Abstract
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with exposure to repetitive head impacts. CTE has been linked to disruptions in cognition, mood, and behavior. Unfortunately, the diagnosis of CTE can only be made post-mortem. Neuropathological evidence suggests limbic structures may provide an opportunity to characterize CTE in the living. Using 3T magnetic resonance imaging, we compared select limbic brain regional volumes – the amygdala, hippocampus, and cingulate gyrus – between symptomatic former National Football League (NFL) players and controls. Moreover, within the group of former NFL players, we examined the relationship between those limbic structures and neurobehavioral functioning. The former NFL group comprised eighty-six men (mean age=55.2±8.0 years) with at least 12 years of organized football experience, at least 2 years of active participation in the NFL, and self-reported declines in cognition, mood, and behavior within the last 6 months. The control group consisted of men (mean age=57.0±6.6 years) with no history of contact-sport involvement or traumatic brain injury. Compared to controls, former NFL players exhibited reduced volumes of the amygdala, hippocampus, and cingulate gyrus. Within the NFL group, reduced bilateral cingulate gyrus volume was associated with worse attention and psychomotor speed (r=0.4 (right), r=0.42 (left); both p<0.001), while decreased right hippocampal volume was associated with worse visual memory (r=0.25, p=0.027). Reduced volumes of limbic system structures in former NFL players are associated with neurocognitive features of CTE. Volume reductions in the amygdala, hippocampus, and cingulate gyrus may be potential biomarkers of neurodegeneration in those at risk for CTE.
Keywords: Volumetric MRI, Chronic Traumatic Encephalopathy, Hippocampus, Limbic System, Repetitive Head Impacts
Introduction
Chronic traumatic encephalopathy (CTE) is a neurodegenerative disease associated with repetitive head impacts (RHI) (McKee et al. 2013). It is often observed among American football players, boxers, and other contact-sport athletes (for reviews, see (Montenigro et al. 2014)). CTE can currently only be diagnosed at postmortem where the pathognomonic lesion of CTE is a perivascular accumulation of hyperphosphorylated tau (p-tau) protein in neurons and astrocytes, most prominent at the depths of the cortical sulci (McKee et al. 2016). As the disease progresses, neuronal loss and atrophy are observed in both frontal and medial temporal regions, including limbic system structures such as the amygdala, hippocampus, and cingulate gyrus (McKee et al. 2016; McKee et al. 2009; McKee et al. 2013).
The clinical features of CTE are not well understood (McKee et al. 2013; Montenigro et al. 2014; Stern et al. 2013). Next-of-kin interviews and medical record reviews of deceased males with neuropathologically-confirmed CTE suggest impaired behavior (e.g., impulsivity, aggression), mood (e.g., depression, hopelessness, apathy), and cognition (e.g., memory and executive dysfunction, eventual dementia) (Stern et al. 2013). The development of biomarkers and criteria that can support a CTE diagnosis during life is critical for the early detection of disease, which can facilitate timely interventions, as they become available (Montenigro et al. 2014).
To date, all cases with neuropathologically-confirmed CTE have had a history of exposure to RHI, making RHI exposure necessary (but not sufficient) for CTE (for review see (Baugh et al. 2012)). A small number of neuroimaging studies of individuals at high risk for CTE (based on extensive exposure to RHI) have shown structure-specific (e.g., cavum septum pellucidum (Koerte et al. 2016)), region-specific (e.g., hippocampus (Singh et al. 2014)), and/or whole brain disruption (for review see (Koerte et al. 2015b)). However, despite postmortem evidence of CTE-related atrophy in amygdala, hippocampus, and cingulate gyrus, there have been few in vivo studies that have examined these structures. One study reported reduced amygdala volumes in boxers and mixed martial arts fighters (Bernick et al. 2015). Similarly, hippocampus atrophy has been described in boxers and martial arts fighters (Orrison et al. 2009), in collegiate American football players (Singh et al. 2014), and in former National Football League (NFL) players (Strain et al. 2015). Finally, although atrophy in the cingulate gyrus has been related to severity of brain injury (Yount et al. 2002), it has yet to be investigated in symptomatic individuals with a history of exposure to RHI who are, therefore, at high risk for CTE. Moreover, across existing RHI studies, there have been only a limited number of studies investigating the association of volumes of limbic system structures with neurobehavioral function assessments (Bernick et al. 2015; Singh et al. 2014; Strain et al. 2015).
The aim of this study was to compare the volumes of the amygdala, hippocampus, and cingulate gyrus in symptomatic former NFL players, relative to asymptomatic controls without a history of RHI or brain trauma. Within the NFL group, we also examined associations between regions of volume reduction and neurocognitive and behavioral functioning.
