Abstract
Background
To predict the active components and potential targets of traditional Chinese medicine and to determine the mechanism behind the curative effect of traditional Chinese medicine, a multitargeted method was used. Jingzhi Guanxin prescriptions expressed a high efficacy for coronary heart disease (CHD) patients of which essential oils from Chuanxiong and Jiangxiang were confirmed to be the most important effective substance. However, the interaction between the active components and the targets for the treatment of CHD has not been clearly explained in previous studies.
Materials and Methods
Genes associated with the disease and the treatment strategy were searched from the electronic database and analyzed by Cytoscape (version 3.2.1). Protein-protein interaction network diagram of CHD with Jiangxiang and Chuanxiong essential oils was constructed by Cytoscape. Pathway functional enrichment analysis was executed by clusterProfiler package in R platform.
Results
121 ingredients of Chuanxiong and Jiangxiang essential oils were analyzed, and 393 target genes of the compositions and 912 CHD-related genes were retrieved. 15 coexpression genes were selected, including UGT1A1, DPP4, RXRA, ADH1A, RXRG, UGT1A3, PPARA, TRPC3, CYP1A1, ABCC2, AHR, and ADRA2A. The crucial pathways of occurrence and treatment molecular mechanism of CHD were analyzed, including retinoic acid metabolic process, flavonoid metabolic process, response to xenobiotic stimulus, cellular response to xenobiotic stimulus, cellular response to steroid hormone stimulus, retinoid binding, retinoic acid binding, and monocarboxylic acid binding. Finally, we elucidate the underlying role and mechanism behind these genes in the pathogenesis and treatment of CHD.
Conclusions
Generally speaking, the nodes in subnetwork affect the pathological process of CHD, thus indicating the mechanism of Jingzhi Guanxin prescriptions containing Chuanxiong and Jiangxiang essential oils in the treatment of CHD.
1. Background
Cardiovascular diseases (CVD) cause more than 17.3 million deaths per year with an estimated mortality increase to 23.6 million by 2030 [1, 2]. Coronary heart disease (CHD) is the leading cause of cardiovascular disease, usually caused by coronary artery occlusion [3], and is the cause of the highest morbidity and mortality in the world [4, 5]. Myocardial infarction (MI), palpitation, and angina pectoris are the main clinical manifestations of CHD [2]. Necropsy analyses of patients who suffered a fatal cerebral stroke indicated that they were often accompanied by a high prevalence of coronary atherosclerosis [6, 7]. Despite the decline in mortality from heart disease in recent years, the social burden of coronary heart disease remains worrisome, particularly in developing countries. However, the potential molecular mechanism of CHD is unclear. Therefore, there is an urgent need for in-depth research and improvement of the treatment of CHD, in order to achieve the purpose of reducing the health and economic burden of patients with coronary heart disease.
Traditional Chinese medicine (TCM) plays a systemic role with multiple targets and multiple ways in treating diseases. Jingzhi Guanxin prescription is a standardized cardiovascular herb medicine from Chinese Pharmacopoeia 2015 editions [8]. Jingzhi Guanxin prescriptions can promote blood circulation and remove blood stasis, which is used to treat angina pectoris and coronary heart disease [8]. These prescriptions contain five herbs, i.e., Salvia miltiorrhiza Bge. (Danshen), Ligusticum chuanxiong Hort. (Chuanxiong), Paeonia lactiflora Pall. (Chishao), Dalbergia odorifera T. (Jiangxiang), and Carthamus tinctorius L. (Honghua), all of which are recorded in Chinese Pharmacopoeia 2015 edition [8]. Several studies have indicated that Jingzhi Guanxin prescriptions have been highly effective for patients with CHD [9, 10], but the specific mechanism is still unclear. Network pharmacology is a field in which network biology and multipharmacology are combined [11]. Principally, the methods are focused on identifying and ranking the targets in biological networks [12]. The network analyses of biological pathways and interactions have revealed that the robustness of biological systems can be obtained from the network structure to a large extent [11, 13, 14]. Coexpressed genes were enriched for searching functionally related genes, and its network showed mutual investigation and mutual relationship [15]. Further, functional enrichment analysis could determine the mechanisms of the putative targets.
In Jingzhi Guanxin prescriptions, Chuanxiong and Jiangxiang essential oils are the most important material basis for promoting blood circulation and removing blood stasis [16, 17]. Previously, our research group had carried out a massive systemic research of the essential oils. On this basis, the related genes of CHD and essential oils were retrieved, and their biological functions were analyzed in order to further clarify the molecular mechanism of the essential oils in the treatment of CHD and to provide a reference for the clinical application of these essential oils and for further drug development.
2. Materials and Methods
The technical strategy of this research is shown in Figure 1. The research strategy was based on network pharmacology of deciphering key pharmacological pathways involved in Chuanxiong and Jiangxiang essential oils acting on CHD.
Figure 1.
Process overview.
3. Data Collections
3.1. Collection of Coronary Heart Disease- (CHD-) Related Genes
Significant genes associated with CHD were obtained from DisGeNET (version 5.0, http://www.disgenet.org/web/DisGeNET/menu/home). DisGeNET is a discovery platform containing collections of 561,119 genes associated with human diseases [18]. In order to collect comprehensive retrieval results, Therapeutic Target Database (TTD, last update by 15 September 2017, https://db.idrblab.org/ttd/) was also used to retrieve genes related to coronary heart disease, which is a database that provides known and explored therapeutic proteins, targeted diseases, corresponding drugs for these targets, and so on [19]. In addition, CHD-related genes were also collected from DrugBank (version 5.1.1, released 03 July 2018, https://www.drugbank.ca/), which is a unique bioinformatics and chemical informatics database, containing 11,628 drugs and related chemical information, drug targets, protein data, and so on [20]. In the study, genes were included from the DisGeNET database with DSI scores above the median, as well as all CHD-related genes from DrugBank and TTD. Through the retrieval of Universal Protein Resource (UniProt, http://www.uniprot.org/), all the genes were normalized into consistent symbols, and the unified information contained UniProt number and gene abbreviation.
3.2. Compositions of Essential Oil from Chuanxiong and Jiangxiang
The essential oil from Chuanxiong was extracted by steam distillation, and the compositions were analyzed by gas chromatography-mass spectrometer (GC-MS). The constituents of Jiangxiang essential oil were obtained from the literature by searching CNKI, Wanfang, and PubMed databases with the keywords of “Jiangxiang volatile oil” and “dalbergiae odoriferae volatile oil”. Studies were referenced which reported compositions of essential oil from Jiangxiang analyzed by GC-MS. Because the chemical composition is usually represented by multiple chemical names, we converted the chemical name to Chemical Abstracts Service (CAS Number) so that TCMSP or PubChem can be used to identify these chemical compounds. The composition name, name code, and CAS Number of compositions are arranged in the supplementary documents.
3.3. Collection of Compositions Associated with the Gene
The main source of composition-targets was obtained from TCMSP (version 2.3, update to 31 May 2014, http://lsp.nwu.edu.cn/tcmsp.php) database [21]. TCMSP database has specific informatics methods to infer drug-disease connection, which were collected from 499 herbs which were all registered in the Chinese Pharmacopoeia (2010) with a total of 12,144 chemicals [21]. Another major database for acquiring composition-targets is STITCH (http://stitch.embl.de/) by searching SMILES structure; STITCH is a database of known and predicted interactions between chemicals and proteins currently containing 9,643,763 proteins from 2,031 organisms [22]. The SMILES structure of compositions obtained from PubChem (https://pubchem.ncbi.nlm.nih.gov/) which is an open chemistry database with 96,502,248 compositions of which 3,151,393 have been tested.
3.4. Network Construction and Analysis
Many common diseases such as cancer and CHD are often caused by multiple molecular abnormalities [23]. In the “network target” theory, the establishment of molecular connections between drug/herbal formulae and diseases/TCM syndromes is crucial [24]. These molecular connections are derived from a disease-specific network, which can be formed due to the interactions of genes or gene products [24]. Meanwhile, the introduction of a “network” in drug discovery incorporates the assessment of network topology, as well as dynamics, and thus offers a quantifiable description of the complex biological system and its response to various drug/herbal treatments [24]. The nodes with high centrality (e.g., network degree) can be viewed as key nodes in a network [24]. The concept attempts to comprehensively describe all of the possible vulnerable targets for clarifying the efficiency of drug treatment [24]. Composition-target-CHD regulatory network was constructed by using Cytoscape (version 3.2.1) software; all node degrees of the network were calculated at the same time. Different expression profiles of genes in relation to the compositions and CHD were filtered by Venny2.1.0 [25]. All nodes whose degrees were more than twice the median were considered as key nodes, which are filtered by the Cytoscape plug-in MCODE [26–28].
