Abstract
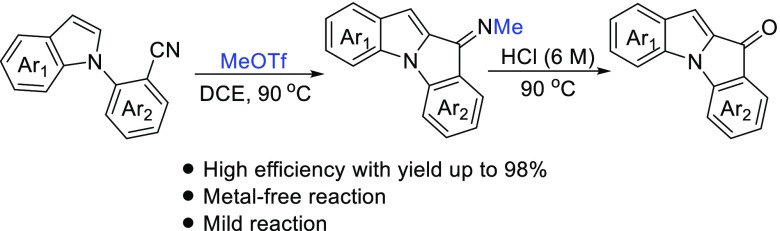
MeOTf-triggered annulation of N-(2-cyanoaryl)indoles to provide indolo[1,2-a]indol-10-imines and the corresponding indolo[1,2-a]indol-10-ones have been described under metal-free conditions. A variety of functional groups are tolerated in their scaffolds with good to excellent yields. The reaction could be carried to gram scale.
Introduction
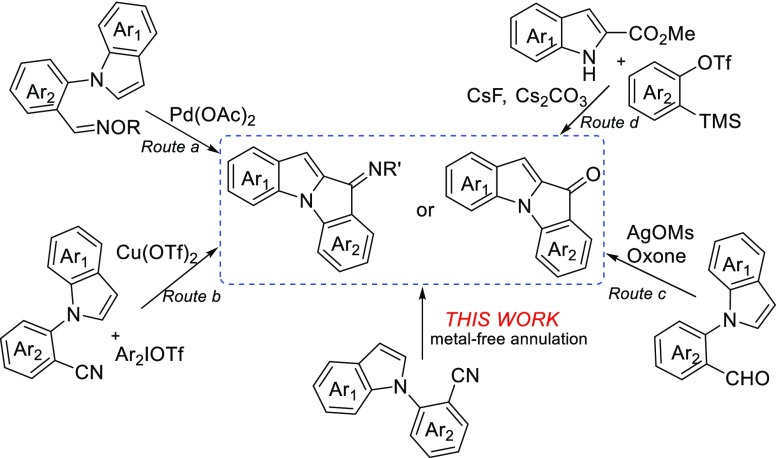
Indole nuclei are the foundation for many well-known biologically active molecules and exhibiting various pharmacological properties.1,2 Indolones are also valuable frameworks in medicinal chemistry and material chemistry.3 Because both indoles and indolones are relevant core structures for medicines, a fusion of two frameworks could theoretically result in a range of biologically active compounds. Therefore, developing a strategy to assemble two biologically and medicinally interesting substructures into one skeletal structure is one of the interesting and important topics in organic synthetic chemistry. Transition-metal-catalyzed syntheses of indole–indolone scaffolds have been developed in recent years.4 For example, Pd-catalyzed or Cu-catalyzed intramolecular annulation of indole-N-arylated aldoximes (Scheme 1, route a) or N-(2-cyanophenyl)indoles (route b) to afford indolo[1,2-a]indol-10-imines has been reported.4a,4b Recently, Rao and co-workers reported an annulation reaction of N-(2-formylaryl)indoles to give indolo[1,2-a]indol-10-ones via silver-catalyzed direct oxidative coupling between aldehyde C–H and sp2 C–H bonds (route c).4c Larock and Ramtohul et al. have developed fluoride and inorganic base-mediated synthesis of indolo[1,2-a]indol-10-ones through the reaction of methyl indole-2-carboxylates with arynes (route d).4d,4e Although significant progresses have been made to date, a new method for the synthesis of diverse indole–indolone scaffolds under metal-free conditions is still highly desirable, particularly in the drug scanning process. Herein, we report the methyl triflate (MeOTf)-induced annulation of N-(2-cyanoaryl)indoles to afford the indolo[1,2-a]indol-10-imines and the corresponding indolo[1,2-a]indol-10-ones under metal-free conditions.
Scheme 1. Synthetic Strategy Leading to Indolo[1,2-a]indol-10-imines or Indolo[1,2-a]indol-10-ones.
Results and Discussion
Learning from our previous studies in the area of triflate-induced annulation,5,6 we initially explored the reaction of 2-(3-methyl-1H-indol-1-yl)benzonitrile 1a and MeOTf 2a with the ratio of 1:1.5 in dichloroethane (DCE) under different temperatures for 12 h (Table 1, entries 1–3), and 90 °C was found to be the optimal temperature for this reaction with the NMR yield up to 75% (entry 2). Then, different solvents were screened such as dichloromethane (CH2Cl2), chloroform (CHCl3), tetrachloromethane (CCl4), and toluene (entries 4–7). DCE was found to be a superior solvent for this reaction (entry 2). Furthermore, the effects of the reaction time were also examined (entry 2 and entries 8–9), the best time for approving yield was 24 h (entry 9). When the ratio of two components was converted to 1:1 and 1:2, the yield of 3a was 79 and 85% (entries 10–11), respectively. We also tried the ratio of 1a and 2a in 1:1.2 and to our delight, 99% yield of 3a was also obtained. On the basis of the above results, the optimal conditions are shown in entry 12.
Table 1. Optimization of the Reaction Conditions for the Formation of 3aa.
| entry | temp (°C) | t (h) | solvent | yield/%b |
|---|---|---|---|---|
| 1 | 60 | 12 | DCE | 64 |
| 2 | 90 | 12 | DCE | 75 |
| 3 | 120 | 12 | DCE | 43 |
| 4 | 90 | 12 | DCM | 54 |
| 5 | 90 | 12 | CHCl3 | 39 |
| 6 | 90 | 12 | CCl4 | 25 |
| 7 | 90 | 12 | toluene | 34 |
| 8 | 90 | 18 | DCE | 89 |
| 9 | 90 | 24 | DCE | 99 (95)c |
| 10d | 90 | 24 | DCE | 79 |
| 11e | 90 | 24 | DCE | 85 |
| 12f | 90 | 24 | DCE | 99 |
Reaction conditions: 2-(3-methyl-1H-indol-1-yl)benzonitrile 1a (0.5 mmol), MeOTf 2a (0.75 mmol, 1.5 equiv) in 0.5 mL solvent under air ambience.
NMR yield based on CH2Br2 as internal standard.
Isolated yield.
MeOTf 2a (0.5 mmol, 1.0 equiv).
MeOTf 2a (1.0 mmol, 2.0 equiv).
MeOTf 2a (0.6 mmol, 1.2 equiv).
