Abstract
Background
Spinal cord injury (SCI) is a severe devastating condition associated with serious disability and neurologic deficits. Aberrant micro RNA (miRNA) expression has been related to a variety of central nervous system diseases including SCI. In the present study, we aimed to discover the role of miR-129-5p on SCI.
Material/Methods
An acute SCI rat model was induced, following the modified Allen method. A total of 36 rats were randomly assigned into 4 groups (n=9 in every group): Sham group; Model group (SCI+saline); SCI+NC group; and SCI+miR-129-5p group (100 nm solution, every 2 days). Basso-Beattie-Bresnahan (BBB) locomotor rating score was carried out to determine functional recovery. TUNEL (terminal dUTP nick-end labeling) staining was used to evaluate cell apoptosis. Hematoxylin and eosin staining was performed to assess the pathological state of spinal cord. Furthermore, western blot assay was conducted to measure the calpain1 and calpain2 expression.
Results
Our data suggested that the expression level of miR-129-5p was markedly reduced in rats after SCI. Then miR-129-5p mimic was injected into the vertebral canal. We found that the SCI+miR-129-5p group had a high score in the BBB test compared with the SCI+NC group and the Model group. The overexpression of miR-129-5p obviously reduced tissue loss, damaged cells, and the number of TUNEL positive cells. Moreover, western blot assay exhibited that overexpression of miR-129-5p decreased calpain1, calpain2, and cleaved caspase-3 expression.
Conclusions
Our findings suggested that overexpression of miR-129-5p improved neurological function by promoting functional recovery, reducing tissue loss and cell apoptosis in rats in an SCI model, possibly through downregulation of calpain1 and calpain2.
MeSH Keywords: Apoptosis, Calpain, MicroRNAs, Spinal Cord Injuries
Background
Spinal cord injury (SCI) is a severe devastating condition associated with serious disability and neurologic deficits, causing permanent disability to at least 180 000 people per year worldwide [1], which brings a heavy financial and psychosocial burden to individuals and their families. The proportion of traumatic SCI from land transport is increasing especially in developing countries due to the poor infrastructure and regulatory challenges of transportation [1]. SCI-induced clinical symptoms, such as spasticity and mechanical allodynia, are difficult to manage and harder to completely overcome [2,3]. Currently, there is no effective treatment for SCI, and treatment is mostly limited to supportive measures. It is of great importance to understand the progress of SCI and explore the underlying molecular mechanism.
MicroRNAs (miRNAs) are a single-stranded non-coding RNA, whose transcripts are about 20 nucleotides long. These small RNAs contribute to the messenger RNA (mRNA) degradation or transcriptional inhibition via binding directly to target mRNAs by specificity [4], and are involved in about 30% of human protein-coding genes regulation [5]. MiRNAs are also critical regulators for the dynamic nature of both the development and activity of the nervous system [6]. A growing body of evidence has suggested that aberrant miRNA expression was related to a variety of central nervous system diseases including SCI [7]. SCI affects miRNAs expression, and aberrant miRNA expression has also emerged as a key regulator of processes of SCI such as inflammation, cell death, and regeneration [7,8]. The recent study disclosed that miR-129-5p expression was obviously downregulated after spinal cord ischemia-reperfusion (IR) [9]. Intrathecal pretreatment with miR-129-5p mimic ameliorates neuroinflammation and blood-spinal cord barrier damage by downregulating HMGB1 expression and inhibiting TLR3-cytokine pathway activation [10]. These findings suggest that miR-129-5p may provide the protective effect in spinal cord IR. However, it still remains unclear as to the role of miR-129-5p in acute SCI. Calpain, a Ca2+-activated ubiquitous intracellular protease, perturbs the integrity and stability of cells through the truncation of cytoskeletal and membrane proteins activated after SCI. Besides, upregulation of calpain promotes apoptosis by activating caspases, most notably caspase-3. Moreover, TargetScan Human 7.1 (www.targetscan.org) was carried out to find that calpain1 is a potential target of miR-129-5p.
