Abstract
The respiratory system is protected from inhaled particles and microbes by the mucociliary system. This system differs between animal species, where pigs and humans have numerous submucosal glands. The polymer-forming mucin, MUC5B, is packed in a highly organized way in granules of the mucus-secreting cells in the glands. Upon secretion, the packed MUC5B is flushed out by a chloride- and bicarbonate-rich fluid from the cystic fibrosis transmembrane conductance regulator–expressing serosal cells located at the most distal part of the gland. The bicarbonate raises the pH and removes calcium from the N terminus of MUC5B, allowing the mucin to be pulled out into a linear polymer. Thousands of such polymers gather in bundles in the submucosal gland duct, and these bundles appear at the opening of the glands. They are moved by the beating cilia, and sweep over the airway surface and are patchily coated with the MUC5AC mucin from the surface goblet cells. The movement of these bundles is controlled by the MUC5AC mucin attachment/detachment to the goblet cells. Thus, higher animals with submucosal glands and large diameters of the proximal airways are efficiently cleaned by the thick mucus bundles sweeping the airway surface and moving particles and bacteria toward the larynx.
Keywords: mucins, mucus, mucociliary clearance, secretory vesicles
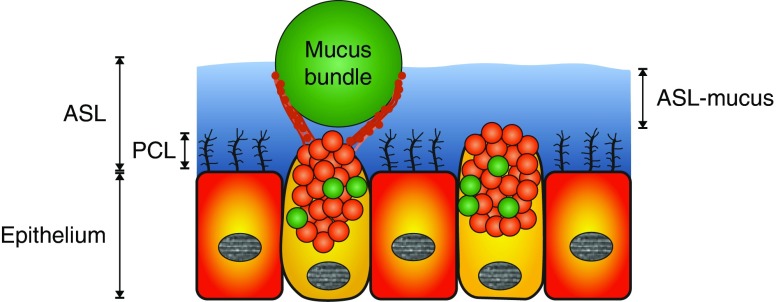
Breathing continuously exposes the airways to inhaled particles, allergens, viruses, bacteria, and noxious chemicals. The lungs are kept clean by the mucociliary system, consisting of the airway surface liquid (ASL) comprising the periciliary liquid (PCL) with the cilia and the surface part of the ASL that we name “ASL-mucus” to separate this from mucus bundles (Figure 1). The normal pig trachea is covered by about 20 μm of ASL-mucus, and the PCL covers 6 μm of that, as measured by microoptical coherence tomography in live pigs (1). A majority of the cells in the conducting airways have 200–300 cilia per cell, performing synchronized beating at 10–20 Hz to generate the transport (2). The PCL is filled with transmembrane mucins, MUC1, MUC4, and MUC16, to normalize the osmotic pressure and to optimize mucociliary clearance (2, 3). To protect the mucosal surface of the lung, the mucus functions as the first line of defense against inhaled particles and pathogens to avoid excessive immune responses and inflammation. Cleaning is accomplished by mucus collecting inhaled particulate matter, and the continuously beating cilia generate movement toward the larynx.
Figure 1.
Schematic drawing of the ciliated epithelium with goblet cells and airway surface liquid (ASL) made up of the periciliary liquid (PCL) and ASL-mucus. Goblet cells secrete MUC5AC (red) and some MUC5B (green). An assumed localization of a mucus bundle (MUC5B; green) is included. Submucosal glands are not included for simplicity.
The major structural components of mammalian respiratory mucus are the gel-forming MUC5AC and MUC5B mucins, highly glycosylated polymeric proteins with similar domain organization (Figure 2). Gel-forming mucins are stored highly organized in secretory granules of mucus-producing cells at low pH and high concentration of calcium ions (4). The high level of organization contributes to facilitating rapid secretion and expansion, as has been shown for the MUC2 mucin (5). The properties of the secreted mucin are highly dependent on the milieu at release, as has been shown for MUC2 in the ileum (6). The unfolding process during secretion requires increase in pH and calcium removal, both of which can be achieved by bicarbonate transported via the cystic fibrosis (CF) transmembrane conductance regulator (CFTR). The disease, CF, is caused by nonfunctional mutations in the gene encoding CFTR, and is an example of abnormal mucus leading to compromised defense against bacteria. Patients with CF suffer chronic bacterial lung infections, inflammation, and lung tissue destruction, due to the thick and sticky mucus where bacteria are trapped. The importance of bicarbonate secretion via CFTR for normal mucus detachment is well illustrated in the CF ileum, where insufficient amounts of bicarbonate lead to poor mucin unfolding. Proper unfolding is required to expose a site required for the protease, meprin β, to cleave MUC2 and thereby detach the mucin (6, 7). We and others have observed that the CF mucin concentration is higher than normal mucus (6, 8). Together with mucin detachment, this illustrates the importance of mucin expansion upon secretion for the formation of normal, transportable mucus.