3.5. GO Enrichment and KEGG Pathway Analysis
In order to better understand the potential biological process of predictive genes, KEGG (Kyoto Encyclopedia of Genes and Genomes, Release 88.0, October 1, 2018) and GO (Gene Ontology, last updated on March 9, 2018) were analyzed for pathway functional enrichment using clusterProfiler software package on R platform [29–32]. The interaction network was constructed by using the Top-Go package of R platform [33].
4. Results
4.1. Collection of 487 Genes Associated with CHD from DisGeNET, TTD, and DrugBank
A total of 912 CHD-related genes were retrieved from DisGeNET and 457 genes with a DSI score higher than the median (0.55) were selected, 28 CHD target genes were found in DrugBank, and one gene was obtained from TTD. Further, 487 genes were identified and an analytical network was established. Details of these genes are provided in Supplementary .
4.2. Compositions and Targets of Chuanxiong and Jiangxiang
As shown in the GC-MS analysis results, 83 ingredients were detected from Chuanxiong essential oil. And 32 compositions of Jiangxiang essential oil were obtained from the literature [34, 35]. An overview of ingredients of Chuanxiong and Jiangxiang essential oils is shown in Supplementary . A total of 315 essential oil-related targets of Chuanxiong essential oil were retrieved from TCMSP and STITCH databases. And 78 related targets of Jiangxiang essential oil were searched from TCMSP, DrugBank, and DisGeNET. Details of all component-related genes can be found in Supplementary .
4.3. Network Analyses
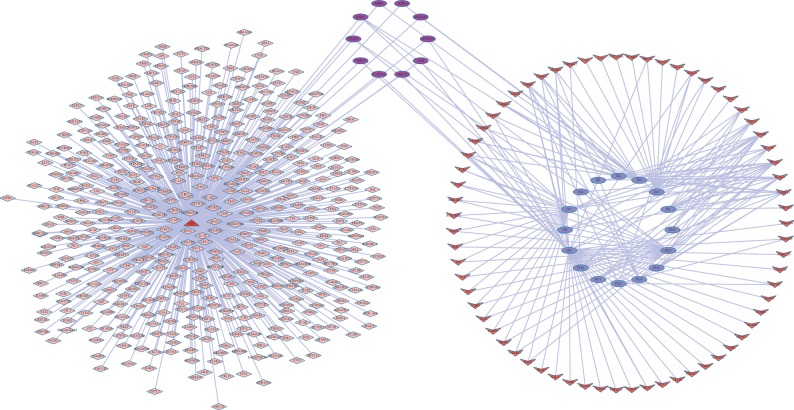
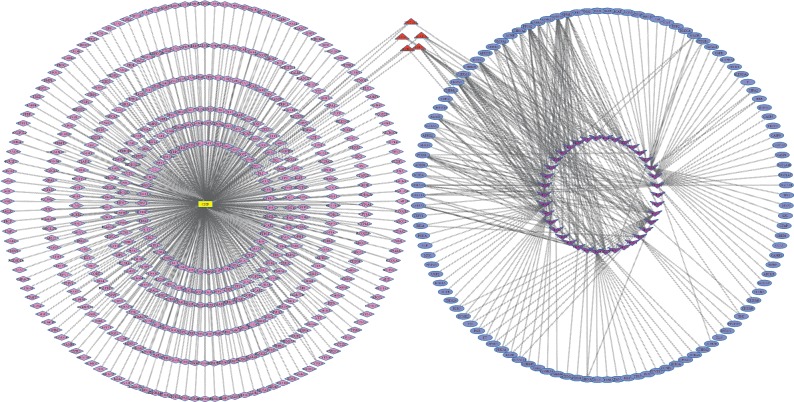
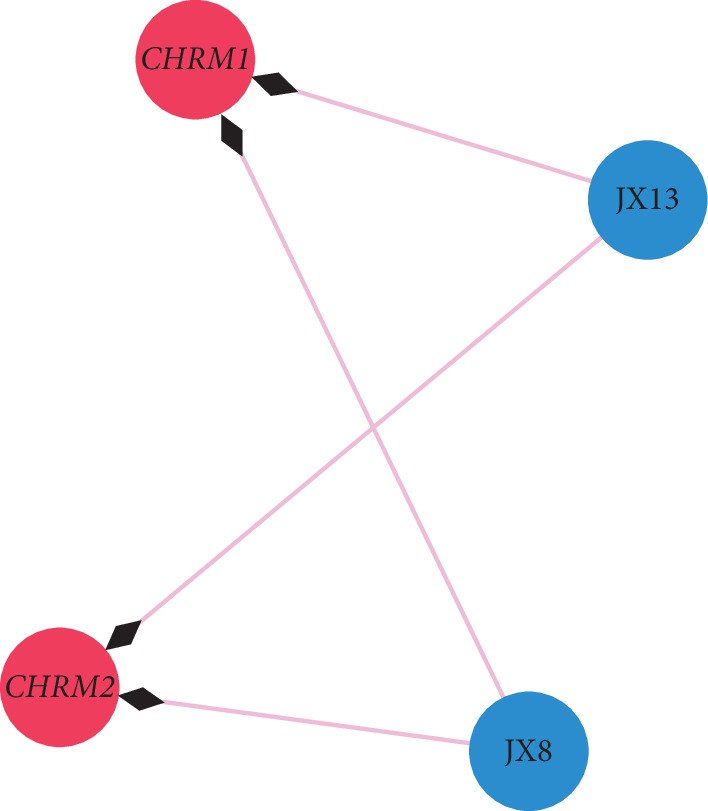
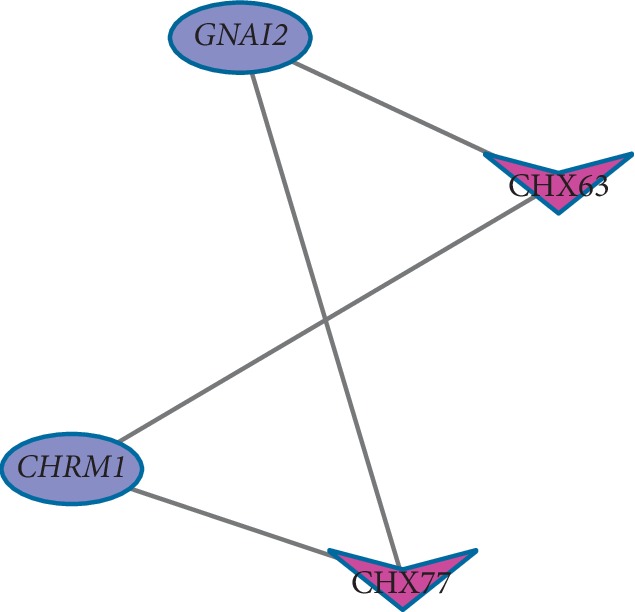
In order to explain the potential pharmacological effects of Chuanxiong and Jiangxiang essential oils in the treatment of CHD, a composition-target-disease network was established (Figures 2 and 3, layout type is “circular layout”). In the network, hub genes with degrees greater than twofold of the median were considered key nodes which were filtered by Cytoscape plug-in MCODE [36, 37]. Key nodes in the pathway were selected by the R language, including CHRM1, CHRM2, JX13, JX8, GNAI2, CHRM1, CHX77, and CHX63, CNET networks as shown in Figures 4 and 5.
Figure 2.
The composition-target-disease networks of Jiangxiang and CHD. The triangle node represents CHD; the diamond nodes represent related genes of CHD; the circular blue nodes represent ingredients of Jiangxiang essential oil; the red V-shape nodes represent related genes of Jiangxiang essential oil; the circular purple nodes represent coexpression of CHD and Jiangxiang essential oil.
Figure 3.
The composition-target-disease networks of Chuanxiong and CHD. The rectangle yellow node represents CHD; the diamond purple nodes represent related genes of CHD; the purple V-shape nodes represent ingredients of Chuanxiong essential oil; the blue oval nodes represent related genes of Chuanxiong essential oil; the red triangle nodes represent coexpression of CHD and Chuanxiong essential oil.
Figure 4.
The cnetplot of Jiangxiang composition-target-disease network. The circular blue nodes represent ingredients of Jiangxiang essential oil; the circular red nodes represent related genes of Jiangxiang essential oil.
Figure 5.
The cnetplot of Chuanxiong composition-target-disease network. The purple V-shape nodes represent ingredients of Chuanxiong essential oil; the blue oval nodes represent related genes of Chuanxiong essential oil.
Apart from these, 12 coexpression genes were selected by Venny2.1.0, including UGT1A1, DPP4, RXRA, ADH1A, RXRG, UGT1A3, PPARA, TRPC3, CYP1A1, ABCC2, AHR, and ADRA2A. The Venny plot is shown in Figure 6.
Figure 6.
