Abstract
Potential celiac disease (PCD) is defined by the presence of positive serum antibodies, HLA-DQ2/DQ8 haplotypes, and a normal small intestinal mucosa (Marsh grade 0-1). This condition occurs in one-fifth of celiac disease (CD) patients and usually represents a clinical challenge. We reviewed genetic, histologic, and clinical features of this specific condition by performing a systematic search on MEDLINE, Embase, and Scholar database. Accordingly, we identified different genetic features in patients with PCD compared to the classical forms. Frequently, signs of inflammation (deposits of immunoglobulin A (IgA) and/or increased number of intraepithelial lymphocytes) can be clearly identify in the mucosa of PCD patients after an accurate histological assessment. Finally, the main challenge is represented by the treatment: the gluten-free diet should be considered only in the presence of gluten-dependent symptoms in both children and adults. What is known: (i) potential celiac disease (PCD) occurs in one-fifth of all celiac diseases (CD), and (ii) despite the absence of classical lesions, clear signs of inflammation are often detectable. What is new: (i) patients with PCD show different genetic features, and (ii) the presence of gluten-dependent symptoms is the main determinant to initiate the gluten-free diet, after a complete diagnostic work-up.
1. Potential Celiac Disease
Celiac disease (CD) is a systemic disorder caused by gluten and characterized by the presence of a variable combination of gluten-dependent clinical manifestations, CD-specific antibodies, HLA-DQ2 or HLA-DQ8 haplotypes, and enteropathy [1]. Potential CD (PCD) is the condition related to people with a normal (Marsh grade 0) or minimally abnormal (Marsh grade 1) intestinal mucosa who are at increased risk of developing CD, as indicated by both positive serum endomysial (EmA) and tissue transglutaminase antibodies (tTGA2) and a positive histocompatibility leukocyte antigen (HLA-DQ2 or HLA-DQ8) genotype [2]. Symptoms and signs of the disease are not always clinically manifest, and even when present, they can range from mild to severe.
The term “potential CD” was first introduced by Ferguson in 1993 [3], and it has long been used interchangeably with “latent CD”; however, the latter has recently been discontinued, as suggested by the Oslo definition [2]. The diagnosis of PCD has significantly increased in the last years as a result of increased CD screening in the general population [4–6]. The number of patients with PCD is now sizeable, and this condition represents about one-fifth of total CD patients [7]. Compared with active classical CD, PCD is characterized by features including lower prevalence of DQ2 and higher prevalence of DQ8 [8]. Patients with PCD more frequently show low-to-moderate HLA-related risk; these cases bear half of the DQ2 heterodimer, either DQB1∗02 or DQA1∗05 only. Furthermore, six polymorphisms have been differently distributed in potential CD; these factors could be implicated with CD pathogenesis maybe with a “gene-dosage” effect as reported for HLA [9]. Establishing a certain diagnosis of PCD is of the utmost importance. False positive values of antibodies can be determined by analytical or random errors in the assay. Conversely, negative histological findings can be generated by a small number of biopsies due to “patchy” involvement of the bulb and duodenal mucosa [10–13], inappropriate biopsy orientation, the lack of the pathologists' expertise [14, 15], and an inadequate gluten intake before the endoscopy [16].
2. Histology Features and Prognostic Biomarkers
In PCD, despite the absence of severe mucosal damages, clear signs of inflammation are often present. There is a remarkable research activity to improve the diagnosis and identify initial mucosal changes in PCD: the four most important prognostic factors for villous atrophy are described in Figure 1. A short history of the most important findings concerning PCD is reported in Table 1, and results from these studies are here described more in detail.
Figure 1.
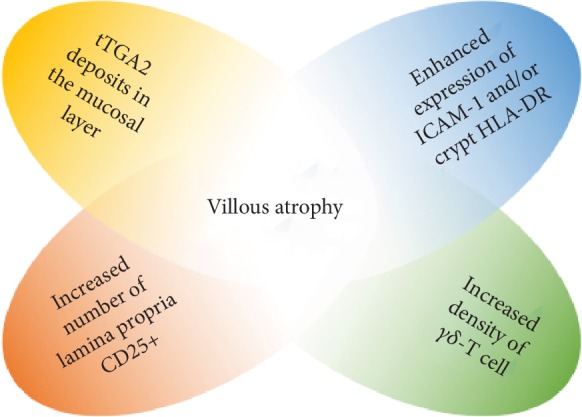
The four most important prognostic factors for villous atrophy in PCD.
