Abstract
Photosynthesis is a process wherein the chromophores in plants and bacteria absorb light and convert it into chemical energy. To mimic this process, an emissive poly(ethylene glycol)-decorated tetragonal prismatic platinum(II) cage was prepared and used as the donor molecule to construct a light-harvesting system in water. Eosin Y was chosen as the acceptor because of its good spectral overlap with that of the metallacage, which is essential for the preparation of light-harvesting systems. Such a combination showed enhanced catalytic activity in catalyzing the cross-coupling hydrogen evolution reaction, as compared with eosin Yalone. This study offers a pathway for using the output energy from the light-harvesting system to mimic the whole photosynthetic process.
Keywords: cage compounds, cross-coupling, hydrogen evolution reaction, light harvesting, photocatalysis
Photosynthesis,[1] as the primary source for the fuel on earth, is a process by which living plants and bacteria absorb, capture, transfer, and store energy from the sun. In this process, the energy from sunlight is captured and funneled by a dense array of chlorophyll molecules to the reaction center, and then converted into chemical energy.[2] So far, many artificial light-harvesting systems mimicking this process have been developed by using a Föster resonance energy-transfer (FRET) process, with the aim of developing clean and sustainable energy.[3-5] Among them, supramolecular systems[5] have received considerable attention not only because of their tunable and functionable molecular structures but also because the energy transfer between chlorophyll and protein in natural systems also relies on supramolecular self-assembly. For example, Yang et al. developed a highly efficient light-harvesting system based on the self-assembly of organic nanocrystals.[5b] Wang, Hu, and co-workers reported light-harvesting systems formed by water-soluble pillar[6]arene-based host-guest interactions.[5g] However, most of these systems just mimicked the FRET process of natural systems. The use of the output energy for photo-catalytic reactions has been rarely addressed. As natural photosynthetic systems also use the transferred energy for chemical reactions, artificial light-harvesting systems with the ability to catalyze chemical reactions for storing and releasing chemical energy are urgently needed.
Metal-organic cages and metallacages[6] represent three-dimensional cagelike structures formed by metal-coordination-driven self-assembly. With precisely controlled inner cavities, such fascinating structures have been widely studied in the past three decades for guest encapsulation, catalysis, and stabilizing reactive intermediates, etc.[7] Recently, Stang et al. developed a series of emissive metallacages[8] through the incorporation of tetraphenylethylene (TPE) derivatives as the building blocks. These metallacages exhibited aggregation-induced emission (AIE) properties[9] because of the restriction of molecular motions that decrease the nonradiative decay. In artificial light-harvesting systems, thousands of donor molecules are generally used for a single acceptor, and may cause fluorescence quenching by dye aggregation. Therefore, the use of AIE-active fluorophores as the donor molecules offers an alternative strategy to avoid the fluorescence quenching upon aggregation and increase the efficiency of light-harvesting systems.
