Summary
Merkel cell carcinoma (MCC) is a rare aggressive skin tumour that appears to be associated with a large number of other tumours. We collected all reported cases in Israel and estimated its association with other tumours, including haematological malignancies. The population based Israel Cancer Registry identified 335 patients with MCC diagnosed between1989 and 2010. Ninety-seven percent were in the Jewish population; median age at diagnosis for Jewish patients was 73·4 and 55·6 years for the Arab population. Other associated malignancies were encountered in 92 patients (27·4%) with MCC (90 Jews, two Arabs). Of the Jewish cases, 66 presented with an associated malignancy before, and 24 after, the diagnosis of MCC. Solid tumours were not significantly increased among patients with MCC. Thirty-one of these associated cancers (34·4%) were haemato-oncological malignancies, 24 were detected before and seven after the diagnosis of MCC. The standardized incidence ratio (SIR) for haematological malignancy was 3·67 for males and 3·62 for females, and the most frequent haemato-oncological neoplasias recorded were chronic lymphocytic leukaemia (45%) and lymphomas (29%). Although MCC is rare, clinicians should be aware of the possible association with B-cell lymphoproliferative disorders when evaluating patients with neuroendocrine skin tumours.
Keywords: Merkel cell carcinoma, haematological neoplasias, chronic/small cell lymphocytic leukaemia (CLL/CLL), lymphomas, malignancies
Merkel cell carcinoma (MCC) is a rare and highly malignant neuroendocrine skin tumour that occurs in elderly patients, with a median age at diagnosis of 70 years. The tumour was first described in the early 1970’s (Toker, 1972), but the current term, ‘MCC’ was adopted in 1980 and thereafter, cases were officially reported to the cancer registry (Tong & Toker, 1978). Since then and during the last decade there has been an increasing prevalence of (Hodgson, 2005; Bichakjian et al, 2007). Based on some series with small numbers of cases, MCC has been reported to be associated with other neoplasms; mostly cutaneous tumours, but also some solid and haematological malignancies (Brenner et al, 2001; Robak et al, 2005; Kaae et al, 2010; Koljonen et al, 2010; Bzhalava et al, 2011). The most prominent association was with B-cell lymphoproliferative disorders, but most of these were recorded as single case reports (Quaglino et al, 1997; Ziprin et al, 2000; Vlad & Woodlock, 2003; Robak et al, 2005).
Recently, advances in the understanding of the pathogenesis of MCC have been made with the discovery of the link between polyomavirus and this rare neuroendocrine tumour (Feng et al, 2008). The virus was termed Merkel cell polyomavirus (MCPyV), and its DNA was shown to be present in approximately 70–80% of MCC cases (Feng et al, 2008). The role of MCPyV in the pathogenesis and development of MCC is still unclear, but some have proposed that it may be reactivated during immunosuppression, which supports the concept of its association with other immunodeficiency disorders (Buell et al, 2002; Feng et al, 2008). Furthermore, a recent epidemiological study showing MCC to be associated with an increased risk of other skin cancers, speculated that ultraviolet light exposure could be an alternative underlying cause for this finding. However, it is also possible that the increased numbers of second skin tumours associated with MCC could merely reflect increased clinical surveillance of the skin in these particular patients (Bzhalava et al, 2011).
After an extensive literature review it was evident that only a few groups have studied the association of MCC with other malignancies. These groups analysed the appearance of second cancers but only reported those occurring after the diagnosis of MCC (Koljonen et al, 2010; Bzhalava et al, 2011). Except for an earlier Israeli report (Brenner et al, 2001), the only study that analysed the association of MCC as a primary or secondary malignancy was from the National Institutes of Health (Howard et al, 2006). In order to update and expand our knowledge on the epidemiological characteristics of MCC in the general population and the association between MCC and other tumours as well as haematological malignancies, we conducted a population-based study based on the data in the Israel National Cancer Registry (INCR).
