences surrounding mutated amino acids resulting from Del19 and L858R EGFR mutations. The affinity of 8–12 mer peptides binding to HLA class I were predicted using the NetMHCPan4.0 binding algorithm [19]. The affinity of 15 mer peptides binding to HLA class II were predicted using the NetMHCIIPan3.1 binding algorithm. Threshold of binding affinity was defined as IC50 < 500 nM. HLA alleles (HLA-A, HLA-B, HLA-C, and HLA-DRB1) with frequency greater than 1% of Chinese population were selected for neoantigen analysis. HLA allele frequency for Chinese population [21] was according to http://www.allelefrequencies.net.
Elisa
Serum from lung adenocarcinoma cancer patients treated in Shanghai Pulmonary Hospital were banked according to protocols approved by Institutional Review Board (K16–245-1). EGFR mutations were detected by ARMS method using ADx EGFR Mutations Detection Kit (Amoy Diagnostics, Xiamen, China) [40].
The recombinant EGFR protein (extracellular part aa 1–645 and intracellular part aa 668–1210) was from Sinobiologicals, China. The EGFR protein was bound to ELISA plates (1 μg/ml) for overnight at 4 °C. 100 μl serum of lung cancer patients were added and incubated for 1 h at room temperature. The plates were washed for 3 times by washing buffer (PBS with 0.05% Tween-20), and incubated with HRP labeled goat anti-human IgG for 1 h, followed by colorimetric detection. PBS 1% BSA was used as blank for determining the cutoff value.
Supplementary information
Additional file 1. Predicted HLA binding epitopes for EGFR delE746_A750.
Additional file 2. Predicted HLA binding epitopes for EGFR delL747_P753insS.
Additional file 3. Predicted HLA binding epitopes for EGFR delL747_T751.
Additional file 4. Predicted HLA binding epitopes for EGFR delL747_A750insP.
Additional file 5. Predicted HLA binding epitopes for EGFR delL747_S752.
Additional file 6. Predicted HLA binding epitopes for EGFR delE746_S752insV.
Additional file 7. Predicted HLA binding epitopes for EGFR delE746_P753insVS.
Additional file 8. Predicted HLA binding epitopes for EGFR delL747_T751insP.
Additional file 9. Predicted HLA binding epitopes for EGFR delE746_T751insA.
Additional file 10. Predicted HLA binding epitopes for EGFR delL747_P753.
Additional file 11. Predicted HLA binding epitopes for EGFR delS752_I759.
Additional file 12. Comparison between EGFR exon Del 19 and EGFR L858R derived peptides.
Acknowledgements
We would like to thank all participants for their participation in this study, as well as the clinical experts without their support this study would not has been possible.
Abbreviations
- ARMS
amplification refractory mutation system
- COSMIC
Catalog Of Somatic Mutations In Cancer
- DC
dendritic cell
- EGFR
epidermal growth factor receptor
- ELISPOT
Enzyme-linked Immunospot
- GM-CSF
granulocyte–macrophage colony-stimulating factor
- HLA
human leukocyte antigen
- HRP
horse radish peroxidase
- MHC
major histocompatibility complex
Authors’ contributions
DZ and YL designed this study. PD, DZ, WC, WW, TW, and CZ contributed to the collection, analysis and interpretation of data. PD and DZ wrote the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Key Research and Development Plan grant 2017YFA0505901, National Natural Science Foundation of China grant 31870792, Fundamental Research Funds for the Central Universities 22120180201, and the Outstanding Clinical Discipline Project of Shanghai Pudong (Grant No.: PWYgy2018–10). Funding bodies have no role in design of the study and collection, analysis, interpretation of data or in writing the manuscript.
Availability of data and materials
The dataset of the current study is available from the corresponding author at a reasonable request.
Ethics approval and consent to participate
This study used patient plasma samples collected after written informed consent from patients, and used in accordance with ethics approval from the Ethics Committee of Shanghai Pulmonary Hospital affiliated with Tongji University School of Medicine (The protocol number is No.K16–245-1, Shanghai Pulmonary Hospital, Shanghai, China).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Deng Pan, Email: dengpan88@163.com.
Dapeng Zhou, Phone: +86-21-65981591, Email: dapengzhoulab@tongji.edu.cn.
Weijing Cai, Email: caiweijing816@163.com.