In the present study, we aimed to investigate the role of miR-129-5p in SCI. We found the expression of miR-129-5p was obviously decreased in a rat model of SCI induced by the modified Allen’s weight drop apparatus. Then miR-129-5p mimic was injected into the vertebral canal of rats. Overexpression of miR-129-5p obviously improved neurological function by promoting functional recovery, reducing tissue loss and neuronal apoptosis after SCI. Meanwhile, the expression of caspase-3, calpain1, and calpain2 were significantly decreased.
Material and Methods
Animals and treatment
Thirty-six adult male Sprague-Dawley rats (weighing between 200 and 250 g), purchased from the Shanghai SLAC Laboratory Animal Co. Ltd, were utilized and kept in a normal light cycle with normal diet. After 4-hour deprivation of water and food, intraperitoneal injection with ketamine (80 mg/kg) and xylazine (40 mg/kg), a dorsal longitudinal incision was made to expose spinal segment T9-T11. Then a laminectomy was performed at T10, spinal cord contusions were induced using a modified Allen’s weight drop apparatus (40 g·cm) to establish a rat model of SCI. Rats of the Sham group received laminectomy only. This study was carefully performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (GB 14925-2001).
The animals were randomly assigned into 4 groups (n=9 in every group): Sham group; Model group (SCI+saline); SCI+NC group (100 nmol negative control mimic); SCI+miR-129-5p group (100 nmol miR-129-5p mimic). For the SCI+NC group and SCI+miR-129-5p group, intrathecal injections were repeatedly performed for every 2 days. 100 nmol of miR-129-5p mimic and negative control mimic with Lipofectamine 2000 (Invitrogen, MA, USA) was injected into the vertebral canal of rats after anesthetization to modulate in vivo miR-129-5p expression as previously described [10]. The sequences of mimic are as follow:
miR-129-5p mimic: 5′-CUUUUUGCGGUCUGGGCUUGC-3′, 5′-AAGCCCAGACCGCAAAAAGUU-3′;
negative control mimic: 5′-UUCUCCGAACGUGUCACGUTT-3, 5′-ACGUGACACGUUCGGAGAATT-3.
At 12 hours, 3 days, and 14 days after BBB score, the spinal cord tissues were excised for further experiments.
Hematoxylin and eosin (H&E) staining
At 12 hours, 3 days, and 14 days after BBB score, the rats were anesthetized and per-fused transcardially with 200 mL of phosphate-buffered saline (PBS) (0.1 mol/L, pH 7.4), followed by 400 mL of PBS (pH 7.4) with 4% paraformaldehyde (PFA). The injury epicenter (about 3-cm-long piece) was excised from the spinal cord and post-fixed in 4% PFA overnight. Then fixed tissues were embedded in paraffin and serially sectioned into 4-μm thick coronal slices and stained with Hematoxylin-Eosin/HE kit (Solarbio Science & Technology, Beijing, China) following standard protocols.
Basso, Beattie and Bresnahan (BBB) score
BBB score was used to evaluate the rats’ neurological function [11]. The BBB score criteria were divided into 21 scores (0 for no observable hind limb movement and 21 for normal locomotion). Scores from 0 to 7 (early stage of recovery), indicated little or no hind limb movement of rats; scores from 8 to 13 (intermediate stage of recovery), indicated uncoordinated steps of rats; scores from 14 to 21 (late stage of recovery) indicated coordination of forelimb and hind limb of rats. BBB testing was performed at 12 hours, 1 day, 3 days, 7 days, and 14 days after spinal cord surgery.
Terminal dUTP nick-end labeling (TUNEL) staining
Cell apoptosis was calculated by using the terminal dUTP nick-end labeling (TUNEL) Assay Kit (Yeasen, Shanghai, China) following the manufacturer’s instructions. The localized green fluorescence of apoptotic cells was observed under fluorescence microscopy (Axiovert 100M, Zeiss, Oberkochen, Germany) at 400× magnification. The results were expressed as average number of TUNEL-positive cells per field in each group. Data were collected from 3 independent experiments.