Materials and methods
The data of the Israeli National Cancer Registry (INCR), a population-based central tumour registry, was used for this analysis. All Israeli citizens diagnosed with MCC (International Classification of Diseases for Oncology [ICD-O] Version 3 code, M82473) from 1989 to 2010 were included. A detailed description of the INCR has been published (Barchana et al, 2004), and formal reporting of malignancies to the registry is now mandatory in Israel. All public and private medical facilities, and pathology laboratories engaged in diagnosing cancer patients are obliged to send a copy of the medical summary and diagnosis to the registry. The INCR also actively collects data on cancer deaths obtained from District Health Authorities and the Central Population Registry (Israel Central Bureau of Statistics, Government Press 2007). All demographic data, as well as place of birth and date of immigration, with residential and other personal data are stored in the Central Population Registry. The INCR is linked to this registry and each cancer patient’s personal data can be retrieved and validated. The last audit concluded that more than 95% of solid tumours and 85–90% of non-solid tumours were registered (Fishler et al, 2003). In addition to demographic data, the INCR registers all of the available information on cancer cases, including the date of diagnosis and tumour location using the ICD-O version-3 codes (http://www.old.health.gov.il/pages/default.asp?maincat=22).
According to data collected in the INCR, between 1989 and 2010, MCC was diagnosed in 335 patients. Histopathological diagnosis was established by light microscopy, immuno-histochemistry and, in some instances, transmission electron microscopy. Demographic data of these patients (using the personal identifier, names and other personal data) was traced back to the INCR database, enabling the retrieval of all other malignancies diagnosed in these patients. Age, gender, ethnic origin, anatomic sites and dates of diagnosis of MCC and accompanying neoplasms were also available. The date and cause of death, when applicable, were recorded for each case.
These 335 MCC patients contributed person-years to the cohort according to the following rules: entry date January 1, 1989 or birth date, whichever was latest; exit date was death, another cancer or December 31, 2010, whichever was first.
The incidence of other cancers in the MCC study cohort was compared to the cancer incidence in the entire Israel population standardized by age, gender and ethnicity for the same period. This yielded a study cohort of 5686 person years (2989 men and 2697 women).The standardized incidence ratio (SIR) of other neoplasms was calculated with a 95% confidence interval (CI).
Survival rates for patients with MCC who had no other tumours were compared with those for patients who had associated tumours. MCC-specific survival, defined as the time from diagnosis of MCC to death, was estimated by Kaplan–Meier product limit method.
Results
Incidence
The annual number of cases of MCC in Israel increased between 1989 and 2010 (Fig. 1), and was in accordance with data reported from others countries, except for the year 2000 (Fig. 1), in which the least number of cases was reported. We speculate that this was due to the 4·5-month long national strike of the entire health system in Israel in 2000, during which less surgery was performed and diagnosis of new cases was not made. In our patient cohort, incidence rates of MCC were similar in males (53%) and females (47%). A total of 335 patients with MCC were diagnosed, mostly in Jews (n = 324; 97%) and there were only 11 cases (3%) in Arabs, and non-Arab-non-Jews (Table I).
Fig 1.
Cases of MCC in Israel in 1989–2010.
Table I.
Characteristics of patients with MCC diagnosed in Israel.
| Variable | N (%) |
|---|---|
| Total, N (%) | 335 (100) |
| Sex, N (%) | |
| Male | 179 (53) |
| Female | 156 (47) |
| Ethnic group, N (%) | |
| Jews | 324 (97) |
| Arabs+ Non Jews/Non Arabs | 11 (3) |
| Age, years; median (range) | |
| Overall | 72·8 (35·2–86·4) |
| Jews | 73·4 (35·2–86·4) |
| Arabs | 55·6 (38·1–64·5) |
| Jews ethnic origin (place of birth) | N (%) |
| Asia or Africa | 51 (16) |
| America or Europe | 212 (65) |
| Israel | 61 (19) |
Epidemiological characteristics
Median age at diagnosis was 73·4 years for the Jewish population (range 35·2–86·4 years) (Table I) and 55·6 years for Arabs (range, 38·1–64·5 years). MCC occurred mostly in patients older than 65 years and its frequency was found to increase with age (Table II). The same trends were found after comparing the number of patients with MCC who had other cancers and those with MCC and haematological malignancies, according to age (Table II); only 6% and 10%, respectively, were 45 years or younger; 24% and 13%, respectively, were aged 45–65 years, while 70% and 77% patients, respectively, were 65 years old or more. Regarding the ethnic origin of Jews: 65% were born in America or Europe and were mostly Ashkenazi, 19% were born in Israel and the remaining 16% originated from in Asia or Africa. Additional epidemiological characteristics are given in Table I.
Table II.