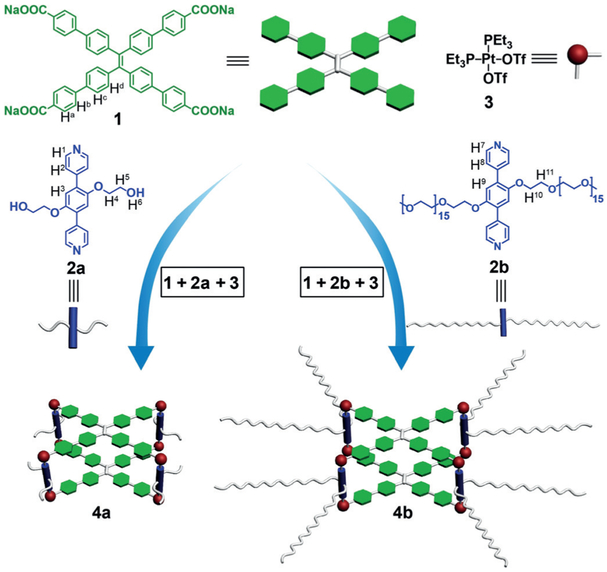
Based on the above discussions, it is inferred that the TPE-based metallacages may behave as good donor molecules for light-harvesting systems because of their highly emissive and AIE-active nature. Through the self-assembly of TPE-based sodium benzoate ligands (1), the dipyridyl ligand 2a or 2b, and cis-Pt(PEt3)2(OTf)2 (3), the metallacages 4a and 4b, respectively, were prepared in good yields (Scheme 1). Although the inner framework of the metallacages is hydrophobic, the introduction of eight poly(ethylene glycol) (PEG) chains affords 4b with good solubility in water (> 15.0 mM). This feature allows us to use it as the donor molecule for the FRET process in aqueous light-harvesting systems. By the further self-assembly of 4b with eosin Y, which serves as the acceptor molecule, through hydrophobic interactions, an efficient light-harvesting system was successfully constructed in water. Because of the intense absorption of 4b in the ultraviolet (UV) region, the light-harvesting system can not only use visible light but also exploit UV light through FRET to activate eosin Y, making it exhibit enhanced photocatalytic activity relative to eosin Y alone. For example, the conversions and yields increased twofold in the cross-coupling hydrogen evolution reaction[10] between benzothiazole and diphenylphosphine oxide (Scheme 2) upon irradiation by a Xe lamp. This study offers an example where the output energy of a supramolecular light-harvesting system can be used for chemical synthesis, which is another step towards artificial photosynthesis.
Scheme 1.
Self-assembly of the metallacages 4a and 4b. Tf = trifluoromethanesulfonyl.
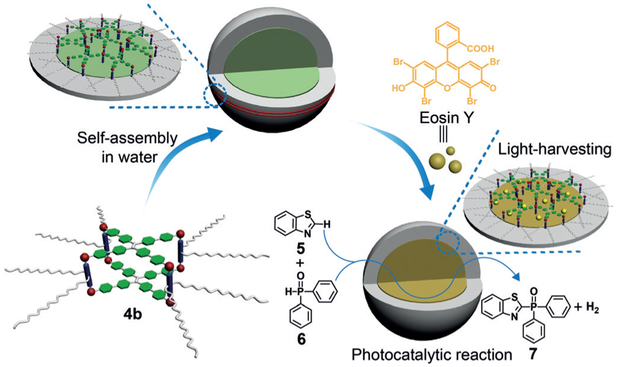
Scheme 2.
Cartoon representations of the light-harvesting system and its application in a photocatalytic reaction.
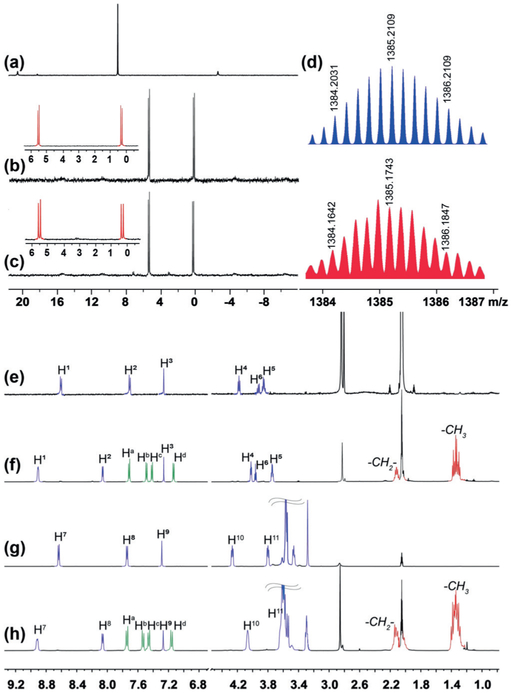
The formation of 4a and 4b was confirmed by multi-nuclear NMR spectroscopy (31P{1H} NMR and 1H NMR) and electrospray ionization time-of-flight mass spectrometry (ESI-TOF-MS). As depicted in Figures 1a-c, the 31P{1H} NMR spectra displayed two doublets of equal intensity with concomitant 195Pt satellites at δ = 5.54 and 0.32 ppm for 4a, δ = 5.45 and 0.31 ppm for 4b, revealing the formation of charge-separated metallacages.