Figure 2.
Schematic drawings of the gel-forming mucins, MUC5B and MUC5AC. BCCK = von Willebrand B and C domains, cystein-knot domain; CysD = CysD domain; D = von Willebrand D domain; PTS = proline, threonine, serine sequences.
MUC5B Packing in Granules
The MUC5B mucin is packed in submucosal gland mucus cell granules in a way that allows for them to be secreted into mucin bundles. The MUC5B mucin has an N-terminal part (MUC5B-N) containing the von Willebrand D1, D2, D′, and D3 assemblies (Figure 2), which are responsible for mucin oligomerization. A covalent dimer was observed when the MUC5B-N was produced as a recombinant protein and studied by gel filtration, small-angle X-ray scattering, and transmission electron microscopy at pH 7.4 (4). In the vesicles of the regulated secretory pathway, the pH is lower and the calcium concentration is higher, and studies at pH 6.2 showed a dimer (D) that formed tetrameric (Dx2) molecules when Ca2+ was added (9). When these tetrameric Dx2 molecules were studied by electron microscopy, the arms formed by the D1 and D2 domains were shown to be interacting noncovalently, forming head-to-head tetramers (Figure 3A). Each MUC5B-N dimer was turned 180° in relation to each other, and the arms were folded inward like hooks and, by this, intertwine the two dimers with each other (9). Further studies suggested that each tetramer was packed to other tetramers side-by-side in a zig-zag manner, where each unit was tilted 20° toward each other (9). The remaining part of the MUC5B mucin (Figure 2), including the extended mucin domains, formed after O-glycosylation of the proline, threonine, and serine (PTS) sequences extend outward from the von Willebrand D3 domain, as illustrated in Figure 3A.
Figure 3.
MUC5B N-terminal packing and mucin bundles made in submucosal glands. (A) MUC5B N-terminal domains and a model of its packing in the secretory granule. The central part of MUC5B is illustrated by a green arrow and the C-terminal part by a black box (see Figure 2). (B) Schematic drawing of a submucosal gland generating a mucus bundle. Serosal cells (blue) secreting chloride- and bicarbonate-rich fluid (blue drops) that flow through the gland ducts. Mucus-producing cells secrete MUC5B (green) that unfolds into linear threads and assemble into bundles exiting the gland. Goblet cells expressing MUC5AC (red) are situated in the outer part of the gland duct and coat the bundle. (C) The MUC5B polymer pulled out into linear thread. Colors as in A and Figure 2. S-S = disulfide bond.
MUC5B Secretion and Unfolding in Submucosal Glands
Pigs and humans have numerous submucosal glands down to the 10th bronchial generation, whereas mice only have a few submucosal glands in the upper trachea. Summarizing what is known about submucosal glands and mucin secretion, these glands are ideal for forming bundles from the linear MUC5B mucin molecules (Figure 3B). The most distal cells in the submucosal glands, the serous cells, express high levels of CFTR and secrete a chloride- and bicarbonate ion–rich fluid that flows through the gland ducts (9). The mucous cells making MUC5B are situated more proximal to the gland opening compared with the serous cells (Figure 3B). When the MUC5B is secreted, it meets the bicarbonate-rich fluid and the bound Ca2+ ions are dissociated from the N termini, allowing the mucin to unfold (9). The directed fluid flow will allow the MUC5B mucin to be pulled into its linear form (Figure 3C). Such flow-mediated unfolding into a linear molecule is similar to the von Willebrand factor unwinding by the pulling forces of the blood flow (10). Transmission electron microscopy revealed that the submucosal gland ducts contained linear threads that likely reflect the MUC5B polymers (9). When the ducts of single human submucosal glands were observed by time-lapse video microscopy, threads moving with the flow were visualized (9). The liquid flow appeared faster than the threads, suggesting that the flow could generate a pulling force that helps the mucin to unfold into linear structures. During the passage through the duct, the MUC5B polymers interact laterally to form thicker and thicker bundles (Figure 3B).
