Abstract
Twenty-two silver nanoparticle (AgNP) consumer products (CPs) were analyzed with respect to their silver speciation. Three CPs and three lab-synthesized particles were selected to simulate environmental fate and transport by simulating their intended usage and disposal methods. Since many of these products are meant for ingestion, we simulated their usage by exposing them to human synthetic stomach fluid followed by exposure to wastewater sludge. We found that during the products individual exposure to wastewater sludge, the conversion rate of silver to AgCl and Ag2S was affected by both the amount of silver ion present and the properties of the AgNP. The rates of conversion of metallic silver to silver sulfide was heavily dependent on the particle size for the lab-synthesized particles, with 90 nm PVP-capped particles reacting to a much lesser extent than the 15 nm PVP-capped or the citrate-capped particles. We observed similar sulfidation rates on two of the tested CPs with the 15 nm lab-synthesized particles despite containing silver nanoparticles >5 times larger, indicating the presence of other influencing factors. Pre-treatment with synthetic stomach fluid modified the rates of Ag2S formation. Due to the variable composition of CPs and the conditions they are exposed to between manufacture, sale, use, and disposal, their final composition may be somewhat unpredictable in the environment. In the present study, we have achieved a more accurate approximation of the expected interactions between silver nanoparticle-containing CPs and environmental media by utilizing real CPs and evaluating them with solid phase and aqueous phase analytical techniques.
Introduction
The worldwide inventory of consumer products (CPs) containing nanomaterials is quickly growing(1). Nanomaterials are incorporated into a wide variety of CPs, ranging from cosmetics to automotive parts. Through CPs, nanomaterials have a variety of pathways to reach the environment, either during their use or after disposal. Understanding the environmental fate of nanomaterials in CPs is crucial to identifying potential risks associated with their release.
Silver nanoparticles (AgNPs) comprise one of the most prevalent and growing inventories of nanomaterial-containing CPs(1, 2). Their rising production and usage is accompanied by increased risk of their release into the environment(3). AgNPs are utilized in CPs primarily for their known antimicrobial properties. Common applications for AgNPs include packaging, clothing, first aid sprays, surface disinfectants, and dietary supplements.
The toxicity of AgNPs is related to their oxidation and the subsequent release of ionic silver(4). The reactivity of AgNPs undergoing dissolution or reaction with environmental media is known to be affected by a variety of factors, including capping agent, particle size, and ionic strength(5–8). These factors have been studied extensively for laboratory synthesized and commercial preparations of AgNPs and their effects on a given system can be predicted. However, the environmental transformation, transport and fate of consumer products containing AgNP suspensions remains largely unexplored.
Most AgNP suspensions will eventually enter the waste stream and make their way to a wastewater treatment plant (WWTP), where they will be exposed to wastewater sludge. Silver (I) sulfide is the primary reaction product from the interaction of silver with wastewater media(9, 10). Ag2S has reduced solubility compared to other forms of silver and past studies have concluded that this lower solubility leads to limited transport and reduced toxicity to certain organisms(11, 12). Nevertheless, the viewpoint that sequestration of Ag+ in the form of Ag2S is an endpoint to silver reaction in the environment is currently being challenged. In two studies performed by Li et. al.(13, 14), Ag2S-NPs were found to dissolve in the presence of either Fe(III) or ClO−, and then form smaller AgNPs. This process could lead to a cycle of Ag2S-NPs -> Ag+ emission -> smaller AgNPs -> smaller Ag2S-NPs -> Ag+ emission in which the toxic effects of Ag+ would likely be observed. Wang et. al.(15) also observed toxicity in plants due to uptake of Ag2S-NPs. If Ag2S is not an endpoint to Ag+ transport in the environment, then further study into its formation is needed.
