Abstract
This study examined factors contributing to increased vascular resistance and plexiform lesion formation in broiler chickens susceptible to idiopathic pulmonary arterial hypertension (IPAH). A diet supplemented with excess tryptophan (high-Trp diet), the precursor for serotonin, was used to accelerate the development of IPAH. Broilers fed the high-Trp diet had higher pulmonary arterial pressures than broilers fed the control diet, and plexiform lesion incidences tended to be higher (P = 0.11) in the high-Trp group than in the control group at 30 d of age. The intrapulmonary arteries were assessed for vasoconstriction in response to serotonin and adenosine triphosphate (ATP) and for activities of key metabolic enzymes for serotonin and ATP. The pulmonary artery (defined as the first major branch of the pulmonary artery inside the lung) and the primary pulmonary arterial rami (defined as the second major branch of the pulmonary artery inside the lung) both exhibited vasoconstriction in response to serotonin and ATP. This is the first study to demonstrate purinergic-mediated vasoconstriction in intrapulmonary arteries from broilers. Arteriole responsiveness did not differ between broilers fed the control diet or the high-Trp diet. Therefore, the high-Trp diet enhanced the development of IPAH but did not affect the artery’s sensitivity to serotonin or ATP. Monoamine oxidase activity, responsible for the breakdown of serotonin, was severely impaired in pulmonary arteries from broilers in the high-Trp group. Accordingly, serotonin may persist longer and elicit an amplified response in broilers fed the high-Trp diet.
Keywords: ATPase, intrapulmonary, monoamine oxidase, purinergic
INTRODUCTION
Idiopathic pulmonary arterial hypertension (IPAH) in broiler chickens is characterized by increased pulmonary arterial pressure and excessively elevated pulmonary vascular resistance. Efforts to clarify the pathogenesis of IPAH have focused on deducing the underlying causes of the excessive resistance to pulmonary blood flow (Peacock et al., 1989; Wideman, 2000, 2001; Chapman and Wideman, 2001, 2006a,b; Wideman et al., 2004, 2007). In addition to chemical mediators of vasoconstriction (Wideman et al., 2004, 2007), increases in pulmonary vascular resistance also may be attributed to pathological changes within the arterioles, including medial hypertrophy (hypertrophy and hyperplasia of smooth muscle), intimal proliferation (endothelial proliferation), and the formation of obstructive plexiform lesions. Indeed, broilers serve as a unique animal model for the spontaneous development of plexogenic arteriopathy (Wideman et al., 2007, 2011;Wideman and Hamal, 2011).
Pulmonary vascular pressure profiles have demonstrated repeatedly that the precapillary vasculature is the predominate site of increased resistance to blood flow in IPAH-susceptible broilers (Chapman and Wideman, 2001; Wideman et al., 2007, 2010; Lorenzoni et al., 2008). Nevertheless, the specific anatomical locations and mechanisms contributing to elevated vascular resistance and lesion formation in the pulmonary arterial tree remain to be elucidated. Serotonin (5-hydroxytryptamine, 5-HT) is a potent pulmonary vasoconstrictor and mitogen that has been implicated in the pathogenesis of IPAH in mammals and broilers. Serotonin can be released from pulmonary neuroendocrine cells or from circulating platelets and thrombocytes (MacLean et al., 2000). Serotonin acts via specific receptors on pulmonary smooth muscle and endothelial cells to cause vasoconstriction and exuberant cellular proliferation, which is thought to precede plexogenic arteriopathy (Tuder et al., 1998a,b; Eddahibi et al., 2001a,b, 2006; Wideman et al., 2010, 2011; Wideman and Hamal, 2011). Adenosine triphosphate (ATP) is released from sympathetic nerves, and purinergic-2 (P2X) receptors have been linked to progression of lung disease in mammalian models (McMillan et al., 1999; Mortaz, et al., 2010; Ohata et al., 2011). However, the presence of purinergic receptors in the resistance vasculature of chicken lungs has not been confirmed, nor is it known if these receptors cause vasoconstriction in broilers. Evidence from mammalian studies suggests that serotonin may act on P2X receptors to elicit excessive vasoconstriction (Zhang et al., 2004; Dergacheva et al., 2008). Therefore, there may be an important interaction between serotonin and ATP that contributes to increased vascular resistance in IPAH.
The purpose of this study was to investigate mechanisms that may contribute to the development of IPAH. To further clarify the anatomical sites of excessive pulmonary vasoconstriction, we evaluated the responsiveness of the pulmonary artery (defined as the first major branch of the pulmonary artery within the lung) and the pulmonary rami (defined as the second major branch of the pulmonary artery within the lung) to graded levels of serotonin and ATP. Chickens require the serotonin precursor tryptophan (Trp) as an essential amino acid in their diet (Corzo et al., 2005a,b; Fatufe et al., 2005; Emadi et al., 2011). Supplemental levels of tryptophan increase the quantities of serotonin that are synthesized and released (Rosebrough, 1996). It was our hypothesis that chickens fed a diet supplemented with high levels of tryptophan should spontaneously develop increased pulmonary arterial pressures, increased plexiform lesion incidences, and greater sensitivity to vasoconstrictors, such as serotonin and ATP. We also evaluated the activity of the enzymes that metabolize serotonin and ATP, as changes in the activities of these enzymes could alter the concentrations of serotonin and ATP available for vasoconstriction.
MATERIALS AND METHODS
Animal procedures were approved by the University of Arkansas Institutional Animal Care and Use Committee. Male broiler chicks were reared in environmental chambers (dimensions: 3.7 m long × 2.5 m wide × 2.5 m high) within the Poultry Environmental Research Lab. Single-pass ventilation was maintained at a constant rate of 6 m3 per minute per chamber. The photoperiod was set for 23L:1D for the first 4 d and 16L:8D thereafter. Thermoneutral temperatures were maintained throughout: 32°C for d 1 to 3, 31°C for d 4 to 6, 29°C for d 7 to10, 26°C for d 11 to 14, and 24°C thereafter. Chicks were initially placed on the floor on wood-shavings litter at a density of 930 cm2/bird, and by 6 wk of age, the bird density had been reduced to ≥ 2,000 cm2/bird. A corn and soybean meal-based feed formulated to meet or exceed the minimum NRC (1994) standards for all ingredients (22.7% CP, 3,059 kcal of ME/kg, 1.5% arginine, and 1.43% lysine) was provided to the control group. The control diet was formulated to contain approximately 0.22% tryptophan (Trp) by weight (2.2 g of Trp/kg of feed). The high-Trp group received the same diet blended with sufficient additional Trp added to achieve 0.88% Trp by weight. Feed and water were available ad libitum throughout the study.