MUC5B Bundles Are Secreted from the Submucosal Glands
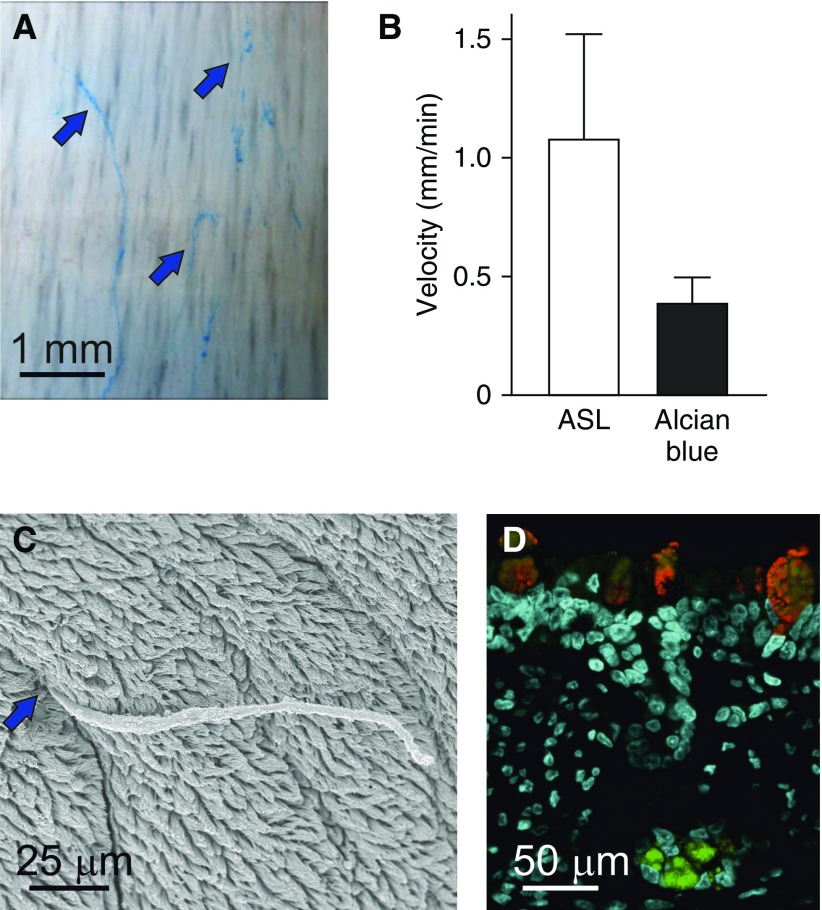
We have used the tracheobronchial tree from newborn pigs as a model for normal mucus transport (11). The trachea and first bronchi were opened from either the ventral or dorsal side and mounted. Studying mucus is difficult, as it is transparent and impossible to track without staining. As the positively charged dye, Alcian blue, is commonly used to stain the negatively charged mucins in tissue section or on electrophoresis gels, we dissolved Alcian blue in physiological buffer, pH 7.4, and added to the explant airways. Within minutes, the linear bundles appeared blue (Figure 4A). Using microscopy and time-lapse recordings, we observed long Alcian blue–stained bundles exiting the submucosal glands and sweeping with uneven speed cephalically across the airways. The bundles appearing at the gland openings had a diameter of 20–30 μm, and can be estimated to contain more than 1,000 MUC5B molecules. The bundles stained positive for MUC5B (11). Scanning electron microscopy also showed long continuous bundles exiting submucosal gland openings (Figure 4C). Similar bundles have also been observed in humans (11). To analyze the nature of the mucus bundles, the bundles were subjected to proteomic analyses and shown to contain both MUC5B and MUC5AC mucins, with a ratio around 1 (11).