Research has shown that AgNP consumer products can undergo drastic physicochemical changes during their use that could have further effects on their future interactions with environmental media(16). AgNP dietary supplements meant for ingestion will be exposed to stomach fluid if used as intended. Studies have shown that human synthetic stomach fluid (SSF) not only affects the AgNPs morphology and promotes aggregation, but also induce their chemical transformation to silver (I) chloride(17, 18). AgCl shows a low solubility in water and precipitates quickly in the presence of Ag+ and Cl−. The AgNP particle size influences both aggregation rate and AgCl transformation(18). The capping agent also affects aggregation rate in SSF(17) with some preventing significant morphological changes for up to 90 days in SSF(19). While there has been some research involving the exposure of AgCl-NPs to wastewater sludge(12), no study has been performed involving sequential exposures to simulate intended usage and subsequent disposal of the same particles.
Some research has been performed to investigate dissolution and reaction of AgNP-containing CPs, although it has primarily focused on AgNPs deposited on solid objects such as textiles and packaging(20–22). This study focused on aqueous products, such as surface sanitizers, disinfectant sprays, and dietary supplements, due to the ease and speed with which they can reach the environment, compared to those products in which the AgNPs are incorporated into a solid matrix. A total of 22 CPs advertising some combination of either colloidal, ionic, or nano-sized silver were obtained. These products represent unique mixtures of different parameters such as particle size, concentration, capping agent, ionic strength, pH, and additional organic or inorganic additives. The fate and transport of AgNPs in the environment has been typically studied using only pristine, lab-synthesized particles and by monitoring their exposure to only one type of media. We intend to more accurately describe the transformations that these products undergo in the environment by using real consumer products (CPs) and sequentially exposing them to multiple types of relevant media. The goal of this work is to utilize X-ray Photoelectron Spectroscopy (XPS) and X-ray Absorption Spectroscopy (XAS) to determine the initial silver chemical speciation and then investigate sulfidation rates when the products are sequentially exposed to SSF and wastewater sludge. A unique experimental setup allows real-time analysis of nanoparticle reactions by XAS. By comparing the chemical properties of these products to well-studied lab-synthesized AgNPs, the interaction and importance of different NP parameters will be determined.
Methods
Physicochemical Characterization of AgNPs
Various parameters, such as particle size, silver concentration, and ionic content for the 22 colloidal silver CPs used for this study, have been measured and discussed in a previous publication(23). A summary of characteristics can be found in the Table S-1, including hydrodynamic diameter (HDD), polydispersity index (PDI), pH, and chloride concentration. Lab-synthesized AgNPs capped with citrate (cit), PVP, and BPEI were synthesized as described in a previous publication(24). Additional PVP-capped AgNPs were synthesized with a size of 90 nm by increasing the initial amount of AgNO3 from 0.08 g to 0.35 g in the reaction mixture. Three PVP-capped AgNP standards were acquired from nanoComposix (San Diego, CA) with nominal sizes of 20 nm (AGPB20-1M), 50 nm (AGPB50-1M), and 100 nm (AGPB100-1M).
XPS Analysis
A glass well plate was used for sample preparation on which samples could be dropcast repeatedly and allowed to dry in a desiccator until sufficient nanomaterial is collected in the bottom of each well to allow analysis. Survey and high-resolution spectra were collected on a PHI Quantera II XPS (Physical Electronics, Chanhassen, MN) equipped with an Al Kα source. Data analysis was performed using CasaXPS version 2.3.16 (Casa Software Ltd). Chemical species were assigned according to both binding energy of Ag 3d5/2 peaks and auger parameters.
XAS Analysis
Spectra were collected at Sector 10-ID of the Advanced Photon Source at Argonne National Laboratory. Aqueous samples were mounted in a sample plate with Kapton tape walls. Spectra were collected from −200 to 1000 eV relative to the K-edge of Ag (25514 eV), with a step size of 0.5 eV. All data were processed and fit using the XAS fitting program Athena, a part of the software package IFEFFIT version 1.2.12.