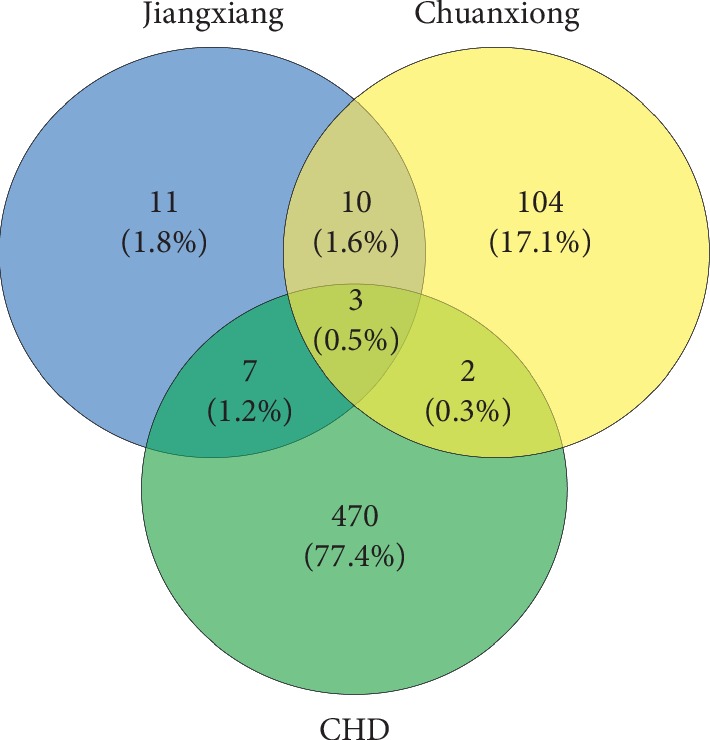
Twelve coexpression genes of CHD and ingredients. The blue represents related genes of Jiangxiang essential oil; the yellow represents related genes of Chuanxiong essential oil; the green represents related genes of CHD.
4.4. Functional Enrichment Analyses
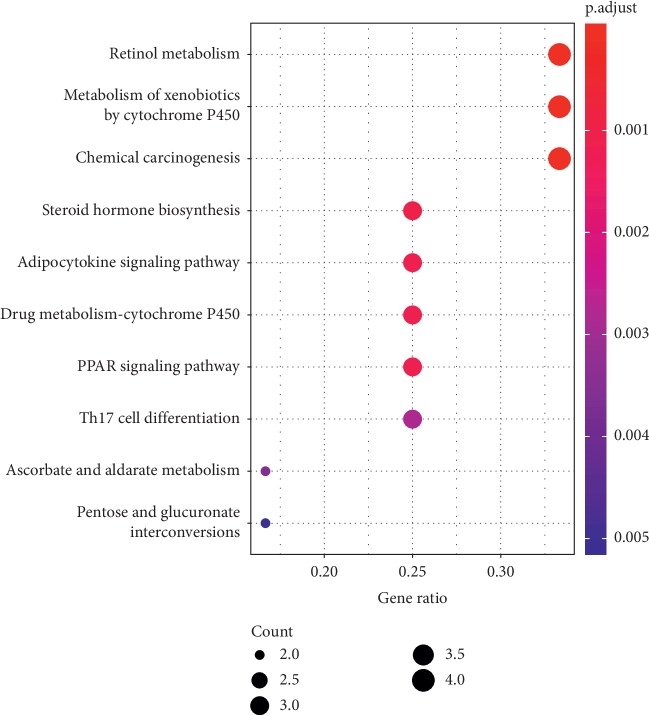
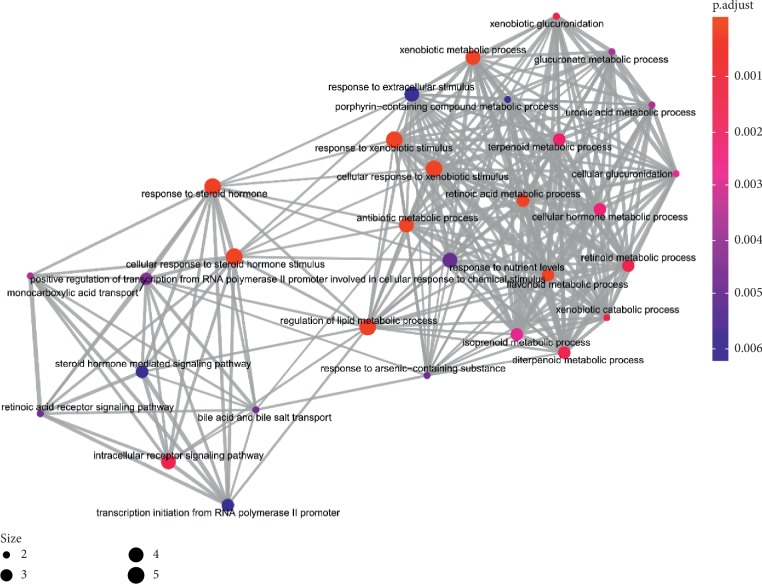
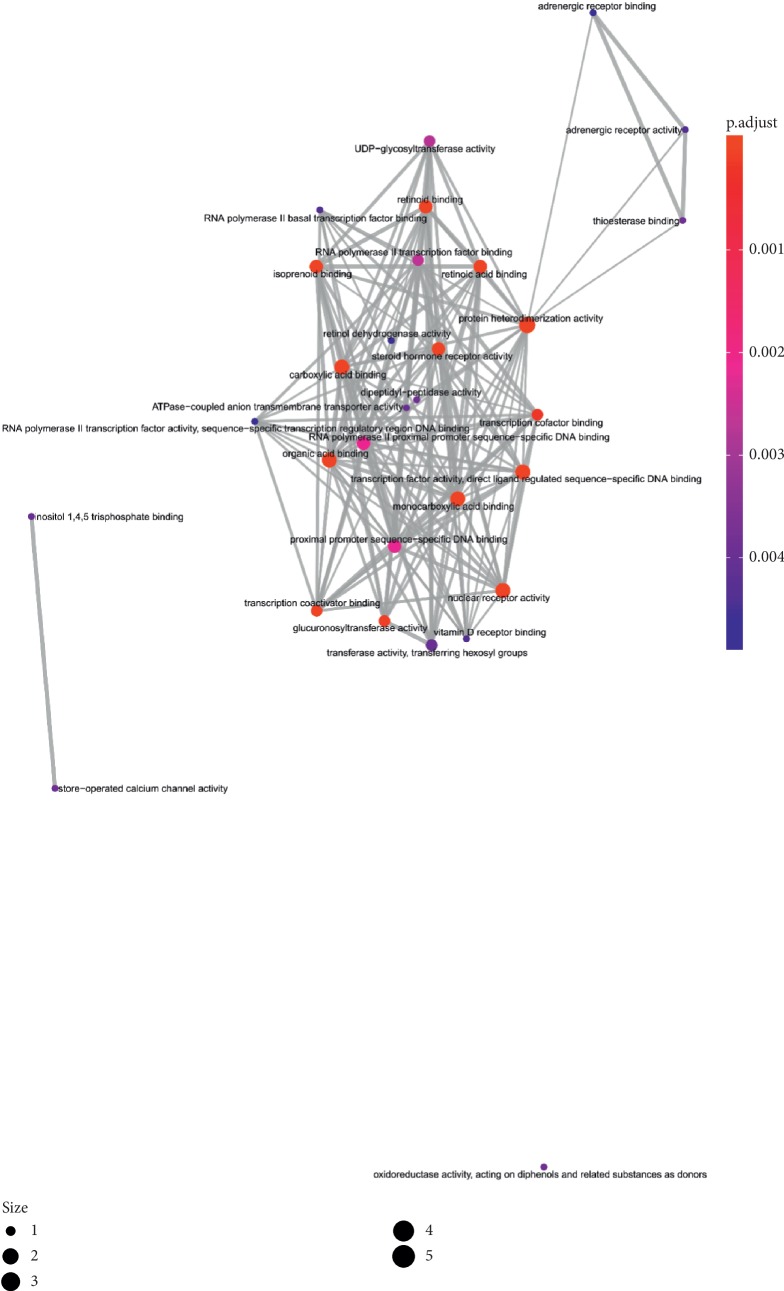
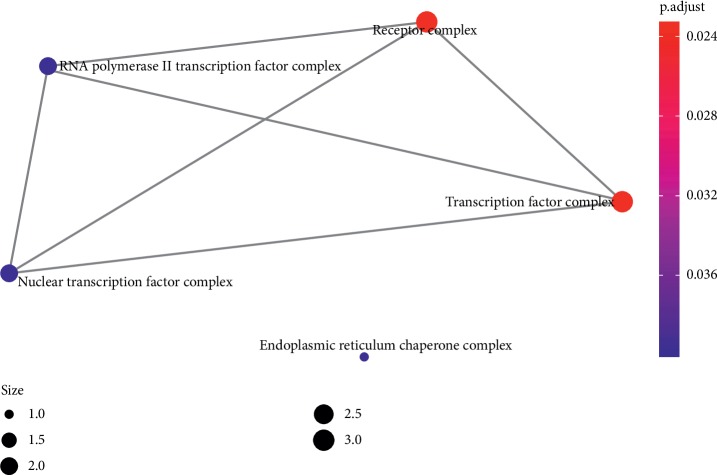
In order to determine the mechanism of predicting the target, KEGG and GO were executed by the clusterProfiler software package in the R language. A total of 944 pathways were enriched and are arranged in Supplementary . Among them, 111 pathways were enriched from KEGG; the first 20 pathways are displayed in Table 1, and Figure 7 shows the top 10 pathways with the highest p value. As shown in Figure 7, retinol metabolism, metabolism of xenobiotics by cytochrome P450, chemical carcinogenesis, steroid hormone biosynthesis, adipocytokine signaling pathway, drug metabolism—cytochrome P450, PPAR signaling pathway, Th17 cell differentiation, ascorbate and aldarate metabolism, pentose and glucuronate interconversions, thyroid cancer, porphyrin and chlorophyll metabolism, non-small-cell lung cancer, bile secretion, drug metabolism—other enzymes, small cell lung cancer, parathyroid hormone synthesis, secretion and action, thyroid hormone signaling pathway, hepatitis C, nonalcoholic fatty liver disease (NAFLD) were involved in the pathological development of CHD. The execution of GO pathways was done using the clusterProfiler software package of R language, including BP (biological process), CC (cellular component), and MF (molecular function) analysis, a total of 833 pathways were enriched [38], and top 10 significant pathways are listed in Table 1. In order to reflect the internal relationship between these GO terms, the clusterProfiler software package was used to reconstruct the interactive network (Figures 8–10).