Figure 4.
Alcian blue staining visualizes mucus bundles essential for airway cleaning. (A) Image from a time-lapse illustrating the Alcian blue–stained bundles moving over the airway surface (blue arrows). Note absence of mucus blanket. Scale bar = 1 mm. (B) Mean velocity of airway surface liquid (ASL) compared with mucus bundles. Because the bundles move slower than the ASL-mucus, the bundles are retained on the airway surface. Data are presented as mean ± SEM. (C) Scanning electron micrograph of a mucus bundle exiting a submucosal gland (blue arrow) and extending over the airway surface. Scale bar = 25 μm. (D) Submucosal gland mucous cells express and secrete MUC5B (green). Surface goblet cells express MUC5AC (red) and, to a lesser extent, MUC5B. Scale bar = 50 μm. SEM = standard error of the mean.
Mucus Bundles Move Separately from the ASL-Mucus
The mucus bundles containing the MUC5B and MUC5AC mucins move ventrally and toward the larynx. It has been shown in live piglets that tantalum microdiscs also move cephalically and to the ventral side of the trachea (12). These authors also used fluorescent nanospheres (40-nm carboxylate-modified beads) in an attempt to label mucus (13, 14). Because the beads are negatively charged, these are unlikely to bind the negatively charged mucins, but may bind to other proteins. When we added the same beads to the trachea and stained the mucus with Alcian blue, separate structures were labeled. In normal piglets, the Alcian blue–stained mucus bundles move with varying speed at an average velocity of around 0.35 mm/min (Figure 4B). The beads, on the other hand, moved in another focal plane and had an average velocity of more than 1 mm/min. This made us conclude that Alcian blue and carboxylated beads illustrate movement of separate entities. The velocity of the fluorescent beads resembled the previously reported transport velocity of the ASL-mucus in live pigs using microoptical coherence tomography (1). This causes us to conclude that the mucus bundles are separate and move slower than the ASL-mucus (11).
The mucus bundles on piglet explants had a diameter of around 27 μm. This is thicker than the ASL-mucus, indicating that the Alcian blue–positive mucus bundles protrude above the ASL-mucus, whereas the beads are in the ASL-mucus. The individual bundles sometimes entangled with each other, and had a tendency to gather into even thicker bundles. Immunostainings of pig airway paraffin sections were done to study the cellular origin of the MUC5B and MUC5AC mucins. The submucosal glands only stained for MUC5B, whereas the surface goblet cells stained for the MUC5AC and, to a lesser extent, the MUC5B mucin (Figure 4D).
The MUC5B Bundles Are Covered with MUC5AC
To illustrate the relationship between the MUC5B and MUC5AC mucins, the different glycosylation of these two mucins in the two compartments were used (11). The Lotus tetragonolobus lectin (LTL) specifically stained the MUC5B mucin from the submucosal glands identical to the anti-MUC5B antibody and the Ulex europaeus A1 (UEA1) lectin stained the MUC5AC mucin in the surface goblet cells. When the mucus bundles exiting the submucosal glands were stained with these lectins, a central core of LTL-stained MUC5B was observed (Figure 5A). Interestingly, this central mucus bundle core was patchily covered with UEA1-stained MUC5AC mucin (Figures 5A and 5B). Together, these results suggest that the mucus bundles coming from the submucosal glands have a core of linear MUC5B mucin that is covered with goblet cell MUC5AC mucins from the gland duct and tracheobronchial surface goblet cells (11). When the openings of submucosal glands were studied by scanning electron microscopy, protrusions typical for goblet cells were observed along the last part of the gland ducts (11). The mucus bundles exiting the glands, as well as mucus bundles on the surface, were covered by lumps of material similar to the protruding mucus from the goblet cells (Figure 5C).
Figure 5.