Methods
This study is part of Diagnosing and Evaluating Traumatic Encephalopathy using Clinical Tests (DETECT; R01NS078337; R56NS078337). The goal of DETECT is to develop methods to characterize the clinical features of CTE, and to develop in vivo biomarkers (further details are provided elsewhere (Stamm et al. 2015a; Alosco et al. 2016; Koerte et al. 2016; Stern et al. 2016; Stamm et al. 2015b)).
Participants and procedure
Participants were recruited via social media, flyers, and word of mouth. Former NFL players met the following inclusion criteria: male, 40 to 69 years of age, at least 12 years of organized football experience with 2 or more years of active participation in the NFL, and self-reported declines in cognition, mood, and behavior within 6 months of study commencement.
Participants in the control group met the following inclusion criteria: male, 40 to 69 years of age, no participation in organized contact sports (e.g., boxing, rugby, football, martial arts, ice hockey) and no previous traumatic brain injury. Exclusion criteria for all subjects included contraindications for MR imaging and lumbar puncture, history or diagnosis of any CNS disease, and English as a second language.
Ninety-six former NFL players were enrolled in DETECT. Neuroimaging data were available for 87 of the 96 NFL players. We excluded one NFL player due to poor data quality. Thus, the final sample size for imaging analyses was 86 (mean age = 55.2 ± 8.0 years). Neurobehavioral data were available for 75 of the 86 players. There were 28 participants enrolled in the control group, with three excluded for poor data quality. We further excluded three controls due to Meniere’s disease, history of sports performance where repetitive mTBI was likely (with new history provided after enrollment), and confirmed history of mTBI (again provided after enrollment), respectively. Thus, the final sample size for controls was 22 (mean age = 57.0 ± 6.6 years).
All participants underwent a comprehensive assessment that included a neurological examination, a structured psychiatric interview, neuropsychological testing, neuroimaging, blood and cerebrospinal fluid sampling, and genetic testing. The present study focused on neuroimaging and neuropsychological testing.
MRI data acquisition
We acquired neuroimaging data on a 3-Tesla MRI Scanner (Verio, Siemens Healthcare, Erlangen, Germany) with a 32-channel head array and the Syngo MR-B17 software suite. T1-weighted images were acquired with a 3D magnetization-prepared-rapid-gradient-echo sequence (MPRAGE): TR=1800 ms, TE=3.36 ms, voxel size=1×1×1mm3, acquisition matrix=256×256, flip angle=7°.
Image processing
We reviewed the quality of the raw T1-weighted images by visually inspecting them for artifacts and intrascan misalignments. We then automatically segmented the volumes of anatomical regions of interest (ROI) from the T1-weighted images using FreeSurfer version 5.3 (http://surfer.nmr.mgh.harvard.edu; Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, USA). This segmentation resulted in an automated Talairach transformation, segmentation of deep gray matter structures (including hippocampus and amygdala), and parcellation of the cerebral cortex (including the cingulate gyrus), based on gyral and sulcal structures (Fischl et al. 2004). Following the automated volumetric segmentation, we conducted a visual quality assessment to ensure the fit and completeness of the obtained FreeSurfer parcellations. For each participant, we obtained an estimated total intracranial volume using the automated method in FreeSurfer. Next, we extracted bilateral ROIs from the automatically created FreeSurfer label maps of the cingulate gyrus, amygdala, and hippocampus.
Manual adjustment of regions of interest
Since the automated FreeSurfer segmentation often provides incomplete ROIs of limbic system structures, ROIs were manually adjusted (see below). Two investigators, trained in neuroanatomy, manually adjusted ROIs using Slicer 4.1 (http://www.slicer.org) (Fedorov et al. 2012). The first investigator adjusted ROIs from 78 participants, while the second investigator adjusted ROIs from 30 participants. A neuroanatomist (N.M.) reviewed, and, when necessary, adjusted the ROIs. Investigators were blind to group membership.
Amygdala and hippocampus
To correct amygdala and hippocampus volumes, we employed an approach described by Gurvits et al. (1996). Briefly, we corrected ROIs on coronal slices, from anterior to posterior, using sagittal slices for verification. The anterior border of the amygdala was often improperly identified by the automated segmentation. Consequently, our manual adjustment focused on including the anterior parts of the amygdala at the height of the frontotemporal junction. We then located the posterior boundary of the amygdala, defined as the last coronal slice before the appearance of the mammillary bodies. Next, we located the anterior border of the hippocampus, defined as the slice where the mammillary bodies first appeared. We identified the posterior boundary of the hippocampus as the coronal slice where the crux of the fornix was seen last. We then extracted volumes from the labels of the left and right amygdalae and hippocampi.