Simulated Usage and Disposal
A sample of anaerobic biosolids was collected from a local WWTP and used with no further treatment. The SSF consisted of 0.4 M HCl and 0.4 M glycine in deionized water adjusted to a pH of 1.5. Reactors were fashioned from polystyrene test tubes by sawing a 1 cm tall window 180° around the side of each tube. The window was covered with Kapton tape, providing a watertight seal while still allowing x-ray transmittance. By placing the tube into a plastic stand on top of a stir plate, the window was positioned in the x-ray beam to allow analysis (photo of a reactor can be seen in Figure S-1). First, 2.0 mL of product solution or lab-synthesized particles was prepared to contain 150 ppm Ag and added into the tube. Second, the micro stir bar was added and preliminary spectra were taken to align the window in the beam. Finally, 1.0 mL of either SSF or wastewater sludge was added and data acquisition initiated. Lag time between addition of wastewater and the start of data acquisition was approximately 35 seconds. For sequential exposure, the AgNP solutions were mixed 1:1 with SSF for 1 hour prior to addition of the wastewater sludge. The energy range was adjusted to allow spectrum collection every 18 seconds, or 200 spectra in 60 min.
Results & Discussion
Speciation of Silver in CPs
One advantage of XAS for the determination of silver speciation is the ability to conduct analysis on aqueous samples, thereby eliminating the need for sample preparation that could generate interferences. XPS was chosen as a complimentary technique for investigating the surface of the nanomaterials, although the need for ultra-high vacuum required the drying of the aqueous products. All observed silver in the 22 CPs was in the form of either elemental silver or silver (I) oxide (Table 1). Silver (I) chloride was not detected with these techniques, which is unexpected, because the concentration of Cl− is high enough to expect AgCl formation in some samples (Table S-1).
Table 1.
Ag speciation in 22 CPs and three lab-synthesized AgNPs as determined by XAS and XPS. (b.d.l. = below detection limit)
| XAS | XPS | |
|---|---|---|
| CP1 | 60% Ag 40% Ag2O | 65% Ag 35% Ag2O |
| CP2 | b.d.l. | b.d.l. |
| CP3 | b.d.l. | b.d.l. |
| CP4 | 100% Ag | 100% Ag |
| CP5 | 60% Ag 40% Ag2O | 100% Ag |
| CP6 | 85% Ag 15% Ag2O | 45% Ag 55% Ag2O |
| CP7 | 100% Ag | b.d.l. |
| CP8 | 100% Ag | b.d.l. |
| CP9 | 100% Ag | 70% Ag 30% Ag2O |
| CP10 | 100% Ag | 65% Ag 35% Ag2O |
| CP11 | b.d.l. | 100% Ag |
| CP12 | b.d.l. | 100% Ag |
| CP13 | 100% Ag | 45% Ag 55% Ag2O |
| CP14 | 90% Ag 10% Ag2O | 100% Ag |
| CP15 | 100% Ag | 40% Ag 50% Ag2O |
| CP16 | 100% Ag | 55% Ag 45% Ag2O |
| CP17 | 90% Ag 10% Ag2O | 55% Ag 45% Ag2O |
| CP18 | b.d.l. | 35% Ag 65% Ag2O |
| CP19 | 100% Ag | 85% Ag 15% Ag2O |
| CP20 | b.d.l. | b.d.l. |
| CP21 | 100% Ag | b.d.l. |
| CP22 | 100% Ag | b.d.l. |
| Cit | 100% Ag | 100% Ag |
| PVP | 100% Ag | 100% Ag |
| BPEI | 100% Ag | 55% Ag 45% Ag2O |
XPS spectra for the Ag 3d region of all CPs with detectable silver can be seen in Figure S-2. XANES of all 22 CPs can be seen in Figure S-3 and Figure S-4. Nine of the products exhibited a higher percentage of Ag2O when analysed by XPS compared to analysis by XAS: CP6, 9, 10, 13, 15, 16, 17, 18, and 19. There may be several reasons for these differences. First, XPS is primarily a surface characterization technique with an analysis depth of approximately 10 nm, and therefore ignores the bulk of larger AgNPs and aggregates. CP6 and CP17 have diameters >10 nm and likely have an outer coating of Ag2O that is closer in concentration to the value determined by XAS, but the value is slightly inflated by XPS analysis that only analyses the surface of the AgNPs. The similarity in Ag2O content determined by XPS and XAS for CP1 indicates the AgNPs contain Ag2O throughout their structure and not just as an outer shell. Second, exposure to air during the evaporation process required for sample preparation may have exposed surface Ag0 to a greater level of oxidation than for the XAS measurement which was performed in aqueous suspension. This explanation, however, would not account for results with the products CP5 and CP14 which showed 100% Ag by XPS as compared to 4% Ag2O and 10% Ag2O, respectively, by XAS. Another explanation for differences in the level of observed oxidation of silver may involve the influence of both stabilizing agents and matrix components which have been shown to influence AgNP reactivity(25, 26).