Weibo Wu, Email: violetwwb@163.com.
Wen Ling Tan, Email: 1593178@tongji.edu.cn.
Caicun Zhou, Email: caicunzhou_dr@163.com.
Yanyan Lou, Email: Lou.Yanyan@mayo.edu.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12865-019-0320-1.
References
- 1.Datta J, Berk E, Cintolo JA, Xu S, Roses RE, Czerniecki BJ. Rationale for a multimodality strategy to enhance the efficacy of dendritic cell-based Cancer immunotherapy. Front Immunol. 2015;6:271. doi: 10.3389/fimmu.2015.00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411–422. doi: 10.1056/NEJMoa1001294. [DOI] [PubMed] [Google Scholar]
- 3.Qu J, Yu H, Li F, Zhang C, Trad A, Brooks C, Zhang B, Gong T, Guo Z, Li Y, et al. Molecular basis of antibody binding to mucin glycopeptides in lung cancer. Int J Oncol. 2016;48(2):587–594. doi: 10.3892/ijo.2015.3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Urban JL, Schreiber H. Tumor antigens. Annu Rev Immunol. 1992;10:617–644. doi: 10.1146/annurev.iy.10.040192.003153. [DOI] [PubMed] [Google Scholar]
- 5.Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, Noguchi T, Ivanova Y, Hundal J, Arthur CD, Krebber WJ, et al. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515(7528):577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gubin MM, Artyomov MN, Mardis ER, Schreiber RD. Tumor neoantigens: building a framework for personalized cancer immunotherapy. J Clin Invest. 2015;125(9):3413–3421. doi: 10.1172/JCI80008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trajanoski Z, Maccalli C, Mennonna D, Casorati G, Parmiani G, Dellabona P. Somatically mutated tumor antigens in the quest for a more efficacious patient-oriented immunotherapy of cancer. Cancer Immunol Immunother. 2015;64(1):99–104. doi: 10.1007/s00262-014-1599-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overwijk WW, Wang E, Marincola FM, Rammensee HG, Restifo NP. Mining the mutanome: developing highly personalized immunotherapies based on mutational analysis of tumors. J Immunother Cancer. 2013;1:11. doi: 10.1186/2051-1426-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 10.Lu YC, Robbins PF. Cancer immunotherapy targeting neoantigens. Semin Immunol. 2016;28(1):22–27. doi: 10.1016/j.smim.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Buuren MM, Calis JJ, Schumacher TN. High sensitivity of cancer exome-based CD8 T cell neo-antigen identification. Oncoimmunology. 2014;3:e28836. doi: 10.4161/onci.28836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duan F, Duitama J, Al Seesi S, Ayres CM, Corcelli SA, Pawashe AP, Blanchard T, McMahon D, Sidney J, Sette A, et al. Genomic and bioinformatic profiling of mutational neoepitopes reveals new rules to predict anticancer immunogenicity. J Exp Med. 2014;211(11):2231–2248. doi: 10.1084/jem.20141308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajasagi M, Shukla SA, Fritsch EF, Keskin DB, DeLuca D, Carmona E, Zhang W, Sougnez C, Cibulskis K, Sidney J, et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124(3):453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Creaney J, Ma S, Sneddon SA, Tourigny MR, Dick IM, Leon JS, Khong A, Fisher SA, Lake RA, Lesterhuis WJ, et al. Strong spontaneous tumor neoantigen responses induced by a natural human carcinogen. Oncoimmunology. 2015;4(7):e1011492. doi: 10.1080/2162402X.2015.1011492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, Ly A, Lie WR, Hildebrand WH, Mardis ER, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348(6236):803–808. doi: 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castro MP, Goldstein N. Mismatch repair deficiency associated with complete remission to combination programmed cell death ligand immune therapy in a patient with sporadic urothelial carcinoma: immunotheranostic considerations. J Immunother Cancer. 2015;3:58. doi: 10.1186/s40425-015-0104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anagnostou V, Smith KN, Forde PM, Niknafs N, Bhattacharya R, White J, Zhang T, Adleff V, Phallen J, Wali N, et al. Evolution of Neoantigen landscape during immune checkpoint blockade in non-Small cell lung Cancer. Cancer Discov. 