Cingulate gyrus
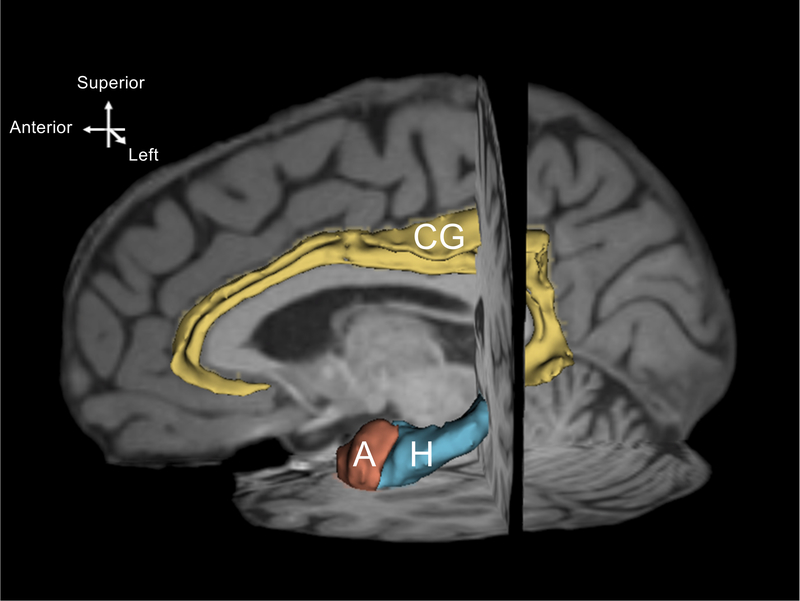
With a sagittal view, we located the cingulate gyrus by identifying the callosomarginal fissure on a paramidsagittal slice. Working from mesial to lateral sections of the brain, we excluded voxels extending into the corpus callosum and paracingulate gyrus. We also eliminated voxels when they extended beyond the rostrum of the corpus callosum into Brodmann’s Area 25. We verified our work by scrolling through coronal slices, working anteriorly to posteriorly. Next, we extracted volumes from the labels of the left and right cingulate gyri. Fig. 1 displays a three-dimensional reconstruction of the ROIs in left hemisphere superimposed on a T1-weighted image.
Fig. 1.
A three-dimensional reconstruction of left hemisphere regions of interest. The model was created from one randomly selected dataset using the model maker module of Slicer 4.1. Yellow = cingulate gyrus (CG), blue = hippocampus (H), terracotta = amygdala (A) The model is shown on a paramidsagittal slice and is superimposed on the individual T1-weighted images.
Neurobehavioral measures: cognition, mood, and behavior
Cognitive function
As part of DETECT, participants completed the following measures of cognition: Trail Making Test Parts A and B (TMT); Digit Span from the Wechsler Adult Intelligence Scale - Revised (WAIS-R); Digit Symbol Coding from the WAIS-R; Wisconsin Card Sorting Test (WCST); Controlled Oral Word Association Test (COWAT); Animal Fluency; Color-Word Interference subtest from the Delis-Kaplan Executive Function System (DKEFS); Boston Qualitative Scoring System (BQSS) for the Rey-Osterrieth Complex Figure; and Story Learning, List Learning, Naming, and Map Reading, from the Neuropsychological Assessment Battery (NAB).
Mood and behavior
Self-report and interview-based measures of mood and behavior were administered to all participants and included: Apathy Evaluation Scale (AES), Barratt Impulsivity Scale (BIS-11), Beck Depression Inventory II (BDI-II), Beck Hopelessness Scale (BHS), Behavior Rating Inventory of Executive Functioning - Adult Version (BRIEF-A), Brown-Goodwin Lifetime History of Aggression (LHA), Center for Epidemiologic Studies - Depression Scale (CES-D), Buss-Durkee Inventory, Hamilton Depression Rating Scale (HDRS), and the Modified Scale for Suicidal Ideation (MSSI).
For all cognitive measures and the BRIEF-A, raw scores were converted to age-, gender-, and education-standardized scores. Next, to reduce the number of analyses and risk of Type I error, a principal components analysis was performed to generate four factors (Alosco et al. 2016): Factor 1 - Mood and Behavior (including AES, BDI-II, BHS, BIS-11, BRIEF-A Behavioral Regulation Index, CES-D, HDRS, LHA); Factor 2 - Attention and Psychomotor Speed (including COWAT, DKEFS Color Word Inhibition/Switching, TMT Parts A and B, Digit Symbol); Factor 3 - Verbal Memory (including NAB Story Learning Phrase Unit Immediate and Delayed Recall, NAB List Learning Short and Long Delayed Recall); and Factor 4: Visual Memory (including BQSS Immediate Copy, Presence and Accuracy, and Delayed Presence and Accuracy).