Age distribution: Jews with MCC and other cancers including haematological malignancies.
| Age (years) | MCC and other cancers No. of patients (%) |
MCC and haematological malignancy No. of patients (%) |
|---|---|---|
| 35–44 | 5 (6) | 3 (10) |
| 45–54 | 9 (10) | 1 (3) |
| 55–64 | 13 (14) | 3 (10) |
| 65–74 | 19 (21) | 2 (6) |
| 75+ | 44 (49) | 22 (71) |
Associated cancers
Of the 335 patients, 92 (27·4%) had associated cancer (52 men, 40 women) including both haematological and solid tumours. Of the Jewish cases, 66 presented with an associated malignancy before, and 24 after, the diagnosis of MCC was established. There was a wide range of solid tumours and the most frequent cancers were: breast (n = 12; 20%), colon (n = 6; 10%), bladder (n = 4; 6·8%), and skin (n = 4; 6·8%). There were two patients with meningioma, and two with pancreas, kidney and gastric cancers (3·3%). Compared to the general population, none of these solid tumours were significantly more frequent among patients with MCC (Table III). The median time period between the detection of the first cancer and the diagnosis of MCC was 9·3 years (range 1·3–17·3 years), while 24 patients developed second tumours after the initial diagnosis of MCC had already been established (median 3·6 years, range 0·3–6·9 years). Full details of the associated cancers in the two Arab patients with MCC were not available.
Table III.
The standardized incidence ratio for non haematological cancers and haematological malignancies associated with MCC. [Presented both before or after the diagnosis of MCC (A), or only after the diagnosis of MCC (B)].
| A | |||||
| 2nd Cancer (before & after) MCC patients vs. All Jews, 1989–2010 | |||||
| Observation | Exp | SIR | 95% CI | ||
| All sites | |||||
| Male | 52 | 49·22 | 1·06 | 0·77 | 1·34 |
| Female | 38 | 36·62 | 1·04 | 0·71 | 1·37 |
| Haematology | |||||
| Male | 18 | 4·90 | 3·67 | 1·98 | 5·37 |
| Female | 13 | 3·59 | 3·62 | 1·65 | 5·59 |
| B | |||||
| 2nd Cancer after MCC patients | vs. All Jews, 1989–2010 | ||||
| All sites | |||||
| Male | 13 | 11·28 | 1·15 | 0·53 | 1·78 |
| Female | 11 | 9·96 | 1·10 | 0·45 | 1·76 |
| Haematology | |||||
| Male | 4 | 1·12 | 3·57 | 0·07 | 7·07 |
| Female | 3 | 1·02 | 2·94 | 0·00 | 6·27 |
MCC, Merkel cell carcinoma; SIR, standardized incidence ratio; 95% CI, 95% confidence interval.
Associated haematological malignancies
Thirty-one of the 90 cancers reported among Jews (34·4%) were haematological malignancies; of these 24 were detected before MCC diagnosis and seven were identified after the diagnosis of MCC had been established. The most frequent haematological neoplasms recorded were chronic lymphocytic leukaemia/small lymphocytic lymphoma (CLL/SLL) (45%) and non-Hodgkin lymphoma (29%). In addition there were three patients with myeloproliferative disorders, two with multiple myeloma, and one each with hairy cell leukaemia, acute myeloid leukaemia and mycosis fungoides (Table IV). The SIR for haematological malignancies in patients with MCC was 3·67 for males (95% CI: range; 1·98–5·37) and 3·62 for females (95% CI: range; 1·65–5·59) (Table III), which was significantly higher than in the age-matched control groups. Of the 30 cases with an haematological malignancy, MCC was present in the following sites: upper limb and shoulder (n = 8; 26·6%), trunk (n = 6; 20%), lower limb and hip (n = 5; 16·7%); scalp and neck (n = 3; 10%); face (n = 3; 10%) external ear and eyelid (n = 2; 7%). Information regarding the site of the MCC was lacking in 3 (10%) cases.
Table IV.
Type of secondary haematological malignancies associated with MCC in Israel during the years 1989–2010.