Histology
All procedures have been described previously (Wideman et al., 2011). At 30 and 42 to 48 d of age, randomly selected birds (control n = 8, high-Trp n = 7 on d 30; control n = 9, high-Trp n = 9 on d 42 to 48) were anesthetized to a surgical plane with intramuscular injections of allobarbitol (5,5-diallylbarbituric acid, 3.0 mL, 25 mg/mL; Sigma-Aldrich, St. Louis, MO) and ketamine HCl (1.0 to 2.5 mL of 100 mg/mL; Henry Schein Inc., Melville, NY). Heparinized saline with papaverine (2 mL per bird of 200 units/mL ammonium heparin in 0.9% NaCl containing 0.4 mg/mL papaverine) was injected intravenously, after which the birds were euthanized by exsanguination. The sternum was retracted to expose the heart, the right atrium was clamped with a hemostat, the left atrium was opened for drainage, and polyethylene tubing (2.5-mm ID) was inserted via a slit in the right ventricle to flush the pulmonary vasculature with 200 mL of 0.9% NaCl containing 50 units/mL ammonium heparin and 0.1 mg/mL papaverine at room temperature. The lungs were then fixed in situ by transcardiac perfusion with 200 to 400 mL of freshly prepared 4% phosphate-buffered paraformaldehyde at room temperature. Carboys containing the saline and the paraformaldehyde were positioned at an elevation sufficient to obtain a gravimetric perfusion pressure of 46 cm of H2O (34 mmHg) at the level of the heart. The thoracic cavity was flooded with 4% paraformaldehyde and the lungs remained fixing in situ for a minimum of 8 h, after which they were removed and cut in the transverse plane at the 4 major rib indentations (costal sulci). These tissues were immersed for 24 h in 4% phosphate-buffered paraformaldehyde, after which they were rinsed briefly in tap water, dehydrated in 25% and 50% ethanol for 30 min each, and stored in 70% ethanol. One inter-rib division from the middle of each lung was embedded in paraffin, sectioned at 5- to 7-μm thickness, and stained with hematoxylin and eosin. One slide per lung division was searched for plexiform lesions using overlapping fields of view and sequential horizontal traverses at 40× total magnification.
Pulmonary Vascular Pressure Profiles
Between 4 and 5 wk of age, broilers (n = 16 for the control group, and n = 16 for the high-Trp group) were anesthetized to a surgical plane with intramuscular injections of allobarbitol (5,5-diallylbarbituric acid, 3.0 mL, 25 mg/mL) and ketamine HCl (1.0 to 2.5 mL of 100 mg/mL). The anesthetized broilers were fastened in dorsal recumbency on a surgical board. Lidocaine HCl (2%) was injected subcutaneously around the basilica vein (major wing vein), and then the proximal end of a Silastic catheter (0.012 in ID, 0.025 in OD; Dow Corning Corp., Midland, MI) filled with heparinized saline (200 IU of heparin/mL of 0.9% NaCl) was inserted into the vein. The distal end of the catheter was attached to a blood pressure transducer interfaced through a Transbridge preamplifier (World Precision Instruments, Sarasota, FL) to a Biopac MP100 data acquisition system using Acqknowledge software (Biopac Systems Inc., Goleta, CA). Venous pressure was recorded with the catheter inserted approximately 2 cm into the basilica vein. Characteristic pulse pressures were monitored to identify the catheter’s location as it was slowly advanced through the wing vein to the right atrium, right ventricle, main trunk of the pulmonary artery, and onward, until a sudden drop in the pressure indicated the tip of the catheter had become wedged in a small arterial branch. A sudden rise in the pressure as the catheter was slightly withdrawn confirmed that the wedge pressure had been obtained. The catheter was then gradually withdrawn to again record the pulmonary arterial, right ventricular, right atrial, and venous pressures. Typical blood pressure recordings from individual broilers undergoing wedge pressure experiments have been published previously (Chapman and Wideman, 2001; Lorenzoni et al., 2008).
Concentration Response Curves
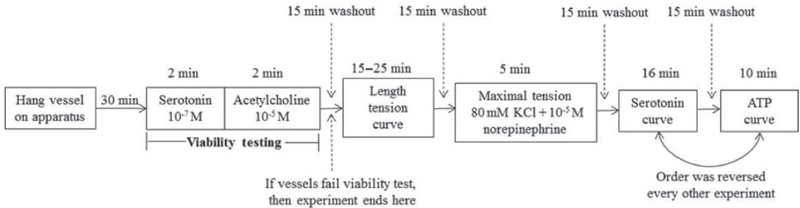
The lungs were removed from euthanized broilers, and the pulmonary artery and pulmonary rami were dissected and prepared for wire myography. The pulmonary arteries were identified as the first major branch within the lung after the pulmonary artery, including the arteria pulmonalis dextra and the arteries pulmonalis sinistra. The pulmonary rami were identified as the second major branch of the pulmonary artery within the lung, including the cranial, caudomedial, and caudolateral rami. Once the vessels were dissected, they were mounted on tungsten triangular hangers connected to force transducers and placed in 15-mL tissue baths containing modified Krebs-Henseleit buffer solution (Sigma, St. Louis, MO) with HEPES (10 mM, Sigma), sodium bicarbonate (25 mM, Sigma-Aldrich), and calcium chloride (0.95 mM, Sigma). The tension of the vessels was set at 0.4 g for 30 min at pH 7.4 and 37°C to allow stabilization. Baths were continuously bubbled with a mixture of 5% CO2 and 30% O2.Viability of the smooth muscle was assessed by at least 10% increase in contraction to serotonin (10−7 M), and the viability of endothelium was assessed by at least 20% relaxation to acetylcholine (10−5 M; see Figure 1 for a diagram of the tension experiments). The vessels were then stimulated by an electrical current delivered via 2 parallel platinum electrodes placed on both sides of the vessel. The electrical current was supplied by a voltage stimulator set at 15 V, using a 2 msec pulse width. Baseline tension was increased by 0.05 g until the developed tension failed to increase by 10% in response to the stimulation. Peak contraction to potassium chloride (80 mM) plus norepinephrine (10−5 M) was also measured. Concentrations response curves of serotonin (10−12 to 10−5 M) and ATP (10−7 to 10−3 M) were recorded and collected using a Powerlab 16/30 with Chart software (version 5.2; ADI instruments, Colorado Springs, CO). Tissue baths were set at 37°C and increasing concentrations were added every 2 min.
Figure 1.
Diagram of vessel tension experiments.
Phosphatase Activity
A QuantiChrom ATPase/GTPase assay kit was used from BioAssay Systems (Hayward, CA) to detect free phosphate in the vessel. The QuantiChrom ATPase/GTPase assay has been shown to be highly sensitive (Samizo et al., 2001) and has previously been used to measure organic and inorganic phosphates (Cogan et al., 1999). The assay included a phosphate standard and reagent (proprietary formula, Bioassay Systems). Free phosphate was detected by measuring absorbance using the PowerWave XS (Bio-Tek Instruments Inc., Winooski, VT) plate-reading spectrophotometer. KCJunior software (Winooski, VT) was used to calculate phosphate concentration of each sample from a standard curve, and phosphate concentration was directly proportional to nucleotidase activity.