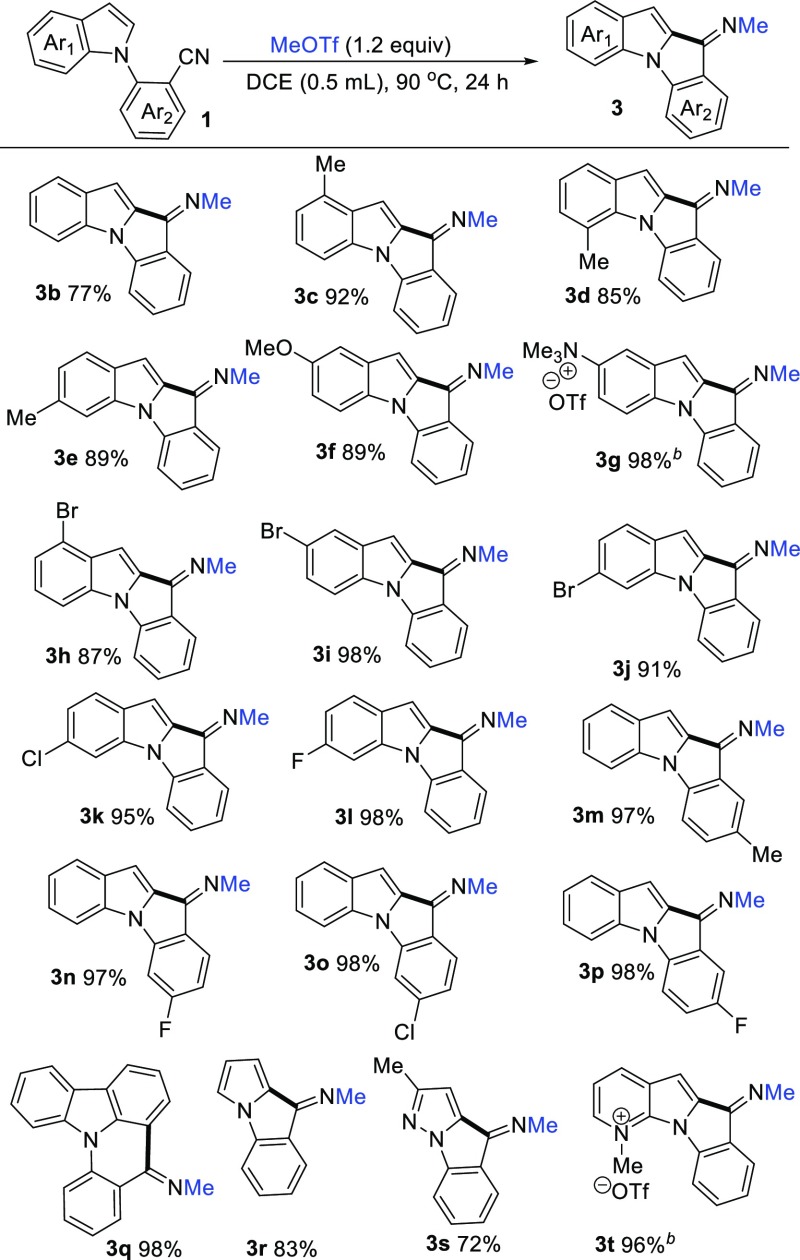
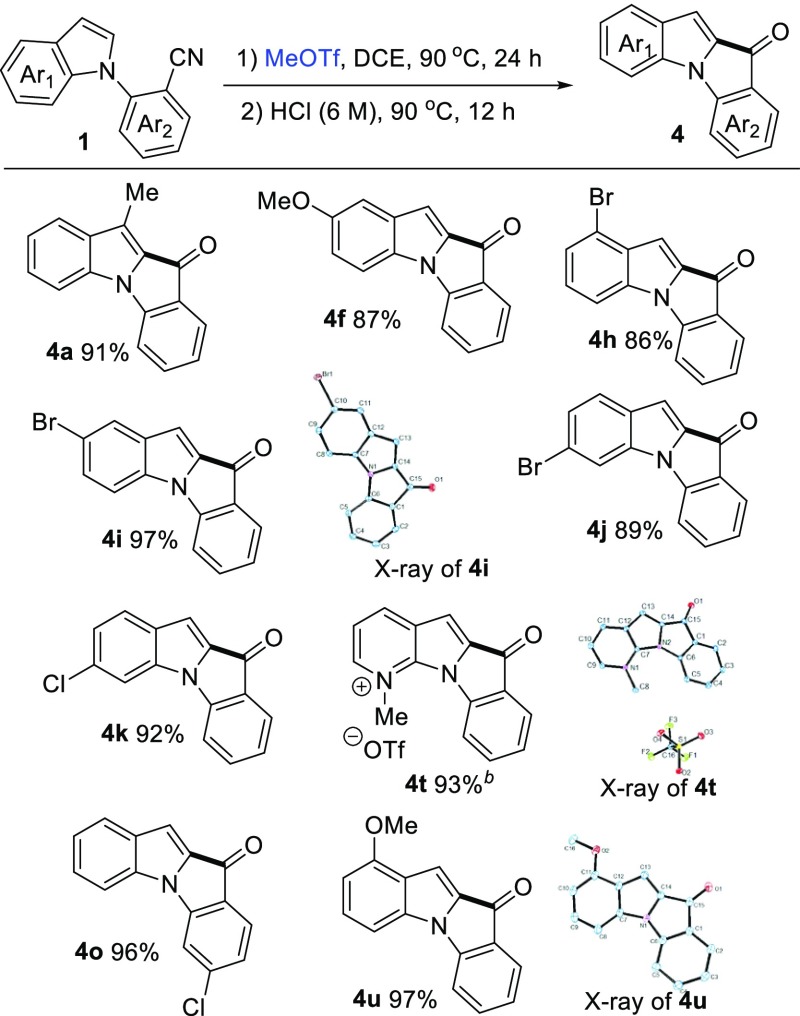
With the optimized reaction conditions in hand (Table 1, entry 12), we probed the substrate scope and the representative results are summarized in Scheme 2. Suitable N-(2-cyanoaryl)indole substrates 1 were prepared by the coupling reaction of commercially available indole derivatives and 2-bromobenzonitrile derivatives.7 Generally, the N-(2-cyanophenyl)-indoles containing electron-rich or electron-deficient substituents could proceed smoothly to afford indolo[1,2-a]indol-10-imines in high yields. For example, N-(2-cyanophenyl)-indoles containing electron-donating groups such as Me, OMe, NMe2 on the phenyl ring displayed higher reactivity to afford the corresponding products in excellent yields. Notably, when 2-(5-(dimethylamino)-1H-indol-1-yl)benzonitrile 2g was employed under the same reaction conditions and N,N,N-trimethyl-10-(methylimino)-10H-indolo[1,2-a]indol-2-aminium trifluoromethanesulfonate 3g was obtained in 75% yield. When 2.2 equiv of MeOTf were added, 3g was obtained quantitatively. The N-(2-cyanophenyl)-indoles containing electron-withdrawing groups such as chlorine and fluorine also displayed higher reactivity to afford the corresponding products in excellent yields (3h–3l). To further expand the substrate scope, the benzonitrile units bearing either electron-donating groups such as methyl or electron-withdrawing groups such as chlorine and fluorine were tested, and all of them readily produce the desired products (3m–3p) in quantitative yields, respectively. The stereochemistries of 3b and 3m were determined by 2-D NMR. The nuclear Overhauser effect spectroscopy spectra revealed that the methyl group and the original indolyl group were in cis-fashion (see Figures S2 and S3 in the Supporting Information). In addition, 2-(9H-carbazol-9-yl)benzonitrile 1q, 2-(1H-pyrrol-1-yl)benzonitrile 1r, and 2-(3-methyl-1H-pyrazol-1-yl)benzonitrile 1s could be used as well to produce the corresponding compounds 3q, 3r, and 3s in good yields, respectively. It is noteworthy that 2-(1H-pyrrolo[2,3-b]pyridin-1-yl)benzonitrile 1t was used in this reaction with 2.2 equiv of MeOTf and 1-methyl-6-(methylimino)-6H-pyrido[3′,2′:4,5]pyrrolo[1,2-a]indol-1-ium trifluoromethanesulfonate 3t was obtained in 96% yield. Notably, when 2-(2-methyl-1H-indol-1-yl)benzonitrile was employed in this reaction, no product was obtained. The starting material remains.
Scheme 2. Scope of Cyclization of (2-Cyanoaryl)indoles.