Western blot analysis
The protein was extracted from spinal cord tissues using a radioimmunoprecipitation assay (RIPA) lysis buffer kit (BioTek, Winooski, VT, USA), and equal amount of protein extractions were separated by 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore Corporation, Billerica, MA, USA). Blots from spinal cord samples were incubated with 5% skimmed milk at room temperature for 1 hour. Membranes were incubated with the primary antibodies overnight at 4°C, followed by secondary antibodies incubation for 2 hours at room temperature. Peroxidase activity was visualized with an electrochemiluminescent (ECL) detection reagent (Millipore). The antibodies used were listed as follow: anti-calpain-1 (sc-271313, Santa Cruz, 1: 500), anti-calpain-2 (sc-373966, Santa Cruz, 1: 500), anti-GAPDH (sc-47724, Santa Cruz, 1: 1000), m-IgGκ BP-HRP (sc-516102, Santa Cruz, 1: 10 000).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated with TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.) according to the supplier’s protocols. The first-strand cDNA was obtained using the TaqMan MicroRNA Reverse Transcription Kit (Takara, Dalian, China) with special stem-loop primers. RT-qPCR was carried out using a Perfect Real Time SYBR Premix Ex Taq Kit (Takara). All procedures were performed according to the protocols of the corresponding manufacturer. U6 was used as control for the expression of miRNA. The reaction conditions for PCR included an initial denaturation of 3 minute at 95°C, followed by 40 cycles of 95°C for 30 seconds, 60°C for 30 seconds. The relative expressions of miRNA were analyzed by the 2−ΔΔCq method. Independent experiments were repeated 3 times.
Statistical analysis
GraphPad Prism version 6 (GraphPad Prism version 6.0, Inc., La Jolla, CA, USA) was used to analyze the statistical data. Data was expressed as mean ± standard deviation. Significant differences were analyzed using Student’s t-test or analysis of variance (ANOVA). P<0.05 was considered to indicate a significant difference.
Results
The expression of miR-129-5p was decreased in SCI rats
RT-qPCR assay was conducted to measure the level of miR-129-5p after BBB locomotor rating score at 12 hours, 3 days, and 14 days following spinal cord surgery. The results showed that the expression of miR-129-5p in the injured group significantly reduced from 12 hours after SCI compared with the Sham group (Figure 1). In order to further explore the role of this miRNA in SCI, miR-129-5p mimic was injected into the vertebral canal of rats after anesthetization every 2 days (Figure 1).
Figure 1.
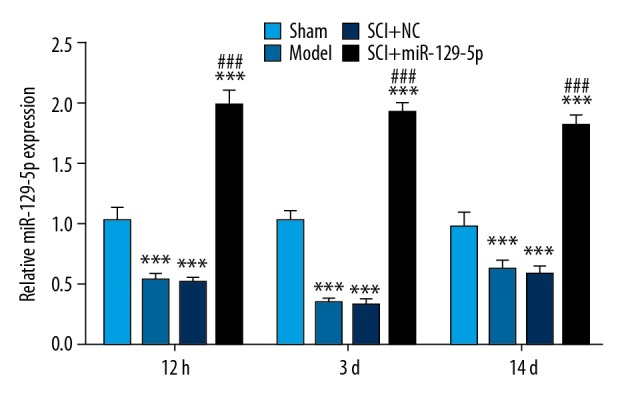
The expression of miR-129-5p was decreased in SCI rats. RT-qPCR was performed to detect the expression of miR-129-5p at 12h, 3d and 14d following spinal cord surgery. *** P<0.001 versus Sham; ### P<0.001 versus SCI+NC. miRNA – microRNA; SCI – spinal cord injury; RT-qPCR – real-time quantitative polymerase chain reaction; NC – negative control mimic.