Statistical Analyses
We used Statistical Analysis System (SAS version 9.4; SAS Institute Inc., North Carolina, USA) for all statistical analyses. We considered results significant when the p-value of our inferential tests was below 0.05. When between-group variances were significant, we used a Satterthwaite approximation(Satterthwaite 1946). We conducted independent samples t-tests for between-group comparisons of age, years of education, and body mass index (BMI). For between-group comparisons of the volumes of the cingulate gyri, amygdalae, and hippocampi, we used mixed effects regression models, controlling for age, BMI, estimated total intracranial volume, and years of education. To minimize variance, we employed a bootstrapping method (Efron and Tibshirani 1994). Specifically, we resampled 500 replicates with replacement, each with a size equal to the original sample, and we re-ran the mixed effect-regression model across all replicates. The resulting confidence intervals were calculated using bias-adjustment correction (Efron 1987). Similarly, we compared standardized neurobehavioral factors between groups using mixed effects regression models. Partial correlations adjusting for age, BMI, estimated total intracranial volume, and years of education examined associations between volume and standardized neurobehavioral factors within the NFL group.
Results
NFL players did not differ from controls in age. However, controls had lower BMI (Table 1). Table 2 displays the standardized mean differences between the groups on the neurobehavioral measures. As expected, based on the inclusion and exclusion criteria, former NFL players had significantly greater dysfunction in mood/behavior (Factor 1) and decreased verbal memory (Factor 3) compared to controls. There were no statistically significant between-group differences in psychomotor speed/executive function (Factor 2) or visual memory (Factor 4). Table 3 summarizes the results of the volumetric analyses. Compared to controls, the NFL group had reduced volumes, bilaterally, in amygdala, hippocampus, and cingulate gyrus. Further, within the NFL group there were statistically significant correlations between select regional volumes and neurobehavioral factor scores (Fig. 2). Specifically, reduced volumes of the left and right cingulate gyrus were associated with worse psychomotor speed/executive function (Factor 2). Additionally, reduced right hippocampus volume was associated with worse visual memory (Factor 4). There were no statistically significant associations between the bilateral amygdalae nor left hippocampus for any of the factor scores.
Table 1.
Demographic Data Comparisons
| NFL (n = 86) |
Controls (n = 22) |
||||
|---|---|---|---|---|---|
| mean (sd) | mean (sd) | t-value | p-value | 95% CI | |
| Age | 54.86 (7.9) | 57.3 (7.0) | 1.30 | 0.1955 | (−1.26, 6.08) |
| Years of education | 16.4 (0.96) | 17.4 (2.2) | 1.97 | .06131 | (−0.05, 1.91) |
| Body mass index | 32.9 (5.0) | 28.5 (3.8) | −3.92 | .0002 | (−6.70, −2.20) |
| No. of concussions2 | 123.1 (580.0) | - | - | - | - |
| No. times lost consciousness | 4.5 (16.5) | - | - | - | - |
Notes: NFL = National Football League.
Satterthwaite approximation due to unequal variances.
Based on self-report after being provided a current definition of concussion (Robbins et al. 2014)
Table 2.
Standardized group differences across neurobehavioral Factor Scores
| Adjusted Mean Difference: Controls - NFL |
Standard Error |
t-value |
p-value |
95% CI |
|
|---|---|---|---|---|---|
| Factor 1: mood and behavior | −1.1225 | 0.2049 | −5.48 | < .001 | (−1.529, −0.716) |
| Factor 2: attention/psychomotor speed | 0.0883 | 0.1958 | 0.45 | .6531 | (−0.300, 0.479) |
| Factor 3: verbal memory | 0.5085 | 0.2384 | 2.13 | .0355 | (0.035, 0.982) |
| Factor 4: visual memory | 0.3153 | 0.2186 | 1.44 | .1524 | (−0.119, 0.749) |
Notes: NFL = National Football League.
Satterthwaite approximation due to unequal variances. Results of mixed effects regression models, controlling for age, body-mass index, estimated total intracranial volume, and years of education. Compared to controls, former NFL players exhibited more mood/behavior symptoms and worse verbal memory. Note that inclusion criteria for the former NFL players required self-reported cognitive, mood, and behavioral dysfunction.
Table 3.