2017;7(3):264–276. doi: 10.1158/2159-8290.CD-16-0828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kobayashi Y, Mitsudomi T. Not all epidermal growth factor receptor mutations in lung cancer are created equal: perspectives for individualized treatment strategy. Cancer Sci. 2016;107(9):1179–1186. doi: 10.1111/cas.12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen M, Lundegaard C, Blicher T, Lamberth K, Harndahl M, Justesen S, Roder G, Peters B, Sette A, Lund O, et al. NetMHCpan, a method for quantitative predictions of peptide binding to any HLA-A and -B locus protein of known sequence. PLoS One. 2007;2(8):e796. doi: 10.1371/journal.pone.0000796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou F, Cao H, Zuo X, et al. Deep sequencing of the MHC region in the Chinese population contributes to studies of complex disease. Nat Genet. 2016;48(7):740–746. doi: 10.1038/ng.3576. [DOI] [PubMed] [Google Scholar]
- 21.Kazandjian D, Suzman DL, Blumenthal G, Mushti S, He K, Libeg M, Keegan P, Pazdur R. FDA approval summary: Nivolumab for the treatment of metastatic non-Small cell lung Cancer with progression on or after platinum-based chemotherapy. Oncologist. 2016;21(5):634–642. doi: 10.1634/theoncologist.2015-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, Howe E, Farago AF, Sullivan RJ, Stone JR, Digumarthy S, Moran T, Hata AN, Yagi Y, Yeap BY, Engelman JA, Mino-Kenudson M. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-Small cell lung Cancer: a retrospective analysis. Clin Cancer Res. 2016;22(18):4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ahn MJ, Sun JM, Lee SH, Ahn JS, Park K. EGFR TKI combination with immunotherapy in non-small cell lung cancer. Expert Opin Drug Saf. 2017;16(4):465–469. doi: 10.1080/14740338.2017.1300656. [DOI] [PubMed] [Google Scholar]
- 24.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M. IMpower150 study group. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 25.Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, Zhou Q, Tu HY, Xu CR, Yan LX, Li YF, Zhong WZ, Wu YL. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6(11):e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azuma K, Komatsu N, Hattori S, Matsueda S, Kawahara A, Sasada T, Itoh K, Hoshino T. Humoral immune responses to EGFR-derived peptides predict progression-free and overall survival of non-small cell lung cancer patients receiving gefitinib. PLoS One. 2014;9(1):e86667. doi: 10.1371/journal.pone.0086667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuan CT, Wikstrand CJ, Bigner DD. EGFRvIII as a promising target for antibody-based brain tumor therapy. Brain Tumor Pathol. 2000;17(2):71–78. doi: 10.1007/BF02482738. [DOI] [PubMed] [Google Scholar]
- 28.Cho J, Chen L, Sangji N, Okabe T, Yonesaka K, Francis JM, et al. Cetuximab response of lung cancer-derived EGF receptor mutants is associated with asymmetric dimerization. Cancer Res. 2013;73:6770–6779. doi: 10.1158/0008-5472.CAN-13-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey KD, Garton AJ, Romero MS, Kahler J, Thomson S, Ross S, et al. Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib. Cancer Res. 2006;66:8163–8171. doi: 10.1158/0008-5472.CAN-06-0453. [DOI] [PubMed] [Google Scholar]
- 30.Wu YL, Sequist LV, Tan EH, Geater SL, Orlov S, Zhang L, et al. Afatinib as first-line treatment of older patients with EGFR mutation-positive non-Small-cell lung Cancer: subgroup analyses of the LUX-lung 3, LUX-lung 6, and LUX-lung 7 trials. Clin Lung Cancer. 2018;19(4):e465–e479. doi: 10.1016/j.cllc.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Reck M, Mok TSK, Nishio M, Jotte RM, Cappuzzo F, Orlandi F, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi: 10.1016/S2213-2600(19)30084-0. [DOI] [PubMed] [Google Scholar]