| N cases (%) | |
|---|---|
| Haematological malignancies | 30 (100) |
| Malignant lymphoma (including small lymphocytic lymphoma) | 9 (29) |
| Mycosis fungoides | 1 (3·3) |
| Multiple myeloma | 2 (6·6) |
| Chronic lymphocytic leukaemia | 14 (45) |
| Acute myeloid leukaemia | 1 (3·3) |
| Chronic myeloid leukaemia | 1 (3·3) |
| Hairy cell leukaemia | 1 (3·3) |
| Chronic myeloproliferative disorders | 2 (6·6) |
Survival
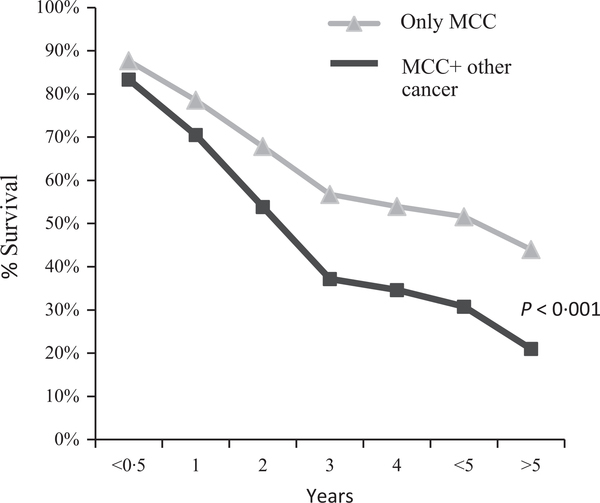
The presence of haematological malignancy, either before or after the diagnosis of MCC, was associated with a significantly higher MCC-specific mortality (RR = 1·68, P < 0·001) (Fig. 2).
Fig 2.
Survival of patients with MCC only vs. MCC and another malignancy.
Discussion
Merkel cell carcinoma is a rare and aggressive skin tumour with apparent increasing prevalence in the last decade (Hodgson, 2005). One of the features of MCC is its tendency to occur in association with other tumours, mostly skin cancers. However, solid-oncological as well as haematological malignancies have also been reported (Brenner et al, 2001; Kaae et al, 2010; Bzhalava et al, 2011), in particular B-cell lymphoproliferative disorders. Very few studies have analysed these associations, and indeed, most of what is known has been derived from cases described as letters to the editor or as single case reports.
Merkel cell polyomavirus (MCPyV), recently described by Feng et al (2008), is detected in approximately 80% of MCC cases, and is believed to represent the missing link for understanding the pathogenesis of this rare tumour (Feng et al, 2008). MCPyV can be reactivated during immunosuppression and may perhaps play an aetiological role in the development of MCC as well as the associated malignancies documented in patients with pre-existing MCC (Pantulu et al, 2010).
In the present study, which is one of the very few performed on a larger scale and with more epidemiological data, the entire database of the Israel Cancer Registry was reviewed to determine the incidence of the association of MCC with other malignancies. The data indicated an increased association between MCC and haematological malignancies, mostly CLL/SLL and lymphomas (Ziprin et al, 2000; Cohen et al, 2002; Vlad & Woodlock, 2003; Teman et al, 2011). In contrast to the Finnish and Scandinavian studies (Koljonen et al, 2010; Bzhalava et al, 2011) the majority of the associated tumours (73%) reported here was already present prior to and not after the diagnosis of MCC was established. Our data imply that if second cancers are looked for only after the diagnosis of MCC, a substantial number will be missed, particularly in patients with B-cell lymphoproliferative disorders who have underlying immunosuppression, which probably provides the appropriate background for the development of MCC (Buell et al, 2002; Tadmor et al, 2011). In this respect it is noteworthy that patients with lymphoproliferative disorders, including CLL, who have been treated with immunosuppressive agents that aggravate the extent of immunosuppression, such as purine-analogs (cladribine, fludarabine) and monoclonal antibodies, have also been reported to develop MCC (Cohen et al, 2002; Robak et al, 2005).
It should be emphasized that MCC was diagnosed as a primary tumour in 27% of the patients in this study, indicating the reciprocal nature of the biological background required for the development and association of both this unusual tumour and the B-cell malignancies.