Reaction conditions: N-(2-cyanoaryl)indoles 1 (0.5 mmol), MeOTf 2a (0.6 mmol, 1.2 equiv) in 0.5 mL of DCE, 90 °C under air ambience, 24 h, isolated yield. bMeOTf 2a (1.1 mmol, 2.2 equiv).
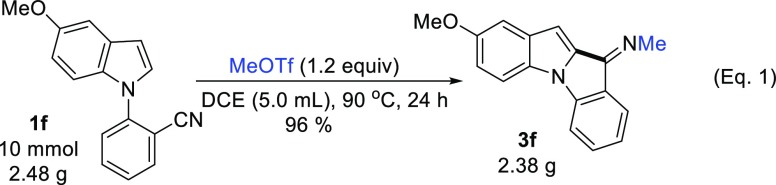
In order to verify the applicability of the current method, the reaction was amplified to 10 mmol scale with the formation of indolo[1,2-a]indole 3f in 96% yield (2.38 g) (eq 1).
 |
1 |
We also performed the reactions with hydrolysis to convert the imine into keto functionality using hydrochloric acid aqueous solution (6 M) at 90 °C for 12 h. The representative results are shown in Scheme 3. The indolo[1,2-a]indol-10-ones 4 were obtained in good to excellent yields. To our delight, the crystals of 4i, 4t, and 4u were suitable for single crystal analysis, and their structures were fully characterized by X-ray diffraction analysis.8 The structures of the compounds were further confirmed in the formation of indole–indolone scaffolds.
Scheme 3. Formation of (2-Cyanoaryl)indoles Subsequent Hydrolysis.
Reaction conditions: (1) N-(2-cyanoaryl)indoles 1 (0.5 mmol), MeOTf 2a (0.6 mmol, 1.2 equiv) in 0.5 mL of DCE under air ambience; (2) HCl (6 M, 5 mL), isolated yield. bMeOTf 2a (1.1 mmol, 2.2 equiv).
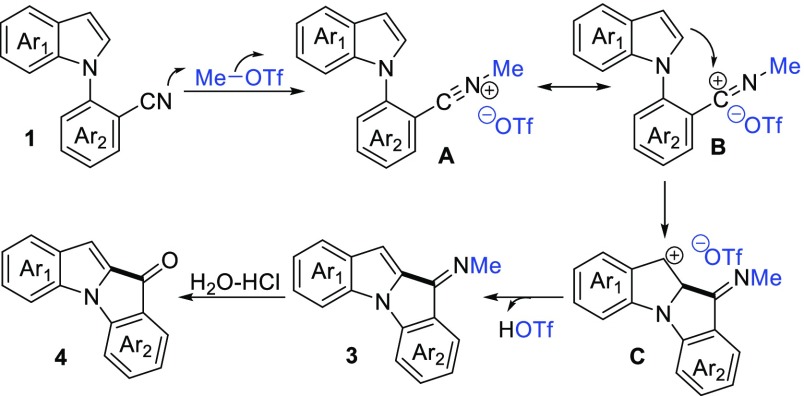
On the basis of the above results and related precedents,5,9 a plausible mechanism is proposed as follows (Scheme 4): first, methylation of the N-(2-cyanoaryl)indole by MeOTf affords carbenium ion A or its resonance B. Then, intramolecular Friedel–Crafts reaction of B with the subsequent deprotonation of intermediate C affords indole–indolone imine 3, which undergoes hydrolysis with HCl aqueous solution to form indole–indolone scaffold 4.
Scheme 4. Plausible Mechanism of the Reaction.
Conclusions
In summary, we have developed a MeOTf-induced intramolecular cyclization of N-(2-cyanoaryl)indoles for the synthesis of indolo[1,2-a]indol-10-imines and the corresponding indolo[1,2-a]indol-10-ones. This annulation represents a general entry for the construction of indole–indolone scaffolds in high yield under metal-free condition.
Experimental Section
General Information
All the reactions were carried out in air ambience. All materials were obtained from commercial sources and used as received. DCE and toluene were freshly distilled, whereas CH2Cl2, EtOAc, MeOH, and Et3N were dried by an activated 4 Å molecular sieve. Column chromatography was performed on a silica column (particle size 300 mesh). 1H NMR and 13C{1H} NMR spectra were recorded on 400 or 600 MHz at ambient temperature with CDCl3 or CD3OD as the solvent. Chemical shifts (δ) were given in ppm, in reference to the residual proton resonance of CDCl3 (7.26) or CD3OD (3.31), to the carbon resonance of CDCl3 (77.16) or CD3OD (49.0). Coupling constants (J) were given in Hertz (Hz). The terms m, q, t, d, s referred to multiplet, quartet, triplet, doublet, singlet. High-resolution mass spectra were recorded on electrospray mass spectrometer (ESI-TOF).
General Procedure for the Synthesis of Starting Materials7a
A sealed tube (25 mL) charged with indole derivatives (5 mmol), K3PO4 (10.2 mmol), CuI (10 mol %), 1,10-phenanthroline hydrate (40 mol %), and toluene (13 mL) was stirred for 10 min. Then, 2-bromobenzonitrile (6 mmol) was slowly added into above tube. The reaction mixture was stirred at 110 °C for 24–36 h under nitrogen atmosphere. After extraction with dichloromethane three times, the combined organic layer was dried by Na2SO4, evaporated, and isolated by silica gel flash chromatography (petroleum ether/ethyl acetate: 5/1) to obtain the starting material 1.
General Procedure for the Synthesis of Product 3
A sealed tube (25 mL) charged with N-(2-cyanoaryl)-indoles (0.5 mmol), MeOTf (0.6 mmol), and DCE (0.5 mL) was stirred at 90 °C for 24 h under. After extraction with dichloromethane three times, the combined organic layer was dried by Na2SO4, evaporated, and isolated by silica gel flash chromatography (petroleum ether/ethyl acetate/triethylamine: 10/1/5) to obtain the corresponding product 3.
Hydrolysis Procedure for Product 4
HCl (6 M, 5 mL) was added to a 25 mL sealed tube with product 3. The solution was stirred at 90 °C for 12 h. After extraction with dichloromethane three times, the combined organic layer was dried by Na2SO4, evaporated, and isolated by silica gel flash chromatography (petroleum ether/EtOAc: 10/1) to obtain the corresponding product 4.
(Z)-N,11-Dimethyl-10H-indolo[1,2-a]indol-10-imine (3a)
Yellow solid, 95% isolated yield (116.9 mg); mp 137–139 °C; 1H NMR (400 MHz, chloroform-d): δ 7.84 (d, J = 7.7 Hz, 1H), 7.56 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.1 Hz, 1H), 7.44–7.38 (m, 2H), 7.32 (t, J = 7.7 Hz, 1H), 7.13 (t, J = 7.5 Hz, 1H), 7.0–6.98 (m, 1H), 3.88 (s, 3H), 2.53 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 157.3, 143.4, 135.3, 134.3, 132.2, 132.0, 128.5, 127.0, 125.1, 122.3, 121.2, 120.8, 114.1, 111.1, 111.0, 41.6, 8.9. IR (CHCl3, cm–1): 3057, 2970, 1640, 1457. HRMS (ESI): [M + H]+ calcd for C17H15N2+, 247.1230; found, 247.1232.