Table 1.
A short history of the most important findings concerning PCD.
| Study | Year | Conclusions |
|---|---|---|
| Holm et al. [29] | 1992 | A healthy person who initially has a normal biopsy, but who also has an increased density of γδ T-cells, may later develop mucosal atrophy compatible with CD. |
| Iltanen et al. [30] | 1999 | 39 of 79 (49%) children with normal jejunal mucosa had an increased density of intraepithelial γδ T-cells. |
| Jarvinen et al. [28] | 2003 | An increase especially in γδ T-cells strengthens the probability of CD. |
| Korponay-Szabo et al. [20] | 2004 | TG2-related IgA deposits in the morphologically normal jejunum were predictive of forthcoming overt coeliac disease with villous atrophy. |
| Jarvinen et al. [27] | 2004 | The villous tip intraepithelial lymphocyte count was statistically significantly higher in patients with early-stage coeliac disease than in nonceliac controls (sensitivity, 0.84; specificity, 0.88). |
| Paparo et al. [17] | 2005 | Increased number of lamina CD25+ and/or enhanced expression of ICAM 1 and crypt HLA DR. |
| Salmi et al. [23] | 2006 | Intestinal coeliac autoantibody deposit had a sensitivity and specificity of 93% and 93%, respectively, in detecting subsequent coeliac disease. |
| Koskinen et al. [21] | 2010 | Mucosal transglutaminase 2-specific autoantibody deposits proved to be accurate gluten-dependent markers of celiac disease. |
| Tosco et al. [25] | 2011 | In most positive cases a patchy distribution of the deposits was observed with areas of clear positivity and areas with absent signal. |
| Bernini et al. [35] | 2011 | Potential CD largely shares the metabolomic signature of overt CD. Results prove that metabolic alterations may precede the development of small intestinal villous atrophy. |
| Biagi et al. [31] | 2013 | In PCD, the intestinal mucosa is maintained architecturally normal thanks to an increased enterocytic proliferation. |
| Borrelli et al. [32] | 2013 | Potential CD patients show a low grade of inflammation that could likely be due to active regulatory mechanism preventing the progression toward a mucosal damage. |
| Borrelli et al. [33] | 2016 | In potential CD, IL-21 is less expressed than that in active CD. |
| Borrelli et al. [22] | 2018 | In CD, the intestinal deposits of anti-tTG2 are a constant presence and appear very early in the natural history of the disease. |
Paparo et al., in 2005, showed immunohistochemical features of immune activation in the epithelium, lamina propria, and crypts in PCD: 70.8% of PCD patients presented an increased number of lamina propria CD25+ and/or enhanced expression of ICAM-1 and crypt HLA-DR [17]. It has been hypothesized that circulating antitissue transglutaminase 2 (tTGA2) may be the result of a “spillover” from the intestinal mucosal layer [18, 19]. Therefore, identifying anti-tTGA2 deposits in the mucosal layer can be a key factor in the histological assessment of CD: such deposits have been reported below the epithelial layer and around blood vessels in both pediatric and adult patients with overt CD [20, 21]. These features could also have a predictive role for villous atrophy, since they have been described in early-stage CD [22]. In 2006, Salmi et al. demonstrated that the detection of anti-tTGA2 deposits in the mucosa seems to be rather specific for CD and might be helpful in predicting the evolution to more severe histological damage [23]. The same data have been discussed in a recent review and, in the same way, have been considered as “markers of existing early disease” [24].