The three lab-synthesized silver AgNPs capped with citrate, PVP, and BPEI were also air-dried prior to XPS analysis. While the citrate- and PVP-capped particles showed the absence of Ag2O by both XAS and XPS, the BPEI-capped particles exhibited significant oxidation as measured by XPS. The amount of Ag2O present did not show any correlation to any measured parameter, including particle size, pH, or ionic content; hence another factor, such as capping agent, age, or additive is responsible for it. Given that surface oxidation of AgNPs resulting in the formation of Ag2O has been shown to dampen surface plasmon resonance (SPR)(27), it is also interesting to note that many of these CPs were clear and did not show SPR even though AgNP were shown to be present(23).
The complex composition of these products presents numerous interactions with AgNPs that are yet to be understood. For example, a short and incomplete list of advertised ingredients in the various products includes: ethyl alcohol, carvacrol, EDTA, hydrogen peroxide, and various essential oils. Some of these are included to add fragrance to the product or enhance their cleaning ability but could have unforeseen effects on AgNPs.
Age is likely a factor that could explain the differences in speciation and reactivity between the various CPs. While manufacturing dates were not reported on most products, 16 of the products advertised an expiration date. All products with manufacturer-suggested expiration dates were analysed prior to these dates. Unfortunately, many of these expiration dates were as far as 3-4 years after the time of purchase which is unrealistically optimistic due to the low stability of AgNP suspensions.
AgNP Exposure to Wastewater Sludge
Based on preliminary analysis by DLS and XAS, a subset of three CPs was chosen to represent the differing composition of AgNPs within the 22 products. CPs 4, 6, and 10 were selected for reaction with wastewater sludge due to their high silver concentration, differing particle sizes, and differing Ag speciation. CP4, 6, and 10 also listed only silver and water as ingredients and, therefore, were free of influence from other matrix components. In addition to these three products, three lab-synthesized AgNPs were also chosen to represent different capping agents and different particle sizes: 10 nm citrate-capped, 10 nm PVP-capped, and 90 nm PVP-capped (Table 2).
Table 2.
Physicochemical parameters for AgNPs selected for reaction with SSF and wastewater sludge.
| % Ag+ | HDD (nm) | PDI | |
|---|---|---|---|
| CP4 | < 2 | 57.46 ± 0.90 | 0.46 |
| CP6 | < 2 | 55.91 ± 15.69 | 0.42 |
| CP10 | 33 | 484.4 ± 224.8 | 0.72 |
| citrate-capped | < 1 | 11.5 | 0.42 |
| PVP-capped | < 1 | 15 | 0.36 |
| < 1 | 93 | 0.56 |
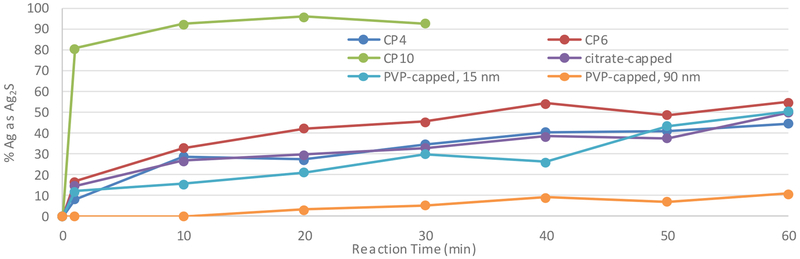
The change in silver speciation during reaction with wastewater sludge only generated Ag2S, which was observed in all evaluated products (Fig. 1). CP10 had the fastest rate of reaction and reached >90% Ag2S. While CP10 contained some large nanoclusters, a large portion of its silver was either ionic or <1 nm and therefore very susceptible to reaction and precipitation within the experimental time frame. The 90 nm PVP-capped AgNPs reacted slowest and had the lowest concentration of Ag2S at the end of 60 min. The lower surface area to volume ratio of the 90 nm PVP-capped particles exposed less area for reaction with sulfide than the 10 nm PVP-capped particles which appeared to be responsible for the lower Ag2S yields over a short time frame.