(Z)-N-Methyl-10H-indolo[1,2-a]indol-10-imine (3b)
Yellow solid, 77% isolated yield (89.3 mg); mp 142–143 °C; 1H NMR (400 MHz, chloroform-d): δ 7.80 (d, J = 7.7 Hz, 1H), 7.68 (d, J = 8.0 Hz, 1H), 7.66 (d, J = 8.1 Hz, 1H), 7.45 (d, J = 3.5 Hz, 2H), 7.43–7.36 (m, 1H), 7.23–7.10 (m, 2H), 6.94 (s, 1H), 3.73 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 156.4, 142.5, 132.9, 132.5, 132.1, 131.8, 131.3, 126.2, 123.7, 123.6, 123.1, 121.5, 111.2, 110.8, 107.3, 42.6. IR (CHCl3, cm–1): 3067, 2976, 1643, 1452. HRMS (ESI): [M + H]+ calcd for C16H13N2+, 233.1073; found, 233.1071.
(Z)-N,1-Dimethyl-10H-indolo[1,2-a]indol-10-imine (3c)
Yellow solid, 92% isolated yield (113.2 mg); mp 145–147 °C; 1H NMR (400 MHz, chloroform-d): δ 7.68 (d, J = 7.5 Hz, 1H), 7.30 (d, J = 7.6 Hz, 1H), 7.25–7.14 (m, 3H), 7.03 (t, J = 7.5 Hz, 1H), 6.84 (d, J = 7.0 Hz, 1H), 6.68 (s, 1H), 3.60 (s, 3H), 2.43 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 156.1, 142.2, 133.0, 132.5, 131.6, 131.6, 131.5, 131.0, 126.0, 123.2, 122.7, 121.6, 110.6, 108.6, 105.5, 42.4, 18.7. IR (CHCl3, cm–1): 3056, 2978, 1648, 1482. HRMS (ESI): [M + H]+ calcd for C17H15N2+, 247.1230; found, 247.1228.
(Z)-N,4-Dimethyl-10H-indolo[1,2-a]indol-10-imine (3d)
Yellow solid, 85% isolated yield (104.6 mg); mp 156–158 °C; 1H NMR (400 MHz, chloroform-d): δ 8.58–8.52 (m, 1H), 8.07 (dd, J = 7.9, 2.8 Hz, 1H), 7.78 (dd, J = 4.0, 2.8 Hz, 1H), 7.72–7.65 (m, 1H), 7.61–7.55 (m, 1H), 7.54–7.48 (m, 1H), 7.42 (d, J = 4.5 Hz, 1H), 7.18 (d, J = 3.1 Hz, 1H), 3.59 (s, 1H), 2.81 (s, 1H). 13C{1H} NMR (101 MHz, chloroform-d): δ 164.6, 139.3, 136.6, 132.8, 129.7, 129.2, 128.6, 128.5, 127.5, 125.2, 123.7, 121.6, 121.2, 120.4, 118.2, 40.3, 16.7. IR (CHCl3, cm–1): 3076, 2970, 1645, 1456. HRMS (ESI): [M + H]+ calcd for C17H15N2+, 247.1230; found, 247.1231.
(Z)-N,3-Dimethyl-10H-indolo[1,2-a]indol-10-imine (3e)
Yellow solid, 89% isolated yield (109.5 mg); mp 152–153 °C; 1H NMR (400 MHz, chloroform-d): δ 7.76 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.1 Hz, 1H), 7.40 (dd, J = 3.6, 2.0 Hz, 2H), 7.37 (s, 1H), 7.11–7.07 (m, 1H), 6.97 (d, J = 8.1 Hz, 1H), 6.84 (s, 1H), 3.67 (s, 3H), 2.50 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 156.4, 142.4, 136.5, 132.5, 132.1, 131.6, 131.3, 130.7, 123.3, 123.3, 123.2, 122.9, 111.2, 110.8, 107.3, 42.5, 22.3. IR (CHCl3, cm–1): 3074, 2973, 1643, 1459. HRMS (ESI): [M + H]+ calcd for C17H15N2+, 247.1230; found, 247.1233.
(Z)-2-Methoxy-N-methyl-10H-indolo[1,2-a]indol-10-imine (3f)
Yellow solid, 89% isolated yield (116.6 mg); mp 149–150 °C; 1H NMR (400 MHz, chloroform-d): δ 7.77 (d, J = 7.5 Hz, 1H), 7.53 (d, J = 8.8 Hz, 1H), 7.43–7.37 (m, 2H), 7.12–7.08 (m, 2H), 7.05–7.03 (m, 1H), 6.85 (s, 1H), 3.87 (s, 3H), 3.71 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 156.5, 155.1, 142.5, 133.5, 132.9, 131.7, 131.1, 127.5, 123.3, 123.0, 116.6, 111.9, 110.3, 106.8, 104.8, 55.9, 42.6. IR (CHCl3, cm–1): 3074, 2962, 1649, 1458. HRMS (ESI): [M + H]+ calcd for C17H15N2O+, 263.1179; found, 263.1180.
(Z)-N,N,N-Trimethyl-10-(methylimino)-10H-indolo[1,2-a]indol-2-aminium (3g)
Yellow solid, 98% isolated yield (215.2 mg); mp 185–186 °C; 1H NMR (400 MHz, methanol-d3): δ 8.11 (d, J = 2.5 Hz, 1H), 7.78 (dd, J = 12.0, 2.6 Hz, 1H), 7.55 (d, J = 9.2 Hz, 1H), 7.33 (d, J = 7.5 Hz, 1H), 7.24 (d, J = 7.7 Hz, 1H), 7.19 (d, J = 7.8 Hz, 1H), 6.92 (t, J = 7.5 Hz, 1H), 6.86 (s, 1H), 4.74 (s, 3H), 3.70 (s, 9H). 13C{1H} NMR (101 MHz, methanol-d3): δ 157.0, 142.1, 134.8, 133.6, 132.1, 130.8, 125.4, 123.9, 119.0, 116.4, 113.8, 112.4, 109.6, 58.4, 42.5, 8.0. IR (CHCl3, cm–1): 3203, 2963, 1642, 1446. HRMS (ESI): [M + H]+ calcd for C20H21F3N3O3S+, 440.1250; found, 440.1253.
(Z)-1-Bromo-N-methyl-10H-indolo[1,2-a]indol-10-imine (3h)
Yellow solid, 87% isolated yield (134.9 mg); mp 150–152 °C; 1H NMR (400 MHz, chloroform-d): δ 7.72 (d, J = 7.5 Hz, 1H), 7.40–7.38 (m, 2H), 7.25–7.27 (m, 2H), 7.16–7.08 (m, 2H), 6.79 (s, 1H), 3.67 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 155.7, 141.8, 133.1, 132.2, 132.0, 131.7, 131.0, 126.7, 124.3, 124.0, 123.1, 117.1, 110.8, 110.1, 106.7, 42.7. IR (CHCl3, cm–1): 3071, 2974, 1647, 1449. HRMS (ESI): [M + H]+ calcd for C16H12BrN2+, 311.0178; found, 311.0180.
(Z)-2-Bromo-N-methyl-10H-indolo[1,2-a]indol-10-imine (3i)
Yellow solid, 98% isolated yield (151.9 mg); mp 154–156 °C; 1H NMR (400 MHz, chloroform-d): δ 7.66 (d, J = 7.5 Hz, 1H), 7.58 (d, J = 1.7 Hz, 1H), 7.38–7.23 (m, 2H), 7.18 (d, J = 8.7 Hz, 1H), 7.13–7.00 (m, 2H), 6.56 (s, 1H), 3.57 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 155.6, 141.6, 134.1, 132.8, 131.6, 130.8, 130.1, 128.7, 125.8, 123.7, 122.9, 114.2, 112.1, 110.5, 105.8, 42.5. IR (CHCl3, cm–1): 3083, 2965, 1643, 1456. HRMS (ESI): [M + H]+ calcd for C16H12BrN2+, 311.0178; found, 311.0181.