tTGA2 deposits were observed by Tosco et al. [25] following a patchy distribution with areas of clear positivity and areas with absent signal, as already described in mucosal damage of active CD [10, 13]; however, these deposits can also be found only in bulb duodenal biopsies [26]. In 2017, an Italian study demonstrated that in at-risk infants for CD, detection of mucosal deposits of anti-tTG2 IgA resulted in 88.3% positive predictive value [22]. The prevalence of γδ T-cell has also been suggested as a histological biomarker of CD. In fact, an increase in intraepithelial lymphocytes at the villus tip and a high γδ+ intraepithelial cell count can be considered good predictors of CD in patient with PCD, as described by two different studies from Finland [27, 28]. Some authors suggest that high density of γδ T-cell receptor-bearing intraepithelial lymphocytes (IELs) can be a prerequisite for developing CD in patients with no morphological abnormality, yet carrying the susceptibility genes; however, despite an increased density of γδ T-cell in potential CD, these findings cannot be considered pathognomonic for celiac disease [29, 30]. It has been hypothesized that in PCD, the intestinal mucosa is maintained architecturally normal by an increased enterocyte proliferation, which will end up in a reduced enterocyte maturity and will thus lead to reduced absorptive capacity of the small bowel [31]. In the same year, another study demonstrated how T-cells seem to be activated and differentiating toward a Th1 pattern, as suggested by high levels of interleukin-2 (IL-2), interferon-γ (IFN-γ), and TGF-β transcription factor. The same study showed an increased density of CD4+CD25+Foxp3+ T regulatory cells, which exert suppressive effect not impaired by IL-15 in potential CD [32]. A recent paper from Borrelli et al. in PCD patients showed reduced expression and increased upregulation in the presence of specific stimuli of interleukin-21 (IL-21), an important cytokine regulating innate and adaptive immune response, differently from active CD. In this study, PCD density of IL-21-producing cells in the lamina propria was found to correlate with serum titer of tTGA2, suggesting a lack of ability of IL-21 to enhance and maintain chronic inflammation in early phases of disease in active or potential CD [33]. In active CD, the overexpression of IL-21 is likely to play a crucial role in the activation of cytotoxic T-cells leading to epithelial cell death and mucosal destruction [34]. Aside from immunological controversies, an overlapping metabolomic signature was found for PCD and active disease, suggesting that common functional-biochemical stigmata might call for the same dietary treatment [35].
3. To Treat or Not to Treat?
The therapeutic management of PCD patients represents the main challenge. The only accepted treatment for CD is gluten-free diet (GFD), but the treatment for potential celiac disease still remains unclear. Likewise, there is no clear consensus in the PCD follow-up [36]. The natural history of PCD, both in adults [7] and children [25], is not sufficient to recommend GFD in any patient. Recently, Auricchio et al. [37] developed a model to predict the evolution to villous atrophy in PCD. They suggested GFD when symptoms of CD can be clearly detected, even without a mucosal damage. This approach aims at reducing symptoms and antibody titers (tTGA2 and EMA), as well as healing minimal alterations in intestinal mucosa [1]. Conversely, the use of GFD in asymptomatic patients is still debated. In 2009 and 2010, two studies from Finland showed that both adults [38] and children [39] with PCD obtained a clinical response to GFD regardless of the presence of small-bowel lesions. According to these studies, the authors suggested to start the dietary treatment as early as possible since treatment would result in reduced risks of delayed puberty and gynecological issues, while avoiding effects on bone mineralization, dental enamel development, and growth. Conversely, in a recent review, Itzlinger et al. considered GFD as inappropriate treatment in asymptomatic patients with PCD [40].
Diverging results emerged from Mandile's work, in which only 54% of PCD symptomatic patients have a positive clinical response during the first 12 months of GFD. However, the authors speculated about irritable bowel syndrome as a significant confounding factor in these patients [41]. In 2014, Auricchio et al. demonstrated that a considerable proportion of PCD patients usually had a fluctuation or decrease of antibody levels, while in those with persistently positive anti-TG2 under a free diet, the mucosal damage was not detectable in 66% of cases until 9 years of follow-up [42]. In 2019, Lionetti et al. reached similar conclusions: in PCD children on free diet, the risk of progression to overt CD is trivial [43].
Previously, Tosco et al. demonstrated that approximately 33% of asymptomatic children with PCD would develop villous atrophy after 3 years without prescribing a GFD [25]. The authors suggested that most children with potential celiac disease remain healthy and for these reason only symptomatic children would start GFD.
In 2012, a decision tree for asymptomatic children with tTGA values lower than 11-fold the upper limit normal was proposed [44]. Symptomless children with a family history of CD and positive CD markers could initially remain on normal free diet, particularly in the case of modest tTGA titer increase. Biopsies should be recommended after a persistent antibody positivity for at least 3-6 months. In 2016, another group indicated that asymptomatic patients can be monitored for the development of new symptoms and/or substantial increase in serum tTGA2 antibodies [45]. These studies are summarized in Table 2 and Figure 2.
Table 2.