All solutions were brought to room temperature. A premix was made by mixing 40 μL of 1 mmol phosphate standard with 960 μL of distilled water. Different concentrations of the phosphate standard were prepared using the premix and distilled water to make 8 concentrations: 40 μmol, 32 μmol, 24 μmol, 16 μmol, 12 μmol, 8 μmol, 4 μmol, and 0 μmol of phosphate. The standards were pipetted (50 μL) in duplicate into a clear-bottom 96-well plate to obtain a standard curve for phosphate concentration from the absorbance read. Duplicate empty wells were used as blanks, and in each well, 100 μL of reagent was added. The wells were mixed at 500 rpm for 60 s using a plate shaker.
The lungs were dissected to remove a 2-mm portion of the pulmonary artery and pulmonary rami (making sure to remove blood within the vessels). Vessel segments were then transferred into sample wells containing 100 μL of reagent (described above) and 0.2 mM of adenosine triphosphate (Sigma-Aldrich). Whole vessel segments were used to reduce the possibility of detecting phosphate from intracellular sources of nucleotidases, such as myosin phosphatase activity within the vessel. The whole segments were left in the well during the incubation period (30 min) and then removed with tweezers before the plate was read. These vessels were assessed for the activity of the enzymes that break down adenine nucleotides using the QuantiChrom ATPase/GTPase assay kit (BioAssay Systems) and manufacturer’s guidelines were followed. The assay is highly sensitive, being able to detect as little as 2 pmol of phosphate. Free phosphate was detected by measuring absorbance at 620 nm using the PowerWave XS (Bio-Tek Instruments Inc.) plate-reading spectrophotometer. KCJunior software was used to calculate phosphate concentration of each sample from a standard curve, and phosphate concentration was directly proportional to phosphatase activity. Phosphatase activity was further normalized by total protein content using a Micro BCA spectrophotometric protein assay. Each vessel was homogenized in saline solution after completion of the QuantiChrom ATPase/GTPase assay. The samples were centrifuged at 2,000 × g for 10 min at 25°C, and then the sample supernatant was removed and the protein assay was conducted per manufacturer’s guidelines. Phosphatase activity was reported as picomoles of phosphate per microgram of protein per minute.
Monoamine Oxidase Activity
Pulmonary arteries and pulmonary rami were also dissected and prepared for an analysis of monoamine oxidase activity using a 2-step luminometric assay (MAO-Glo, Promega, Madison, WI). Arteries were cut into 2-mm segments, homogenized in 120 μL of enzyme buffer (100 mM of HEPES, 5% glycerol; pH = 7.4), and the supernatant was removed for MAO analysis after centrifugation at 2,000 × g for 10 min at 25°C. In the first step, an aminopropylether analog to methyl ester luciferin is oxidized to form an imine, followed by hydrolysis to an aldehyde, and then spontaneous conversion to methyl ester luciferin. The second step involves the addition of esterase and luciferase, with the former converting methyl ester luciferin to luciferin, and the latter converting luciferin to oxyluciferin and light. Relative luminescence units were proportional to MAO activity. The plates were read using a Biotek Luminometer (Biotek Instruments Inc.) using a sensitivity setting of 125, a delay before measurement of 1,000 ms, and a delay between measurements of 1,250 ms. The values from the assay are expressed as relative luminescent units because the absolute values from one luminometer to another vary depending on the manufacturer and model of the luminometer.
Data Analysis
All data are reported as mean ± standard deviation. Plexiform lesion incidences were calculated as (number of lung sections with ≥ 1 plexiform lesion)/(number of lung sections examined). Plexiform lesion incidences were compared between diet groups within each age using a Z-test (Jandel Scientific, 1994). Statistical comparisons of vascular pressures across different anatomical locations and between groups within an anatomical location were assessed by one-way ANOVA. Differences in tension by diet group were evaluated using a 2-way ANOVA (group by concentration). When comparing pulmonary arteries versus pulmonary rami, the data were reported as a percentage of the peak tension during the potassium chloride plus norepinephrine challenge to normalize for differences in arterioles. Baseline was set to zero by subtracting the normalized baseline from the values at each concentration. The data were then analyzed using a 2-way ANOVA (vessel by concentration). Phosphatase and monoamine oxidase activity were analyzed using a 2-way ANOVA (vessel by group). For all ANOVA, a Tukey post hoc analysis was used when appropriate. A P < 0.05 was used as the level of significance.
RESULTS
Histology
The plexiform lesion incidence at 30 d of age was 78.6% (11/14 lungs had lesions) in the high-Trp group and 43.8% (7/16 lungs) in the control group (P = 0.11). No difference was detected at 42 to 48 d of age, when lungs from birds fed the high-Trp diet exhibited a 55.6% incidence of plexiform lesions (10/18 lungs), as compared with a 61.1% incidence of lesions (11/18 lungs) in the lungs from birds fed the control diet (P = 0.79). Figure 2 is a representative photomicrograph of a plexiform lesion from a 30-d-old broiler. No differences in the histology of plexiform lesions were apparent between the control and high-Trp groups. Plexiform lesions primarily develop at the points where muscularized interparabronchial arteries branch from their parent arteries. Mature lesions typically consisted of a matrix of intimal proliferating cells and embedded macrophages having a foam-type appearance that tend to be arrayed adjacent to the remnants of the dilated, often indistinct remnants of the arterial wall. Sparse, slit-like vascular channels within the lesions generally appeared to be free of erythrocytes in well-perfused lungs, giving the lesions their characteristic glomeruloid appearance (Figure 2).
Figure 2.
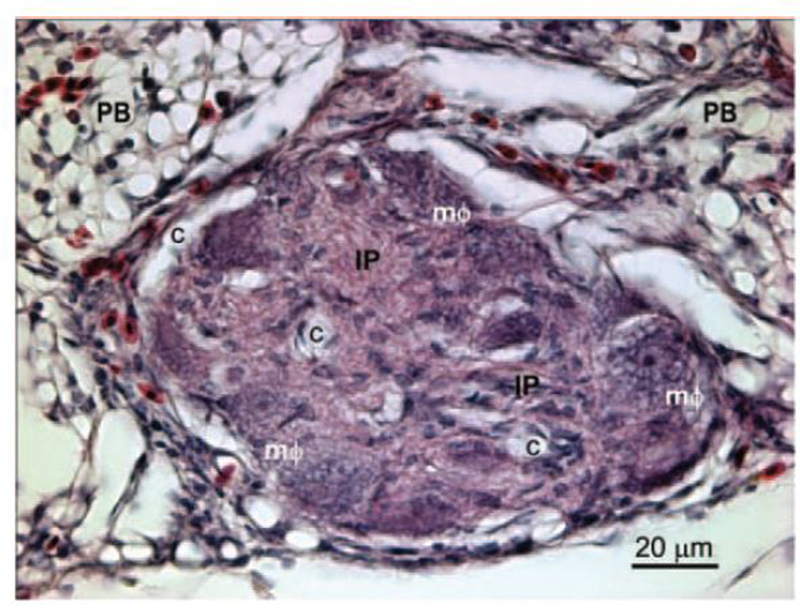
Lung section from a 30-d-old broiler showing an arteriole with its lumen occluded by a plexiform lesion. The affected arteriole lies in the connective tissue septum separating 2 adjacent parabronchi (PB). The lesion consists of a matrix of proliferating intimal cells (IP) with foam-type macrophages (mΦ) arrayed around the remnants of the vascular wall. The perivascular connective tissue and gas exchange parenchyma contain nucleated avian erythrocytes and heterophils. The glomeruloid-like structure of this mature plexiform lesion is indicated by the multiple vascular channels (c) that have been cleared of erythrocytes by perfusion fixation. The 5-μm-thick section is stained with hematoxylin and eosin. Color version available in the online version of this paper.
