Abstract
Background
Eupatilin, an active flavone separated from Artemisia species, has various biological activity such as anti-inflammatory activity. The aim of the present study was to find out the influence of eupatilin on lipopolysaccharide (LPS)-induced acute lung injury (ALI) in rats.
Material/Methods
The administration of LPS was used to induce ALI; eupatilin was given 1 hour before the LPS administration. Lung structural damage of rats was analyzed by hematoxylin and eosin staining and the wet/dry lung ratio. The related inflammatory factors and lung injury markers were examined by enzyme-linked immunosorbent assay. The oxidative stress factors were analyzed by corresponding kits. The expression of peroxisome proliferator-activated receptor-α (PPAR-α) was assayed by western blot and immunohistochemical staining.
Results
The results showed that eupatilin alleviated LPS-induced structural damage and decreased the wet/dry lung ratio concentration-dependently. Eupatilin decreased the level of surfactant protein (SP)-A, SP-D, and inflammatory factors such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and monocyte chemo-attractant protein (MCP)-1. LPS trigged nitric oxide (NO) generation, improved the production of malondialdehyde (MDA) and lactate dehydrogenase (LDH) and decreased the activity of superoxide dismutase (SOD), which were reversed when rats treated with eupatilin in a concentration-dependent way. Besides, the expression of PPAR-α was increased under the treatment of eupatilin.
Conclusions
Collectively, eupatilin alleviated LPS-induced ALI through inhibiting inflammation and oxidative stress in a concentration-dependent way, which was likely to be closely related with the activation of PPAR-α.
MeSH Keywords: Acute Lung Injury, Eupatorium, Lipopolysaccharides, Oxidative Stress, PPAR alpha
Background
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) (ALI/ARDS), characterized as serious pulmonary inflammation and pulmonary edema resulted from the destruction of the capillary-alveolar barrier, are mainly common pulmonary disease clinically [1]. ALI/ARDS are usually trigged by various lung pathologies, such as sepsis, pneumonia, and ischemia-reperfusion [2]. More and more evidence has demonstrated that oxidative stress, apoptosis, and inflammation are regarded as critical events involved in the pathological progression of ALI/ARDS [3–5]. In spite of the advances in the development in technology of respiratory support and management of critical care patients, ALI/ARDS still has a high mortality rate of up to 40% [2]. Therefore, seeking for innovative therapies and effective medications is in an urgent need.
Lipopolysaccharide (LPS) is a type of gram-negative bacterial product that induces pulmonary infection [6]. LPS exposure can lead to ALI. Thus, LPS has been applied to a mouse model established to simulate humans in various studies [7]. In the LPS-stimulated ALI mouse model study, LPS could activate nuclear factor kappa B (NF-κB) signaling and nuclear factor erythroid-2 related factor-2 (Nrf2)/hemeoxygenase-1 (HO-1) pathway, and accelerate the production of proinflammatory cytokines, and eventually result in inflammation response in lung tissue [3]. Thus, inhibiting the secretion of inflammatory cytokines and alleviating inflammatory damage are keys to ALI treatment.
Currently, herbal drugs have been popular and widespread. Increasing amounts of herbs and compounds isolated from them have been reported to be anti-inflammatory [8,9]. Eupatilin (5,7-dihydroxy-3,4,6-trimethoxyflavone), a pharmacologically active flavone isolated from Artemisia species, has a variety of biological activities such as neuroprotection, anti-cancer, anti-oxidation, and anti-inflammation [10–13]. It has been reported that eupatilin could inhibit NF-κB mediated inflammatory response [14], and suppress inflammatory cytokines production such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β in LPS-stimulated macrophages [15]. Besides, eupatilin acts as a selective peroxisome proliferator-activated receptor-α (PPAR-α) agonist and increases the transitivity and expression of PPAR-α in HaCaT cells [16]. However, as far as we know, there has been no relative research concern on the effect and function of eupatilin on ALI. As a consequence, the current study focused on exploring the role of eupatilin in LPS-stimulated ALI and finding out the potential mechanism of actions.