Results of available evidence in support or against GFD in PCD asymptomatic patients.
| Study | About GFD | Study population | Conclusions | Limitations |
|---|---|---|---|---|
| Tosco et al. [25] | Against GFD | 106 children | 33% of incidence of villous atrophy after 3 years in with PCD | Unknown number of patients lost at follow-up |
| Lionetti et al. [44] | Against GFD | 24 asymptomatic children | CD markers disappear in most young children with potential CD despite a regular diet | Small sample size |
| Silvester et al. [45] | Against GFD | Review paper | In the absence of symptoms or villous atrophy, treatment with a GFD does not appear to be necessary in most cases | N/A |
| Mandile et al. [41] | Against GFD | 47 children | Association between CD and irritable bowel syndrome may be a significant confounding factor | Irritable bowel syndrome is overlapping with CD |
| Lionetti et al. [43] | Against GFD | 23 asymptomatic children | Risk of progression to overt CD while on a gluten-containing diet is very low in the long-term. | Age of the study group and study design |
| Kurppa et al. [38] | Supports GFD | 23 adults | Patients with endomysial antibodies benefit from a GFD regardless of the degree of enteropathy. | Marsh II included in study population |
| Kurppa et al. [39] | Supports GFD | 17 children | Children benefit from early treatment despite normal mucosal structure | Small sample size |
Figure 2.
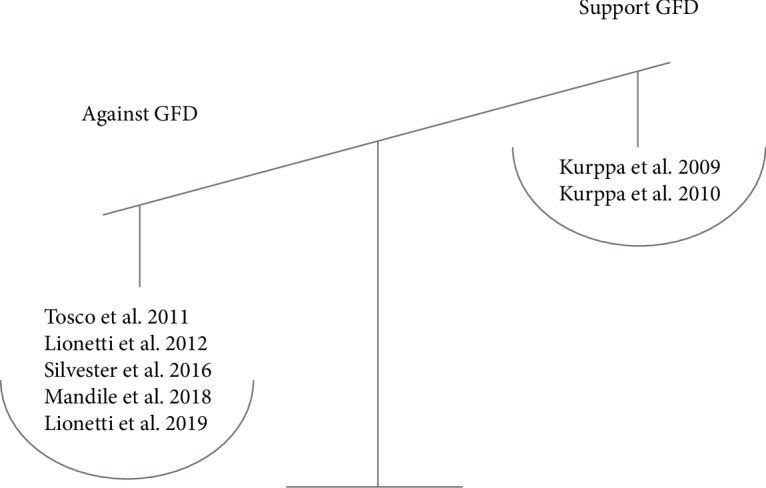
Results of available evidence in support or against GFD in PCD asymptomatic patients.
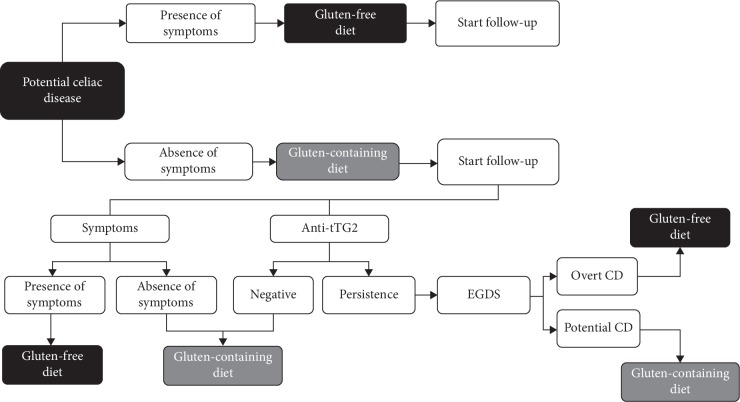
In conclusion, the presence of symptoms in both adults and children should be considered as the main determinant to prescribe a GFD in potential celiac disease. It is important to remember that all symptoms have to be considered important for the beginning of a GFD. There is no difference in the decision tree, in fact, if patient has gastrointestinal (diarrhea, constipation, abdominal pain) or extraintestinal manifestation (anemia, osteoporosis, migraine), as suggested by Popp and Maki in a recent review too [24]. As the timing of flattening is totally unpredictable, asymptomatic patients with PCD should undergo a comprehensive follow-up in order to detect early symptoms and promptly start a GFD. A conclusive algorithm is proposed in Figure 3 with the aim to provide valuable information in the management of this challenging condition.