Overexpression of miR-129-5p improved neurological function in SCI rats
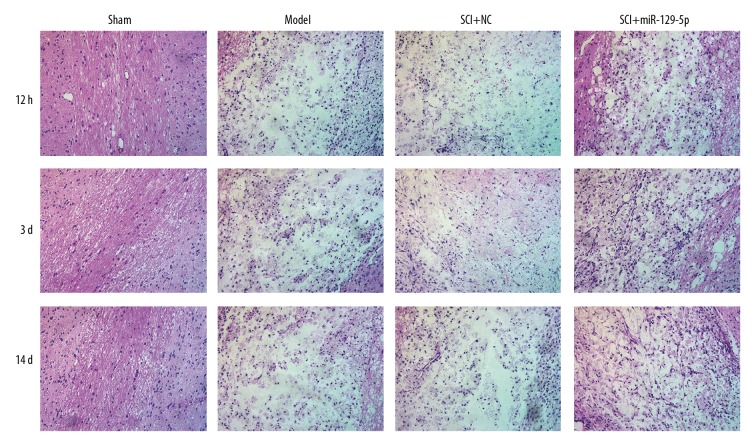
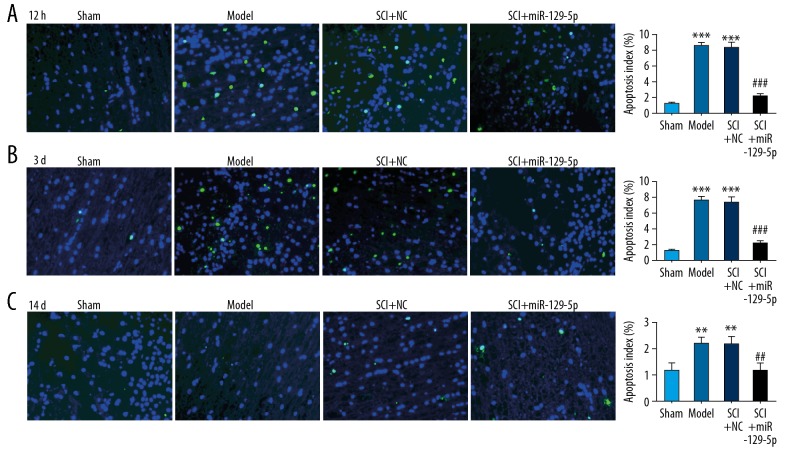
The BBB locomotor test was performed to assess neurological function of rats’ hind limbs. We found that the SCI+miR-129-5p group exhibited functional recovery at 1 day, and the effect improved with time increased lasted until the 14 days after SCI, compared with the SCI+NC group (Figure 2). However, there was no significant difference between the SCI+NC group and Model group. The results of H&E staining demonstrated that SCI induced and histological changes of cells including neuronal swelling and shrunken neurons with darkly stained, condensed nuclei (Figure 3). Meanwhile, the tissue loss and irregularly arranged were widely observed in SCI+NC group and Model group (Figure 3). While in SCI+miR-129-5p group, the number of damaged cells was markedly decreased, and the morphology of the injured segments showed reduced diameters with the formation of cavities (Figure 3). TUNEL staining was performed to assess cell apoptosis. The results showed that in SCI+NC and Model groups, positive cells were markedly increased and widely distributed, compared with Sham group (Figure 4). Compared with the SCI+NC group, overexpression of miR-129-5p dramatically reduced the number of TUNEL-positive neurons (Figure 4).
Figure 2.
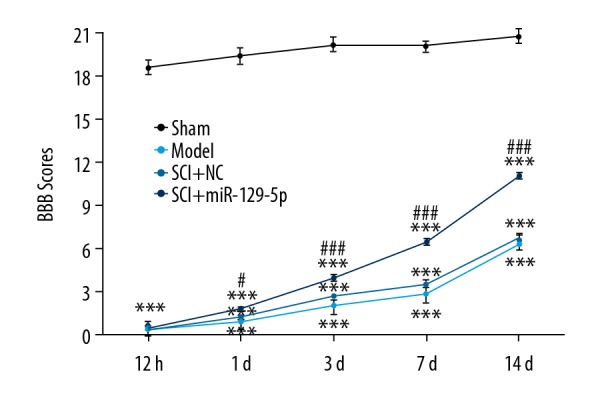
Overexpression of miR-129-5p improved functional recovery in rats after SCI. BBB locomotor rating score was performed to assess functional recovery of rats’ hind limbs at 12 hours, 1 day, 3 days, 7 days, and 14 days following spinal cord surgery. *** P<0.001 versus Sham; # P<0.05, ### P<0.001 versus SCI+NC. miRNA – microRNA; SCI – spinal cord injury; BBB – Basso-Beattie-Bresnahan; NC – negative control mimic.