[8] In the 1H NMR spectra, significant downfield shifts were observed for α-pyridyl protons H1 (from δ = 8.61 to 8.92 ppm) and β-pyridyl protons H2 (from δ = 7.72 to 8.06 ppm; Figures 1e,f), as compared with the free dipyridyl ligand 2a, because of the coordination of the pyridyl moieties to the PtII ions. Analogous downfield shifts were also observed for 4b, as α-pyridyl protons H7 shifted from δ = 8.65 to 8.95 ppm and β-pyridyl protons H8 shifted from δ = 7.75 to 8.09 ppm (Figures 1 g,h). Ethoxy protons H4 and H5 of 4a and H10 and H11 of 4b shifted upfield, and is probably due to the shielding effect of the cage. ESI-TOF-MS affirmed the presumptive stoichiometry of 4a by showing isotopically well-resolved peaks at m/z = 2407.7954, 1768.7821, 1385.1743, 1129.1058 and 809.5500 (Figure 1d; see Figure S9 in the Supporting Information), corresponding to [M–3 OTf]3+, [M–4OTf]4+, [M–5OTf]5+, [M–6OTf]6+ and [M–8OTf]8+, respectively. Although all the attempts to get the ESI-TOF-MS of 4b failed, probably because of the polydispersity of PEG chains along with multiple charge states, we can compare the 31P{1H} NMR and 1H NMR spectra of 4a and 4b to conclude that both the metallacages were successfully prepared.
Figure 1.
Partial 31P {1H} NMR spectra (121.4 MHz, CD3COCD3, 295 K) of 3 (a), 4a (b), and 4b (c). d) Experimental (red) and calculated (blue) ESI-TOF-MS spectra of 4a: [M-5OTf]5+. Partial 1H NMR spectra (400 MHz, CD3COCD3, 295 K) of 2a (e), 4a (f), 2b (g), and 4b (h).
The photophysical properties of 4a and 4b in different solvents were collected (see Figure S12). Both cages show an intense absorption peak centered at about 292 nm with a shoulder at 356 nm in almost all the tested solvents, indicating that the solvents have little influence on the absorption. The metallacages 4a and 4b are emissive even in dilute solutions because the metal-coordination bonds restrict the rotation of the phenyl groups on the TPE units,[8] thus decreasing the nonradiative decay to give bright emission. It is worth noting that 4b shows a strong emission peak centered at 511 nm with quantum yield (Φf) of 23.8% in water. Moreover, similar to previously reported TPE-based metallacages,[8] it is also AIE-active (see Figure S12). These characteristics make it a good energy donor for aqueous light-harvesting systems.