Material and Methods
Rats and ALI rat model establishment
Six to 8-week old male Sprague Dawley rats were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China). These rats were kept under standard conditions according to the animal experimental guideline set by the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Rats were randomized to 5 groups (n=10 for each group): control group, LPS group, and 3 LPS+eupatilin groups at 5,10, and 15 mg/kg LPS. Rats were treated intraperitoneally with 4 mg/kg LPS (Sigma, St. Louis, MO, USA) to stimulate ALI or an equal volume of normal saline (NS) as a vehicle control. Eupatilin (Chengdu Biopurity Phytochemicals Ltd., Sichuan, China) was given 1 hour before LPS treatment. All rats were euthanized by an intravenous injection of thiopental 24 hours following ALI induction. Then the thoraxes were opened, and blood was collected by cardiac puncture. The lungs were lavaged twice with 0.8 mL sterile saline each time to obtain bronchoalveolar lavage fluid (BALF). The BALF and blood were centrifuged at 2000 g for 10 minutes, and the supernatant and plasma were stored for further investigation.
Histological changes
After the rats were sacrificed, lungs were fixed in 4% paraformaldehyde for 24 hours embedded in paraffin, and cut at a thickness of 4 μm. Finally, histological changes of lungs were determined by hematoxylin and eosin (H&E) staining under a light microscope (Nikon, Tokyo, Japan). The severity of the lung injury was evaluated according to the methods described previously [17]. Six fields for each tissue were observed and evaluated. The average of values was regarded as the severity of lung injury.
Lung edema determination
The severity of pulmonary edema was determined by the lung wet/dry weight ratio (W/D). The right lungs were weighed and then dehydrated at 60°C for 72 hours in an oven to obtain the dry weight.
Enzyme-linked immunosorbent assay (ELISA)
The levels of IL-6, TNF-α, and monocyte chemo-attractant protein-1 (MCP-1) in BALF and pulmonary surfactant protein A (SP-A) and SP-D in blood were determined using the enzyme-linked immunosorbent assay (ELISA) kits for the corresponding cytokines (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Western blot
Proteins were isolated from the lungs via a tissue homogenizer with RIPA and PMSF. The concentration of protein was detected with a BCA Protein Assay Kit (Thermo Fisher Scientific, USA). Then proteins were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked and incubated at 4°C overnight with primary antibodies. Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies. Finally, bands were visualized by an enhanced chemiluminescence (ECL) kit (Amersham Biosciences, Buckinghamshire, UK).
Oxidative stress level assay
NO concentration in blood was determined by released NO metabolites (nitrates and nitrites) with the assay kit in accordance with the manufacturer’s instructions (Biovision Inc., USA). The lactate dehydrogenase (LDH) activity was determined via the Sigma LDH determination kit (Sigma-Aldrich, USA). The activity of superoxide dismutase (SOD) and content of malondialdehyde (MDA) in BALF were spectrophotometrically assessed according to the test kits following the manufacturer’s instructions (Nanjing Jiancheng Biotechnology Institute, China).
Immunohistochemical staining
Lungs were fixed in 4% paraformaldehyde, embedded in paraffin and cut into sections of 4 μm in thickness. Sections were incubated with primary antibodies against PPAR-α (Cell Signaling Technology). After washing with phosphate-buffered saline (PBS), these sections were incubated with EnVision+HRP/Rb (DAKO, Glostrup, Denmark) at room temperature for 30 minutes. The positive signals of tissue sections were detected using the DAB (3,3′-diaminobenzidine) substrate kit (ZSGB-BIO, China) following the manufacturer’s instructions. The images were caught under a light microscope (Nikon, Tokyo, Japan).