Pulmonary Vascular Pressures
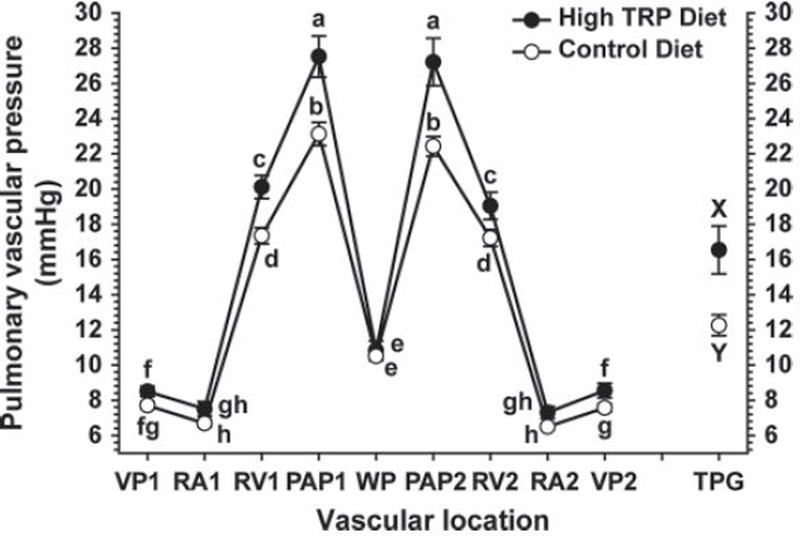
Pulmonary vascular pressure profiles are shown for the control and high-Trp groups in Figure 3. These profiles include the pressures recorded as a catheter was advanced from the wing vein forward through the vascular tree: venous pressure 1 (VP1); right atrial pressure 1 (RA1); right ventricular pressure 1 (RV1); pulmonary arterial pressure 1 (PAP1); and the wedge pressure (WP). The pressures were recorded again as the catheter was withdrawn from the vascular tree (PAP2, RV2, RA2, and RV2). The transpulmonary pressure gradient (TPG) was calculated as the difference between the PAP and WP: (TPG = PAP − WP). Broilers fed the high-Trp diet had higher values for PAP1, PAP2, RV1, RV2, and VP2 than broilers fed the control diet. Values for VP1, RA1, RA2, and WP did not differ between the control and high-Trp groups. In both groups, the PAP was higher than the RV pressure, which in turn was higher than the WP. Venous pressures were lower than WP but higher than RA pressures within each group. The TPG was markedly higher in the high-Trp group than in the control group. Ascites fluid accumulation was detected in one bird in the high-Trp group and none of the birds in the control group.
Figure 3.
Pulmonary vascular pressure profiles for the control (n = 16) and high-Trp (n = 16) groups. Pressures (mmHg) were recorded as a catheter was advanced through the basilica vein (VP1), into the right atrium (RA1), right ventricle (RV1), and pulmonary artery (PAP1), until the wedge pressure (WP) was obtained. Vascular pressures were also recorded as the catheter was withdrawn (PAP2, RV2, RA2, and VP2). The transpulmonary pressure gradient (TPG) was calculated as PAP − WP. a–hValues with different letters differed significantly (P < 0.05).
Concentration Response Curves
One of the pulmonary arteries was judged to be nonviable and was not used for data collection. There were no significant differences in baseline tensions by group (control vs. high-Trp) or vessel type (pulmonary artery vs. pulmonary rami). The average baseline tension for the pulmonary artery was 0.39 ± 0.02 g and for the pulmonary rami was 0.40 ± 0.02 g. The peak tension produced by the addition of potassium chloride + norepinephrine was not different between the control and high-Trp group. However, pulmonary arteries produced more tension (1.60 ± 0.86 g) than pulmonary rami (0.84 ± 0.26 g; P < 0.03). As a result, when comparing pulmonary arteries and pulmonary rami, the data are expressed as a percentage of peak potassium chloride + norepinephrine tension.