Figure 3.
Diagnostic algorithm for PCD.
Further research is necessary in order to establish the optimal frequency of testing the antibodies and clinical evaluation for PCD patients (both adults and children) continuing after initial evaluations on gluten-containing diet. Dietary habits and gluten intake during clinical evaluation should be routinely checked during clinical evaluation, as following a diagnosis of PCD, the patient or his family could decrease the amount of gluten, resulting in false negative serology and fluctuating antibodies.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Husby S., et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. Journal of Pediatric Gastroenterology and Nutrition. 2012;54(1):136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 2.Ludvigsson J. F., Leffler D. A., Bai J. C., et al. The Oslo definitions for coeliac disease and related terms. Gut. 2013;62(1):43–52. doi: 10.1136/gutjnl-2011-301346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferguson A., Arranz E., O'Mahony S. Clinical and pathological spectrum of coeliac disease--active, silent, latent, potential. Gut. 1993;34(2):150–151. doi: 10.1136/gut.34.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ludvigsson J. F., et al. Screening for celiac disease in the general population and in high-risk groups. United European Gastroenterology Journal. 2015;3(2):106–120. doi: 10.1177/2050640614561668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonamico M., Nenna R., Montuori M., et al. First salivary screening of celiac disease by detection of anti-transglutaminase autoantibody radioimmunoassay in 5000 Italian primary schoolchildren. Journal of Pediatric Gastroenterology and Nutrition. 2011;52(1):17–20. doi: 10.1097/MPG.0b013e3181e6f2d0. [DOI] [PubMed] [Google Scholar]
- 6.Catassi C., et al. The coeliac iceberg in Italy. A multicentre antigliadin antibodies screening for coeliac disease in school-age subjects. Acta Paediatrica. Supplement. 1996;412:29–35. doi: 10.1111/j.1651-2227.1996.tb14244.x. [DOI] [PubMed] [Google Scholar]
- 7.Volta U., et al. Features and progression of potential celiac disease in adults. Clin Gastroenterol Hepatol. 2016;14(5):686–93 e1. doi: 10.1016/j.cgh.2015.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Biagi F., Bianchi P. I., Vattiato C., et al. Influence of HLA-DQ2 and DQ8 on severity in celiac disease. Journal of Clinical Gastroenterology. 2012;46(1):46–50. doi: 10.1097/MCG.0b013e318221077e. [DOI] [PubMed] [Google Scholar]
- 9.Sperandeo M. P., Tosco A., Izzo V., et al. Potential celiac patients: a model of celiac disease pathogenesis. PLoS One. 2011;6(7, article e21281) doi: 10.1371/journal.pone.0021281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ravelli A., Villanacci V., Monfredini C., Martinazzi S., Grassi V., Manenti S. How patchy is patchy villous atrophy?: distribution pattern of histological lesions in the duodenum of children with celiac disease. The American Journal of Gastroenterology. 2010;105(9):2103–2110. doi: 10.1038/ajg.2010.153. [DOI] [PubMed] [Google Scholar]
- 11.Bonamico M., Mariani P., Thanasi E., et al. Patchy villous atrophy of the duodenum in childhood celiac disease. Journal of Pediatric Gastroenterology and Nutrition. 2004;38(2):204–207. doi: 10.1097/00005176-200402000-00019. [DOI] [PubMed] [Google Scholar]
- 12.Bonamico M., Thanasi E., Mariani P., et al. Duodenal bulb biopsies in celiac disease: a multicenter study. Journal of Pediatric Gastroenterology and Nutrition. 2008;47(5):618–622. doi: 10.1097/MPG.0b013e3181677d6e. [DOI] [PubMed] [Google Scholar]
- 13.Valitutti F., di Nardo G., Barbato M., et al. Mapping histologic patchiness of celiac disease by push enteroscopy. Gastrointestinal Endoscopy. 2014;79(1):95–100. doi: 10.1016/j.gie.2013.06.012. [DOI] [PubMed] [Google Scholar]