(Z)-3-Bromo-N-methyl-10H-indolo[1,2-a]indol-10-imine (3j)
Yellow solid, 91% isolated yield (141.1 mg); mp 148–149 °C; 1H NMR (400 MHz, chloroform-d): δ 7.78–7.77 (m, 1H), 7.73 (s, 1H), 7.51–7.49 (m, 1H), 7.47–7.43 (m, 1H), 7.38–7.36 (m, 1H), 7.26–7.24 (m, 1H), 7.17–7.13 (m, 1H), 6.86 (s, 1H), 3.70 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 155.9, 141.8, 132.7, 132.4, 131.9, 131.6, 131.1, 124.8, 124.7, 124.0, 123.2, 119.8, 114.2, 110.9, 106.9, 42.6. IR (CHCl3, cm–1): 3065, 2970, 1643, 1450. HRMS (ESI): [M + H]+ calcd for C16H12BrN2+, 311.0178; found, 311.0177.
(Z)-3-Chloro-N-methyl-10H-indolo[1,2-a]indol-10-imine (3k)
Yellow solid, 95% isolated yield (126.4 mg); mp 146–147 °C; 1H NMR (400 MHz, chloroform-d): δ 7.71 (d, J = 7.6 Hz, 1H), 7.46 (d, J = 8.5 Hz, 1H), 7.42–7.36 (m, 2H), 7.22 (d, J = 7.8 Hz, 1H), 7.14–7.02 (m, 2H), 6.73 (s, 1H), 3.63 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 155.9, 141.8, 132.7, 132.4, 131.9, 131.6, 131.1, 124.8, 124.7, 124.0, 123.2, 119.8, 114.2, 110.9, 106.9, 42.6. IR (CHCl3, cm–1): 3074, 2973, 1641, 1454. HRMS (ESI): [M + H]+ calcd for C16H12ClN2+, 267.0684; found, 267.0686.
(Z)-3-Fluoro-N-methyl-10H-indolo[1,2-a]indol-10-imine (3l)
Yellow solid, 98% isolated yield (122.5 mg); mp 137–138 °C; 1H NMR (400 MHz, chloroform-d): δ 7.72 (d, J = 7.5 Hz, 1H), 7.52–7.48 (m, 1H), 7.40–7.36 (m, 1H), 7.21 (d, J = 7.9 Hz, 1H), 7.15–7.07 (m, 2H), 6.89–6.84 (m, 1H), 6.76 (s, 1H), 3.62 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 161.99 (d, J = 243.7 Hz), 155.8, 141.7, 132.97 (d, J = 3.8 Hz), 131.8, 131.7, 130.11 (d, J = 191.8 Hz), 124.51 (d, J = 10.4 Hz), 123.8, 123.0, 110.6, 110.23 (d, J = 24.8 Hz), 107.0, 97.76 (d, J = 27.2 Hz), 42.5. 19F NMR (565 MHz, chloroform-d): δ −113.92 (td, J = 9.5, 5.5 Hz). IR (CHCl3, cm–1): 3058, 2966, 1649, 1439. HRMS (ESI): [M + H]+ calcd for C16H12FN2+, 251.0979; found, 251.0982.
(Z)-N,8-Dimethyl-10H-indolo[1,2-a]indol-10-imine (3m)
Yellow solid, 97% isolated yield (119.3 mg); mp 134–135 °C; 1H NMR (400 MHz, chloroform-d): δ 7.63 (d, J = 8.1 Hz, 1H), 7.54 (d, J = 8.8 Hz, 2H), 7.38–7.29 (m, 1H), 7.27–7.21 (m, 1H), 7.20–7.08 (m, 2H), 6.85 (s, 1H), 3.67 (s, 3H), 2.34 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 156.5, 140.2, 133.1, 132.7, 132.6, 132.1, 132.0, 131.2, 126.0, 123.6, 123.5, 121.2, 111.1, 110.4, 106.9, 42.4, 21.1. IR (CHCl3, cm–1): 3047, 2987, 1644, 1465. HRMS (ESI): [M + H]+ calcd for C17H15N2+, 247.1230; found, 247.1232.
(Z)-7-Fluoro-N-methyl-10H-indolo[1,2-a]indol-10-imine (3n)
Yellow solid, 97% isolated yield (121.3 mg); mp 124–126 °C; 1H NMR (400 MHz, chloroform-d): δ 7.71–7.63 (m, 2H), 7.51–7.49 (m, 1H), 7.40–7.36 (m, 1H), 7.19–7.15 (m, 1H), 7.05–7.06 (m, 1H), 6.88 (s, 1H), 6.80–6.75 (m, 1H), 3.67 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 165.4 (d, J = 249.7 Hz), 155.1, 143.3 (d, J = 12.3 Hz), 132.9 (d, J = 6.2 Hz), 131.9, 127.1, 126.4, 124.3 (d, J = 10.5 Hz), 123.8, 121.9, 111.0, 110.1 (d, J = 23.1 Hz), 107.6, 99.3 (d, J = 28.1 Hz), 42.5. 19F NMR (565 MHz, chloroform-d): δ −107.03. IR (CHCl3, cm–1): 3069, 2954, 1639, 1469. HRMS (ESI): [M + H]+ calcd for C16H12FN2+, 251.0979; found, 251.0981.
(Z)-7-Chloro-N-methyl-10H-indolo[1,2-a]indol-10-imine (3o)
Yellow solid, 98% isolated yield (130.4 mg); mp 135–136 °C; 1H NMR (400 MHz, chloroform-d): δ 7.59–7.55 (m, 2H), 7.40–7.38 (m, 1H), 7.35–7.25 (m, 1H), 7.18 (s, 1H), 7.15–7.11 (m, 1H), 7.01–6.98 (m, 1H), 6.77 (s, 1H), 3.60 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 155.1, 142.7, 137.4, 132.8, 132.4, 131.7, 129.6, 126.4, 123.7, 123.6, 123.4, 121.8, 111.2, 111.1, 107.6, 42.5. IR (CHCl3, cm–1): 3061, 2984, 1638, 1459. HRMS (ESI): [M + H]+ calcd for C16H12ClN2+, 267.0684; found, 267.0686.
(Z)-8-Fluoro-N-methyl-10H-indolo[1,2-a]indol-10-imine (3p)
Yellow solid, 98% isolated yield (122.5 mg); mp 129–130 °C; 1H NMR (400 MHz, chloroform-d): δ 7.58–7.56 (m, 1H), 7.38–7.36 (m, 2H), 7.33–7.29 (m, 1H), 7.14–7.09 (m, 2H), 7.03–7.01 (m, 1H), 6.75 (s, 1H), 3.59 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 159.6 (d, J = 242.2 Hz), 155.5, 138.5, 132.6, 132.2 (d, J = 73.3 Hz), 126.3, 123.8, 121.5, 118.1, 118.0 (d, J = 24.7 Hz), 111.2, 110.8, 110.7, 110.4, 110.52 (d, J = 24.9 Hz), 42.5. 19F NMR (565 MHz, chloroform-d): δ −119.20 to −119.26 (m). IR (CHCl3, cm–1): 3063, 2944, 1646, 1456. HRMS (ESI): [M + H]+ calcd for C16H12FN2+, 251.0979; found, 251.0979.