Serotonin- and ATP-Mediated Vasoconstriction
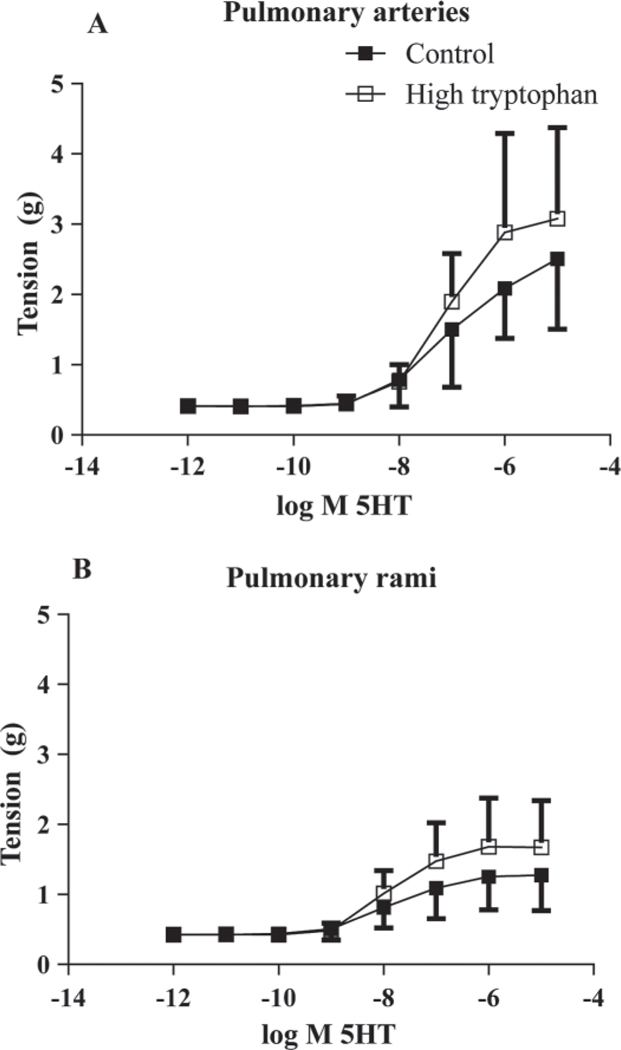
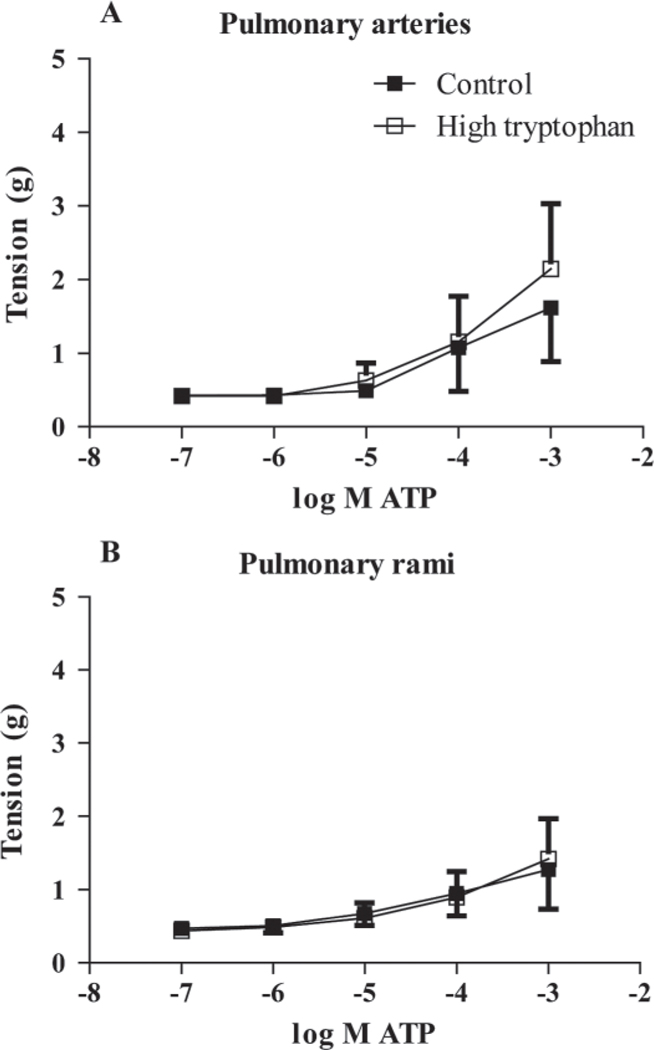
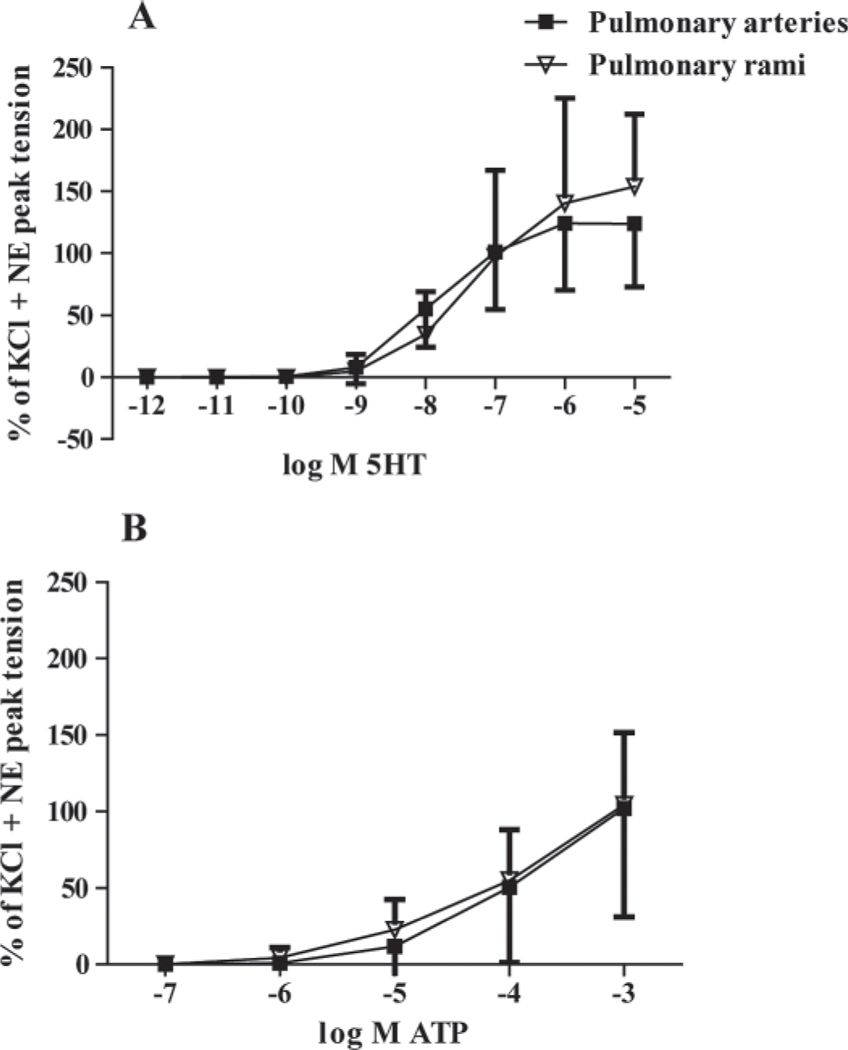
A cumulative concentration response curve to serotonin for the pulmonary arteries for both groups is represented in Figure 4A. Both the high-Trp (n = 9) and the control group (n = 10) showed an increase in tension as serotonin concentrations increased within the pulmonary arteries (P < 0.05 different from baseline). The pulmonary rami in both groups showed similar increases in tension when serotonin concentrations increased (Figure 4B). The pulmonary arteries (control, n = 10; high-Trp, n = 9) showed an increasing tension in both groups as ATP concentrations increased (P < 0.05 different from baseline), with most of the tension developing between 10−5 M to 10−3 M ATP (Figure 5A). Both groups showed a similar responsiveness to ATP within the pulmonary rami (control, n = 11; high-Trp, n = 9; Figure 5B). As the ATP concentrations increased, both groups exhibited increased tension. To assess the type of purinergic receptor present, α,β-methylene ATP (10−5 M), an ATP analog specific to P2X receptors, was added to pulmonary arteries and pulmonary rami (n = 2, each). The α,β-methylene ATP resulted in vasoconstriction in the pulmonary arteries (average peak tension: 1.26 ± 0.24 g) and in the pulmonary rami (average peak tension: 0.64 ± 0.01 g). The serotonin-mediated and ATP-mediated contraction in pulmonary arteries and pulmonary rami are compared in Figure 6. Because there were no differences in the diet groups, the groups were combined for the following data (pulmonary arteries, n = 19; pulmonary rami, n = 20).When data was reported as a percentage of potassium chloride + norepinephrine peak tension and corrected for the baseline, there were no differences in response to serotonin (Figure 6A) or ATP (Figure 6B).