- 14.Ravelli A., Villanacci V. Tricks of the trade: how to avoid histological pitfalls in celiac disease. Pathology, Research and Practice. 2012;208(4):197–202. doi: 10.1016/j.prp.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Green P. H., Jabri B. Coeliac disease. Lancet. 2003;362(9381):383–391. doi: 10.1016/S0140-6736(03)14027-5. [DOI] [PubMed] [Google Scholar]
- 16.Andersson H., et al. Influence of the amount of dietary gluten on gastrointestinal morphology and function in dermatitis herpetiformis. Human Nutrition. Clinical Nutrition. 1984;38(4):279–285. [PubMed] [Google Scholar]
- 17.Paparo F., Petrone E., Tosco A., et al. Clinical, HLA, and small bowel immunohistochemical features of children with positive serum antiendomysium antibodies and architecturally normal small intestinal mucosa. The American Journal of Gastroenterology. 2005;100(10):2294–2298. doi: 10.1111/j.1572-0241.2005.41134.x. [DOI] [PubMed] [Google Scholar]
- 18.Marzari R., Sblattero D., Florian F., et al. Molecular dissection of the tissue transglutaminase autoantibody response in celiac disease. Journal of Immunology. 2001;166(6):4170–4176. doi: 10.4049/jimmunol.166.6.4170. [DOI] [PubMed] [Google Scholar]
- 19.Kaukinen K., Peräaho M., Collin P., et al. Small-bowel mucosal transglutaminase 2-specific IgA deposits in coeliac disease without villous atrophy: a prospective and randomized clinical study. Scandinavian Journal of Gastroenterology. 2005;40(5):564–572. doi: 10.1080/00365520510023422. [DOI] [PubMed] [Google Scholar]
- 20.Korponay-Szabo I. R., et al. In vivo targeting of intestinal and extraintestinal transglutaminase 2 by coeliac autoantibodies. Gut. 2004;53(5):641–648. doi: 10.1136/gut.2003.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koskinen O., et al. Usefulness of small-bowel mucosal transglutaminase-2 specific autoantibody deposits in the diagnosis and follow-up of celiac disease. Journal of Clinical Gastroenterology. 2010;44(7):483–488. doi: 10.1097/MCG.0b013e3181b64557. [DOI] [PubMed] [Google Scholar]
- 22.Borrelli M., Maglio M., Korponay-Szabó I. R., et al. Intestinal anti-transglutaminase 2 immunoglobulin A deposits in children at risk for coeliac disease (CD): data from the prevent CD study. Clinical and Experimental Immunology. 2018;191(3):311–317. doi: 10.1111/cei.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salmi T. T., et al. Immunoglobulin A autoantibodies against transglutaminase 2 in the small intestinal mucosa predict forthcoming coeliac disease. Alimentary Pharmacology & Therapeutics. 2006;24(3):541–552. doi: 10.1111/j.1365-2036.2006.02997.x. [DOI] [PubMed] [Google Scholar]
- 24.Popp A., Maki M. Gluten-induced extra-intestinal manifestations in potential celiac disease-celiac trait. Nutrients. 2019;11(2):p. 320. doi: 10.3390/nu11020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tosco A., et al. Natural history of potential celiac disease in children. Clin Gastroenterol Hepatol. 2011;9(4):320–325. doi: 10.1016/j.cgh.2010.09.006. quiz e36. [DOI] [PubMed] [Google Scholar]
- 26.De Leo L., et al. Immunohistologic analysis of the duodenal bulb: a new method for celiac disease diagnosis in children. Gastrointestinal Endoscopy. 2018;88(3):521–526. doi: 10.1016/j.gie.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 27.Jarvinen T. T., et al. Villous tip intraepithelial lymphocytes as markers of early-stage coeliac disease. Scandinavian Journal of Gastroenterology. 2004;39(5):428–433. doi: 10.1080/00365520310008773. [DOI] [PubMed] [Google Scholar]
- 28.Jarvinen T. T., et al. Intraepithelial lymphocytes in celiac disease. The American Journal of Gastroenterology. 2003;98(6):1332–1337. doi: 10.1111/j.1572-0241.2003.07456.x. [DOI] [PubMed] [Google Scholar]
- 29.Holm K., Mäki M., Savilahti E., Laippala P., Lipsanen V., Koskimies S. Intraepithelial gamma delta T-cell-receptor lymphocytes and genetic susceptibility to coeliac disease. Lancet. 1992;339(8808):1500–1503. doi: 10.1016/0140-6736(92)91262-7. [DOI] [PubMed] [Google Scholar]