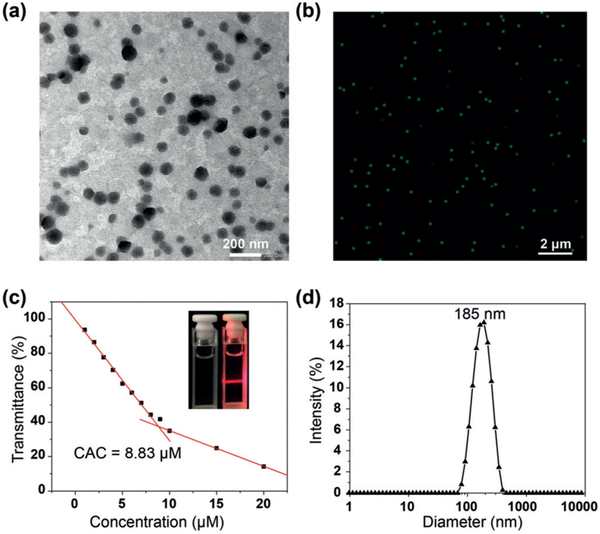
The self-assemblies formed by 4b in water were studied by transmission electron microscopy (TEM), confocal laser scanning microscopy (CLSM), and dynamic light scattering (DLS) measurements. It can be seen clearly that 4b forms spherical micelles with an average diameter of 140 nm at 10.0 μm (Figure 2a). Moreover, because 4b is emissive in water, nanoparticles with bright emission and diameter of 190 nm were visualized by CLSM (Figure 2b). The critical aggregation concentration (CAC) of 4b was measured to be 8.83 × 10−6 m by plotting the optical transmittance versus the concentration of 4b at 400 nm (Figures 3c; see Figure S13). DLS measurements showed that 4b formed large-sized aggregates with averaged hydrodynamic diameters (Dh) of 185 nm (Figure 2d), consistent with the TEM and CLSM results. In the micelles, the hydrophobic inner framework of 4b forms the core and the PEG chains form the corona, and they further assembled into spherical nanoparticles through hydrophobic interactions (Scheme 2).
Figure 2.
a) TEM and b) CLSM images of 4b in water (c = 10.0 μm, λex = 405 nm). c) Concentration-dependent optical transmittance at 400 nm of 4b in water. Inset: Tyndall effect of 4b in water (c = 10.0 μm). d) DLS data of 4b in water (c = 10.0 μm).
Figure 3.
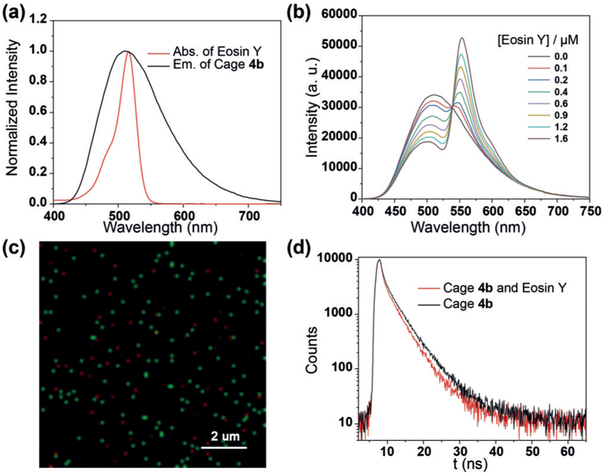
a) Normalized absorption spectrum of eosin Y and emission spectrum of 4b. b) Fluorescence spectra of 4b (c = 30.0 μm) in water with different concentrations of eosin Y (λex = 365 nm). c) CLSM image of 4b (c = 10.0 μm) and eosin Y (c = 5.0 μm) in water (λex = 405 nm). d) Fluorescence decay profiles of 4b (blank line), 4b and eosin Y (red line). ([4b] = 30.0 μm, [eosin Y] = 1.6 μm).