(Z)-N-Methyl-8H-indolo[3,2,1-de]acridin-8-imine (3q)
Yellow solid, 98% isolated yield (138.2 mg); mp 196–197 °C; 1H NMR (400 MHz, chloroform-d): δ 8.39–8.38 (m, 1H), 8.04–8.02 (m, 3H), 7.99–7.95 (m, 2H), 7.50–7.44 (m, 2H), 7.33–7.27 (m, 2H), 7.25–7.22 (m, 1H), 3.84 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 154.0, 138.2, 138.0, 136.8, 130.4, 127.9, 127.5, 127.1, 126.7, 125.8, 124.1, 123.7, 121.9, 121.8, 121.3, 121.0, 116.7, 114.4, 113.9, 41.7. IR (CHCl3, cm–1): 3021, 2868, 2674, 1627, 1460. HRMS (ESI): [M + H]+ calcd for C20H15N2+, 283.1230; found, 283.1233.
(Z)-N-Methyl-9H-pyrrolo[1,2-a]indol-9-imine (3r)
Yellow solid, 83% isolated yield (75.5 mg); mp 133–135 °C; 1H NMR (400 MHz, chloroform-d): δ 7.73–7.71 (m, 1H), 7.35–7.33 (m, 1H), 7.16–7.09 (m, 2H), 7.07 (m, 1H), 6.59–6.58 (m, 1H), 6.38–6.3 (m, 1H), 3.62 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 155.7, 141.6, 132.0, 131.0, 128.6, 124.7, 122.8, 115.2, 114.8, 112.8, 109.9, 42.3. IR (CHCl3, cm–1): 1641, 1492, 1457, 1269, 752. HRMS (ESI): [M + H]+ calcd for C12H11N2+, 183.0917; found, 183.0915.
(Z)-N,2-Dimethyl-4H-pyrazolo[1,5-a]indol-4-imine (3s)
Brown solid, 72% isolated yield (70.9 mg); mp 105–106 °C; 1H NMR (400 MHz, methanol-d3): δ 8.76–8.75 (m, 1H), 8.28–8.26 (m, 1H), 8.19–8.17 (m, 1H), 8.14–8.10 (m, 1H), 7.14 (s, 1H), 3.99 (s, 3H), 2.81 (s, 3H). 13C{1H} NMR (101 MHz, methanol-d3): δ 152.1, 139.7, 136.3, 135.9, 134.7, 131.6, 123.3, 120.1, 113.7, 110.5, 35.1, 12.3. IR (CHCl3, cm–1): 3021, 2674, 1647, 1460. HRMS (ESI): [M + H]+ calcd for C12H12N3+, 198.1026; found, 198.1029.
(Z)-1-Methyl-6-(methylimino)-6H-pyrido[3′,2′:4,5]pyrrolo[1,2-a]indol-1-ium Trifluoromethanesulfonate (3t)
Yellow solid, 96% isolated yield (190.6 mg); mp 172–173 °C; 1H NMR (600 MHz, methanol-d3): δ 8.90–8.89 (m, 1H), 8.81–8.80 (m, 1H), 8.38 (s, 1H), 8.23–8.82 (m, 1H), 8.14–8.13 (m, 1H), 7.92–7.89 (m, 1H), 7.76–7.73 (m, 1H), 7.53–7.51 (m, 1H), 4.92 (s, 3H), 3.80 (s, 3H). 13C{1H} NMR (151 MHz, methanol-d3): δ 161.4, 147.8, 144.5, 143.6, 141.7, 140.7, 136.3, 133.5, 129.0, 128.4, 126.2, 123.2, 121.0, 120.3, 120.0, 118.4, 36.6. IR (CHCl3, cm–1): 3076, 2970, 1645, 1451. HRMS (ESI): [M + H]+ calcd for C17H15F3N3O3S+, 398.0781; found, 398.0783.
11-Methyl-10H-indolo[1,2-a]indol-10-one (4a)10
Yellow solid, 91% isolated yield (106.1 mg); mp 121–123 °C; 1H NMR (400 MHz, chloroform-d): δ 7.59–7.58 (m, 1H), 7.54–7.52 (m, 1H), 7.46–7.42 (m, 1H), 7.37–7.36 (m, 2H), 7.26–7.22 (m, 1H), 7.10–7.06 (m, 1H), 7.03–7.01 (m, 1H), 2.51 (s, 3H). 13C{1H} 13C NMR (101 MHz, chloroform-d): δ 182.0, 145.0, 135.1, 134.2, 133.6, 132.8, 129.9, 128.3, 124.9, 123.2, 122.9, 122.4, 121.4, 111.3, 111.1, 9.4. IR (CHCl3, cm–1): 3069, 2971, 1634, 1465. HRMS (ESI): [M + H]+ calcd for C16H12NO+, 234.0913; found, 234.0915.
2-Methoxy-10H-indolo[1,2-a]indol-10-one (4f)4e
Yellow solid, 87% isolated yield (108.3 mg); mp 132–133 °C; 1H NMR (600 MHz, chloroform-d): δ 7.61–7.60 (m, 1H), 7.49–7.46 (m, 1H), 7.39–7.37 (m, 1H), 7.27–7.25 (m, 1H), 7.07–7.02 (m, 4H), 3.84 (s, 3H). 13C{1H} NMR (151 MHz, chloroform-d): δ 181.7, 155.4, 145.7, 136.3, 135.5, 133.3, 129.8, 129.5, 125.2, 123.8, 119.2, 112.2, 110.9, 107.6, 105.6, 55.8. IR (CHCl3, cm–1): 3049, 2964, 1637, 1475. HRMS (ESI): [M + H]+ calcd for C16H12NO2+, 250.0863; found, 250.0862.
1-Bromo-10H-indolo[1,2-a]indol-10-one (4h)4c
Yellow solid, 86% isolated yield (128.1 mg); mp 134–135 °C; 1H NMR (400 MHz, chloroform-d): δ 7.69–7.68 (m, 1H), 7.58–7.54 (m, 1H), 7.50–7.48 (m, 1H), 7.39–7.37 (m, 1H), 7.33–7.26 (m, 2H), 7.21 (s, 1H), 7.16–7.13 (m, 1H). 13C{1H} NMR (151 MHz, DMSO-d6): δ 179.9, 144.2, 135.8, 135.1, 133.6, 131.9, 128.7, 128.0, 124.5, 124.4, 124.3, 116.7, 112.0, 111.0, 105.9. IR (CHCl3, cm–1): 3054, 2985, 1627, 1460. HRMS (ESI): [M + H]+ calcd for C15H9BrNO+, 297.9862; found, 297.9860.
2-Bromo-10H-indolo[1,2-a]indol-10-one (4i)4e
Yellow solid, 97% isolated yield (144.0 mg); mp 136–137 °C; 1H NMR (400 MHz, chloroform-d): δ 7.76–7.65 (m, 1H), 7.65–7.62 (m, 1H), 7.52–7.45 (m, 2H), 7.37–7.26 (m, 2H), 7.13–7.09 (m, 1H), 7.02–7.00 (m, 1H). 13C{1H} NMR (101 MHz, chloroform-d): δ 181.4, 145.3, 136.6, 135.8, 134.2, 132.7, 131.0, 129.3, 127.5, 125.5, 124.4, 115.1, 112.6, 111.5, 106.8. IR (CHCl3, cm–1): 3078, 2981, 1631, 1456. HRMS (ESI): [M + H]+ calcd for C15H9BrNO+, 297.9862; found, 297.9863.