Table 1.
Top 20 enriched KEGG pathways and top 10 enriched GO pathways.
| Description | p value | p.adjust | q value | Count | Source |
|---|---|---|---|---|---|
| Retinol metabolism | 2.77E – 06 | 8.12E – 05 | 3.51E – 05 | 4 | KEGG |
| Metabolism of xenobiotics by cytochrome P450 | 4.60E – 06 | 8.12E – 05 | 3.51E – 05 | 4 | KEGG |
| Chemical carcinogenesis | 6.25E – 06 | 8.12E – 05 | 3.51E – 05 | 4 | KEGG |
| Steroid hormone biosynthesis | 9.79E – 05 | 9.54E – 04 | 4.12E – 04 | 3 | KEGG |
| Adipocytokine signaling pathway | 1.56E – 04 | 1.07E – 03 | 4.63E – 04 | 3 | KEGG |
| Drug metabolism—cytochrome P450 | 1.77E – 04 | 1.07E – 03 | 4.63E – 04 | 3 | KEGG |
| PPAR signaling pathway | 1.93E – 04 | 1.07E – 03 | 4.63E – 04 | 3 | KEGG |
| Th17 cell differentiation | 5.72E – 04 | 2.79E – 03 | 1.20E – 03 | 3 | KEGG |
| Ascorbate and aldarate metabolism | 8.12E – 04 | 3.52E – 03 | 1.52E – 03 | 2 | KEGG |
| Pentose and glucuronate interconversions | 1.29E – 03 | 5.03E – 03 | 2.17E – 03 | 2 | KEGG |
| Thyroid cancer | 1.53E – 03 | 5.41E – 03 | 2.34E – 03 | 2 | KEGG |
| Porphyrin and chlorophyll metabolism | 1.97E – 03 | 6.39E – 03 | 2.76E – 03 | 2 | KEGG |
| Non-small-cell lung cancer | 4.79E – 03 | 1.44E – 02 | 6.21E – 03 | 2 | KEGG |
| Bile secretion | 5.53E – 03 | 1.54E – 02 | 6.65E – 03 | 2 | KEGG |
| Drug metabolism—other enzymes | 6.81E – 03 | 1.77E – 02 | 7.64E – 03 | 2 | KEGG |
| Small cell lung cancer | 9.33E – 03 | 2.27E – 02 | 9.82E – 03 | 2 | KEGG |
| Parathyroid hormone synthesis, secretion, and action | 1.20E – 02 | 2.75E – 02 | 1.19E – 02 | 2 | KEGG |
| Thyroid hormone signaling pathway | 1.43E – 02 | 3.09E – 02 | 1.33E – 02 | 2 | KEGG |
| Nonalcoholic fatty liver disease (NAFLD) | 2.29E – 02 | 4.25E – 02 | 1.83E – 02 | 2 | KEGG |
| Hepatitis C | 1.80E – 02 | 3.69E – 02 | 1.59E – 02 | 2 | KEGG |
| Cellular response to xenobiotic stimulus | 6.58E – 08 | 3.75E – 05 | 1.97E – 05 | 5 | GO-BP |
| Flavonoid metabolic process | 1.09E – 07 | 3.75E – 05 | 1.97E – 05 | 3 | GO-BP |
| Retinoic acid metabolic process | 4.82E – 07 | 8.37E – 05 | 4.41E – 05 | 3 | GO-BP |
| Cellular response to steroid hormone stimulus | 4.85E – 07 | 8.37E – 05 | 4.41E – 05 | 5 | GO-BP |
| Response to xenobiotic stimulus | 6.64E – 07 | 9.16E – 05 | 4.82E – 05 | 5 | GO-BP |
| Xenobiotic metabolic process | 9.64E – 07 | 1.11E – 04 | 5.83E – 05 | 4 | GO-BP |
| Antibiotic metabolic process | 1.74E – 06 | 1.71E – 04 | 9.00E – 05 | 4 | GO-BP |
| Regulation of lipid metabolic process | 2.62E – 06 | 2.26E – 04 | 1.19E – 04 | 5 | GO-BP |
| Response to steroid hormone | 3.62E – 06 | 2.78E – 04 | 1.46E – 04 | 5 | GO-BP |
| Xenobiotic catabolic process | 2.32E – 05 | 1.46E – 03 | 7.67E – 04 | 2 | GO-BP |
| Transcription factor complex | 1.21E – 03 | 2.37E – 02 | 1.45E – 02 | 3 | GO-CC |
| Receptor complex | 1.53E – 03 | 2.37E – 02 | 1.45E – 02 | 3 | GO-CC |
| RNA polymerase II transcription factor complex | 4.38E – 03 | 3.97E – 02 | 2.43E – 02 | 2 | GO-CC |
| Nuclear transcription factor complex | 6.15E – 03 | 3.97E – 02 | 2.43E – 02 | 2 | GO-CC |
| Endoplasmic reticulum chaperone complex | 6.40E – 03 | 3.97E – 02 | 2.43E – 02 | 1 | GO-CC |
| Invadopodium | 1.02E – 02 | 5.28E – 02 | 3.23E – 02 | 1 | GO-CC |
| Lamellipodium membrane | 1.40E – 02 | 5.90E – 02 | 3.61E – 02 | 1 | GO-CC |
| Apical plasma membrane | 1.52E – 02 | 5.90E – 02 | 3.61E – 02 | 2 | GO-CC |
| Cell projection membrane | 1.84E – 02 | 6.24E – 02 | 3.82E – 02 | 2 | GO-CC |
| Cytochrome complex | 2.10E – 02 | 6.24E – 02 | 3.82E – 02 | 1 | GO-CC |
| Nuclear receptor activity | 2.61E – 08 | 1.45E – 06 | 7.15E – 07 | 4 | GO-MF |
| Transcription factor activity, direct ligand-regulated sequence-specific DNA binding | 2.61E – 08 | 1.45E – 06 | 7.15E – 07 | 4 | GO-MF |
| Monocarboxylic acid binding | 6.41E – 08 | 2.37E – 06 | 1.17E – 06 | 4 | GO-MF |
| Retinoic acid binding | 1.98E – 07 | 5.50E – 06 | 2.71E – 06 | 3 | GO-MF |
| Retinoid binding | 1.44E – 06 | 2.92E – 05 | 1.44E – 05 | 3 | GO-MF |
| Isoprenoid binding | 1.58E – 06 | 2.92E – 05 | 1.44E – 05 | 3 | GO-MF |
| Carboxylic acid binding | 6.03E – 06 | 8.72E – 05 | 4.30E – 05 | 4 | GO-MF |
| Organic acid binding | 6.29E – 06 | 8.72E – 05 | 4.30E – 05 | 4 | GO-MF |
| Steroid hormone receptor activity | 7.77E – 06 | 9.59E – 05 | 4.73E – 05 | 3 | GO-MF |
| Protein heterodimerization activity | 1.24E – 05 | 1.37E – 04 | 6.76E – 05 | 5 | GO-MF |
Figure 7.
Top 10 enriched KEGG pathways with p value.
Figure 8.
The GO interaction network of coexpression genes (the emapplot of coexpression genes from BP enriched pathway).
Figure 9.
The GO interaction network of coexpression genes (the emapplot of coexpression genes from MF enriched pathway).
Figure 10.
The GO interaction network of coexpression genes (the emapplot of coexpression genes from CC enriched pathway).