Fig. 1.
Silver (I) sulfide formation in the reaction of three AgNP-containing CPs and the three lab-synthesized AgNPs with wastewater .sludge.
CP4, CP6, 15 nm PVP-capped, and citrate-capped AgNPs all had similar rates of reaction and final concentrations of Ag2S after 60 min. Although the HDD values for CP4 and CP6 are in the range of 50-60 nm, they show a significant degree of polydispersity. In addition, TEM micrographs show the presence of smaller particles with less than 10 nm diameter (Figure S-5). These CPs contain particles that react at different rates and a variety of compounds are present in their matrices that may affect the reaction rates. Consequently, these mixtures do not necessarily behave as the average diameter of the particles would predict.
AgNP Exposure to SSF
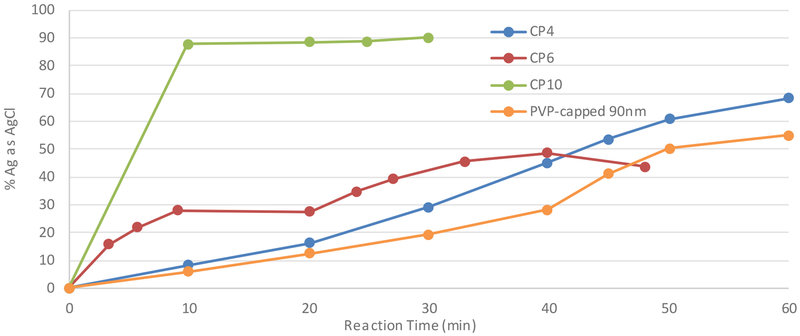
Similar to the rates of formation of Ag2S, the transformation of AgNPs to AgCl during exposure to SSF was observed in the four tested AgNPs (CP4, CP6, CP10, and PVP-90). CP10 showed a more rapid formation than the other three particle suspensions (Figure 2). Again, this is most likely due to the higher percentage of free silver ion present in this suspension. The observed similarity in chloride formation towards the end of the reaction among CP4, CP6, and PVP-capped (90 nm) in the presence of SSF is likely due to either the formation of large aggregates which would normalize the available surface area or a similarity in capping agent. The formation of aggregates and presence of AgCl at the particle surface has been previously reported(17) when AgNP were incubated in SFF. After aggregation, the differences in available surface area were less pronounced between the tested AgNPs and the chlorination rates were similar. On the timescale of digestion processes, most AgNPs would not fully transform to AgCl, but would likely gain an AgCl shell that may inhibit further dissolution or reaction in other media that they may encounter.
Fig. 2.
Silver (I) chloride formation in the reaction of three AgNP CPs and a lab-synthesized AgNP with SSF.
Simulated Usage and Disposal
The use of AgNPs as dietary supplements leads to their exposure to stomach fluid and, inevitably, wastewater. These AgNPs will likely undergo physical and chemical transformations during their use and may not resemble their original forms when entering the waste water system. The sequential exposure to SSF followed by wastewater may approximate the reactions that these AgNPs undergo during their use and disposal. Three PVP-capped OECD AgNP standards (20, 50, and 100 nm) were subjected to sequential exposure experiments to allow comparison of different particle sizes. CP4 and PVP-capped AgNPs (15 nm) were also included for comparison to individual exposure experiments.