- 30.Iltanen S., Holm K., Partanen J., Laippala P., Mauki M. Increased density of jejunal gammadelta+ T cells in patients having normal mucosa--marker of operative autoimmune mechanisms? Autoimmunity. 1999;29(3):179–187. doi: 10.3109/08916939908998533. [DOI] [PubMed] [Google Scholar]
- 31.Biagi F., et al. Prevalence and natural history of potential celiac disease in adult patients. Scandinavian Journal of Gastroenterology. 2013;48(5):537–542. doi: 10.3109/00365521.2013.777470. [DOI] [PubMed] [Google Scholar]
- 32.Borrelli M., Salvati V. M., Maglio M., et al. Immunoregulatory pathways are active in the small intestinal mucosa of patients with potential celiac disease. The American Journal of Gastroenterology. 2013;108(11):1775–1784. doi: 10.1038/ajg.2013.303. [DOI] [PubMed] [Google Scholar]
- 33.Borrelli M., et al. In the intestinal mucosa of children with potential celiac disease IL-21 and IL-17A are less expressed than in the active disease. The American Journal of Gastroenterology. 2016;111(1):134–144. doi: 10.1038/ajg.2015.390. [DOI] [PubMed] [Google Scholar]
- 34.Strengell M., Matikainen S., Sirén J., et al. IL-21 in synergy with IL-15 or IL-18 enhances IFN-gamma production in human NK and T cells. Journal of Immunology. 2003;170(11):5464–5469. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 35.Bernini P., Bertini I., Calabrò A., et al. Are patients with potential celiac disease really potential? The answer of metabonomics. Journal of Proteome Research. 2011;10(2):714–721. doi: 10.1021/pr100896s. [DOI] [PubMed] [Google Scholar]
- 36.Valitutti F., Trovato C. M., Montuori M., Cucchiara S. Pediatric celiac disease: follow-up in the spotlight. Advances in Nutrition. 2017;8(2):356–361. doi: 10.3945/an.116.013292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Auricchio R., Mandile R., del Vecchio M. R., et al. Progression of celiac disease in children with antibodies against tissue transglutaminase and normal duodenal architecture. Gastroenterology. 2019;157(2):413–420.e3. doi: 10.1053/j.gastro.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 38.Kurppa K., Collin P., Viljamaa M., et al. Diagnosing mild enteropathy celiac disease: a randomized, controlled clinical study. Gastroenterology. 2009;136(3):816–823. doi: 10.1053/j.gastro.2008.11.040. [DOI] [PubMed] [Google Scholar]
- 39.Kurppa K., et al. Celiac disease without villous atrophy in children: a prospective study. J Pediatr. 2010;157(3):373–380. doi: 10.1016/j.jpeds.2010.02.070. 380 e1. [DOI] [PubMed] [Google Scholar]
- 40.Itzlinger A., Branchi F., Elli L., Schumann M. Gluten-free diet in celiac disease-forever and for all? Nutrients. 2018;10(11):p. 1796. doi: 10.3390/nu10111796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandile R., Discepolo V., Scapaticci S., et al. The effect of gluten-free diet on clinical symptoms and the intestinal mucosa of patients with potential celiac disease. Journal of Pediatric Gastroenterology and Nutrition. 2018;66(4):654–656. doi: 10.1097/MPG.0000000000001745. [DOI] [PubMed] [Google Scholar]
- 42.Auricchio R., Tosco A., Piccolo E., et al. Potential celiac children: 9-year follow-up on a gluten-containing diet. The American Journal of Gastroenterology. 2014;109(6):913–921. doi: 10.1038/ajg.2014.77. [DOI] [PubMed] [Google Scholar]
- 43.Lionetti E., Castellaneta S., Francavilla R., et al. Long-term outcome of potential celiac disease in genetically at-risk children: the prospective CELIPREV cohort study. Journal of Clinical Medicine. 2019;8(2):p. 186. doi: 10.3390/jcm8020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lionetti E., Castellaneta S., Pulvirenti A., et al. Prevalence and natural history of potential celiac disease in at-family-risk infants prospectively investigated from birth. The Journal of Pediatrics. 2012;161(5):908–914.e2. doi: 10.1016/j.jpeds.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 45.Silvester J. A., Kelly C. P. The potential for treatment of potential celiac disease. Clinical Gastroenterology and Hepatology. 2016;14(5):694–695. doi: 10.1016/j.cgh.2016.01.001. [DOI] [PubMed] [Google Scholar]