Eosin Y was used as the acceptor for 4b to construct an aqueous light-harvesting system because the absorption of eosin Y overlaps well with the emission of 4b in water (Figure 3a). With the gradual addition of eosin Y to the aqueous solution of 4b, the emission intensity at 511 nm, derived from 4b, decreased, while a new emission band at 550 nm, ascribed to eosin Y, appeared and increased when excited at 365 nm (Figure 3b). These phenomena suggested that efficient energy transfer took place from 4b to eosin Y. This process was also evidenced by the images taken by CLSM. When the light-harvesting system was irradiated at 405 nm, a FRET process took place from 4b to eosin Y, therefore some red nanoparticles derived from eosin Y were observed even though eosin Y itself cannot be excited at 405 nm (Figure 3c; see Figure S14). The fluorescence decay measurements were also carried out to study the light-harvesting process. The fluorescence lifetime (τ) of the assembly formed by 4b and eosin Y (τ1 = 2.45 ns, τ2 = 5.69 ns) was shorter than that of 4b (τ1 = 2.76 ns, τ2 = 6.30 ns), giving more evidence for the efficient energy transfer from 4b to eosin Y.[5] When the molar ratio of 4b/eosin Y is 20, the energy-transfer efficiency reached 45%, which is comparable to previously reported supramolecular light-harvesting systems in aqueous solution.[5g]
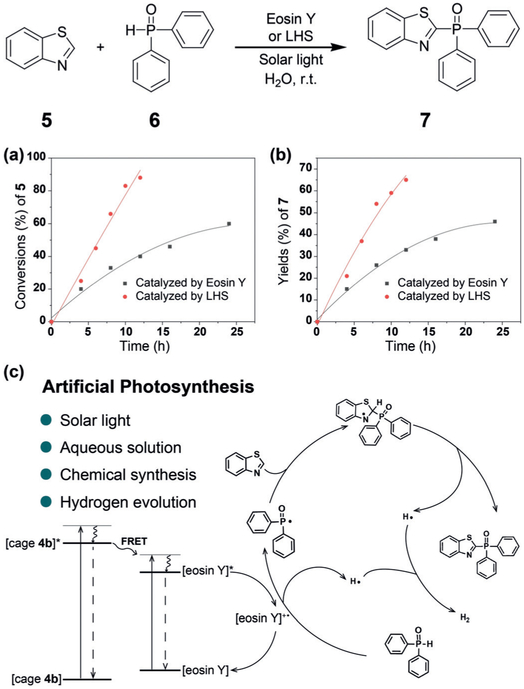
It is known that eosin Y can be used as a photosensitizer to catalyze C–H arylation of heteroarenes and cross-coupling hydrogen evolution reactions.[10] To use the output energy to mimic the whole photosynthetic process, we employed the above light-harvesting system as a photocatalyst for the cross-coupling reaction between benzothiazole (5) and diphenylphosphine oxide (6) in water. For eosin-Y-based photocatalytic reactions, green light or visible light is generally used as the light source[10b] because eosin Y shows little absorption in the UV region. However, in our system, because 4b exhibits strong absorption in the UV region, it can serve as an antenna to transfer the energy from the UV light to eosin Y, indicating that we can directly use solar light (here we used Xe lamp as the solar light simulator) to trigger the photocatalytic reaction. As shown in Figures 4 a and b, both the conversion of 5 and the yield of 7 increased dramatically by using the light-harvesting system compared with those of eosin Y alone. After a 12 hour reaction, the conversion of 5 reached 88% and the yield of 7 reached 65% when using the light-harvesting system as the photocatalyst. In comparison only 40% conversion and 33% yield were obtained by using eosin Y alone. Nearly no catalytic activity was observed for 4b. The FRET process increases the number of excited photocatalysts (eosin Y*) which undergo the catalytic cycle (Figure 4c) to increase the catalytic activity. Meanwhile, hydrogen was produced by the combination of dissociated hydrogen radicals, offering a pathway to store the energy from the light. Moreover, the photobleaching effect of eosin Y upon long-time irradiation was also decreased because of the formation of supramolecular micelles, which trap eosin Y and protect it from the damage of trace singlet oxygen.[11] All these results suggest that a light-harvesting system with good ability to catalyze organic reactions in water was successfully constructed. We believe that this strategy can also be applied in other light-harvesting systems to increase the photocatalytic activities of dye photosensitizers.
Figure 4.
a) Conversions of 5 versus reaction time. b) Yields of 7 versus reaction time. c) Plausible mechanism of cross-coupling hydrogen evolution reaction using LHS as photocatalyst. LHS = light-harvesting system.