XANES spectra of all samples during sequential exposure can be seen in Figure S-6. Particle size appeared to be a major factor in determining the effect of SSF exposure prior to wastewater addition. None of the three standards showed any AgCl presence or Ag2S formation after SSF incubation for 1 hr and after the first minute of reaction in waste water (Table 3). While this is expected for the 50 and 100 nm standards, it is surprising that the 20 nm particles, having the most surface area of the three nanoComposix standards, showed no AgCl presence. Still, both the 20 and 50 nm standards exhibited some dissolution, likely caused by the exposure to SSF. The extent of Ag2S formation after 1 hour was also influenced by particle size, with the 100 nm standard exhibiting the least amount of Ag2S formation.
Table 3.
Silver speciation in AgNP solutions exposed to WWTP sludge following exposure to SSF for 1 hour.
| WWTP sludge exposure (min) | Ag0 (%) | Ag+ (%) | AgCl (%) | Ag2S (%) | |
|---|---|---|---|---|---|
| N20 | 1 | 93 | 7 | ||
| 60 | 59 | 11 | 30 | ||
| N50 | 1 | 94 | 6 | ||
| 60 | 66 | 34 | |||
| N100 | 1 | 100 | |||
| 60 | 80 | 20 | |||
| CP4 | 1 | 91 | 9 | ||
| 60 | 90 | 10 | |||
| PVP (15nm) | 1 | 89 | 11 | ||
| 60 | 6 | 22 | 72 |
After exposure of CP4 to SSF for 1 hr, there was significant formation of AgCl (see Figure 2), but initial exposure to SSF followed by secondary waste water exposure for 1 min resulted in lower amounts of AgCl (Table 3). In contrast to direct exposure of CP4 to wastewater converting most of the silver to Ag2S, after 60 min of secondary waste water exposure (following initial SSF exposure) resulted in only a small portion of the silver being converted to Ag2S. A comparison of XANES spectra for both individual and sequential exposure of CP4 to SSF and wastewater sludge can be seen in Figure S-7. The change in rates of sulfidation and chlorination in the sequential exposure is likely due to increased precipitation upon mixture of the two media. This may have led to uneven sampling of the various silver species due to precipitation in the reaction cell. This could also be due to aggregation leading to a decrease in available particle surface area, such that the resulting silver aggregates could also be shielded by AgCl coatings that inhibit sulfidation. The 15 nm PVP-capped AgNPs exhibited a different effect, showing a higher sulfidation rate after sequential exposure to SSF and wastewater. Smaller AgNPs will typically react at faster rates due to their higher surface area to volume ratios. The results do not strictly follow this relationship between particle size and reactivity, indicating the presence of other controlling factors such as variable capping agent protocols and particle age.
Conclusion
As the worldwide inventory of AgNP-containing CPs grows, the release of AgNPs to the environment will increase. Aqueous products such as dietary supplements, first aid sprays, and surface sanitizers represent a simple pathway to silver release as their entire content of silver enters our wastewater system. In this work, 22 AgNP-containing CPs were analyzed to determine the chemical speciation of their silver as purchased and selected products were analyzed with respect to when they are used and enter the waste stream. While particle size was found to strongly influence the sulfidation rate in lab-synthesized AgNPs, polydisperse CPs did not behave according to their average particle diameter. This work has shown that CPs are typically complex mixtures and our lab-synthesized model particles sometimes fail to imitate their behavior. By incorporating actual CPs in our future work, we will be better able to adjust our models to address and explain the differences between pristine particles and CP suspensions. The sequential exposures that AgNPs undergo during their use and disposal must also be considered when studying these environmental transformations. The AgNPs in new CPs may be completely changed in both chemical speciation and morphology before they enter the waste stream.
Supplementary Material
Environmental Significance.