Figure 3.
Overexpression of miR-129-5p reduce tissue loss and damaged cells. H&E staining was performed to assess histological changes at 12h, 3d and 14d following spinal cord surgery. H&E – hematoxylin and eosin.
Figure 4.
Overexpression of miR-129-5p reduce cell apoptosis. TUNEL staining was performed at 12 hours (A), 3 days (B), and 14 days (C) following spinal cord surgery. ** P<0.01, *** P<0.001 versus Sham; ## P<0.01, ### P<0.001 versus SCI+NC. TUNEL – terminal dUTP nick-end labeling; SCI – spinal cord injury; NC – negative control mimic.
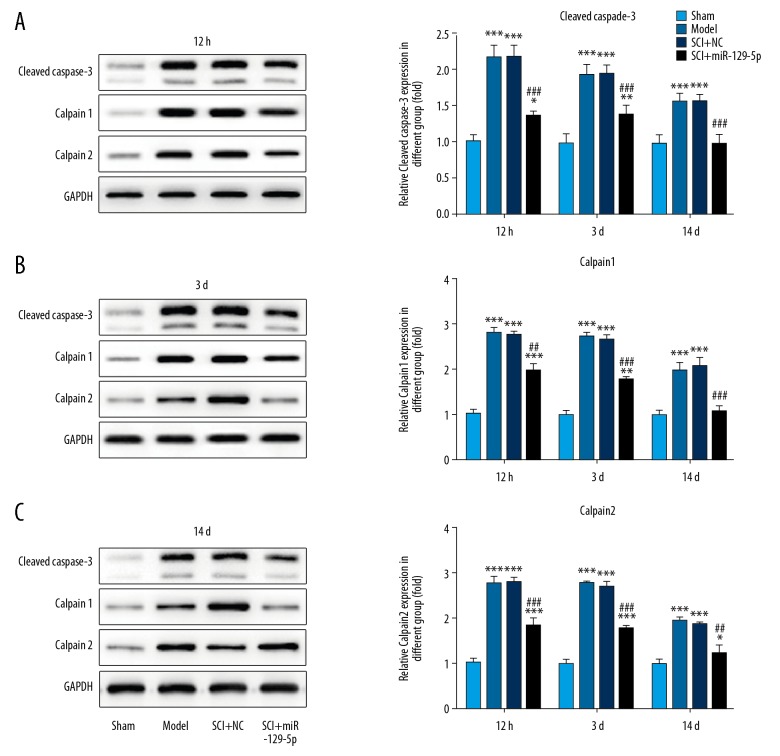
Overexpression of miR-129-5p decreased caspase-3, calpain1, and calpain2 expression
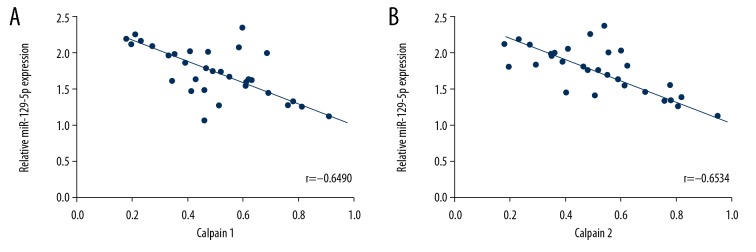
To further investigate the potential mechanism of protective effect of miR-129-5p on SCI, western bolt assay was performed. The results of western bolt assays showed that the expression of cleaved caspase-3, calpain1, and calpain2 protein were significantly increased after injury. While, upregulation of miR-129-5p markedly decreased cleaved caspase-3, calpain1, and calpain2 expression (Figure 5). Besides, an inverse relationship between calpain1 (r=−0.6490) or calpain2 (r=−0.6534) expression and miR-129-5p were observed in spinal cord tissues (Figure 6).
Figure 5.