Mucus bundle morphology. (A) Mucus bundles from submucosal glands in live pig airway tissue consist of a core of MUC5B (LTL, green) and are coated by MUC5AC (UEA1, red). Scale bar = 20 μm. (B) A net-like structure can be observed attached to mucus bundles viewed with scanning electron microscopy. Scale bar = 2 μm. (C) The same net-like material comes from a surface goblet cell. Scale bar = 2 μm. (D) Mucus bundles (LTL, green) are retained by material from goblet cells (UEA1, red). The white arrow points to a goblet cell secreting material attached to the bundle. Scale bar = 10 μm. (E) Mucus bundle attached to goblet cell. Scale bar = 1 μm. LTL = Lotus tetragonolobus lectin; UEA1 = Ulex europaeus A1.
The MUC5B in the core of the bundles had MUC5AC coating that stretched out from the surface goblet cells (Figure 5D) (11). Protrusions typical for goblet cells were also observed binding to the mucus bundles by electron microscopy (Figure 5E). Together, these observations suggest that the MUC5AC mucin anchored the bundles to the surface. The movement of the mucus bundles was typically intermittent in contrast to the continuous beating of the cilia and flow of ASL-mucus. This suggested a transient attachment/detachment of the mucus bundles to the surface goblet cells. This organization could also be understood as important for retaining the sweeping long bundles onto the airway surface, and will allow control of bundle motion uncoupled from ciliary beating and ASL-mucus movement. The work described here is for the pig and in relation to MUC5B secreted from gland cells, and not surface goblet cells. The secretion of MUC5B from surface cells could be studied in distal airways of pigs or airways of mice.
Conclusions
The MUC5B mucin covalent dimers are stored in the mucus-secreting cells at low pH and high Ca2+ as head-to-head tetramers arranged into linear assemblies. When the fluid created by CFTR in the serous cells most distal in the glands flows by the secreted MUC5B, the mucin is pulled out into linear threads that form thick bundles with more than 1,000 linear MUC5B polymers. Emerging from the glands are 27-μm-thick bundles that dip into the ASL-mucus and are moved by the cilia. These mucus bundles sweep the airway surface, thereby collecting bacteria and debris. These mucus bundles are thicker than the depth of the ASL-mucus, as measured in live pigs (1), and move slower than the ASL-mucus. Our observations suggest that the goblet cell MUC5AC mucin anchors the mucus bundles, and thus controls their transport.
The bundle movement being controlled by attachment/detachment of MUC5AC from the surface goblet cells, as we suggest, is similar to the mucus system of the small intestine. Here, the MUC2 mucin is secreted anchored to the goblet cell, and its detachment requires bicarbonate-mediated unfolding and meprin β protease cleavage (7). We suggest that a similar mechanism is active in the respiratory tract.
The different organization of the mucus systems in mice and humans can explain some of the controversy of how the mucociliary clearance functions. Another issue is the use of human bronchial epithelial cells cultured at air–liquid interface to study mucus transport. In this system, the secreted mucus forms a blanket transported in a circular pattern (15). The traditional model demonstrating how the mucociliary system is organized contains cilia and the ASL, which, in its turn, has two parts: the PCL and the mucus layer. The mucus layer is suggested to be composed of MUC5AC and MUC5B, conceived as entangled strands. A continuous mucus blanket, like the one in the intestine, should be difficult to transport, especially as the surface area shrinks from the periphery to trachea, in agreement with the discussion by van As (16). In contrast, we have shown that normal mucus emanating from the normal glands has a sophisticated organization, with linear MUC5B molecules interacting laterally to form mucus bundles capable of cleaning proximal airways. Surface goblet cell MUC5AC controls the movement of the bundles, creating a highly organized and tightly controlled system in higher animals and humans. Exactly how these bundles interact with the more homogenous ASL-mucus remains to be fully defined.
Supplementary Material
Footnotes
Supported by the Swedish Research Council, the Swedish Cancer Foundation, the Knut and Alice Wallenberg Foundation, IngaBritt and Arne Lundberg Foundation, Sahlgrenska University Hospital, Wilhelm and Martina Lundgren’s Foundation, National Institute of Allergy and Infectious Diseases grant U01AI095473, Erica Lederhausen’s Foundation, Magnus Bergvall’s Foundation, the Swedish Heart–Lung Foundation, the Swedish Cystic Fibrosis Foundation, Lederhausen’s Center for CF Research at University of Gothenburg, and the Cystic Fibrosis Foundation grant Hansso14X0.