In summary, two TPE-based tetragonal prismatic platinum(II) cages were prepared and characterized by 31P NMR and 1H NMR spectroscopy, ESI-TOF-MS, UV absorption, and fluorescence spectroscopy. By the incorporation of PEG chains on the pillars of the metallacage, it exhibits good water-solubility and is highly emissive in water. The metallacage was further used as an energy donor for eosin Y to prepare an efficient light-harvesting system in water through a FRET process. More importantly, the light-harvesting system shows good photocatalytic activity for cross-coupling hydrogen evolution reaction, offering the products in yields almost twofold that of using eosin Y alone. This study not only gives a type of metallacage-based structure as an energy donor for the construction of efficient light-harvesting systems, but also utilizes the output energy for photocatalytic reactions to prepare useful hydrocarbons and release gas, and will pave the way for the fabrication of artificial photosynthetic systems.
Supplementary Material
Acknowledgements
This work was supported by the National Natural Science Foundation of China (21801203 to M.Z.), National Science Foundation (CHE-1506722 to X. L.), National Institutes of Health (R01GM128037 to X. L.). M.Z. is thankful for start-up funds from Xi’an Jiaotong University. We thank Dr. Gang Chang and Yu Wang at Instrument Analysis Center and Dr. Aqun Zheng and Junjie Zhang at the Provincial Demonstration Center for Experimental Chemistry Education of Xi’an Jiaotong University for NMR and fluorescence measurements.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supporting information and the ORCID identification number(s) for the author(s) of this article can be found under: https://doi.org/10.1002/anie.201904407.
Contributor Information
Zeyuan Zhang, State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049 (P. R. China).
Zhengqing Zhao, State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049 (P. R. China).
Yali Hou, State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049 (P. R. China).
Heng Wang, Department of Chemistry, University of South Florida Tampa, FL 33620 (USA).
Xiaopeng Li, Department of Chemistry, University of South Florida Tampa, FL 33620 (USA).
Gang He, Frontier Institute of Science and Technology, Xi’an Jiaotong University, Xi’an 710049 (P. R. China).
Mingming Zhang, State Key Laboratory for Mechanical Behavior of Materials, Xi’an Jiaotong University, Xi’an 710049 (P. R. China).
References
- [1] a).Barber J, Chem. Soc. Rev 2009, 38, 185–196; [DOI] [PubMed] [Google Scholar]; b) Lu H, Kobayashi N, Chem. Rev 2016, 116, 6184–6261; [DOI] [PubMed] [Google Scholar]; c) Mora SJ, Odella E, Moore GF, Gust D, Moore TA, Moore AL, Acc. Chem. Res 2018, 51, 445–453; [DOI] [PubMed] [Google Scholar]; d) Zhang B, Sun L, Chem. Soc. Rev 2019, 48, 2216–2264. [DOI] [PubMed] [Google Scholar]
- [2].Scholes GD, Fleming GR, Olaya-Castro A, van Grondelle R, Nat. Chem 2011, 3, 763–774. [DOI] [PubMed] [Google Scholar]