Statistical analysis
All data was shown as mean ± standard deviation of at least 3 biological replicates. The differences were analyzed by one-way analysis of variance using GraphPad Prism (GraphPad Software, La Jolla, CA. USA). * P<0.05 was considered as statistically significant.
Results
Eupatilin attenuated LPS-induced pulmonary histopathologic changes in rats
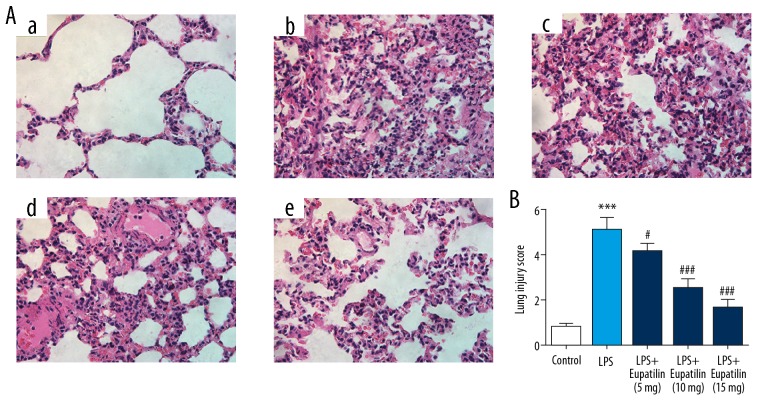
To observe the influence of eupatilin on LPS-induced ALI, the pulmonary histopathologic changes were detected by H&E staining. As seen in Figure 1A and 1B, compared to the control group, the LPS group exhibited severe histopathologic changes: thickened peri-bronchial wall, congested vascular in the lungs, and infiltrated inflammatory cells into the alveolar space. With the treatment of eupatilin, theses histopathologic changes were significantly reduced with a dose-dependent manner.
Figure 1.
Eupatilin attenuated LPS-induced pulmonary histopathologic changes in rats. (A) Histological changes of lung in different groups observed by H&E staining (400×). (a) Control group; (b) LPS group; (c) LPS+eupatilin (5 mg/kg) group; (d) LPS+eupatilin (10 mg/kg) group; (e) LPS+eupatilin (15 mg/kg) group. (B) Lung injury score of the differentially treated rats. *** P<0.001 versus control group; #, ### represented P<0.05, 0.001 versus LPS group, respectively. LPS – lipopolysaccharide; H&E – hematoxylin and eosin.
Eupatilin decreased lung injury in rats
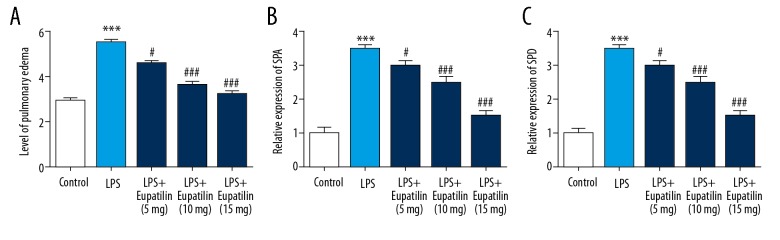
To observe the influence of eupatilin on lung injury induced by LPS, lung edema and lung surfactant were assessed. Lung edema was determined by lung wet/dry weight ratio. As shown in Figure 2A, LPS notably improved lung wet/dry weight ratio, while eupatilin obviously decreased lung wet/dry weight ratio. A change of expression level of SP-A and SP-D in the blood was a characteristic of lung injury. As shown in Figure 2B and 2C, the treatment of eupatilin was obviously decreased and the increased levels of SP-A and SP-D were dose-dependently caused by the stimulation of LPS.
Figure 2.
To explore the effect of eupatilin in LPS-induced lung injury, the pulmonary edema condition was assesed by lung wet/dry weight ratio (A). Besides, the lung injury markers such as SPA and SPD. The activity of SPA (B) and SPD (C) were determined by ELISA. The data are presented from 3 independent experiments. * Represented P<0.05 versus control group; #, ### represented P<0.05, 0.001 versus LPS group, respectively. LPS – lipopolysaccharide.