Volumetric group differences
| Adjusted Mean Difference: Controls – NFL (mm3) |
95% confidence interval (bias adjusted) |
p-value |
||
|---|---|---|---|---|
| Left cingulate gyrus | 575.014 | 29.6806 | 1289.02 | 0.036 |
| Right cingulate gyrus | 475.282 | 21.5682 | 1192.07 | 0.032 |
| Left amygdala | 176.216 | 86.059 | 313.24 | <.005 |
| Right amygdala | 157.425 | 49.7234 | 305.24 | 0.012 |
| Left hippocampus | 158.439 | 21.643 | 349.7 | 0.024 |
| Right hippocampus | 146.091 | 5.2748 | 343.66 | 0.032 |
Notes: NFL = National Football League. Results of mixed effects regression models, controlling for age, body-mass index, estimated total intracranial volume, and years of education. Former NFL players exhibited lower volumes of limbic system structures.
Fig. 2.
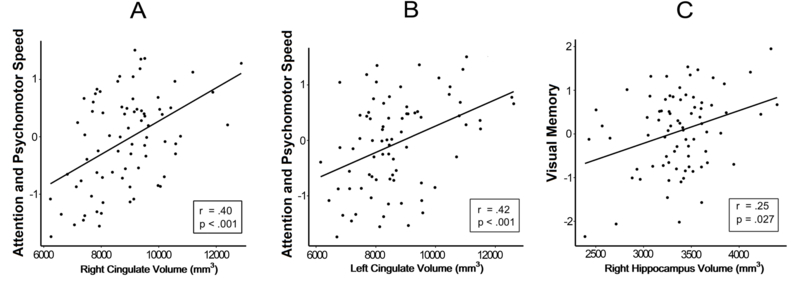
Significant associations between neurobehavioral factors and cingulate gyrus and hippocampus in the NFL group. A displays the association between volume in the right cingulate and Factor 2 (attention and psychomotor speed). B displays the association between the volume of the left cingulate gyrus and Factor 2. C displays the association between the right hippocampus and Factor 4 (visual memory). All analyses are based on partial correlations, adjusting for age, body-mass index, estimated total intracranial volume, and years of education.
Discussion
This study evaluated the volumes of the amygdala, hippocampus, and cingulate gyrus in a cohort of symptomatic former NFL players and a same-age control group. Relative to controls, former NFL players exhibited reduced volumes in bilateral amygdalae, hippocampi, and cingulate gyri. The structural changes in the limbic system in symptomatic former NFL players may be due to either RHI (i.e., microgliosis (Robinson et al. 2016)), neuropathological processes (i.e., tau-related neurodegeneration (McKee et al. 2013)), or a combination of both. Secondary findings from this study indicate that, within the NFL group, reduced volumes of the left and right cingulate gyri were associated with worse psychomotor speed/executive function, whereas reduced volume of the right hippocampus was associated with worse visual memory. The diversity of symptoms reported in CTE may be potentially related to the involvement of limbic system structures.
Cingulate Gyrus
In neuropathologically confirmed cases of CTE, cingulate gyrus atrophy has been observed (McKee et al. 2009; McKee et al. 2013). In vivo studies of former contact-sport athletes with a history of extensive RHI have demonstrated abnormal neurochemistry (Koerte et al. 2015a; Lin et al. 2015) and glucose metabolism (Provenzano et al. 2010) in the posterior cingulate gyrus. Cingulate gyrus atrophy has also been positively associated with severity of a single TBI (Yount et al. 2002). Given the history of RHI in our cohort of former NFL players, our results suggest that RHI, and not just injury severity, may impact the volume of the cingulate gyrus.
The NFL group demonstrated significantly reduced psychomotor speed/executive function relative to controls, and reduced performance in this domain was associated with reduced bilateral cingulate gyrus volumes. The cingulate gyrus is associated with diverse cognitive processes, including psychomotor speed/executive function (Davis et al. 2000; Drummond et al. 2005). Moreover, the cingulate gyrus is an important component of the default mode network (DMN) (Greicius et al. 2003). Abnormal DMN connectivity has been revealed in the acute post-injury phase after mild TBI, which was observed to resolve within five months (Mayer et al. 2011). Taken together, evidence of RHI vulnerability of the cingulate gyrus, along with its neurobehavioral correlates and neuropathology in confirmed CTE (Stein et al. 2014), suggest that the volume of this brain region is a potential neuroimaging marker for those at risk of CTE.