In addition, our epidemiological data relates specifically to the Israeli population and contains novel data which may add to the understanding of the pathogenesis of MCC. Indeed, here as in other earlier reports, MCC occurred more frequently in individuals with a, ‘lighter skin’ (Kaae et al, 2010; Tadmor et al, 2011) and was more commonly encountered in Jews than in Arabs, mostly among ‘Ashkenazi’s’ or Jews emigrating from the USA or Europe. In contrast to other reports, an increased incidence of associated solid tumours was not noted in our cohort of patients with MCC. However, there was a significantly higher incidence of haematological malignancies in patients with MCC compared to the healthy normal population. One explanation for these findings could relate to the fact that our patient cohort was smaller compared to those studies utilizing the Surveillance, Epidemiology and End Results (SEER) program or the Finnish cancer registry data (Kaae et al, 2010; Koljonen et al, 2010). On the other hand, our results correspond to other studies that showed an increased incidence of MCC in patients with existing haematological malignancies (Ziprin et al, 2000; Cohen et al, 2002; Vlad & Woodlock, 2003). In this respect, patients with CLL/SLL and lymphomas appear to have the highest relative risk of developing MCC. Indeed, second malignancies are well recognized as one of the complications occurring in the course of CLL but in most of reports, MCC was not listed as an associated second tumour (Tsimberidou et al, 2009). In this regard, it is interesting to note that Pantulu et al (2010) reported increased expression of MCPyV in highly purified CLL cells, implying that it may perhaps function as an oncogenic virus in B-lymphocytes. These results are in contrast with those of a recent study of 18 patients with CLL (Tolstov et al, 2010), which showed that there is insufficient evidence for direct involvement of MCPyV in this form of leukaemia.
Taking all these results into consideration, it is apparent that more studies need to be done to more clearly define the role of MCPyV in haematological malignancies, and to understand the linkage and possible mechanisms involved in patients developing both MCC and CLL. We also report for the first time, three MCC patients associated with myeloproliferative disorders, one with hairy cell leukaemia and an additional patient with T-cell lymphoma, indicating that non B-cell derived haematological malignancies may also be linked with this very rare skin tumour.
In conclusion we underline two important aspects of our observations: Firstly, that, a greater awareness of the association of these disorders amongst haematologists and oncologists could perhaps result in more routine examinations of suspected skin lesions being performed in these patients. This could contribute to an earlier diagnosis of MCC, which is often a very aggressive and locally invasive tumour, particularly when diagnosed late and after regional spread. The second aspect relates to the understanding of the pathogenesis of both MCC and the associated haematological malignancies and the common immunological and infectious pathways involved. This is particularly interesting in the light of our results, which showed that in 73% of the patients with MCC, the haematological malignancy preceded the occurrence of the skin tumour. This observation provides further support for the hypothesis that implies an association of MCC with immunodeficiency. Future studies investigating this association and the link between the virus and the pathogenesis of MCC and haematological neoplasias, particularly CLL and SLL, are still needed. In the future, pooled studies involving much larger cohorts of patients on an international basis may well provide more convincing data with greater statistical power than cohorts from a single country with smaller sized populations. Global epidemiological studies on CLL and MCC would clearly contribute to the accumulation of more accurate incidence data worldwide and reveal any possible geopathological and ethnic correlations as implied by the results of this study.
Footnotes
Conflict of interest
The authors have no conflicts of interest to declare.
References
- Barchana M, Liphshitz I & Rozen P. (2004) Trends in colorectal cancer incidence and mortality in the Israeli Jewish ethnic populations. Familial Cancer, 3, 207–214. [DOI] [PubMed] [Google Scholar]
- Bichakjian CK, Lowe L, Lao CD, Sandler HM, Bradford CR, Johnson TM & Wong SL(2007) Merkel cell carcinoma: critical review with guidelines for multidisciplinary management. Cancer, 110, 1–12. [DOI] [PubMed] [Google Scholar]
- Brenner B, Sulkes A, Rakowsky E, Feinmesser M, Yukelson A, Bar-Haim E, Katz A, Idelevich E, Neuman A, Barhana M & Fenig E. (2001) Second neoplasms in patients with Merkel cell carcinoma. Cancer, 91, 1358–1362. [DOI] [PubMed] [Google Scholar]
- Buell JF, Trofe J, Hanaway MJ, Beebe TM, Gross TG, Alloway RR, First MR & Woodle ES (2002) Immunosuppression and merkel cell cancer. Transplantation proceedings, 34, 1780–1781. [DOI] [PubMed] [Google Scholar]
- Bzhalava D, Bray F, Storm H & Dillner J. (2011) Risk of second cancers after the diagnosis of Merkel cell carcinoma in Scandinavia. British Journal of Cancer, 104, 178–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Amir G & Polliack A. (2002) Development and rapid dissemination of Merkel-cell carcinomatosis following therapy with fludarabine and rituximab for relapsing follicular lymphoma. European Journal of Haematology, 68, 117–119. [DOI] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y & Moore PS (2008) Clonal Integration of a polyomavirus in human merkel cell carcinoma. Science, 319, 1096–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishler Y, Chitrit A, Barchana M & Modan B. (2003) Assessment of the Completeness of the Israel Cancer Registry Database — Methods and Findings [in Hebrew]. Israel Center for Disease Control, Tel Hashomer, Israel. [Google Scholar]
- Hodgson NC (2005) Merkel cell carcinoma: changing incidence trends. Journal of Surgical Oncology, 89, 1–4. [DOI] [PubMed] [Google Scholar]
- Howard RA, Dores GM, Curtis RE, Anderson WF & Travis LB (2006) Merkel cell carcinoma and multiple primary cancers. Cancer Epidemiology Biomarkers & Prevention, 15, 1545–1549. [DOI] [PubMed] [Google Scholar]
- Israel Central Bureau of Statistics (2007) Death causes in Israel – 2007. WWW document. URL: http://www1.cbs.gov.il/reader/newhodaot/hodaa_template.html?hodaa=200905179.