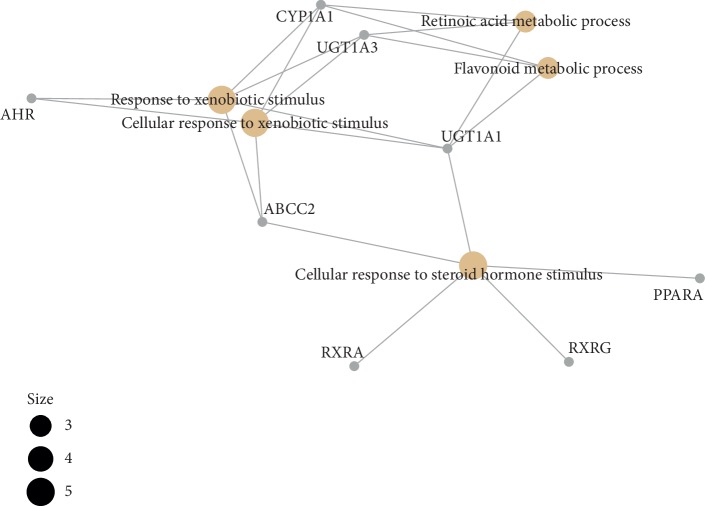
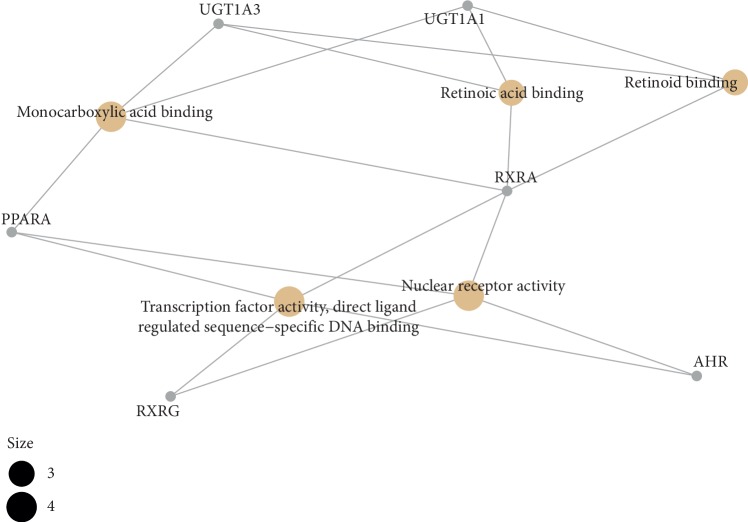
The pathway network of hub genes was filtered, and the cnetplot is shown in Figures 11 and 12. The cnetplot indicated that the subnetwork could participate in the pathological development processes of CHD retinoic acid metabolic process, flavonoid metabolic process, response to xenobiotic stimulus, cellular response to xenobiotic stimulus, cellular response to steroid hormone stimulus, retinoid binding, retinoic acid binding, monocarboxylic acid binding, transcription factor activity, and direct ligand-regulated sequence-specific DNA binding. The target genes participating in the core network include AHR, CYP1A1, UGT1A3, UGT1A1, ABCC2, RXRA, RXRG, and PPARA.
Figure 11.
The GO interaction network of coexpression genes (the cnetplot of coexpression genes from BP enriched pathway).
Figure 12.
The GO interaction network of coexpression genes (the cnetplot of coexpression genes from MF enriched pathway).
5. Discussion
The overall and local burden of CHD is enormous. On an annual basis, about 785,000 Americans have new coronary attacks and 470,000 have a recurrence [5]. Based on the theoretical principles of TCM, CHD belongs to the category of chest arthralgia and heartache [39]. Jingzhi Guanxin prescription is a classical prescription for the treatment of angina pectoris of CHD [40]. It is worth mentioning that Jiangxiang and Chuanxiong are important components of decoctions and Chinese patent medicine for the treatment of CHD and angina pectoris [8]. Wei et al. [41] indicated that Jingzhi Guanxin prescription can reduce the range of myocardial infarction in rats with acute myocardial ischemia. Jiangxiang essential oil and water extract can protect rats from ischemia/reperfusion injury, which may play a role by regulating sugar metabolism, lipid metabolism, and amino acid metabolic pathways [42]. Meanwhile, Jiangxiang has been observed to reduce the degree of atherosclerosis and erythrocyte deformability in experimental atherosclerotic rabbits [43]. A large number of experimental results showed that Jiangxiang volatile oil could inhibit thrombosis and increase platelet cAPM in incubated rabbits [44, 45]. In addition, the flavonoids in Jiangxiang have the effects of antioxidation, anticancer, anti-inflammation, analgesia, and antiplatelet aggregation [46–48]. The volatile oil of Ligusticum chuanxiong can significantly slow down the heart rate and weaken the myocardial contractility [17]. These effects may be attributed to the mechanism of volatile oil in the treatment of CHD.
Networks may provide a scaffold for the integration of omics data [15]. Network target can provide predictive and quantitative measures to the mechanistic role of drugs or herbal formulae in the treatment of diseases [24]. With the rapid advancement in bioinformatics, systems biology, and polypharmacology, “network pharmacology,” there is a shift from “one target, one drug” paradigm to the “network target, multicomponent” strategy, as it can not only reveal the underlying complex interactions between a herbal formula and cellular proteins, but also detect the influence of their interactions on the function and behavior of the human system. This key idea is in line with the holistic theory of TCM [36]. In order to study the relationship between drugs and disease targets and to clarify the targets of volatile oils in the treatment of CHD, a component-target-disease network was constructed, which provided a basis for further understanding the changes of disease tissues and revealed the molecular mechanism of CHD. The coexpressed genes in the target network represent the potential target of Jiangxiang and Chuanxiong volatile oils in the treatment of coronary heart disease. In the results, twelve coexpression genes were selected by Venny2.1.0, including UGT1A1, DPP4, RXRA, ADH1A, RXRG, UGT1A3, PPARA, TRPC3, CYP1A1, ABCC2, AHR, and ADRA2A. Many studies have shown a significant association between low serum bilirubin levels and CVD [49]. UGT1A1 is the only enzyme that contributes substantially to bilirubin glucuronidation and thus enhances bilirubin elimination (catalyzed by UGT1A1 enzyme) [50, 51]. Further, TA repeat polymorphism may be a key characteristic in the gene controlling bilirubin level [52]. Studies found that expression of DPP4 in the heart was coordinated with a set of gene expression signature characteristic for whole blood proliferation, which is enriched for genes involved in cell cycle control and DNA replication, potentially impacting peripheral stem cell mobilization [53]. Ku et al. [54] found that DPP4 can protect the heart from ischemia/reperfusion through GLP-1 receptor-dependent and receptor-independent mechanisms. Methylation of RXRA gene promoter may be one of the reasons for the downregulation of the expression of right subventricular bundle myocardium in patients with tetralogy of Fallot [55]. Research shows that drugs can improve lipid metabolism in ischemic heart model by regulating transcriptional factors such as RXRA and PPARs [56]. And ADH1A makes an important impact on the omega oxidation pathway [57]. Further, studies have identified a human-specific subnetwork regulated by RXRG, which has been validated to play a different role in hyperlipidemia and type 2 diabetes between human and mouse [58]. Familial combined hyperlipidemia (FCHL) is the most common atherogenic disorder of lipid metabolism [59]. Variation in the RXRG gene may contribute to genetic dyslipidemia in FCHL subjects [59]. UGT1A3 serves as potential therapeutic targets for CHD risk and has an effect on the function of high-density lipoprotein [60]. PPARA is an important gene that controls lipid metabolism. Studies have found that reduced PPARA expression during heart failure leads to reduced fatty acid oxidation and myocardial energy deficiency [61, 62]. Volatile oil may affect the lipid metabolism in the heart by acting on the expression of PPARA in the heart tissue. TRPC3 is highly expressed in the heart and participates in the pathogenesis of cardiac hypertrophy and heart failure as a pathological response to chronic mechanical stress [38]. Meanwhile, TRPC3 channel is an indispensable regulator of fibrosis development, by promoting fibroblasts to transition into myofibroblasts via intracellular Ca2+ overload, and it plays an important role in the process of myocardial fibrosis [63]. The members of CYP1 family (1A1, 1A2, and 1B1) play a major role in the bioactivation of PAHs to genotoxic metabolites which lead to DNA adducts, atherosclerosis, and carcinogenesis [64]. Ko and Shin [65] demonstrated that cardio-sulfa caused aberrant heart development in zebrafish and was activated through the AhR signaling pathway in a CYP1A-independent manner. Research results indicate that variations in the ABCC2 gene might influence the left ventricular parameters [66]. Additionally, AHR is highly correlated with heart defects. Studies have shown that when AHR is activated, it can lead to heart malformations in zebrafish embryos [67]. Furthermore, a study indicated that the cardiac developmental toxicity of PM2.5 might be prevented by targeting AHR or wnt/β-catenin signaling [67]. This study suggested that fetal ADRA2A may be important for normal heart development. However, it has been suggested that adult cardiac myocytes are virtually devoid of postsynaptic ADRA2A [68]. These results provide an important reference for the further study of the pathogenesis of CHD and the pathway mechanism for the treatment of CHD. Overall, these coexpressed genes participate in the process of lipid metabolism, myocardial fibrosis, ischemia/reperfusion, and other physiological changes in the heart to varying degrees, thus affecting the development of CHD. Jiangxiang and Chuanxiong volatile oils may play a role in the treatment of coronary heart disease by acting on these targets and giving full play to the corresponding biological effects.