The environmental fate of silver nanoparticles is typically studied using pristine lab-synthesized nanoparticles. The nanoparticles emitted by nano-enabled consumer products differ from lab-synthesized particles in many properties, specifically particle size distribution and silver speciation. Using a unique experimental setup that allows real-time monitoring of silver speciation by X-ray absorption spectroscopy, we have shown that nano-enabled consumer products behave differently from their lab-synthesized counterparts. The complex matrices and various usage pathways lead to drastically different particles entering the waste stream and raise new concerns over the fate and transport of silver nanoparticles in the environment.
Acknowledgements
This research was funded and conducted by the National Risk Management Research Laboratory of the U.S. Environmental Protection Agency (EPA), Cincinnati, OH. This project was supported, in part, by appointments in the Research Participation Program at the Office of Research and Development (ORD), EPA administered by the Oak Ridge Institute for Science and Education (92431601) through an interagency agreement between the DOE and EPA. This manuscript was subjected to EPA internal reviews and quality assurance approval. The research results presented in this paper do not necessarily reflect the views of the Agency or its policy. Mention of trade names or products does not constitute endorsement or recommendation for use.
Footnotes
Conflicts of interest
There are no conflicts to declare.
References
- 1.Vance ME, Kuiken T, Vejerano EP, McGinnis SP, Hochella MF Jr., Rejeski D, et al. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J Nanotechnol. 2015;6:1769–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tolaymat TM, El Badawy AM, Genaidy A, Scheckel KG, Luxton TP, Suidan M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: a systematic review and critical appraisal of peer-reviewed scientific papers. Sci Total Environ. 2010;408(5):999–1006. [DOI] [PubMed] [Google Scholar]
- 3.Keller AA, McFerran S, Lazareva A, Suh S. Global life cycle releases of engineered nanomaterials. J Nanopart Res. 2013;15(6). [Google Scholar]
- 4.Sotiriou GA, Pratsinis SE. Antibacterial Activity of Nanosilver Ions and Particles. Environmental Science & Technology. 2010;44(14):5649–54. [DOI] [PubMed] [Google Scholar]
- 5.Zhou W, Liu YL, Stallworth AM, Ye C, Lenhart JJ. Effects of pH, Electrolyte, Humic Acid, and Light Exposure on the Long-Term Fate of Silver Nanoparticles. Environ Sci Technol. 2016;50(22):12214–24. [DOI] [PubMed] [Google Scholar]
- 6.Gitipour A, Thiel SW, Scheckel KG, Tolaymat T. Anaerobic toxicity of cationic silver nanoparticles. Sci Total Environ. 2016;557-558:363–8. [DOI] [PubMed] [Google Scholar]
- 7.Levard C, Hotze EM, Lowry GV, Brown GE. Environmental Transformations of Silver Nanoparticles: Impact on Stability and Toxicity. Environmental Science & Technology. 2012;46(13):6900–14. [DOI] [PubMed] [Google Scholar]
- 8.Choi O, Hu ZQ. Size dependent and reactive oxygen species related nanosilver toxicity to nitrifying bacteria. Environmental Science & Technology. 2008;42(12):4583–8. [DOI] [PubMed] [Google Scholar]
- 9.Kaegi R, Voegelin A, Sinnet B, Zuleeg S, Hagendorfer H, Burkhardt M, et al. Behavior of Metallic Silver Nanoparticles in a Pilot Wastewater Treatment Plant. Environmental Science & Technology. 2011;45(9):3902–8. [DOI] [PubMed] [Google Scholar]
- 10.Kent RD, Oser JG, Vikesland PJ. Controlled Evaluation of Silver Nanoparticle Sulfidation in a Full-Scale Wastewater Treatment Plant. Environmental Science & Technology. 2014;48(15):8564–72. [DOI] [PubMed] [Google Scholar]