Figure 4.
Serotonin (5HT)-mediated vasoconstriction in the pulmonary arteries and pulmonary rami. In the pulmonary arteries (A) and the pulmonary rami (B), increasing concentrations of serotonin caused an increase in vessel tension. There was no difference between diet groups (control n = 10; high-Trp, n = 9) in the pulmonary arteries or the pulmonary rami.
Figure 5.
Adenosine triphosphate (ATP)-mediated vasoconstriction in the intrapulmonary arteries. There were no differences between the control (n = 10) and high-Trp groups (n = 9) with ATP-mediated vasoconstriction. The ATP caused vasoconstriction in the pulmonary arteries (A) and in the pulmonary rami (B).
Figure 6.
Comparison of the agonist response curve in the pulmonary arteries versus the pulmonary rami. Diet groups are combined because there was no diet-group effect. Serotonin (5HT)-mediated vasoconstriction (A) was not different in pulmonary rami (n = 19) and pulmonary arteries (n = 20). The addition of adenosine triphosphate (ATP) produced an increase in relative tension in the pulmonary artery and rami (B). NE = norepinephrine.
Enzyme Activities
There was no effect of diet (control vs. high-Trp) on phosphatase activity in the pulmonary artery and the pulmonary rami. However, the pulmonary rami had greater phosphatase activity (8.35 ± 9.34 μM phosphate/μg of protein per minute; range: 0.65–39.57 μM phosphate/μg of protein per minute, n = 20) compared with the pulmonary artery (3.80 ± 2.43 μM phosphate/μg of protein per minute; P < 0.05; range: 0.50–9.34 μM phosphate/μg of protein per minute, n = 20). Monoamine oxidase activity was also assessed in the pulmonary arteries and the pulmonary rami. In the control group, 5 of the pulmonary arteries were excluded because they had too much blood embedded in the vessel to accurately assess monoamine oxidase activity in the vessel wall. Monoamine oxidase activity in the control group (3,559 ± 1,169 relative luminescence units, n = 3) was greater in magnitude than in the high-Trp group (83 ± 186 relative luminescence units, P < 0.05, n = 5) in the pulmonary arteries. In the high-Trp group, 4 pulmonary arteries exhibited no detectible monoamine oxidase activity, but all of the vessels in the control group had measureable monoamine oxidase activity. For the pulmonary rami, monoamine oxidase activities did not differ between the control group (340 ± 602 relative luminescence units, n = 7) and the high-Trp group (0 ± 0 relative luminescence units, n = 8). There was no detectible monoamine oxidase activity in any of the pulmonary rami from the high-Trp group or in 5 of the arteries from the control group.
DISCUSSION
In the present study, broilers in the high-Trp group tended to develop a higher incidence of plexiform lesions than broilers in the control group at 30 d of age (P = 0.11), but no difference was evident between the groups at 42 to 48 d of age. Clearly, plexiform lesions developed rapidly in relatively immature birds, during the exponential phase of growth when broilers are most likely to succumb to spontaneous IPAH (Wideman and Hamal, 2011). The relatively low incidence of vascular obstruction by plexiform lesions in broiler lungs does not seem likely to have substantially increased the pulmonary vascular resistance, because an extremely low proportion of the vascular channels are obstructed. Accordingly, plexogenic arteriopathy appears to be a consequence rather than the proximate cause of the pulmonary hypertension in broilers that develop IPAH (Wideman et al., 2011). In agreement with previous studies, the results of the present study demonstrated that broilers from the high-Trp group had pulmonary arterial pressures that were higher than those of the control group, yet the wedge pressures did not differ between the groups. These observations, in combination with the higher transpulmonary pressure gradient for the high-Trp group, indicate that the pulmonary hypertension attributable to supplemental dietary tryptophan was the net result of elevated precapillary resistance to blood flow (pulmonary arterial hypertension) rather than to postcapillary resistance (pulmonary venous hypertension; Chapman and Wideman, 2001; Lorenzoni et al., 2008; Wideman et al., 2010).
Serotonin and ATP caused contraction in intrapulmonary arteries. The finding that ATP causes contraction in chicken intrapulmonary arteries has not been shown previously and has not been explored previously as a potential mechanism for IPAH. For serotonin and ATP, the high-Trp diet did not significantly alter pulmonary artery and pulmonary rami responses or receptor sensitivity. There were no differences in tension development between the pulmonary rami and the pulmonary arteries for serotonin or ATP. In the Wistar rat, pulmonary arteries and pulmonary rami were similar in response to serotonin, suggesting that the number of receptors and their sensitivity were comparable in both vessels. However, when an agonist for 5-HT1 receptor was used, tension development was greater in the pulmonary rami than in the arteries, suggesting that regional differences exist in the intrapulmonary vasculature of mammals (Rodat-Despoix et al., 2008). Human pulmonary arteries also have 5-HT1 and 5-HT2 receptor-mediated vasoconstriction that is modulated by a combination of intracellular and extracellular calcium (Rodat-Despoix et al., 2009). Calcium sources for serotonin-mediated contraction also appear to be an important regional difference in the pulmonary arterial tree. Previous work in rats indicates that serotonin-mediated vasoconstriction in pulmonary arteries was more sensitive to blockade of calcium from the sarcoplasmic reticulum, when compared with second order arteries (Rodat-Despoix et al., 2008). If there is a level of the arterial tree that is more responsible for the development of pulmonary hypertension, further investigation into the mechanisms of vasoconstriction and the receptors involved is critically important. The serotonin transporter and the 5-HT1A, 5-HT2A, 5-HT-1B, and 5-HT2B receptors are present in broiler lungs, and increased expression of the 5-HT1A and 5-HT2B receptors was correlated with pulmonary hypertension in broilers (Hamal et al., 2010).
Purinergic vasoconstriction in broilers is a unique finding and suggests that some of the purinergic receptors are expressed in the intrapulmonary arteries. Previous work in rats showed that, α,β-methylene ATP, a specific P2X agonist, was the most potent in causing constriction in small and large intrapulmonary arteries (Chootip et al., 2002). Smaller arteries exhibited greater tension development to the specific P2X agonist, α,β-methylene ATP, compared with larger intrapulmonary arteries. These results demonstrate pharmacological evidence for P2X receptors on intrapulmonary arteries in mammals (Chootip et al., 2002). Pilot data collected for this study (reported in the Results) indicate that P2X receptors are also present within broiler pulmonary arteries and pulmonary rami. Although α,β-methylene ATP was not used for dose-response curves in the current study, increasing ATP concentrations did elicit increased vasoconstriction within broiler pulmonary artery and pulmonary rami, suggesting the presence of purinergic receptors in the broiler intrapulmonary arteries. The purinergic 2 receptor subtype remains to be identified. Vasoconstriction in response to ATP should be considered as a potential mechanism contributing to excessive vascular resistance in broilers susceptible to IPAH.