GO analysis provides the most comprehensive resource which is currently available for computable knowledge regarding the functions of genes and gene products, used to recognize shared associations between proteins and annotations to GO [69]. KEGG is a database resource for the understanding of high-level functions and utilities of the biological system. We used KEGG and GO to enrich 944 pathways, which revealed the molecular mechanism of CHD and better explained the variation between healthy and diseased tissues. Hence, this can be used to develop effective treatment strategies. Key nodes in the pathway were selected by R language; the results suggest CHRM2, GNAI2, CHRM1, JX8, JX13, CHX63, and CHX77 may play an important role in the pathological and therapeutic mechanism of CHD. Adrenaline can restore heartbeat by increasing coronary and cerebral perfusion pressure [70]. β-Blockers are first-line drugs for the treatment of coronary heart disease and can increase the survival rate of patients with acute myocardial infarction [1]. G (i) protein can affect the response of cyclase to β-adrenergic stimulation by participating in the hormone regulation of adenylate cyclase [71]. The activation of β1-adrenergic receptor can produce positive myocardial effect, which leads to the aggravation of contraction, the acceleration of cardiac ejection velocity, and the increase of heart rate, and β2-adrenergic receptor can cause smooth muscle relaxation [72]. GNAI2 is guanine nucleotide-binding protein G (i) subunit alpha-2, and it may affect the role of epinephrine in the heart by inhibiting the stimulation of β-epinephrine by cyclase. K+ plays an important role in maintaining the normal operation of cardiac electrophysiology, and the abnormal change in the K+ channel is an important factor leading to various heart diseases [73]. Muscarinic acetylcholine receptors play a role in regulating cardiac function and smooth muscle contraction [74]. Studies have shown that CHRM2 is closely related to the expression of human cardiac function and plays an important role in the regulation of cardiovascular functions [75, 76]. CHRM1 plays an important role in the regulation of IK, Ach atrial repolarization [77]. CHRM1 and CHRM2 can regulate K+ channels through the action of G protein. Meanwhile, CHRM1 can increase heart rate [78, 79] and contractile force [80], CHRM2 can modulate pacemaker activity, atrioventricular conduction, and force of contractility [81] and sympathetic neurotransmitter release in atria [82]. It is worth mentioning that isobornyl acetate (CHX63) has a clear analgesic and anti-inflammatory effect, which may be an important role in the treatment of coronary heart disease. JX13 and JX8 are positive and negative isomers of each other and are the main components of volatile oil. The CHX77, CHX63, JX13, and JX8 components act on GNAI2, and CHRM1 and CHRM2 and thus activate the important molecular mechanisms for the treatment of CHD with volatile oils. Further, its main function may be in regulating heart rate, myocardial contraction, and ion channels. These studies provide indirect evidence to support our predictions.
6. Conclusions
Taken together, bioinformatics data show that the positive effect of Jiangxiang and Chuanxiong volatile oils on CHD may be predominantly due to its effect on ischemia/reperfusion, lipid metabolism, and myocardial fibrosis and may be related to the regulation of ion channels, myocardial contraction, and heart rate. These results highlight that the predicted therapeutic target may be a potential biomarker for the treatment of CHD with Jiangxiang and Chuanxiong volatile oils. However, systematic and rigorous experiments are needed to verify our findings.
Acknowledgments
This work was supported by the Natural Science Foundation of China (grant number: 81703720); Key Research and Development Program of Shaanxi Province of China (grant number: 2017ZDXM–SF–008); Chinese Medicine Pharmaceutical Key Discipline of Shaanxi Province (grant number: 303061107); Key Research and Development Plan of Shaanxi Province (grant number: 2018SF-314); and Discipline Innovation Team Project of Shaanxi University of Chinese Medicine (2019-YL11). The authors would like to acknowledge the Shaanxi Province Key Subject of Pharmacy Engineering of Shaanxi Provincial Traditional Chinese Medicine Administration.
Abbreviations
- CVD:
Cardiovascular diseases
- CHD:
Coronary heart disease
- MI:
Myocardial infarction
- TCM:
Traditional Chinese medicine
- UniProt:
Universal Protein Resource
- GC-MS:
Gas chromatography-mass spectrometer
- CAS Number:
Chemical Abstracts Service
- KEGG:
Kyoto Encyclopedia of Genes and Genomes
- GO:
Gene Ontology
- BP:
biological process
- CC:
cellular component
- MF:
molecular function
- NAFLD:
Nonalcoholic fatty liver disease.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Jia Tai, Junbo Zou, and Yu Wang searched articles in electronic databases and wrote the manuscript. Yulin Liang, Xiaofei Zhang, Dongyan Guo, and Mei Wang analyzed the data. Chunli Cui, Jing Wang, and Jiangxue Cheng performed the data extraction. Yajun Shi designed the study and amended the paper. Jia Tai, Junbo Zou, and Xiaofei Zhang contributed equally to this work and are co-first authors.
Supplementary Materials
Supplementary Table 1: genes of CHD. Supplementary Table 2: ingredients of Jiangxiang and Chuanxiong essential oils. Supplementary Table 3: related genes of essential oil compounds. Supplementary Table 4: pathways from KEGG and GO.
References
- 1.Dalen J. E., Alpert J. S., Goldberg R. J., Weinstein R. S. The epidemic of the 20th century: coronary heart disease. The American Journal of Medicine. 2014;127(9):807–812. doi: 10.1016/j.amjmed.2014.04.015. [DOI] [PubMed] [Google Scholar]
- 2.Wong N. D. Epidemiological studies of CHD and the evolution of preventive cardiology. Nature Reviews Cardiology. 2014;11(5):276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 3.Lazaro V. L. 2014 PHA clinical practice guidelines for the diagnosis and management of patients with coronary heart disease. ASEAN Heart Journal. 2016;24(1) doi: 10.7603/s40602-016-0003-6. [DOI] [Google Scholar]
- 4.Berry C., Corcoran D., Hennigan B., Watkins S., Layland J., Oldroyd K. G. Fractional flow reserve-guided management in stable coronary disease and acute myocardial infarction: recent developments. European Heart Journal. 2015;36(45):3155–3164. doi: 10.1093/eurheartj/ehv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kones R. Primary prevention of coronary heart disease: integration of new data, evolving views, revised goals, and role of rosuvastatin in management. A comprehensive survey. Drug Design, Development and Therapy. 2011;5:325–380. doi: 10.2147/dddt.s14934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gongora-Rivera F., Labreuche J., Jaramillo A., Steg P. G., Hauw J.-J., Amarenco P. Autopsy prevalence of coronary atherosclerosis in patients with fatal stroke. Stroke. 2007;38(4):1203–1210. doi: 10.1161/01.str.0000260091.13729.96. [DOI] [PubMed] [Google Scholar]
- 7.Chen Z., Venkat P., Seyfried D., Chopp M., Yan T., Chen J. Brain-heart interaction. Circulation Research. 2017;121(4):451–468. doi: 10.1161/circresaha.117.311170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji L. Pharmacology of Traditional Chinese Medical Formulae. Beijing, China: Traditional Chinese Medicine Publishing House; 2012. [Google Scholar]
- 9.Shujun Q. Therapeutic effect of purified Guanxin soft capsule in the treatment of angina of coronary heart disease. Journal of Practical Medical Techniques. 2007;14:1425–1426. [Google Scholar]
- 10.Yuanxiang L., Shenchun D. Application of Jingzhi Guanxin pills in the treatment of angina pectoris. Chinese and Foreign Health Digests. 2009;22:248–249. [Google Scholar]
- 11.Hopkins A. L. Network pharmacology: the next paradigm in drug discovery. Nature Chemical Biology. 2008;4(11):682–690. doi: 10.1038/nchembio.118. [DOI] [PubMed] [Google Scholar]
- 12.Bezhentsev V., Ivanov S., Kumar S., Goel R., Poroikov V. Identification of potential drug targets for treatment of refractory epilepsy using network pharmacology. Journal of Bioinformatics and Computational Biology. 2018;16(1) doi: 10.1142/s0219720018400024.1840002 [DOI] [PubMed] [Google Scholar]
- 13.Barabási A.-L., Oltvai Z. N. Network biology: understanding the cell’s functional organization. Nature Reviews Genetics. 2004;5(2):101–113. doi: 10.1038/nrg1272. [DOI] [PubMed] [Google Scholar]