Amygdala
In advanced stages of CTE, the medial temporal regions are marked by extensive neurofibrillary pathology and regional atrophy (Stein et al. 2014; McKee et al. 2009; McKee et al. 2013). In our sample of former NFL players, bilateral amygdalae were significantly reduced in volume, compared to controls. Previous studies of combat-sport athletes have reported amygdala atrophy associated with RHI (Banks et al. 2014; Bernick et al. 2015). The amygdala presumably subserves many important neurobehavioral processes, including fear conditioning and memory modulation, and it has a role in regulating social behavior (Kilpatrick and Cahill 2003; Amaral et al. 2003; Rogan et al. 1997). Yet, in RHI studies, amygdala volume is both understudied and infrequently associated with cognitive dysfunction (Banks et al. 2014; Bernick et al. 2015). To our knowledge, in the context of RHI, amygdala volume has only been associated with processing speed, which was also associated with other brain regions, including limbic and basal ganglia structures (Bernick et al. 2015). In the present study, reduced amygdala volumes in the NFL group were not associated with neurobehavioral function. Given the limited evidence of association between RHI-related amygdala atrophy and neurobehavioral dysfunction, future work is needed to understand better the implications of amygdala atrophy following RHI.
Hippocampus
In in vivo studies of RHI, the hippocampus is emerging as a structure that may be particularly vulnerable to RHI (Bernick et al. 2015; Singh et al. 2014; Strain et al. 2015; Mannix et al. 2016). Neuropathological studies of CTE highlight the medial temporal lobe, including the hippocampus, as a region that is atrophied in later stages of the disease (McKee et al. 2013). Compared to controls, the NFL group exhibited reduced bilateral hippocampal volumes. Furthermore, volume reduction in the right hippocampus was associated with impaired visual memory, consistent with expected brain-behavior relationships. In the context of RHI studies, hippocampal atrophy has been associated with information processing speed and verbal memory (Bernick et al. 2015; Strain et al. 2015).
Limitations
There are limitations that need to be considered when interpreting our results. For example, the size of the NFL group was larger than the size of the control group. This may have diminished our power to detect effects among controls. Our findings also may not generalize to other groups frequently exposed to RHI, including former American football players who only played through high school or college. Future studies also need to investigate the effects of RHI in both sexes and across different sports. Further, although less likely, it cannot be ruled out that the observed smaller size of the limbic system structures in former NFL players (even after correction for BMI and intracranial volume) was present prior to their football careers. Longitudinal studies are thus needed to determine changes in volume over time. This is also important in following the trajectory of changes that may inform more the subgroup of players who develop neurodegenerative diseases such as CTE. Finally, not all athletes participating in contact sports experience CTE (Hazrati et al. 2013); thus, without postmortem analysis, it is not possible to state that our findings are specific to CTE. Nonetheless, our findings of disrupted limbic system structures, given their associated neurocognitive disturbances, may be candidate biomarkers of CTE in individuals who are at risk for CTE. Future research is needed to confirm these findings and refine their implications.
Conclusion
Symptomatic former NFL players, who are at high risk to develop CTE, exhibited reduced volumes of the amygdala, hippocampus, and cingulate gyrus, compared to controls. Within the NFL group, reduced volumes of limbic system structures were associated with worse neurocognitive function. Our findings suggest the diversity of symptoms reported in CTE may potentially be related to the involvement of limbic system structures, and the neurodegeneration of those structures may be a potential biomarker of CTE.
Funding and Acknowledgements:
The authors extend their appreciation to the study participants who make this work possible. This study was supported by the NIH (R01 NS 078337 (RAS, YT, CMB, NGF, BMM, CC); F31 NS 081957 (JMS); 1F32NS096803–01 (MLA), P30 AG13846 (RAS, YT, MLA, CMB, BMM, CC); UL1-TR000157 (RAS); P41 EB015902 (OP); T32GM074905 (IW)), and participant travel was partially funded by JetBlue Airlines, the National Football League, and the NFL Players Association. This study was also partly supported by the Else Kröner-Fresenius Foundation, Germany (IK, MM) and by a VA Merit Award (MES, MC). VS was supported by the German Academic Exchange Service PROMOS award. CL was supported by the Canadian Institutes of Health Research Frederick Banting and Charles Best Doctoral Award. The sponsors had no role in the design and conduct of the study, the collection, analysis, or the interpretation of the data, nor in the preparation, review, or approval of the manuscript, or the decision of submission for publication. This work was completed in partial fulfillment of JH’s Ph.D. dissertation.
Footnotes
Compliance with Ethical Standards: All procedures performed in this study were in accordance with the ethical standards of the Boston University Medical Campus Institutional Review Board, the Partners Institutional Review Board, and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
For all other authors, no competing financial interests exist.