Overexpression of miR-129-5p decreased caspase-3, calpain1, and calpain2 expression. Western blot assay was conducted to detect the protein expression of caspase-3, calpain1, and calpain2. * P<0.05, *** P<0.001 versus Sham; ## P<0.05, ### P<0.001 versus SCI+NC. SCI – spinal cord injury; NC – negative control mimic.
Figure 6.
The inverse correlation between calpain1 (r=−0.6490, P<0.001) (A) or calpain2 (r=−0.6534, P<0.001) (B) expression with miR-129-5p.
Discussion
In recent years, the role of miRNAs in SCI has been highlighted due to their potential in diagnosis and treatment. In present study, we found that the level of miR-129-5p was obviously reduced with the injury time increased. Then miR-129-5p mimic was injected into the vertebral canal of rats after anesthetization. Overexpression of miR-129-5p obviously improved neurological function by promoting functional recovery, reducing tissue loss and neuronal apoptosis after SCI. In follow-up experiments, upregulation of miR-129-5p significantly decreased caspase-3, calpain1, and calpain2 expression. Importantly, through data analysis, we found an inverse correlation between calpain1 or calpain2 expression with miR-129-5p in spinal cord tissues. These results imply that the expression of miR-129-5p was markedly reduced after SCI. And overexpression of miR-129-5p promoted functional recovery, inhibited tissue loss and cell apoptosis in rats after SCI, possibly through downregulation of calpain1 and calpain2.
SCI affects miRNAs expression, and aberrant expression of miRNAs is closely associated with various secondary injury responses including inflammation, oxidative stress, and apoptosis [7]. A growing body of evidence has suggested that various miRNAs are dysregulated in SCI animal models. For example, miR-137 is downregulated in rats after SCI, overexpression of miR-137 reduce the inflammation level, apoptosis and oxidative stress of the secondary injury responses [12,13]. Juan et al. compared miRNA levels between the Mexican axolotl salamander (Ambystoma mexicanum) and rat following spinal cord injury. MiR-125b was chosen for further study, and the authors found that miR-125b was essential for functional recovery, and it guides correct regeneration of axons through the lesion site via direct downstream target Sema4D in axolotls [14]. MiR-129-5p acts as a tumor suppressor and has been widely studied in different cancer including colon cancer [15], non-small cell lung cancer [16], bone cancer [17], and breast cancer [18]. However, the role of miR-129-5p in the acute SCI still remains unclear. A recent study suggested that miR-129-5p expression was obviously downregulated after spinal cord IR. Moreover, increasing miR-129-5p levels played a protective role against IR [10]. Consistent with the previous study, in the present study, the expression of miR-129-5p showed a rapid ~40% reduction at 12 hours after injury, which was found to decrease until 14 days. Thereby, miR-129-5p, might be a promising biomarker of the development of SCI. And the functional assays have demonstrated that miR-129-5p provided the protective effect of the spinal cord injury in rats, so it could be a potentially therapeutic target against SCI. Moreover, western blot assays showed that the expression of calpain1, calpain2, and cleaved caspase-3 were decreased after overexpression of miR-129-5p. Calpain, which activated by aberrant Ca2+ homeostasis following SCI, has been shown to play a crucial role in the pathophysiology of SCI [19]. Administration of specific inhibitors of calpain has provided significant neuroprotection in experimental animal models of SCI [20]. Yu et al. demonstrated that calpain1 knockdown significantly reduced pathological damage and improved total tissue sparing through pre-injury intraspinal administration of LV-CAPN1shRNA [21]. Our data showed that overexpression of miR-129-5p significantly reduced the levels of calpain1, calpain2, and caspase-3. And there was an inverse correlation between calpain1 or calpain2 expression with miR-129-5p in spinal cord tissues, indicating that overexpression of miR-129-5p exerted the functions likely through the negative-regulation of calpain1 and calpain2.