Author Contributions: A.E. and G.C.H. wrote the manuscript; A.E., S.T.-M., and G.C.H. approved the manuscript.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Chu KK, Unglert C, Ford TN, Cui D, Carruth RW, Singh K, et al. In vivo imaging of airway cilia and mucus clearance with micro-optical coherence tomography. Biomed Opt Express. 2016;7:2494–2505. doi: 10.1364/BOE.7.002494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tilley AE, Walters MS, Shaykhiev R, Crystal RG. Cilia dysfunction in lung disease. Annu Rev Physiol. 2015;77:379–406. doi: 10.1146/annurev-physiol-021014-071931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widdicombe JH, Wine JJ. Airway gland structure and function. Physiol Rev. 2015;95:1241–1319. doi: 10.1152/physrev.00039.2014. [DOI] [PubMed] [Google Scholar]
- 4.Ridley C, Kouvatsos N, Raynal BD, Howard M, Collins RF, Desseyn J-L, et al. Assembly of the respiratory mucin MUC5B: a new model for a gel-forming mucin. J Biol Chem. 2014;289:16409–16420. doi: 10.1074/jbc.M114.566679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ambort D, Johansson MEV, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci USA. 2012;109:5645–5650. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gustafsson JK, Ermund A, Ambort D, Johansson MEV, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–1272. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schütte A, Ermund A, Becker-Pauly C, Johansson MEV, Rodriguez-Pineiro AM, Bäckhed F, et al. Microbial-induced meprin β cleavage in MUC2 mucin and a functional CFTR channel are required to release anchored small intestinal mucus. Proc Natl Acad Sci USA. 2014;111:12396–12401. doi: 10.1073/pnas.1407597111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Henderson AG, Ehre C, Button B, Abdullah LH, Cai L-H, Leigh MW, et al. Cystic fibrosis airway secretions exhibit mucin hyperconcentration and increased osmotic pressure. J Clin Invest. 2014;124:3047–3060. doi: 10.1172/JCI73469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trillo-Muyo S, Nilsson HE, Recktenwald CV, Ermund A, Ridley C, Meiss LN, et al. Granule-stored MUC5B mucins are packed by the non-covalent formation of N-terminal head-to-head tetramers. J Biol Chem. 2018;293:5746–5754. doi: 10.1074/jbc.RA117.001014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng Y, Chen J, López JA. Flow-driven assembly of VWF fibres and webs in in vitro microvessels. Nat Commun. 2015;6:7858. doi: 10.1038/ncomms8858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ermund A, Meiss LN, Rodriguez-Pineiro AM, Bähr A, Nilsson HE, Trillo-Muyo S, et al. The normal trachea is cleaned by MUC5B mucin bundles from the submucosal glands coated with the MUC5AC mucin. Biochem Biophys Res Commun. 2017;492:331–337. doi: 10.1016/j.bbrc.2017.08.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoegger MJ, Awadalla M, Namati E, Itani OA, Fischer AJ, Tucker AJ, et al. Assessing mucociliary transport of single particles in vivo shows variable speed and preference for the ventral trachea in newborn pigs. Proc Natl Acad Sci USA. 2014;111:2355–2360. doi: 10.1073/pnas.1323633111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ostedgaard LS, Moninger TO, McMenimen JD, Sawin NM, Parker CP, Thornell IM, et al. Gel-forming mucins form distinct morphologic structures in airways. Proc Natl Acad Sci USA. 2017;114:6842–6847. doi: 10.1073/pnas.1703228114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoegger MJ, Fischer AJ, McMenimen JD, Ostedgaard LS, Tucker AJ, Awadalla MA, et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science. 2014;345:818–822. doi: 10.1126/science.1255825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khelloufi M-K, Loiseau E, Jaeger M, Molinari N, Chanez P, Gras D, et al. Spatiotemporal organization of cilia drives multiscale mucus swirls in model human bronchial epithelium. Sci Rep. 2018;8:2447. doi: 10.1038/s41598-018-20882-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van As A. Pulmonary airway clearance mechanisms: a reappraisal. Am Rev Respir Dis. 1977;115:721–726. doi: 10.1164/arrd.1977.115.5.721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.