Eupatilin inhibited LPS-induced inflammatory cytokines secretion in rats
To explore the anti-inflammation of eupatilin in LPS-induced ALI, the production level of inflammatory cytokines such as TNF-α, IL-6, and chemotactic factor MCP-1 were analyzed by ELISA and western blot. The result revealed that the levels of IL-6, TNF-α, and MCP-1 obviously increased by LPS. However, secretion of IL-6, TNF-α, and MCP-1 significantly decreased with the treatment of eupatilin in a dose-dependent way (Figure 3).
Figure 3.
Eupatilin inhibited LPS-induced inflammatory cytokines production. The inflammatory cytokines such as IL-6, TNF-α, MCP-1 in BALF were detected by ELISA (A–C). The inflammatory cytokines such as IL-6, TNF-α, MCP-1 of lung tissue were analyzed by western blot (D). *** P<0.001 versus control group; #, ##, ### P<0.05, 0.01, and 0.001 versus LPS group, respectively. LPS – lipopolysaccharide; IL – interleukin; TNF – tumor necrosis factor; MCP – monocyte chemo-attractant protein.
Eupatilin alleviated LPS-induced oxidative stress in rats
As we can see from Figure 4, LPS triggered NO generation, and treatment with eupatilin decreased NO generation. Besides, the production of MDA and the activity of LDH were promoted, and the activity of SOD was decreased after LPS stimulation in rats. However, the treatment of eupatilin significantly decreased the production of MDA and the activity of LDH, and improved the activity of SOD dose-dependently.
Figure 4.
Eupatilin alleviated LPS-induced oxidative stress in lung. To investigate the effect of eupatilin in LPS-induced oxidative stress, the contents of NO (A), LDH (B) and MDA (C), and the activity of SOD (D) were detected in accordance with the manufactory’s instructions, respectively. *** P<0.001 versus control group; #, ##, ### P<0.05, 0.01, and 0.001 versus LPS group, respectively. LPS – lipopolysaccharide; LDH – lactate dehydrogenase; MDA – malondialdehyde; SOD – superoxide dismutase.
Eupatilin activated PPAR-α expression in rats
To assay the anti-lung injury mechanism of eupatilin, western blot and immunohistochemical staining were performed to analyze the effect of eupatilin on LPS-stimulated PPAR-α expression in lung tissues. As shown in Figure 5, both western blot and immunohistochemical staining results indicated that LPS inhibited expression of PPAR-α, and eupatilin promoted its expression in a dose-dependent manner.
Figure 5.
Eupatilin activated PPAR-α. The expression of PPAR-α of lung tissue was analyzed by western blot (A) and immunohistochemical staining (B), respectively. (a) Control group; (b) LPS group; (c) LPS+ Eupatilin (5 mg/kg) group; (d) LPS+ Eupatilin (10 mg/kg) group; (e) LPS+ Eupatilin (15 mg/kg) group. *** P<0.001 versus control group; ##, ### P<0.01, 0.001 versus LPS group, respectively. PPAR-α – peroxisome proliferator-activated receptor-α; LPS – lipopolysaccharide.
Discussion
ALI has been a major clinical problem worldwide, with a high incidence and mortality in recent years [18,19]. To date, the clinical practice of ALI mainly focuses on conservative fluid management and supportive ventilatory treatment, however, the optimal treatment strategy has still not been established [20]. Traditional medicines, such as phytopharmaceuticals, have been widely used for the treatment of various diseases for a long time, especially in Asia, and these plants are considered as Chinese Traditional Medicine (TCM). TCM has a variety of pharmacological effects, and there have been many studies that have demonstrated TCM protective role in ALI. Eriodictyol, a flavonoid extracted from Dracocephalum rupestre, was reported to attenuate ALI through its antioxidant activity [17]. Gastrodin, a phenolic glucoside isolated from Gastrodiaelata blume, showed its anti-inflammatory activity via inhibiting NF-κB pathway to function in ALI [19]. Thus, the development of TCM might be a suitable and effective strategy for the treatment of ALI.