Due to the serious situation of SCI, researcher have been focusing more and more attention on SCI therapy, and made some progress in basic research and/or clinical trials [22]. For example, Yan X et al. found that botulinum toxin A, derived from Clostridium botulinum, might be an effective therapeutic option for SCI-induced spasticity in clinical trials [23]. Wu Y et al. reported a therapeutic effect of Erbin inhibitor on spinal cord contusion in mice [24]. Besides, with the advancement in biotechnology, cell therapy using cell transplantation is also under investigation for SCI treatment [2,22]. Nevertheless, more efforts should be devoted to diagnosis, therapy, and prognosis to improve the living environment of SCI patients. In this study, we discovered a novel protective miRNA against SCI. The role of miR-129-5p in acute SCI was first reported in our study. Besides, we disclosed the potential molecular mechanism of improved acute SCI by miR-129-5p overexpression. The relation of miR-129-5p and calpain was also first disclosed in this study, laying a foundation for the further exploration concerning on miR-129-5p, calpain1, and calpain2 in acute SCI, even other relative diseases, and providing a new diagnosed marker or therapy strategy for SCI. However, there were some limits to this study. Firstly, in our study, we used H&E staining and BBB score to determine the degree of SCI, more detection methods, such as immunofluorescence staining and Nissl staining, could also be performed to show more detail and data concerned the changes of each experimental group, enriching the content of this study. Besides, this study was conducted as an in vivo experiment only; experiments in vitro are also needed to verify these findings and provide more in-depth research on potential molecular mechanism. For example, just as we stated before, the relation of miR-129-5p and calpain was reported in this study, and whether calpain1 or calpain2 was a direct target of miR-129-5p needs to be identified in vitro. Finally, propulsion research is needed to promote the application of miR-129-5p and more miRNAs in clinical.
Conclusions
MiR-129-5p was found to decrease in a time-dependent manner after SCI. Overexpression of miR-129-5p obviously improved neurological function by promoting functional recovery, and reducing tissue loss and neuronal apoptosis after SCI, possibly through downregulation of calpain1 and calpain2. Although further experiments are needed, miR-129-5p could be a potentially therapeutic target and a biomarker of against SCI.
Footnotes
Source of support: Departmental sources
References
- 1.Lee BB, Cripps RA, Fitzharris M, Wing PC. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord. 2014;52(2):110–16. doi: 10.1038/sc.2012.158. [DOI] [PubMed] [Google Scholar]
- 2.Zeng Y, Han H, Tang B, et al. Transplantation of recombinant vascular endothelial growth factor (VEGF) 189-neural stem cells downregulates transient receptor potential vanilloid 1 (TRPV1) and improves motor outcome in spinal cord injury. Med Sci Monit. 2018;24:1089–96. doi: 10.12659/MSM.905264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hefter H, Jost WH, Reissig A, et al. Classification of posture in poststroke upper limb spasticity: A potential decision tool for botulinum toxin A treatment? Int J Rehabil Res. 2012;35(3):227–33. doi: 10.1097/MRR.0b013e328353e3d4. [DOI] [PubMed] [Google Scholar]
- 4.Winter J, Jung S, Keller S, et al. Many roads to maturity: MicroRNA biogenesis pathways and their regulation. Nature Cell Biol. 2009;11(3):228–34. doi: 10.1038/ncb0309-228. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136(2):215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochella L, Hobert O. Diverse functions of microRNAs in nervous system development. Curr Top Dev Biol. 2012;99:115–43. doi: 10.1016/B978-0-12-387038-4.00005-7. [DOI] [PubMed] [Google Scholar]