- Kaae J, Hansen AV, Biggar RJ, Boyd HA, Moore PS, Wohlfahrt J & Melbye M. (2010) Merkel cell carcinoma: incidence, mortality, and risk of other cancers. Journal of the National Cancer Institute, 102, 793–801. [DOI] [PubMed] [Google Scholar]
- Koljonen V, Kukko H, Tukiainen E, Böhling T, Sankila R, Joensuu H & Pukkala E. (2010) Second cancers following the diagnosis of Merkel cell carcinoma: a nationwide cohort study. Cancer Epidemiology, 34, 62–65. [DOI] [PubMed] [Google Scholar]
- Pantulu ND, Pallasch CP, Kurz AK, Kassem A, Frenzel L, Sodenkamp S, Kvasnicka HM, Wendtner CM & zur Hausen A. (2010) Detection of a novel truncating Merkel cell polyomavirus large T antigen deletion in chronic lymphocytic leukemia cells. Blood, 116,5280–5284. [DOI] [PubMed] [Google Scholar]
- Quaglino D, Di Leonardo G, Lalli G, Pasqualoni E, Di Simone S, Vecchio L & Ventura T. (1997) Association between chronic lymphocytic leukaemia and secondary tumours: unusual occurrence of a neuroendocrine (Merkell cell) carcinoma. European Review for Medical and Pharmacological Sciences, 1, 11–16. [PubMed] [Google Scholar]
- Robak E, Biernat W, Krykowski E, Jeziorski A & Robak T. (2005) Merkel cell carcinoma in a patient with B-cell chronic lymphocytic leukemia treated with cladribine and rituximab. Leukemia & Lymphoma, 46, 909–914. [DOI] [PubMed] [Google Scholar]
- Tadmor T, Aviv A & Polliack A. (2011) Merkel cell carcinoma, chronic lymphocytic leukemia and other lymphoproliferative disorders: an old bond with possible new viral ties. Annals of Oncology, 22, 250–256. [DOI] [PubMed] [Google Scholar]
- Teman CJ, Tripp SR, Perkins SL & Duncavage EJ (2011) Merkel cell polyomavirus (MCPyV) in chronic lymphocytic leukemia/ small lymphocytic lymphoma. Leukemia Research, 35, 689–692. [DOI] [PubMed] [Google Scholar]
- Toker C. (1972) Trabecular carcinoma of the skin. Archives of Dermatology, 105, 107–110. [PubMed] [Google Scholar]
- Tolstov YL, Arora R, Scudiere SC, Busam K, Chaudhary PM, Chang Y & Moore PS (2010) Lack of evidence for direct involvement of Merkel cell polyomavirus (MCV) in chronic lymphocytic leukemia (CLL). Blood, 115, 4973–4974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong C & Toker C. (1978) Trabecular carcinoma of the skin: an ultrastructural study. Cancer, 42, 2311–2321. [DOI] [PubMed] [Google Scholar]
- Tsimberidou A-M, Wen S, McLaughlin P, O’Brien S, Wierda WG, Lerner S, Strom S, Freireich EJ, Medeiros LJ, Kantarjian HM & Keating MJ (2009) Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. Journal of Clinical Oncology, 27, 904–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlad R & Woodlock TJ (2003) Merkel cell carcinoma after chronic lymphocytic leukemia: case report and literature review. American Journal of Clinical Oncology, 26, 531–534. [DOI] [PubMed] [Google Scholar]
- Ziprin P, Smith S, Salerno G & Rosin RD (2000) Two cases of Merkel cell tumour arising in patients with chronic lymphocytic leukaemia. British Journal of Dermatology, 142, 525–528. [DOI] [PubMed] [Google Scholar]