In the current study, we focused on the roles of eupatilin, a flavonoid compound from Artemisia species, and provided some new ideas about the function of eupatilin and the treatment of ALI. First, a mouse model of ALI was established by LPS. Eupatilin (5, 10, and 15 mg/kg) were utilized to verify its effect. Here, we found that LPS lead to a great change of histopathology including destruction of cellular structures and infiltration of inflammatory cells. Eupatilin attenuated these histological alterations in a dose-dependent way. Besides, eupatilin also alleviated pulmonary edema by decreasing lung wet/dry weight ratio. Thus, eupatilin could inhibit histopathologic changes, lung injury, inflammation, and oxidative stress in rats induced by LPS, suggesting that eupatilin might be a protective drug for LPS-induce ALI.
A large amount of evidence demonstrated that oxidative stress and inflammation play an important role in the pathogenesis of ALI [21]. In this study, the levels of inflammatory cytokines (TNF-α and IL-6) and chemotactic factor (MCP-1) were detected using ELISA kits. As expected, all inflammatory mediators were dramatically increased with the stimulation of LPS, and they were obviously decreased with the treatment of eupatilin. The results suggested that eupatilin effectively alleviated pulmonary inflammation in LPS-stimulated ALI. Accumulation of neutrophil leads to the production of granular enzymes and reactive oxygen species (ROS), resulting in oxidative stress and lung tissue injury by activating an inflammatory cascade. Under oxidative stress, oxidative and antioxidative balance was disordered with the production of various oxidative and antioxidative products, such as NO, MDA, LDH, and SOD [22]. NO, a critical member in oxidative system, was significantly increased with the induction of LPS, which indicated an imbalance between oxidation system and antioxidant system. Meanwhile, as biomarkers of oxidative stress, MDA, LDH, and SOD changed with the induction of LPS. In LPS-stimulated ALI, the content of MDA and LDH increased, and the activity of SOD decreased. All these changes were significantly reversed by eupatilin. Thus, these results illustrated that the mechanism eupatilin protected from ALI might account for the inhibition of inflammatory response and oxidative stress.
PPAR receptors (PPARs), ligand-activated transcription factors, are members of the nuclear hormone receptor superfamily and exist as 3 isotypes: PPAR-α, PPAR-β (or PPARδ), and PPARγ [23,24]. All of the PPARs have been shown exert anti-inflammatory activity in diseases including lung disorders [25–27]. Thus far, the majority of studies focus on the function of PPAR-γ on ALI/ARDS, but only little data are published on the role of PPAR-α and pulmonary disease. PPAR-α−/− rats showed more amount of neutrophil and macrophage in BALF, as well as increased TNF-α and MCP-1 in LPS-induced airway injury, suggesting that PPAR-α activation might play a positive role in inflammatory airway and pulmonary disorders [23]. PPAR-α could be activated by kinds of natural occurring ligands to exhibit anti-inflammatory activity in vivo and in vitro [28]. It is interesting that previous studies have demonstrated that eupatilin could act as a selective PPAR-α agonist and specially activate PPAR-α through direct binding in HaCaT cells [16]. Besides, eupatilin could activate PPAR-α to block NF-κB p65 nuclear translocation, which acts as a major controller of inflammatory response. In this study, we also found that in LPS-induced ALI, the activity of PPAR-α was suppressed, while eupatilin could activate PPAR-α and increase its expression significantly. Hence, we considered that eupatilin play a protective role in ALI by inhibiting oxidative stress and inflammation, which was closely linked with the activation of PPAR-α, and PPAR-α might play a critical role in the treatment of ALI.