- 11.Levard C, Hotze EM, Colman BP, Dale AL, Truong L, Yang XY, et al. Sulfidation of Silver Nanoparticles: Natural Antidote to Their Toxicity. Environmental Science & Technology. 2013;47(23):13440–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lombi E, Donner E, Taheri S, Tavakkoli E, Jamting AK, McClure S, et al. Transformation of four silver/silver chloride nanoparticles during anaerobic treatment of wastewater and post-processing of sewage sludge. Environmental Pollution. 2013;176:193–7. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Xu Z, Wimmer A, Tian Q, Wang X. New Insights into the Stability of Silver Sulfide Nanoparticles in Surface Water: Dissolution through Hypochlorite Oxidation. Environ Sci Technol. 2017;51(14):7920–7. [DOI] [PubMed] [Google Scholar]
- 14.Li L, Zhou Q, Geng F, Wang Y, Jiang G. Formation of Nanosilver from Silver Sulfide Nanoparticles in Natural Waters by Photoinduced Fe(II, III) Redox Cycling. Environ Sci Technol. 2016;50(24):13342–50. [DOI] [PubMed] [Google Scholar]
- 15.Wang P, Lombi E, Sun SK, Scheckel KG, Malysheva A, McKenna BA, et al. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ Sci-Nano. 2017;4(2):448–60. [PMC free article] [PubMed] [Google Scholar]
- 16.Gitipour A, Al-Abed SR, Thiel SW, Scheckel KG, Tolaymat T. Nanosilver as a disinfectant in dental unit waterlines: Assessment of the physicochemical transformations of the AgNPs. Chemosphere. 2017;173:245–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mwilu SK, El Badawy AM, Bradham K, Nelson C, Thomas D, Scheckel KG, et al. Changes in silver nanoparticles exposed to human synthetic stomach fluid: Effects of particle size and surface chemistry. Science of the Total Environment. 2013;447:90–8. [DOI] [PubMed] [Google Scholar]
- 18.Rogers KR, Bradham K, Tolaymat T, Thomas DJ, Hartmann T, Ma LZ, et al. Alterations in physical state of silver nanoparticles exposed to synthetic human stomach fluid. Science of the Total Environment. 2012;420:334–9. [DOI] [PubMed] [Google Scholar]
- 19.Atta AM, Al-Lohedan HA, Ezzat AO. Synthesis of Silver Nanoparticles by Green Method Stabilized to Synthetic Human Stomach Fluid. Molecules. 2014;19(5):6737–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environmental Science & Technology. 2008;42(11):4133–9. [DOI] [PubMed] [Google Scholar]
- 21.Pourzahedi L, Vance M, Eckelman MJ. Life Cycle Assessment and Release Studies for 15 Nanosilver-Enabled Consumer Products: Investigating Hotspots and Patterns of Contribution. Environmental Science & Technology. 2017;51(12):7148–58. [DOI] [PubMed] [Google Scholar]
- 22.Quadros ME, Pierson R, Tulve NS, Willis R, Rogers K, Thomas TA, et al. Release of Silver from Nanotechnology-Based Consumer Products for Children. Environmental Science & Technology. 2013;47(15):8894–901. [DOI] [PubMed] [Google Scholar]
- 23.Rogers KR, Navratilova J, Stefaniak A, Bowers L, Knepp AK, Al-Abed SR, et al. Characterization of engineered nanoparticles in commercially available spray disinfectant products advertised to contain colloidal silver. Science of the Total Environment. 2018;619:1375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.El Badawy AM, Luxton TP, Silva RG, Scheckel KG, Suidan MT, Tolaymat TM. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environmental Science & Technology. 2010;44(4):1260–6. [DOI] [PubMed] [Google Scholar]
- 25.Toh HS, Jurkschat K, Compton RG. The influence of the capping agent on the oxidation of silver nanoparticles: nano-impacts versus stripping voltammetry. Chemistry. 2015;21(7):2998–3004. [DOI] [PubMed] [Google Scholar]
- 26.Jimenez-Lamana J, Slaveykova VI. Silver nanoparticle behaviour in lake water depends on their surface coating. Sci Total Environ. 2016;573:946–53. [DOI] [PubMed] [Google Scholar]
- 27.Wu QM, Si MT, Zhang B, Zhang K, Li HH, Mi LF, et al. Strong damping of the localized surface plasmon resonance of Ag nanoparticles by Ag2O. Nanotechnology. 2018;29(29). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.