- [3].For reviews, see: a) Ajayaghosh A, Praveen VK, Vijayakumar C, Chem. Soc. Rev 2008, 37, 109–122; [DOI] [PubMed] [Google Scholar]; b) Frischmann PD, Mahata K, Wurthner F, Chem. Soc. Rev 2013, 42, 1847–1870; [DOI] [PubMed] [Google Scholar]; c) Peng H-Q, Niu L-Y, Chen Y-Z, Wu L-Z, Tung C-H, Yang Q-Z, Chem. Rev 2015, 115, 7502–7542; [DOI] [PubMed] [Google Scholar]; d) McCusker JK, Science 2019, 363, 484–488; [DOI] [PubMed] [Google Scholar]; e) Xiao T, Zhong W, Zhou L, Xu L, Sun X-Q, Elmes RBP, Hu X-Y, Wang L, Chin. Chem. Lett 2019, 30, 31–36. [Google Scholar]
- [4].For light-harvesting systems based on covalent bonds, see: a) Miao L, Han J, Zhang H, Zhao L, Si C, Zhang X, Hou C, Luo Q, Xu J, Liu J, ACS Nano 2014, 8, 3743–3751; [DOI] [PubMed] [Google Scholar]; b) Zou Q, Zhang L, Yan X, Wang A, Ma G, Li J, Moehwald H, Mann S, Angew. Chem. Int. Ed 2014, 53, 2366–2370; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2014, 126, 2398–2402; [Google Scholar]; c) Liu K, Xing R, Chen C, Shen G, Yan L, Zou Q, Ma G, Moehwald H, Yan X, Angew. Chem. Int. Ed 2015, 54, 500–505 [DOI] [PubMed] [Google Scholar]; Angew. Chem 2015, 127, 510–515; [Google Scholar]; d) Xu J, Zhang B, Jansen M, Goerigk L, Wong WWH, Ritchie C, Angew. Chem. Int. Ed 2017, 56, 13882–13886; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 14070–14074. [Google Scholar]
- [5].For light-harvesting systems based on supramolecular self-assembly, see: a) Peng H-Q, Chen Y-Z, Zhao Y, Yang Q-Z, Wu L-Z, Tung C-H, Zhang L-P, Tong Q-X, Angew. Chem. Int. Ed 2012, 51, 2088–2092; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2012, 124, 2130–2134; [Google Scholar]; b) Chen PZ, Weng YX, Niu LY, Chen YZ, Wu LZ, Tung CH, Yang QZ, Angew. Chem. Int. Ed 2016, 55, 2759–2763; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 2809–2813; [Google Scholar]; c) Liu Y, Jin J, Deng H, Li K, Zheng Y, Yu C, Zhou Y, Angew. Chem. Int Ed 2016, 55, 7952–7957; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2016, 128, 8084–8089; [Google Scholar]; d) Xu Z, Peng S, Wang Y-Y, Zhang J-K, Lazar AI, Guo D-S, Adv. Mater 2016, 28, 7666–7671; [DOI] [PubMed] [Google Scholar]; e) Ke X-S, Kim T, Lynch VM, Kim D, Sessler JL, J. Am. Chem. Soc 2017, 139, 13950–13956; [DOI] [PubMed] [Google Scholar]; f) Li J-J, Chen Y, Yu J, Cheng N, Liu Y, Adv. Mater 2017, 29, 1701905; [DOI] [PubMed] [Google Scholar]; g) Guo S, Song Y, He Y, Hu X-Y, Wang L, Angew. Chem. Int. Ed 2018, 57, 3163–3167; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2018, 130, 3217–3221; [Google Scholar]; h) Sun C-L, Peng H-Q, Niu L-Y, Chen Y-Z, Wu L-Z, Tung C-H, Yang Q-Z, Chem. Commun 2018, 54, 1117–1120; [DOI] [PubMed] [Google Scholar]; i) Li C, Zhang J, Zhang S, Zhao Y, Angew. Chem. Int. Ed 2019, 58, 1643–1647; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2019, 131, 1657–1661. [Google Scholar]