Conclusions
The treatment of eupatilin could alleviate LPS-induced lung injury by suppressing inflammation and oxidative stress in a concentration-dependent manner. The protective role of eupatilin in ALI might be closely linked with the activation of PPAR-α. However, the deeply mechanism action and clinical application of eupatilin are deserved to be further investigated.
Footnotes
Conflict of interests
None.
Source of support: This study was supported by the funding from Sanming Project of Medicine in Shenzhen (No. SZSM201812056)
References
- 1.Matthay MA, Ware LB, Zimmerman GA. The acute respiratory distress syndrome. J Clin Invest. 2012;122(8):2731–40. doi: 10.1172/JCI60331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(19):1904–5. doi: 10.1056/NEJMc1711824. [DOI] [PubMed] [Google Scholar]
- 3.Jiang W, Luo F, Lu Q, et al. The protective effect of Trillin LPS-induced acute lung injury by the regulations of inflammation and oxidative state. Chem Biol Interact. 2016;243:127–34. doi: 10.1016/j.cbi.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Lin WC, Chen CW, Huang YW, et al. Kallistatin protects against sepsis-related acute lung injury via inhibiting inflammation and apoptosis. Sci Rep. 2015;5:12463. doi: 10.1038/srep12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jing W, Chunhua M, Shumin W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-kappaB pathway in vivo and in vitro. Toxicol Appl Pharmacol. 2015;285(2):128–35. doi: 10.1016/j.taap.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Tao Z, Yuan Y, Liao Q. Alleviation of lipopolysaccharides-induced acute lung injury by mir-454. Cell Physiol Biochem. 2016;38(1):65–74. doi: 10.1159/000438609. [DOI] [PubMed] [Google Scholar]
- 7.Yang YI, Jung SH, Lee KT, Choi JH. 8,8′-Bieckol, isolated from edible brown algae, exerts its anti-inflammatory effects through inhibition of NF-kappaB signaling and ROS production in LPS-stimulated macrophages. Int Immunopharmacol. 2014;23(2):460–68. doi: 10.1016/j.intimp.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 8.He DY, Dai SM. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora Pall., a traditional Chinese herbal medicine. Front Pharmacol. 2011;2:10. doi: 10.3389/fphar.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MJ, Vargnolle N, McKnight W, et al. Inhibition of neurogenic inflammation by the Amazonian herbal medicine sangre de grado. J Invest Dermatol. 2001;117(3):725–30. doi: 10.1046/j.0022-202x.2001.01446.x. [DOI] [PubMed] [Google Scholar]
- 10.Nageen B, Sarfraz I, Rasul A, et al. Eupatilin: A natural pharmacologically active flavone compound with its wide range applications. J Asian Nat Prod Res. :2018. doi: 10.1080/10286020.2018.1492565. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Cheong JH, Hong SY, Zheng Y, Noh SH. Eupatilin inhibits gastric cancer cell growth by blocking STAT3-mediated VEGF expression. J Gastric Cancer. 2011;11(1):16–22. doi: 10.5230/jgc.2011.11.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai M, Phan PT, Hong JG, et al. The neuroprotective effect of eupatilin against ischemia/reperfusion-induced delayed neuronal damage in mice. Eur J Pharmacol. 2012;689(1–3):104–10. doi: 10.1016/j.ejphar.2012.05.042. [DOI] [PubMed] [Google Scholar]
- 13.Yu K, Li XM, Xu XL, et al. Eupatilin protects against tumor necrosis factor-alpha-mediated inflammation inhuman umbilical vein endothelial cells. Int J Clin Exp Med. 2015;8(12):22191–97. [PMC free article] [PubMed] [Google Scholar]