Conflict of interest: Dr. Robert Stern is a paid consultant to Avanir Pharmaceuticals, Inc. (Aliso Viejo, CA), Biogen (Cambridge, MA), and Eli Lilly (Indianapolis, IN). He receives royalties for published neuropsychological tests from Psychological Assessment Resources, Inc. (Lutz, FL, USA). CMB receives research funding through the Harvard Football Players Health Study, which is funded by the NFL Players’ Association.
References
- Alosco ML, Jarnagin J, Tripodis Y, Platt M, Martin B, Chaisson CE, et al. (2016). Olfactory Function and Associated Clinical Correlates in Former National Football League Players. J Neurotrauma, doi: 10.1089/neu.2016.4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Capitanio JP, Jourdain M, Mason WA, Mendoza SP, & Prather M. (2003). The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia, 41(2), 235–240. [DOI] [PubMed] [Google Scholar]
- Banks SJ, Mayer B, Obuchowski N, Shin W, Lowe M, Phillips M, et al. (2014). Impulsiveness in professional fighters. [Paper]. J Neuropsychiatry Clin Neurosci, 26(1), 44–50, doi: 10.1176/appi.neuropsych.12070185. [DOI] [PubMed] [Google Scholar]
- Baugh CM, Stamm JM, Riley DO, Gavett BE, Shenton ME, Lin A, et al. (2012). Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav, 6(2), 244–254, doi: 10.1007/s11682-012-9164-5. [DOI] [PubMed] [Google Scholar]
- Bernick C, Banks SJ, Shin W, Obuchowski N, Butler S, Noback M, et al. (2015). Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the Professional Fighters’ Brain Health Study. Br J Sports Med, 49(15), 1007–1011, doi: 10.1136/bjsports-2014-093877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KD, Hutchison WD, Lozano AM, Tasker RR, & Dostrovsky JO (2000). Human anterior cingulate cortex neurons modulated by attention-demanding tasks. J Neurophysiol, 83(6), 3575–3577. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, & Meloy MJ (2005). The neural basis of the psychomotor vigilance task. Sleep, 28(9), 1059–1068. [PubMed] [Google Scholar]
- Efron B. (1987). Better Bootstrap Confidence Intervals. Journal of the American Statistical Association, 82(397), 171–185. [Google Scholar]
- Efron B, & Tibshirani RJ (1994). An Introduction to the Bootstrap. Boca Raton: Chapman & Hall/CRC Press. [Google Scholar]
- Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. (2012). 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging, 30(9), 1323–1341, doi: 10.1016/j.mri.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. (2004). Automatically parcellating the human cerebral cortex. Cereb Cortex, 14(1), 11–22. [DOI] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, & Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A, 100(1), 253–258, doi: 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurvits TV, Shenton ME, Hokama H, Ohta H, Lasko NB, Gilbertson MW, et al. (1996). Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry, 40(11), 1091–1099, doi: 10.1016/S0006-3223(96)00229-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazrati LN, Tartaglia MC, Diamandis P, Davis KD, Green RE, Wennberg R, et al. (2013). Absence of chronic traumatic encephalopathy in retired football players with multiple concussions and neurological symptomatology. Front Hum Neurosci, 7, 222, doi: 10.3389/fnhum.2013.00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick L, & Cahill L. (2003). Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage, 20(4), 2091–2099. [DOI] [PubMed] [Google Scholar]
- Koerte IK, Hufschmidt J, Muehlmann M, Tripodis Y, Stamm JM, Pasternak O, et al. (2016). Cavum Septi Pellucidi in Symptomatic Former Professional Football Players. J Neurotrauma, 33(4), 346–353, doi: 10.1089/neu.2015.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Lin AP, Muehlmann M, Merugumala S, Liao H, Starr T, et al. (2015a). Altered Neurochemistry in Former Professional Soccer Players without a History of Concussion. J Neurotrauma, doi: 10.1089/neu.2014.