A unique aspect of this study was to include metabolic activity of the key family of enzymes that metabolize serotonin and ATP. We measured the activity of phosphatases that are responsible for breaking down ATP, and monoamine oxidase that is responsible for breaking down serotonin. Phosphatase activity was not influenced by the diet treatment, but pulmonary rami did have more relative phosphatase activity when compared with the pulmonary arteries. Phosphatase activity may play an important role in modulating the amount of ATP that reaches the purinergic receptors and thus blunt some of the vasoconstriction caused by ATP.
In contrast, there was a significant effect of diet on monoamine oxidase activity such that the high-Trp group had little to no detectible activity in the pulmonary arteries and the pulmonary rami. Low monoamine oxidase activity implies that serotonin may linger longer in the high-Trp group compared with the control group. Considering that supplemental levels of tryptophan increase the quantities of serotonin that are synthesized and released (Rosebrough, 1996), even in the absence of higher serotonin receptor sensitivity, broilers in the high-Trp group may exhibit amplified serotoninmediated vasoconstriction because locally acting serotonin is not as easily neutralized. This hypothesis was not tested in this study, but previous work suggests that genetic deletion or inhibition of monoamine oxidase does result in enhanced serotonin-mediated vasoconstriction and mitogenic activity (Lairez et al., 2009; Seto et al., 2009).There is no previous data suggesting that tryptophan itself inhibits monoamine oxidase activity. It is more likely that the inhibition of activity or reduced expression of monoamine oxidase in the highTrp group was a secondary effect of higher pulmonary pressures. These results should be interpreted with caution because monoamine oxidase is not the only enzyme that breaks down serotonin, and the sample size for these experiments was small.
In conclusion, broilers fed a commercial diet supplemented with excess tryptophan developed higher pulmonary arterial pressures than broilers fed a control diet. Plexiform lesion incidences tended to be higher (P = 0.11) in the high-Trp group than in the control group at 30 d of age, although lesion incidences for both groups had converged by 42 to 48 d of age. The pulmonary arteries and pulmonary rami both exhibited constriction in response to serotonin and ATP. This is the first study to demonstrate purinergic-mediated constriction in broiler intrapulmonary arteries. Metabolic activity of ATP phosphatases was greater in pulmonary rami.
ACKNOWLEDGMENTS
The authors thank Ryan Bailey (Department of Health Science, Kinesiology, Recreation and Dance, University of Arkansas, Fayetteville) for his technical assistance during this project. This project was supported by the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlements Proceeds Acts of 2000. Supported by NIH/National Heart Lung Blood Institute Grant 1R15HL092517-01.
REFERENCES
- Chapman ME, and Wideman RF Jr. 2001. Pulmonary wedge pressures confirm pulmonary hypertension in broilers is initiated by an excessive pulmonary arterial (precapillary) resistance. Poult. Sci. 80:468–473. [DOI] [PubMed] [Google Scholar]
- Chootip K, Ness KF, Wang Y, Gurney AM, and Kennedy C. 2002. Regional variation in P2 receptor expression in the rat pulmonary arterial circulation. Br. J. Pharmacol. 137:637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ME, and Wideman RF. 2006a. Evaluation of the serotonin receptor blockers ketanserin and methiothepin on the pulmonary hypertensive responses of broilers to intravenously infused serotonin. Poult. Sci. 85:777–786. [DOI] [PubMed] [Google Scholar]
- Chapman ME, and Wideman RF. 2006b. Evaluation of the serotonin receptor blocker methiothepin in broilers injected intravenously with lipopolysaccharide and microparticles. Poult. Sci. 85:2222–2230. [DOI] [PubMed] [Google Scholar]
- Cogan EB, Birrell GB, and Griffith OH. 1999. A robotics-based automated assay for inorganic and organic phosphates. Anal. Biochem. 271:29–35. [DOI] [PubMed] [Google Scholar]
- Corzo A, Kidd MT, Thaxton JP, and Kerr BJ. 2005a. Dietary tryptophan effects on growth and stress responses of male broiler chicks. Br. Poult. Sci. 46:478–484. [DOI] [PubMed] [Google Scholar]
- Corzo A, Moran ET Jr, Hoehler D, and Lemmell A. 2005b. Dietary tryptophan need of broiler males from forty-two to fifty-six days of age. Poult. Sci. 84:226–231. [DOI] [PubMed] [Google Scholar]
- Dergacheva O, Wang X, Kamendi H, Cheng Q, Pinol RM, Jameson H, Gorini C, and Mendelowitz D. 2008. 5HT2 receptor activation facilitates P2X receptor mediated excitatory neurotransmission to cardiac vagal neurons in the nucleus ambiguus. Neuropharmacology 54:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddahibi S, Adnot S, Frisdal E, Levame M, Hamon M, and Raffestin B. 2001a. Dexfenfluramine-associated changes in 5-hydroxytryptamine transporter expression and development of hypoxic pulmonary hypertension in rats. J. Pharmacol. Exp. Ther. 297:148–154. [PubMed] [Google Scholar]
- Eddahibi S, Guignabert C, Barlier-Mur AM, Dewachter L, Fadel E, Dartevelle P, Humbert M, Simonneau G, Hanoun N, Saurini F, Hamon M, and Adnot S. 2006. Cross talk between endothelial and smooth muscle cells in pulmonary hypertension: Critical role for serotonin-induced smooth muscle hyperplasia. Circulation 113:1857–1864. [DOI] [PubMed] [Google Scholar]
- Eddahibi S, Humbert M, Fadel E, Raffestin B, Darmon M, Capron F, Simonneau G, Dartevelle P, Hamon M, and Adnot S. 2001b. Serotonin transporter overexpression is responsible for pulmonary artery smooth muscle hyperplasia in primary pulmonary hypertension. J. Clin. Invest. 108:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadi M, Jahanshiri F, Kaveh K, Hair-Bejo M, Ideris A, and Alimon AR. 2011. Nutrition and immunity: The effects of the combination of arginine and tryptophan on growth performance, serum parameters and immune response in broiler chickens challenged with infectious bursal disease vaccine. Avian Pathol. 40:63–72. [DOI] [PubMed] [Google Scholar]