- 32.Ebben JD, Lubet RA, Gad E, Disis ML, You M. Epidermal growth factor receptor derived peptide vaccination to prevent lung adenocarcinoma formation: an in vivo study in a murine model of EGFR mutant lung cancer. Mol Carcinog. 2016;55(11):1517–1525. doi: 10.1002/mc.22405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li G, Wong AJ. EGF receptor variant III as a target antigen for tumor immunotherapy. Expert Rev Vaccines. 2008;7(7):977–985. doi: 10.1586/14760584.7.7.977. [DOI] [PubMed] [Google Scholar]
- 34.Hartley ML, Bade NA, Prins PA, Ampie L, Marshall JL. Pancreatic cancer, treatment options, and GI-4000. Hum Vaccin Immunother. 2015;11(4):931–937. doi: 10.1080/21645515.2015.1011017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaft JE, Litvak A, Arcila ME, Patel P, D'Angelo SP, Krug LM, Rusch V, Mattson A, Coeshott C, Park B, et al. Phase II study of the GI-4000 KRAS vaccine after curative therapy in patients with stage I-III lung adenocarcinoma harboring a KRAS G12C, G12D, or G12V mutation. Clin Lung Cancer. 2014;15(6):405–410. doi: 10.1016/j.cllc.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 36.D'Angelo SP, Pietanza MC, Johnson ML, Riely GJ, Miller VA, Sima CS, Zakowski MF, Rusch VW, Ladanyi M, Kris MG. Incidence of EGFR exon 19 deletions and L858R in tumor specimens from men and cigarette smokers with lung adenocarcinomas. J Clin Oncol. 2011;29(15):2066–2070. doi: 10.1200/JCO.2010.32.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vos van Steenwijk PJ, van Poelgeest MI, Ramwadhdoebe TH, Lowik MJ, van der Meer DM B, van der Minne CE, Loof NM, Stynenbosch LF, Fathers LM, Valentijn AR, et al. The long-term immune response after HPV16 peptide vaccination in women with low-grade pre-malignant disorders of the uterine cervix: a placebo-controlled phase II study. Cancer Immunol Immunother. 2014;63(2):147–160. doi: 10.1007/s00262-013-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Welters MJ, Kenter GG, Piersma SJ, Vloon AP, Lowik MJ, der Meerdm B-v, Drijfhout JW, Valentijn AR, Wafelman AR, Oostendorp J, et al. induction of tumor-specific CD4+ and CD8+ T-cell immunity in cervical cancer patients by a human papillomavirus type 16 E6 and E7 long peptides vaccine. Clin Cancer Res. 2008;14(1):178–187. doi: 10.1158/1078-0432.CCR-07-1880. [DOI] [PubMed] [Google Scholar]
- 39.Turajlic S, Litchfield K, Xu H, Rosenthal R, McGranahan N, Reading JL, Wong YNS, Rowan A, Kanu N, Al Bakir M, et al. Insertion-and-deletion-derived tumour-specific neoantigens and the immunogenic phenotype: a pan-cancer analysis. Lancet Oncol. 2017;18(8):1009–1021. doi: 10.1016/S1470-2045(17)30516-8. [DOI] [PubMed] [Google Scholar]
- 40.Liu Y, Liu B, Li XY, Li JJ, Qin HF, Tang CH, Guo WF, Hu HX, Li S, Chen CJ, et al. A comparison of ARMS and direct sequencing for EGFR mutation analysis and tyrosine kinase inhibitors treatment prediction in body fluid samples of non-small-cell lung cancer patients. J Exp Clin Cancer Res. 2011;30:111. doi: 10.1186/1756-9966-30-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Predicted HLA binding epitopes for EGFR delE746_A750.
Additional file 2. Predicted HLA binding epitopes for EGFR delL747_P753insS.
Additional file 3. Predicted HLA binding epitopes for EGFR delL747_T751.
Additional file 4. Predicted HLA binding epitopes for EGFR delL747_A750insP.
Additional file 5. Predicted HLA binding epitopes for EGFR delL747_S752.
Additional file 6. Predicted HLA binding epitopes for EGFR delE746_S752insV.
Additional file 7. Predicted HLA binding epitopes for EGFR delE746_P753insVS.
Additional file 8. Predicted HLA binding epitopes for EGFR delL747_T751insP.
Additional file 9. Predicted HLA binding epitopes for EGFR delE746_T751insA.
Additional file 10. Predicted HLA binding epitopes for EGFR delL747_P753.
Additional file 11. Predicted HLA binding epitopes for EGFR delS752_I759.
Additional file 12. Comparison between EGFR exon Del 19 and EGFR L858R derived peptides.
Data Availability Statement
The dataset of the current study is available from the corresponding author at a reasonable request.