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerte IK, Lin AP, Willems A, Muehlmann M, Hufschmidt J, Coleman MJ, et al. (2015b). A review of neuroimaging findings in repetitive brain trauma. Brain Pathol, 25(3), 318–349, doi: 10.1111/bpa.12249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin AP, Ramadan S, Stern RA, Box HC, Nowinski CJ, Ross BD, et al. (2015). Changes in the neurochemistry of athletes with repetitive brain trauma: preliminary results using localized correlated spectroscopy. Alzheimers Res Ther, 7(1), 13, doi: 10.1186/s13195-015-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannix R, Berkner J, Mei Z, Alcon S, Hashim J, Robinson S, et al. (2016). Adolescent Mice Demonstrate a Distinct Pattern of Injury after Repetitive Mild Traumatic Brain Injury. J Neurotrauma, doi: 10.1089/neu.2016.4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AR, Mannell MV, Ling J, Gasparovic C, & Yeo RA (2011). Functional connectivity in mild traumatic brain injury. Hum Brain Mapp, 32(11), 1825–1835, doi: 10.1002/hbm.21151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cairns NJ, Dickson DW, Folkerth RD, Keene CD, Litvan I, et al. (2016). The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol, 131(1), 75–86, doi: 10.1007/s00401-015-1515-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol, 68(7), 709–735, doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain, 136(Pt 1), 43–64, doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenigro PH, Baugh CM, Daneshvar DH, Mez J, Budson AE, Au R, et al. (2014). Clinical subtypes of chronic traumatic encephalopathy: literature review and proposed research diagnostic criteria for traumatic encephalopathy syndrome. Alzheimers Res Ther, 6(5), 68, doi: 10.1186/s13195-014-0068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orrison WW, Hanson EH, Alamo T, Watson D, Sharma M, Perkins TG, et al. (2009). Traumatic brain injury: a review and high-field MRI findings in 100 unarmed combatants using a literature-based checklist approach. J Neurotrauma, 26(5), 689–701, doi: 10.1089/neu.2008.0636. [DOI] [PubMed] [Google Scholar]
- Provenzano FA, Jordan B, Tikofsky RS, Saxena C, Van Heertum RL, & Ichise M. (2010). F-18 FDG PET imaging of chronic traumatic brain injury in boxers: a statistical parametric analysis. [Paper]. Nucl Med Commun, 31(11), 952–957, doi: 10.1097/MNM.0b013e32833e37c4. [DOI] [PubMed] [Google Scholar]
- Robinson S, Berglass JB, Denson JL, Berkner J, Anstine CV, Winer JL, et al. (2016). Microstructural and microglial changes after repetitive mild traumatic brain injury in mice. J Neurosci Res, doi: 10.1002/jnr.23848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogan MT, Staubli UV, & LeDoux JE (1997). Fear conditioning induces associative long-term potentiation in the amygdala. Nature, 390(6660), 604–607, doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- Satterthwaite FE (1946). An approximate distribution of estimates of variance components. Biometrics, 2(6), 110–114. [PubMed] [Google Scholar]
- Singh R, Meier TB, Kuplicki R, Savitz J, Mukai I, Cavanagh L, et al. (2014). Relationship of collegiate football experience and concussion with hippocampal volume and cognitive outcomes. JAMA, 311(18), 1883–1888, doi: 10.1001/jama.2014.3313. [DOI] [PubMed] [Google Scholar]
- Stamm JM, Bourlas AP, Baugh CM, Fritts NG, Daneshvar DH, Martin BM, et al. (2015a). Age of first exposure to football and later-life cognitive impairment in former NFL players. Neurology, 84(11), 1114–1120, doi: 10.1212/WNL.0000000000001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamm JM, Koerte IK, Muehlmann M, Pasternak O, Bourlas AP, Baugh CM, et al. (2015b). Age at First Exposure to Football is Associated with Altered Corpus Callosum White Matter Microstructure in Former Professional Football Players. J Neurotrauma, doi: 10.1089/neu.2014.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein TD, Alvarez VE, & McKee AC (2014). Chronic traumatic encephalopathy: a spectrum of neuropathological changes following repetitive brain trauma in athletes and military personnel. Alzheimers Res Ther, 6(1), 4, doi: 10.1186/alzrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Daneshvar DH, Baugh CM, Seichepine DR, Montenigro PH, Riley DO, et al. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology, 81(13), 1122–1129, doi: 10.1212/WNL.0b013e3182a55f7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern RA, Tripodis Y, Baugh CM, Fritts NG, Martin BM, Chaisson C, et al. (2016). Preliminary Study of Plasma Exosomal Tau as a Potential Biomarker for Chronic Traumatic Encephalopathy. J Alzheimers Dis, doi: 10.3233/JAD-151028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain JF, Womack KB, Didehbani N, Spence JS, Conover H, Hart J Jr., et al. (2015). Imaging Correlates of Memory and Concussion History in Retired National Football League Athletes. JAMA Neurol, 72(7), 773–780, doi: 10.1001/jamaneurol.2015.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, et al. (2002). Traumatic brain injury and atrophy of the cingulate gyrus. J Neuropsychiatry Clin Neurosci, 14(4), 416–423. [DOI] [PubMed] [Google Scholar]