3-Bromo-10H-indolo[1,2-a]indol-10-one (4j)4e
Yellow solid, 89% isolated yield (132.2 mg); mp 128–129 °C; 1H NMR (400 MHz, chloroform-d): δ 7.63–7.61 (m, 2H), 7.53–7.44 (m, 2H), 7.29–7.25 (m, 1H), 7.21–7.19 (m, 1H), 7.11–7.08 (m, 1H), 7.03 (s, 1H). 13C{1H} NMR (101 MHz, chloroform-d): δ 181.2, 145.1, 136.2, 135.7, 134.6, 131.4, 129.4, 126.1, 125.5, 125.4, 124.5, 122.2, 114.4, 111.5, 107.7. IR (CHCl3, cm–1): 3064, 2969, 1631, 1443. HRMS (ESI): [M + H]+ calcd for C15H9BrNO+, 297.9862; found, 297.9861.
3-Chloro-10H-indolo[1,2-a]indol-10-one (4k)4c
Yellow solid, 92% isolated yield (116.3 mg); mp 125–126 °C; 1H NMR (400 MHz, chloroform-d): δ 7.62 (m, 1H), 7.52–7.48 (m, 2H), 7.43 (s, 1H), 7.27–7.25 (m, 1H), 7.11–7.08 (m, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 181.2, 145.1, 136.4, 135.7, 134.3, 134.2, 131.1, 129.4, 125.8, 125.4, 124.5, 122.9, 111.5, 111.4, 107.7. IR (CHCl3, cm–1): 3074, 2986, 1630, 1454. HRMS (ESI): [M + H]+ calcd for C15H9ClNO+, 254.0367; found, 254.0366.
7-Chloro-10H-indolo[1,2-a]indol-10-one (4o)
Yellow solid, 96% isolated yield (121.4 mg); mp 119–120 °C; 1H NMR (400 MHz, chloroform-d): δ 7.63 (d, J = 8.1 Hz, 1H), 7.54 (d, J = 8.0 Hz, 1H), 7.46–7.40 (m, 2H), 7.30 (s, 1H), 7.17–7.10 (m, 2H), 7.04 (dd, J = 8.0 Hz, 1H). 13C{1H} NMR (101 MHz, chloroform-d): δ 180.3, 146.2, 141.8, 136.1, 134.3, 132.8, 128.6, 128.0, 126.1, 125.3, 124.1, 122.6, 112.1, 111.4, 108.9. IR (CHCl3, cm–1): 3061, 2930, 1627, 1446. HRMS (ESI): [M + H]+ calcd for C15H9ClNO+, 254.0367; found, 254.0369.
1-Methyl-6-oxo-6H pyrido[3′,2′:4,5]pyrrolo[1,2-a]indol-1-ium Trifluoromethane-Sulfonate (4t)
Yellow solid, 93% isolated yield (178.6 mg); mp 141–142 °C; 1H NMR (400 MHz, methanol-d3): δ 8.88 (d, J = 8.0 Hz, 1H), 8.87 (d, J = 8.0 Hz, 1H), 8.23 (d, J = 8.4 Hz, 1H), 7.93–7.76 (m, 2H), 7.74–7.72 (m, 2H), 7.55–7.51 (m, 1H), 4.99 (s, 3H). 13C{1H} NMR (151 MHz, methanol-d3): δ 180.6, 145.6, 145.3, 143.4, 141.0, 140.2, 138.4, 133.8, 131.0, 128.8, 126.9, 122.8, 120.7, 119.9, 117.8, 107.6. IR (CHCl3, cm–1): 3045, 2964, 1633, 1459. HRMS (ESI): [M + H]+ calcd for C16H12F3N2O4S+, 385.0464; found, 385.0467.
1-Methoxy-10H-indolo[1,2-a]indol-10-one (4u)4d
Yellow solid, 97% isolated yield (121.3 mg); mp 144–145 °C; 1H NMR (400 MHz, chloroform-d): δ 7.64 (d, J = 8.1 Hz, 1H), 7.49 (s, 1H), 7.35–7.31 (m, 2H), 7.25 (d, J = 8.0 Hz, 1H), 7.10–7.08 (m, 2H), 6.50 (d, J = 8.0 Hz, 1H), 3.94 (s, 3H). 13C{1H} NMR (101 MHz, chloroform-d): δ 181.4, 156.2, 145.5, 135.6, 135.3, 134.6, 129.8, 129.7, 125.2, 124.0, 123.6, 111.5, 106.1, 104.4, 101.8, 55.6. IR (CHCl3, cm–1): 3058, 2985, 1629, 1450. HRMS (ESI): [M + H]+ calcd for C16H12NO2+, 250.0863; found, 250.0865.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (nos. 91645120, 21871163, and 21911530097).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b02679.
Accession Codes
CCDC (1921810 for 4i), (1921809 for 4t), and (1921808 for 4u) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif, or by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.
The authors declare no competing financial interest.
Supplementary Material
References
- a Somei M.; Yamada F. Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit. Nat. Prod. Rep. 2004, 21, 278–311. 10.1039/b212257j. [DOI] [PubMed] [Google Scholar]; b Cacchi S.; Fabrizi G. Synthesis and Functionalization of Indoles Through Palladium-catalyzed Reactions. Chem. Rev. 2005, 105, 2873–2920. 10.1021/cr040639b. [DOI] [PubMed] [Google Scholar]; c Somei M.; Yamada F. Simple Indole Alkaloids and Those with a Nonrearranged Monoterpenoid Unit. Nat. Prod. Rep. 2005, 22, 73–103. 10.1039/b316241a. [DOI] [PubMed] [Google Scholar]; d Garg N. K.; Caspi D. D.; Stoltz B. M. Development of an Enantiodivergent Strategy for the Total Synthesis of (+)- and (-)-Dragmacidin F from a Single Enantiomer of Quinic Acid. J. Am. Chem. Soc. 2005, 127, 5970–5978. 10.1021/ja050586v. [DOI] [PubMed] [Google Scholar]; e Humphrey G. R.; Kuethe J. T. Practical Methodologies for the Synthesis of Indoles. Chem. Rev. 2006, 106, 2875–2911. 10.1021/cr0505270. [DOI] [PubMed] [Google Scholar]; f Krajewski K.; Zhang Y.; Parrish D.; Deschamps J.; Roller P. P.; Pathak V. K. New HIV-1 Reverse Transcriptase Inhibitors based on a Tricyclic Benzothiophene Scaffold: Synthesis, Resolution, and Inhibitory Activity. Bioorg. Med. Chem. Lett. 2006, 16, 3034–3038. 10.1016/j.bmcl.2006.02.049. [DOI] [PubMed] [Google Scholar]; g Chen Z.-Z.; Li S.-Q.; Zhang Y.-J.; Tang D.-Y.; Meng J.-P.; Lei J.; Li H.-Y.; Xu Z.-G. Synthesis of Pyridodiindoles with Anticancer Activity by a Three-Component Cascade Condensation. Org Lett 2018, 20, 7811–7815. 10.1021/acs.orglett.8b03245. [DOI] [PubMed] [Google Scholar]
- Sundberg R.-J. In Comprehensive Heterocyclic Chemistry; Katritzky A.-R., Rees C. W., Eds.; Pergamon: Oxford, 1984; Vol. 4, p 313. [Google Scholar]