- Fatufe AA, Hirche F, and Rodehutscord M. 2005. Estimates of individual factors of the tryptophan requirement based on protein and tryptophan accretion responses to increasing tryptophan supply in broiler chickens 8–21 days of age. Arch. Anim. Nutr. 59:181–190. [DOI] [PubMed] [Google Scholar]
- Hamal KR, Wideman RF, Anthony NB, and Erf GF. 2010. Differential expression of vasoactive mediators in microparticle-challenged lungs of chickens that differ in susceptibility to pulmonary arterial hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 298:R235–242. [DOI] [PubMed] [Google Scholar]
- Jandel Scientific. 1994. SigmaStat Statistical Software User’s Manual. Jandel Scientific Software, San Rafael, CA. [Google Scholar]
- Lairez O, Calise D, Bianchi P, Ordener C, Spreux-Varoquaux O, Guilbeau-Frugier C, Escourrou G, Seif I, Roncalli J, Pizzinat N, Galinier M, Parini A, and Mialet-Perez J. 2009. Genetic deletion of MAO-A promotes serotonin-dependent ventricular hypertrophy by pressure overload. J. Mol. Cell Cardiol. 46:587–595. [DOI] [PubMed] [Google Scholar]
- Lorenzoni AG, Anthony NB, and Wideman RF Jr.. 2008. Transpulmonary pressure gradient verifies pulmonary hypertension is initiated by increased arterial resistance in broilers. Poult. Sci. 87:125–132. [DOI] [PubMed] [Google Scholar]
- MacLean MR, Herve P, Eddahibi S, and Adnot S. 2000. 5-hydroxytryptamine and the pulmonary circulation: Receptors, transporters and relevance to pulmonary arterial hypertension. Br. J. Pharmacol. 131:161–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan MR, Burnstock G, and Haworth SG. 1999. Vasoconstriction of intrapulmonary arteries to P2-receptor nucleotides in normal and pulmonary hypertensive newborn piglets. Br. J. Pharmacol. 128:549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortaz E, Folkerts G, Nijkamp FP, and Henricks PA. 2010. ATP and the pathogenesis of COPD. Eur. J. Pharmacol. 638:1–4. [DOI] [PubMed] [Google Scholar]
- NRC. 1994. Nutrient Requirements of Poultry. 9th rev. ed National Academy Press, Washington, DC. [Google Scholar]
- Ohata Y, Ogata S, Nakanishi K, Kanazawa F, Uenoyama M, Hiroi S, Tominaga S, and Kawai T. 2011. Expression of P2X4R mRNA and protein in rats with hypobaric hypoxia-induced pulmonary hypertension. Circ. J. 75:945–954. [DOI] [PubMed] [Google Scholar]
- Peacock AJ, Picket C, Morris K, and Reeves JT. 1989. The relationship between rapid growth and pulmonary hemodynamics in the fast-growing broiler chicken. Am. Rev. Respir. Dis. 139:1524–1530. [DOI] [PubMed] [Google Scholar]
- Rodat-Despoix L, Aires V, Ducret T, Marthan R, Savineau JP, Rousseau E, and Guibert C. 2009. Signalling pathways involved in the contractile response to 5-HT in the human pulmonary artery. Eur. Respir. J. 34:1338–1347. [DOI] [PubMed] [Google Scholar]
- Rodat-Despoix L, Crevel H, Marthan R, Savineau JP, and Guibert C. 2008. Heterogeneity in 5-HT-induced contractile and proliferative responses in rat pulmonary arterial bed. J. Vasc. Res. 45:181–192. [DOI] [PubMed] [Google Scholar]
- Rosebrough RW 1996. Crude protein and supplemental dietary tryptophan effects on growth and tissue neurotransmitter levels in the broiler chicken. Br. J. Nutr. 76:87–96. [DOI] [PubMed] [Google Scholar]
- Samizo K, Ishikawa R, Nakamura A, and Kohama K. 2001. A highly sensitive method for measurement of myosin ATPase activity by reversed-phase high-performance liquid chromatography. Anal. Biochem. 293:212–215. [DOI] [PubMed] [Google Scholar]
- Seto SW, Lam HY, Lau WS, Au AL, Lam TY, Chim SS, Ngai SM, Chan SW, Leung TY, Yeung JH, Kong SK, Leung GP, Lee SM, and Kwan YW. 2009. Role of monoamine oxidases in the exaggerated 5-hydroxytryptamine-induced tension development of human isolated preeclamptic umbilical artery. Eur. J. Pharmacol. 605:129–137. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Lee SD, and Cool CC. 1998a. Histopathology of pulmonary hypertension. Chest 114:1S–6S. [DOI] [PubMed] [Google Scholar]
- Tuder RM, Radisavljevic Z, Shroyer KR, Polak JM, and Voelkel NF. 1998b. Monoclonal endothelial cells in appetite suppressant-associated pulmonary hypertension. Am. J. Respir. Crit. Care Med. 158:1999–2001. [DOI] [PubMed] [Google Scholar]
- Wideman RF 2000. Cardio-pulmonary hemodynamics and ascites in broiler chickens. Poult. Avian Biol. Rev. 11:21–43. [Google Scholar]
- Wideman RF 2001. Pathophysiology of heart/lung disorders: Pulmonary hypertension syndrome in broiler chickens. World’s Poult. Sci. J. 57:289–307. [Google Scholar]
- Wideman RF, Chapman ME, Hamal KR, Bowen OT, Lorenzoni AG, Erf GF, and Anthony NB. 2007. An inadequate pulmonary vascular capacity and susceptibility to pulmonary arterial hypertension in broilers. Poult. Sci. 86:984–998. [DOI] [PubMed] [Google Scholar]
- Wideman RF, Chapman ME, Wang W, and Erf GF. 2004. Immune modulation of the pulmonary hypertensive response to bacterial lipopolysaccharide (endotoxin) in broilers. Poult. Sci. 83:624–637. [DOI] [PubMed] [Google Scholar]
- Wideman RF Jr., Eanes ML, Hamal KR, and Anthony NB. 2010. Pulmonary vascular pressure profiles in broilers selected for susceptibility to pulmonary hypertension syndrome: Age and sex comparisons. Poult. Sci. 89:1815–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RF Jr., and Hamal KR. 2011. Idiopathic pulmonary arterial hypertension: an avian model for plexogenic arteriopathy and serotonergic vasoconstriction. J. Pharmacol. Toxicol. Methods 63:283–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman RF Jr., Hamal KR, Bayona MT, Lorenzoni AG, Cross D, Khajali F, Rhoads DD, Erf GF, and Anthony NB. 2011. Plexiform lesions in the lungs of domestic fowl selected for susceptibility to pulmonary arterial hypertension: Incidence and histology. Anat. Rec. (Hoboken) 294:739–755. [DOI] [PubMed] [Google Scholar]
- Zhang S, Remillard CV, Fantozzi I, and Yuan JX. 2004. ATP-induced mitogenesis is mediated by cyclic AMP response element-binding protein-enhanced TRPC4 expression and activity in human pulmonary artery smooth muscle cells. Am. J. Physiol. Cell Physiol. 287:C1192–1201. [DOI] [PubMed] [Google Scholar]