- [6] a).Chakrabarty R, Mukherjee PS, Stang PJ, Chem. Rev 2011, 111, 6810–6918; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Han M, Engelhard DM, Clever GH, Chem. Soc. Rev 2014, 43, 1848–1860; [DOI] [PubMed] [Google Scholar]; c) Cook TR, Stang PJ, Chem. Rev 2015, 115, 7001–7045; [DOI] [PubMed] [Google Scholar]; d) Brown CJ, Toste FD, Bergman RG, Raymond KN, Chem. Rev 2015, 115, 3012–3035; [DOI] [PubMed] [Google Scholar]; e) Wang W, Wang YX, Yang HB, Chem. Soc. Rev 2016, 45, 2656–2693; [DOI] [PubMed] [Google Scholar]; f) Zhang D, Ronson TK, Nitschke JR, Acc. Chem. Res 2018, 51, 2423–2436; [DOI] [PubMed] [Google Scholar]; g) Chakraborty S, Newkome GR, Chem. Soc. Rev 2018, 47, 3991–4016; [DOI] [PubMed] [Google Scholar]; h) Jing X, He C Zhao L, Duan C, Acc. Chem. Res 2019, 52, 100–109. [DOI] [PubMed] [Google Scholar]
- [7] a).Wang M, Zheng Y-R, Ghosh K, Stang PJ, J. Am. Chem. Soc 2010, 132, 6282–6283; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kishi N, Li Z, Yoza K, Akita M, Yoshizawa M, J. Am. Chem. Soc 2011, 133, 11438–11441; [DOI] [PubMed] [Google Scholar]; c) Li K, Zhang L-Y, Yan C, Wei S-C, Pan M, Zhang L, Su C-Y, J. Am. Chem. Soc 2014, 136, 4456–4459; [DOI] [PubMed] [Google Scholar]; d) Fujita D, Ueda Y, Sato S, Mizuno N, Kumasaka T, Fujita M, Nature 2016, 540, 563–566; [DOI] [PubMed] [Google Scholar]; e) Cullen W, Misuraca MC, Hunter CA, Williams NH, Ward MD, Nat. Chem 2016, 8, 231–236; [DOI] [PubMed] [Google Scholar]; f) Zhang L, Lin L, Liu D, Lin YJ, Li ZH, Jin GX, J. Am. Chem. Soc 2017, 139, 1653–1660; [DOI] [PubMed] [Google Scholar]; g) Song B, Zhang Z, Wang K, Hsu CH, Bolarinwa O, Wang J, Li Y, Yin GQ, Rivera E, Yang HB, Liu C, Xu B, Li X, Angew. Chem. Int. Ed 2017, 56, 5258–5262; [DOI] [PubMed] [Google Scholar]; Angew. Chem 2017, 129, 5342–5346; [Google Scholar]; h) Howlader P, Mondal B, Purba PC, Zangrando E, Mukherjee PS, J. Am. Chem. Soc 2018, 140, 7952–7960; [DOI] [PubMed] [Google Scholar]; i) Cai L-X, Li S-C, Yan D-N, Zhou L-P, Guo F, Sun Q-F, J. Am. Chem. Soc 2018, 140, 4869–4876. [DOI] [PubMed] [Google Scholar]
- [8] a).Yan X, Cook TR, Wang P, Huang F, Stang PJ, Nat. Chem 2015, 7, 342–348; [DOI] [PubMed] [Google Scholar]; b) Zhang M, Saha ML, Wang M, Zhou Z, Song B, Lu C, Yan X, Li X, Huang F, Yin S, Stang PJ, J. Am. Chem. Soc 2017, 139, 5067–5074; [DOI] [PubMed] [Google Scholar]; c) Lu C, Zhang M, Tang D, Yan X, Zhang Z, Zhou Z, Song B, Wang H, Li X, Yin S, Sepehrpour H, Stang PJ, J. Am. Chem. Soc 2018, 140, 7674–7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9] a).Zhao Z, Lam JWY, Tang BZ, J. Mater. Chem 2012, 22, 23726–23740; [Google Scholar]; b) Mei J, Leung NL, Kwok RT, Lam JW, Tang BZ, Chem. Rev 2015, 115, 11718–11940. [DOI] [PubMed] [Google Scholar]
- [10] a).Meng Q-Y, Zhong J-J, Liu Q, Gao X-W, Zhang H-H, Lei T, Li Z-J, Feng K, Chen B, Tung C-H, Wu L-Z, J. Am. Chem. Soc 2013, 135, 19052–19055; [DOI] [PubMed] [Google Scholar]; b) Hari DP, Konig B, Chem. Commun 2014, 50, 6688–6699; [DOI] [PubMed] [Google Scholar]; c) Luo K, Chen Y-Z, Yang W-C, Zhu J, Wu L, Org. Lett 2016, 18, 452–455. [DOI] [PubMed] [Google Scholar]
- [11].DeRosa MC, Crutchley RJ, Coord. Chem. Rev 2002, 233–234, 351–371. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.