- 14.Albert R., Jeong H., Barabási A.-L. Error and attack tolerance of complex networks. Nature. 2000;406(6794):378–382. doi: 10.1038/35019019. [DOI] [PubMed] [Google Scholar]
- 15.Lee S., Zhang C., Liu Z., et al. Network analyses identify liver-specific targets for treating liver diseases. Molecular Systems Biology. 2017;13(8):p. 938. doi: 10.15252/msb.20177703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bo Z., Jia L., Hongyan L., Yanli P. Research progress on Jiangxiang essential oils. China Pharmacist. 2014;17(8):1403–1406. [Google Scholar]
- 17.Dalie Z, Guisheng L. Research progress on volatile oil of Chuanxiong. Lishizhen Medicine and Materia Medica Research. 2005;7:664–666. [Google Scholar]
- 18.Piñero J., Queralt-Rosinach N., Bravo A., et al. DisGeNET: a discovery platform for the dynamical exploration of human diseases and their genes. Database (Oxford) 2015;2015 doi: 10.1093/database/bav028.bav028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Yu C., Li X., et al. Therapeutic target database update 2018: enriched resource for facilitating bench-to-clinic research of targeted therapeutics. Nucleic Acids Research. 2018;46(D1):D1121–D1127. doi: 10.1093/nar/gkx1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wishart D. S., Feunang Y. D., Marcu A., et al. HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Research. 2018;46(D1):D608–D617. doi: 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ru J., Li P., Wang J., et al. TCMSP: a database of systems pharmacology for drug discovery from herbal medicines. Journal of Cheminformatics. 2014;6(1):p. 13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szklarczyk D., Santos A., Von Mering C., Jensen L. J., Bork P., Kuhn M. STITCH 5: augmenting protein-chemical interaction networks with tissue and affinity data. Nucleic Acids Research. 2016;44(D1):D380–D384. doi: 10.1093/nar/gkv1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boezio B., Audouze K., Ducrot P., Taboureau O. Network-based approaches in pharmacology. Molecular Informatics. 2017;36(10) doi: 10.1002/minf.201700048.1700048 [DOI] [PubMed] [Google Scholar]
- 24.Li S., Zhang B. Traditional Chinese medicine network pharmacology: theory, methodology and application. Chinese Journal of Natural Medicines. 2013;11(2):110–120. doi: 10.1016/s1875-5364(13)60037-0. [DOI] [PubMed] [Google Scholar]
- 25.Oliveros J. C. Venny. An Interactive Tool for Comparing Lists with Venn’s Diagrams. 2007. , 2007, https://bioinfogp.cnb.csic.es/tools/venny/index.html. [Google Scholar]
- 26.Li S., Zhang Z. Q., Wu L. J., Zhang X. G., Wang Y. Y., Li Y. D. Understanding Zheng in traditional Chinese medicine in the context of neuro-endocrine-immune network. IET Systems Biology. 2007;1(1):51–60. doi: 10.1049/iet-syb:20060032. [DOI] [PubMed] [Google Scholar]
- 27.Song C., Havlin S., Makse H. A. Self-similarity of complex networks. Nature. 2005;433(7024):392–395. doi: 10.1038/nature03248. [DOI] [PubMed] [Google Scholar]
- 28.Jeong H., Tombor B., Albert R., Oltavi Z. N., Barabasi A. L. The large-scale organization of metabolic networks. Nature. 2000;407(6804):651–654. doi: 10.1038/35036627. [DOI] [PubMed] [Google Scholar]
- 29.Ogata H., Goto S., Sato K., Fujibuchi W., Bono H., Kanehisa M. KEGG: kyoto Encyclopedia of genes and genomes. Nucleic Acids Research. 1999;27(1):29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kanehisa M., Sato Y., Kawashima M., Furumichi M., Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Research. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M., Furumichi M., Tanabe M., Sato Y., Morishima K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Research. 2017;45(D1):D353–D361. doi: 10.1093/nar/gkw1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu G., Wang L.-G., Han Y., He Q.-Y. ClusterProfiler: an R package for comparing biological themes among gene clusters. OMICS: A Journal of Integrative Biology. 2012;16(5):284–287. doi: 10.1089/omi.2011.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alexa A., Rahnenfuhrer J., Lengauer T. Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics. 2006;22(13):1600–1607. doi: 10.1093/bioinformatics/btl140. [DOI] [PubMed] [Google Scholar]
- 34.Hongling L. Analysis the compounds of essential Oil by GC-MS. Chinese Traditional Patent Medicine. 2009;31(6):915–917. [Google Scholar]
- 35.Shengxiang Z., Jianhe W., Bingchun G., Hui M., Jindong F. Study on GC fingerprint of Jiangxiang essential oil. Chinese Journal of Modern Applied Pharmacy. 2011;(11):995–999. [Google Scholar]
- 36.Yanqiong Z., Yuting L., Mao X., et al. Thyroid hormone synthesis: a potential target of a Chinese herbal formula Haizao Yuhu Decoction acting on iodine-deficient goiter. Oncotarget. 2016;7(32) doi: 10.18632/oncotarget.10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su M., Guo C., Liu M., Liang X., Yang B. Therapeutic targets of vitamin C on liver injury and associated biological mechanisms: a study of network pharmacology. International Immunopharmacology. 2019;66:383–387. doi: 10.1016/j.intimp.2018.11.048. [DOI] [PubMed] [Google Scholar]
- 38.Mossio M., Montévil M., Longo G. Theoretical principles for biology: organization. Progress in Biophysics and Molecular Biology. 2016;122(1):24–35. doi: 10.1016/j.pbiomolbio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Qingyong H., Jie W., Zhan S., Xingjiang X. The conception of theoretical structure of PRO efficacy scale of traditional Chinese medicine in coronary heart disease. China Journal of Traditional Chinese Medicine and Pharmacy. 2010;25(1):42–45. [Google Scholar]
- 40.Pharmacopoeia Commission. Chinese Pharmacopoeia. Ghaziabad, India: Pharmacopoeia Commission; 2015. [Google Scholar]
- 41.Wei Z., Lanying C., Jie Z., Xiao C., Jun L. Comparative studies on protective effects of guanxindanshen recipe, Jingzhiguanxin recipe and shuxiong recipe in acute myocardial ischemia rats. Journal of Jiangxi University of Traditional Chinese Medicine. 2013;(5):63–65. [Google Scholar]
- 42.Fei M., Jialin D., Haixu B., et al. Metabolomics study on the preventive effects of Jiangxiang extract and essential oil on rats with myocardial ischemia/reperfusion injury. Chinese Pharmacological Bulletin. 2016;32(10):1377–1382. [Google Scholar]
- 43.Ran X., Ma L., Peng C., Zhang H., Qin L.-P. Ligusticum chuanxiongHort: a review of chemistry and pharmacology. Pharmaceutical Biology. 2011;49(11):1180–1189. doi: 10.3109/13880209.2011.576346. [DOI] [PubMed] [Google Scholar]
- 44.Mou F., Duan J., Bian H., et al. Metabonomics study on the preventive effects of perfume extract and volatile oil on myocardial ischemia/reperfusion injury in rats. Chinese Pharmacological Bulletin. 2016;32(10):1377–1382. [Google Scholar]
- 45.Wang X., Zhang Y. Preparation of Jiangxiang volatile oil-HP-β-CD and its protective effect on acute myocardial ischemia in rats. Journal of Shandong University of Traditional Chinese Medicine. 2010;34(3):256–257. [Google Scholar]
- 46.Zhao X., Mei W., Gong M., Zuo W., Bai H., Dai H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules. 2011;16(12):9775–9782. doi: 10.3390/molecules16129775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee D.-S., Li B., Im N.-K., Kim Y.-C., Jeong G.-S. 4,2′,5′-Trihydroxy-4′-methoxychalcone from Dalbergia odorifera exhibits anti-inflammatory properties by inducing heme oxygenase-1 in murine macrophages. International Immunopharmacology. 2013;16(1):114–121. doi: 10.1016/j.intimp.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 48.Fan Z., Wang Y., Xie R., Wang D., Zhou X. Research progress on chemical constituents and pharmacological effects of fructus rehmanniae. Lishizhen Medicine and Materia Medica Research. 2016;27(10):2478–2480. [Google Scholar]
- 49.Schwertner H. A., Jackson W. G., Tolan G. Association of low serum concentration of bilirubin with increased risk of coronary artery disease. Clinical Chemistry. 1994;40(1):18–23. [PubMed] [Google Scholar]
- 50.Baudhuin L. M., Miller W. L., Train L., et al. Relation of ADRB1, CYP2D6, and UGT1A1 polymorphisms with dose of, and response to, carvedilol or metoprolol therapy in patients with chronic heart failure. The American Journal of Cardiology. 2010;106(3):402–408. doi: 10.1016/j.amjcard.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Lin J.-P., O’Donnell C. J., Schwaiger J. P., et al. Association between the UGT1A1 ∗ 28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation. 2006;114(14):1476–1481. doi: 10.1161/circulationaha.106.633206. [DOI] [PubMed] [Google Scholar]