- 14.Chang JW, Hwang HS, Kim YS, et al. Protective effect of Artemisia asiatica (Pamp.) Nakai ex Kitam ethanol extract against cisplatin-induced apoptosis of human HaCaT keratinocytes: Involvement of NF-kappa B- and Bcl-2-controlled mitochondrial signaling. Phytomedicine. 2015;22(6):679–88. doi: 10.1016/j.phymed.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Choi EJ, Lee S, Chae JR, et al. Eupatilin inhibits lipopolysaccharide-induced expression of inflammatory mediators in macrophages. Life Sci. 2011;88(25–26):1121–26. doi: 10.1016/j.lfs.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Jung Y, Kim JC, Choi Y, et al. Eupatilin with PPARalpha agonistic effects inhibits TNFalpha-induced MMP signaling in HaCaT cells. Biochem Biophys Res Commun. 2017;493(1):220–26. doi: 10.1016/j.bbrc.2017.09.043. [DOI] [PubMed] [Google Scholar]
- 17.Zhu GF, Guo HJ, Huang Y, et al. Eriodictyol, a plant flavonoid, attenuates LPS-induced acute lung injury through its antioxidative and anti-inflammatory activity. Exp Ther Med. 2015;10(6):2259–66. doi: 10.3892/etm.2015.2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brun-Buisson C, Minelli C, Bertolini G, et al. Epidemiology and outcome of acute lung injury in European intensive care units. Results from the ALIVE study. Intensive Care Med. 2004;30(1):51–61. doi: 10.1007/s00134-003-2022-6. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Z, Zhou J, Song D, et al. Gastrodin protects against LPS-induced acute lung injury by activating Nrf2 signaling pathway. Oncotarget. 2017;8(19):32147–56. doi: 10.18632/oncotarget.16740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson ER, Matthay MA. Acute lung injury: Epidemiology, pathogenesis, and treatment. J Aerosol Med Pulm Drug Deliv. 2010;23(4):243–52. doi: 10.1089/jamp.2009.0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lang JD, McArdle PJ, O’Reilly PJ, Matalon S. Oxidant-antioxidant balance in acute lung injury. Chest. 2002;122(6 Suppl):314S–20S. doi: 10.1378/chest.122.6_suppl.314s. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigo S, Rodriguez L, Otero P, et al. Fructose during pregnancy provokes fetal oxidative stress: The key role of the placental heme oxygenase-1. Mol Nutr Food Res. 2016;60(12):2700–11. doi: 10.1002/mnfr.201600193. [DOI] [PubMed] [Google Scholar]
- 23.Delayre-Orthez C, Becker J, Guenon I, et al. PPARalpha downregulates airway inflammation induced by lipopolysaccharide in the mouse. Respir Res. 2005;6:91. doi: 10.1186/1465-9921-6-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker J, Delayre-Orthez C, Frossard N, Pons F. Regulation of inflammation by PPARs: A future approach to treat lung inflammatory diseases? Fundam Clin Pharmacol. 2006;20(5):429–47. doi: 10.1111/j.1472-8206.2006.00425.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang YL, Gou XY, He W, et al. Effects of alliin on LPS-induced acute lung injury by activating PPARγ. Microb Pathog. 2017;110:375–79. doi: 10.1016/j.micpath.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Bao XC, Fang YQ, You P, et al. Protective role of peroxisome proliferator-activated receptor beta/delta in acute lung injury induced by prolonged hyperbaric hyperoxia in rats. Respir Physiol Neurobiol. 2014;199:9–18. doi: 10.1016/j.resp.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Yoo SH, Abdelmegeed MA, Song BJ. Activation of PPAR-alpha by Wy-14643 ameliorates systemic lipopolysaccharide-induced acute lung injury. Biochem Biophys Res Commun. 2013;436(3):366–71. doi: 10.1016/j.bbrc.2013.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyama T, Nakamura H, Harano Y, et al. PPARalpha ligands activate antioxidant enzymes and suppress hepatic fibrosis in rats. Biochem Biophys Res Commun. 2004;324(2):697–704. doi: 10.1016/j.bbrc.2004.09.110. [DOI] [PubMed] [Google Scholar]