- 7.Nieto-Diaz M, Esteban FJ, Reigada D, et al. MicroRNA dysregulation in spinal cord injury: Causes, consequences and therapeutics. Front Cell Neurosci. 2014;8:53. doi: 10.3389/fncel.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Gasperi R, Graham ZA, Harlow LM, et al. The signature of microRNA dysregulation in muscle paralyzed by spinal cord injury includes downregulation of microRNAs that target myostatin signaling. PLoS One. 2016;11(12):e0166189. doi: 10.1371/journal.pone.0166189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhai F, Zhang X, Guan Y, et al. Expression profiles of microRNAs after focal cerebral ischemia/reperfusion injury in rats. Neural Regen Res. 2012;7(12):917–23. doi: 10.3969/j.issn.1673-5374.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li XQ, Chen FS, Tan WF, et al. Elevated microRNA-129-5p level ameliorates neuroinflammation and blood-spinal cord barrier damage after ischemia-reperfusion by inhibiting HMGB1 and the TLR3-cytokine pathway. J Neuroinflammation. 2017;14(1):205. doi: 10.1186/s12974-017-0977-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rong W, Pan YW, Cai X, et al. The mechanism of naringin-enhanced remyelination after spinal cord injury. Neural Regen Res. 2017;12(3):470–77. doi: 10.4103/1673-5374.202923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao L, Dai C, Feng Z, et al. MiR-137 inhibited inflammatory response and apoptosis after spinal cord injury via targeting of MK2. J Cell Biochem. 2018;119(4):3280–92. doi: 10.1002/jcb.26489. [DOI] [PubMed] [Google Scholar]
- 13.Dai J, Xu LJ, Han GD, et al. MiR-137 attenuates spinal cord injury by modulating NEUROD4 through reducing inflammation and oxidative stress. Eur Rev Med Pharmacol Sci. 2018;22(7):1884–90. doi: 10.26355/eurrev_201804_14709. [DOI] [PubMed] [Google Scholar]
- 14.Diaz Quiroz JF, Tsai E, Coyle M, et al. Precise control of miR-125b levels is required to create a regeneration-permissive environment after spinal cord injury: A cross-species comparison between salamander and rat. Dis Model Mech. 2014;7(6):601–11. doi: 10.1242/dmm.014837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu Q, Meng WY, Jie Y, Zhao H. LncRNA MALAT1 induces colon cancer development by regulating miR-129-5p/HMGB1 axis. J Cell Physiol. 2018;233(9):6750–57. doi: 10.1002/jcp.26383. [DOI] [PubMed] [Google Scholar]
- 16.Ma Z, Cai H, Zhang Y, et al. MiR-129-5p inhibits non-small cell lung cancer cell stemness and chemoresistance through targeting DLK1. Biochem Biophys Res Commun. 2017;490(2):309–16. doi: 10.1016/j.bbrc.2017.06.041. [DOI] [PubMed] [Google Scholar]
- 17.Yu SN, Liu GF, Li LY, et al. Analgesic effects of microRNA-129-5p against bone cancer pain through the EphB1/EphrinB2 signaling pathway in mice. J Cell Biochem. 2017;120(3):2876–85. doi: 10.1002/jcb.26605. [DOI] [PubMed] [Google Scholar]
- 18.Meng R, Fang J, Yu Y, et al. miR-129-5p suppresses breast cancer proliferation by targeting CBX4. Neoplasma. 2018;65(4):572–78. doi: 10.4149/neo_2018_170814N530. [DOI] [PubMed] [Google Scholar]
- 19.Ray SK, Shields DC, Saido TC, et al. Calpain activity and translational expression increased in spinal cord injury. Brain Res. 1999;816(2):375–80. doi: 10.1016/s0006-8993(98)01128-7. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Huang Z, Dai H, et al. Therapeutic efficacy of E-64-d, a selective calpain inhibitor, in experimental acute spinal cord injury. Biomed Res Int. 2015;2015 doi: 10.1155/2015/134242. 134242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu CG, Li Y, Raza K, et al. Calpain 1 knockdown improves tissue sparing and functional outcomes after spinal cord injury in rats. J Neurotrauma. 2013;30(6):427–33. doi: 10.1089/neu.2012.2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim YH, Ha KY, Kim SI. Spinal cord injury and related clinical trials. Clin Orthop Surg. 2017;9(1):1–9. doi: 10.4055/cios.2017.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan X, Lan J, Liu Y, Miao J. Efficacy and safety of botulinum toxin type A in spasticity caused by spinal cord injury: A randomized, controlled trial. Med Sci Monit. 2018;24:8160–71. doi: 10.12659/MSM.911296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu Y, Chen H, Tan Z, et al. Therapeutic effects of Erbin inhibitor on spinal cord contusion in mice. Am J Transl Res. 2019;11(4):2570–79. [PMC free article] [PubMed] [Google Scholar]