- a Goetz M. A.; Lopez M.; Monaghan R. L.; Chang R. S. L.; Lotti V. J.; Chen T. B. Asperlicin, a Novel non-Peptidal Cholecystokinin Antagonist from Aspergillus Alliaceus Fermentation, Isolation and Bilolgical Properties. J. Antibiot. 1985, 38, 1633–1637. 10.7164/antibiotics.38.1633. [DOI] [PubMed] [Google Scholar]; b Liesch J. M.; Hensens O. D.; Springer J. P.; Chang R. S. L.; Lotti V. J. Asperlicin, a Novel non-Peptidal Cholecystokinin Antagonist from Aspergillus Alliaceus Structure elucldation. J. Antibiot. 1985, 38, 1638–1641. 10.7164/antibiotics.38.1638. [DOI] [PubMed] [Google Scholar]; c Sun H. H.; Byard S. J.; Cooper R. Revised NMR assignments for the cholecystokinin antagonist asperlicin. J. Antibiot. 1994, 47, 599–601. 10.7164/antibiotics.47.599. [DOI] [PubMed] [Google Scholar]; d Wong S.-M.; Musza L. L.; Kydd G. C.; Kullnig R.; Gillum A. M.; Cooper R. Fiscalins: New Substance P Inhibitors Produced by the Fungusn Neosartorya fischeri Taxonomy, Fermentation, structure, and Biological Properties. J. Antibiot. 1993, 46, 545–553. 10.7164/antibiotics.46.545. [DOI] [PubMed] [Google Scholar]
- a Hazra S.; Mondal B.; De R. N.; Roy B. Pd-catalyzed Dehydrogenative C–H Activation of Iminyl hydrogen with the Indole C3–H and C2–H bond: an Elegant Synthesis of Indeno[1,2-b]indoles and Indolo[1,2-a]indoles. RSC Adv. 2015, 5, 22480–22489. 10.1039/c4ra16661b. [DOI] [Google Scholar]; b Zhang L.; Wang Y.; Zheng L.; Guo B.; Hua R.–M. Synthesis of Fused Polycyclic indoles via Cu(II)-catalyzed Intramolecular Cyclization of N-(2-cyanophenyl)indoles in the Presence of Diaryliodonium Salts. Tetrahedron 2017, 73, 395–402. 10.1016/j.tet.2016.12.022. [DOI] [Google Scholar]; c Wang X.; Li Z.; Cao S.; Rao H. Silver-Catalyzed Intramolecular C-2 Selective Acylation of Indoles with Aldehydes: An Atom-Economical Entry to Indole-Indolone Scaffolds. Adv. Synth. Catal. 2016, 358, 2059–2065. 10.1002/adsc.201600108. [DOI] [Google Scholar]; d Rogness D. C.; Larock R. C. Rapid Synthesis of the Indole-indolone Scaffold via [3+2] Annulation of Arynes by Methyl Indole-2-carboxylates. Tetrahedron Lett. 2009, 50, 4003–4008. 10.1016/j.tetlet.2009.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Ramtohul Y.; Giacometti R. Synthesis of Polycyclic Indolone and Pyrroloindolone Heterocycles via the Annulation of Indole- and Pyrrole-2-Carboxylate Esters with Arynes. Synlett 2009, 2010–2016. 10.1055/s-0029-1217535. [DOI] [Google Scholar]
- a Liu Y.; Yi X.; Luo X.; Xi C. MeOTf-Mediated Annulation of Alkylnitriles and Arylalkynes Leading to Polysubstituted NH-Pyrroles. J. Org. Chem. 2017, 82, 11391–11398. 10.1021/acs.joc.7b01845. [DOI] [PubMed] [Google Scholar]; b Yan X.; Zou S.; Zhao P.; Xi C. MeOTf-induced Carboannulation of Arylnitriles and Aromatic Alkynes: a New Metal-free Strategy to Construct Indenones. Chem. Commun. 2014, 50, 2775–2777. 10.1039/c4cc00088a. [DOI] [PubMed] [Google Scholar]
- a Zhang X.; Wang S.; Liu Y.; Xi C. Triflates-Triggered Intermolecular Cyclization of Carbodiimides Leading to 2-Aminoquinazolinone and 2,4-Diaminoquinazoline Derivatives. Org. Lett. 2018, 20, 2148–2151. 10.1021/acs.orglett.8b00314. [DOI] [PubMed] [Google Scholar]; b Yan X.; Yi X.; Xi C. Direct Cleavage of the N=N Bond of Azobenzenes by MeOTf leading to N-arylbenzimidazoles. Org. Chem. Front. 2014, 1, 657–660. 10.1039/c4qo00056k. [DOI] [Google Scholar]; c Zhao P.; Liu Y.; Xi C. MeOTf-Induced Carboannulation of Isothiocyanates and Aryl Alkynes with C=S Bond Cleavage: Access to Indenones. Org. Lett. 2015, 17, 4388–4391. 10.1021/acs.orglett.5b02201. [DOI] [PubMed] [Google Scholar]; d Zhao P.; Yan X.; Yin H.; Xi C. Alkyltriflate-Triggered Annulation of Arylisothiocyanates and Alkynes Leading to Multiply Substituted Quinolines through Domino Electrophilic Activation. Org. Lett. 2014, 16, 1120–1123. 10.1021/ol500221u. [DOI] [PubMed] [Google Scholar]; e Liu Y.; Zhao P.; Zhang B.; Xi C. MeOTf-catalyzed Annulation of Aldehydes and Arylalkynes Leading to 2,3-disubstituted Indanones. Org. Chem. Front. 2016, 3, 1116–1119. 10.1039/c6qo00253f. [DOI] [Google Scholar]; f Liu Y.; Zhang X.; Xi C. MeOTf-induced Annulation of Arylisocyanates and Arylalkynes leading to 4-methoxyl-2,3-diarylquinolines. Tetrahedron Lett. 2018, 59, 2440–2442. 10.1016/j.tetlet.2018.05.030. [DOI] [Google Scholar]; g Wang S.; Shao P.; Du G.; Xi C. MeOTf- and TBD-Mediated Carbonylation of ortho-Arylanilines with CO2 Leading to Phenanthridinones. J. Org. Chem. 2016, 81, 6672–6676. 10.1021/acs.joc.6b01318. [DOI] [PubMed] [Google Scholar]; h Zou S.; Wang S.; Xi C. ROTf-induced Annulation of Heteroatom Reagents and Unsaturated Substrates Leading to Cyclic Compounds. R. Soc. Open Sci. 2018, 5, 181389. 10.1098/rsos.181389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Antilla J. C.; Baskin J. M.; Barder T. E.; Buchwald S. L. Copper-Diamine-Catalyzed N-Arylation of Pyrroles, Pyrazoles, Indazoles, Imidazoles, and Triazoles. J. Org. Chem. 2004, 69, 5578–5587. 10.1021/jo049658b. [DOI] [PubMed] [Google Scholar]; b Patra T.; Agasti S.; Akanksha A.-D.; Maiti D. Nickel-catalyzed Decyanation of Inert Carbon–cyano Bonds. Chem. Commun. 2013, 49, 69–71. 10.1039/c2cc36883h. [DOI] [PubMed] [Google Scholar]
- CCDC number for three compounds: 4i (1921810), 4t (1921809), and 4u (1921808).
- Booth B. L.; Jibodu K. O.; Proença M. F. The Synthesis and Some Reactions of N-Methylnitrilium Trifluoromethanesulphonate Salts. J. Chem. Soc., Chem. Commun. 1980, 1151–1153. 10.1039/c39800001151. [DOI] [Google Scholar]
- Motiwala H. F.; Vekariya R. H.; Aubé J. Intramolecular Friedel-Crafts Acylation Reaction Promoted by 1,1,1,3,3,3-Hexafluoro-2-propanol. Org. Lett. 2015, 17, 5484–5487. 10.1021/acs.orglett.5b02851. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.