- 52.Lin J.-P., Schwaiger J. P., Cupples L. A., et al. Conditional linkage and genome-wide association studies identify UGT1A1 as a major gene for anti-atherogenic serum bilirubin levels-the framingham heart study. Atherosclerosis. 2009;206(1):228–233. doi: 10.1016/j.atherosclerosis.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Long Q., Argmann C., Houten S. M., et al. Inter-tissue coexpression network analysis reveals DPP4 as an important gene in heart to blood communication. Genome Medicine. 2016;8(1):p. 15. doi: 10.1186/s13073-016-0268-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ku H.-C., Chen W.-P., Su M.-J. DPP4 deficiency preserves cardiac function via GLP-1 signaling in rats subjected to myocardial ischemia/reperfusion. Naunyn-Schmiedeberg’s Archives of Pharmacology. 2011;384(2):197–207. doi: 10.1007/s00210-011-0665-3. [DOI] [PubMed] [Google Scholar]
- 55.Zhang J., Ma X., Wang H., Ma D., Huang G. Elevated methylation of the RXRA promoter region may be responsible for its downregulated expression in the myocardium of patients with TOF. Pediatric Research. 2014;75(5):588–594. doi: 10.1038/pr.2014.17. [DOI] [PubMed] [Google Scholar]
- 56.Wang Q., Li C., Zhang Q., et al. The effect of Chinese herbs and its effective components on coronary heart disease through PPARs-PGC1alpha pathway. BMC Complement Altern Med. 2016;16(1):p. 514. doi: 10.1186/s12906-016-1496-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Menni C., Metrustry S. J., Ehret G., et al. Molecular pathways associated with blood pressure and hexadecanedioate levels. PLoS One. 2017;12(4) doi: 10.1371/journal.pone.0175479.e0175479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang K., Narayanan M., Zhong H., et al. Meta-analysis of inter-species liver co-expression networks elucidates traits associated with common human diseases. PLoS Computational Biology. 2009;5(12) doi: 10.1371/journal.pcbi.1000616.e1000616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sentinelli F., Minicocci I., Montali A., et al. Association of RXR-gamma gene variants with familial combined hyperlipidemia: genotype and haplotype analysis. J Lipids. 2013;2013:7. doi: 10.1155/2013/517943.517943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oates C. P., Koenig D., Rhyne J., et al. Novel polymorphisms associated with hyperalphalipoproteinemia and apparent cardioprotection. Journal of Clinical Lipidology. 2018;12(1):110–115. doi: 10.1016/j.jacl.2017.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Drosatos K., Pollak N. M., Pol C. J., et al. Cardiac myocyte KLF5 regulates ppara expression and cardiac function. Circulation Research. 2016;118(2):241–253. doi: 10.1161/circresaha.115.306383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sugden M. C., Caton P. W., Holness M. J. PPAR control: it’s SIRTainly as easy as PGC. Journal of Endocrinology. 2010;204(2):93–104. doi: 10.1677/joe-09-0359. [DOI] [PubMed] [Google Scholar]
- 63.Han L., Li J. Canonical transient receptor potential 3 channels in atrial fibrillation. European Journal of Pharmacology. 2018;837:1–7. doi: 10.1016/j.ejphar.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 64.Messina A., Puccinelli E., Gervasi P. G., Longo V. Expression and inducibility of CYP1A1, 1A2, 1B1 by β-naphthoflavone and CYP2B22, CYP3As by rifampicin in heart regions and coronary arteries of pig. Research in Veterinary Science. 2013;94(1):77–83. doi: 10.1016/j.rvsc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Ko S.-K., Shin I. Cardiosulfa induces heart deformation in zebrafish through the AhR-mediated, CYP1A-independent pathway. Chembiochem. 2012;13(10):1483–1489. doi: 10.1002/cbic.201200177. [DOI] [PubMed] [Google Scholar]
- 66.Sági J. C., Egyed B., Kelemen A., et al. Possible roles of genetic variations in chemotherapy related cardiotoxicity in pediatric acute lymphoblastic leukemia and osteosarcoma. BMC Cancer. 2018;18(1):p. 704. doi: 10.1186/s12885-018-4629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang H., Yao Y., Chen Y., et al. Crosstalk between AhR and wnt/β-catenin signal pathways in the cardiac developmental toxicity of PM2.5 in zebrafish embryos. Toxicology. 2016;355-356:31–38. doi: 10.1016/j.tox.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 68.Porter A. C., Svensson S. P. S., Stamer W. D., Bahl J. J., Richman J. G., Regan J. W. Alpha-2 adrenergic receptors stimulate actin organization in developing fetal rat cardiac myocytes. Life Sciences. 2003;72(13):1455–1466. doi: 10.1016/s0024-3205(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 69.Gan Y., Zheng S., Baak J. P. A., et al. Prediction of the anti-inflammatory mechanisms of curcumin by module-based protein interaction network analysis. Acta Pharmaceutica Sinica B. 2015;5(6):590–595. doi: 10.1016/j.apsb.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gough C. J. R., Nolan J. P. The role of adrenaline in cardiopulmonary resuscitation. Critical Care. 2018;22(1):p. 139. doi: 10.1186/s13054-018-2058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cho H., Kehrl J. H. Localization of Giαproteins in the centrosomes and at the midbody: implication for their role in cell division. The Journal of Cell Biology. 2007;178(2):245–255. doi: 10.1083/jcb.200604114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zuo L., Du Y., Ma J., et al. Pro-arrhythmic action of autoantibodies against the second extracellular loop of β1-adrenoceptor and its underlying molecular mechanisms. International Journal of Cardiology. 2015;198:251–258. doi: 10.1016/j.ijcard.2015.06.144. [DOI] [PubMed] [Google Scholar]
- 73.Jeevaratnam K., Chadda K. R., Huang C. L.-H., Camm A. J. Cardiac potassium channels: physiological insights for targeted therapy. Journal of Cardiovascular Pharmacology and Therapeutics. 2018;23(2):119–129. doi: 10.1177/1074248417729880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kruse A. C., Ring A. M., Manglik A., et al. Activation and allosteric modulation of a muscarinic acetylcholine receptor. Nature. 2013;504(7478):101–106. doi: 10.1038/nature12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brodde O. E., Michel M. C. Adrenergic and muscarinic receptors in the human heart. Pharmacological Reviews. 1999;51(4):651–690. [PubMed] [Google Scholar]
- 76.Hautala A. J., Rankinen T., Kiviniemi A. M., et al. Heart rate recovery after maximal exercise is associated with acetylcholine receptor M2 (CHRM2) gene polymorphism. American Journal of Physiology-Heart and Circulatory Physiology. 2006;291(1):H459–H466. doi: 10.1152/ajpheart.01193.2005. [DOI] [PubMed] [Google Scholar]
- 77.Heijman J., Kirchner D., Kunze F., et al. Muscarinic type-1 receptors contribute to I K,ACh in human atrial cardiomyocytes and are upregulated in patients with chronic atrial fibrillation. International Journal of Cardiology. 2018;255:61–68. doi: 10.1016/j.ijcard.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Islam A., Nojima H., Kimura I. Muscarinic M1 receptor activation reduces maximum upstroke velocity of action potential in mouse right atria. European Journal of Pharmacology. 1998;346(2-3):227–236. doi: 10.1016/s0014-2999(98)00055-7. [DOI] [PubMed] [Google Scholar]
- 79.Colecraft H. M., Egamino J. P., Sharma V. K., Sheu S. S. Signaling mechanisms underlying muscarinic receptor-mediated increase in contraction rate in cultured heart cells. The Journal of Biological Chemistry. 1998;273(48):32158–32166. doi: 10.1074/jbc.273.48.32158. [DOI] [PubMed] [Google Scholar]
- 80.Gallo M. P., Alloatti G., Eva C., Oberto A., Levi R. C. M1 muscarinic receptors increase calcium current and phosphoinositide turnover in Guinea-pig ventricular cardiocytes. The Journal of Physiology. 1993;471(1):41–60. doi: 10.1113/jphysiol.1993.sp019890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Dhein S., Van Koppen C. J., Brodde O.-E. Muscarinic receptors in the mammalian heart. Pharmacological Research. 2001;44(3):161–182. doi: 10.1006/phrs.2001.0835. [DOI] [PubMed] [Google Scholar]
- 82.Trendelenburg A.-U., Gomeza J., Klebroff W., Zhou H., Wess J. Heterogeneity of presynaptic muscarinic receptors mediating inhibition of sympathetic transmitter release: a study with M2- and M4-receptor-deficient mice. British Journal of Pharmacology. 2003;138(3):469–480. doi: 10.1038/sj.bjp.0705053. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: genes of CHD. Supplementary Table 2: ingredients of Jiangxiang and Chuanxiong essential oils. Supplementary Table 3: related genes of essential oil compounds. Supplementary Table 4: pathways from KEGG and GO.
Data Availability Statement
The data used to support the findings of this study are included within the article.