Key Points
Metformin use was associated with significantly fewer SCD-related health care utilization encounters and clinical events.
Our findings provide the first evidence to suggest potential clinical benefits associated with metformin use in patients with SCD.
Abstract
Metformin was recently found to increase fetal hemoglobin, which is protective in sickle cell disease (SCD). We tested the hypothesis that, among adults with SCD and diabetes mellitus (DM), metformin use is associated with fewer adverse SCD clinical outcomes and lower health care utilization. This is a retrospective cohort study using the MarketScan Medicaid claims database for 2006 to 2016, comparing metformin users and nonusers. Patients on hydroxyurea, insulin, or iron chelation were excluded. Main outcomes included annual rates of all-cause inpatient encounters, all-cause emergency department (ED) encounters, inpatient and ED encounters with SCD codes, vaso-occlusive episodes (VOEs), strokes, acute chest syndrome (ACS), avascular necrosis (AVN), and gallstones. Of 457 adults (median age [interquartile range], 43 years [33-52 years]; 72% female), 142 (31%) were treated with metformin. Adjusted for age, sex, and Charlson Comorbidity Index, metformin users had significantly lower rate ratios of all-cause inpatient encounters (0.68; 95% confidence interval [CI], 0.52-0.88; P < .01), inpatient encounters with SCD codes (0.45; 95% CI, 0.30-0.66; P < .01), ED encounters with SCD codes (0.34; 95% CI, 0.21-0.54; P < .01), VOE (0.22; 95% CI, 0.12-0.41; P < .01), ACS (0.17; 95% CI, 0.05-0.60; P = .01), and AVN (0.30; 95% CI, 0.11-0.87; P = .03). A subgroup analysis of 54 enrollees preinitiation and postinitiation of metformin did not indicate significant changes in rates of clinical events. Metformin was associated with significantly fewer inpatient and ED SCD encounters in adults with SCD and DM; however, confounding of underlying SCD severity cannot be excluded.
Visual Abstract
Introduction
Sickle cell disease (SCD) is an inherited hemoglobin disorder affecting ∼100 000 individuals in the United States, primarily those of African American descent.1 SCD was estimated to affect 312 000 neonates with homozygous sickle hemoglobin globally in 2010 alone, suggesting an important public health problem worldwide.2 SCD is a chronic debilitating health condition that extends from childhood to adulthood and has been associated with substantial morbidity and mortality.3-5 Patients with SCD suffer from intermittent sickling with vaso-occlusion, which leads to a number of complications, including acute and chronic pain, lung and heart disease, cerebrovascular disease, kidney injury, and avascular necrosis (AVN) of joints.3,4 Patients with SCD have increased health care utilization, with frequent hospitalizations and emergency department (ED) visits.6-9 Hydroxyurea is efficacious in preventing these complications and decreasing health care utilization in this population by increasing fetal hemoglobin (HbF) levels.10,11 However, hydroxyurea has a number of side effects, and its uptake and adherence is suboptimal.12-15
Although the prevalence of diabetes mellitus (DM) in the United States is relatively high, affecting nearly 30.3 million individuals,16 earlier studies suggested that DM is relatively rare in patients with SCD,17-23 which was not supported in a recent study by Zhou et al that reported a prevalence of 16.5% of DM in SCD patients.24 Therefore, the prevalence of DM in patients with SCD remains unclear. Metformin is a commonly used drug for the initial treatment of type 2 DM25,26 as well as for the management of overweight and obese nondiabetic patients.27,28 Metformin was recently suggested to have potential benefits for SCD through HbF induction with upregulation of FOXO3 and γ globin expression, anti-inflammatory properties, and increased nitric oxide production.29,30 However, these findings are based on laboratory experiments with no clinical data. Two recent retrospective studies reported in vivo evidence of an increase in HbF level in response to metformin use in patients with SCD (N = 18)31 and those without SCD (N = 7),32 although neither reported statistically significant findings. Both studies were limited to a small number of patients, and neither examined the relationship between metformin use and SCD-related outcomes or health care utilization.31,32 Therefore, the relation of metformin utilization to clinical outcomes and health care utilization in adults with SCD and DM, to date, has not been studied.
Our objectives were to identify prevalent cases of DM in a cohort of publicly insured adult patients with SCD and to assess the association of metformin with clinical outcomes and health care utilization in this population. We hypothesized that adults with SCD and DM who take metformin have less frequent SCD-related complications and lower utilization of hospital care for those complications than similar adults with SCD who do not take metformin.
Methods
Data source
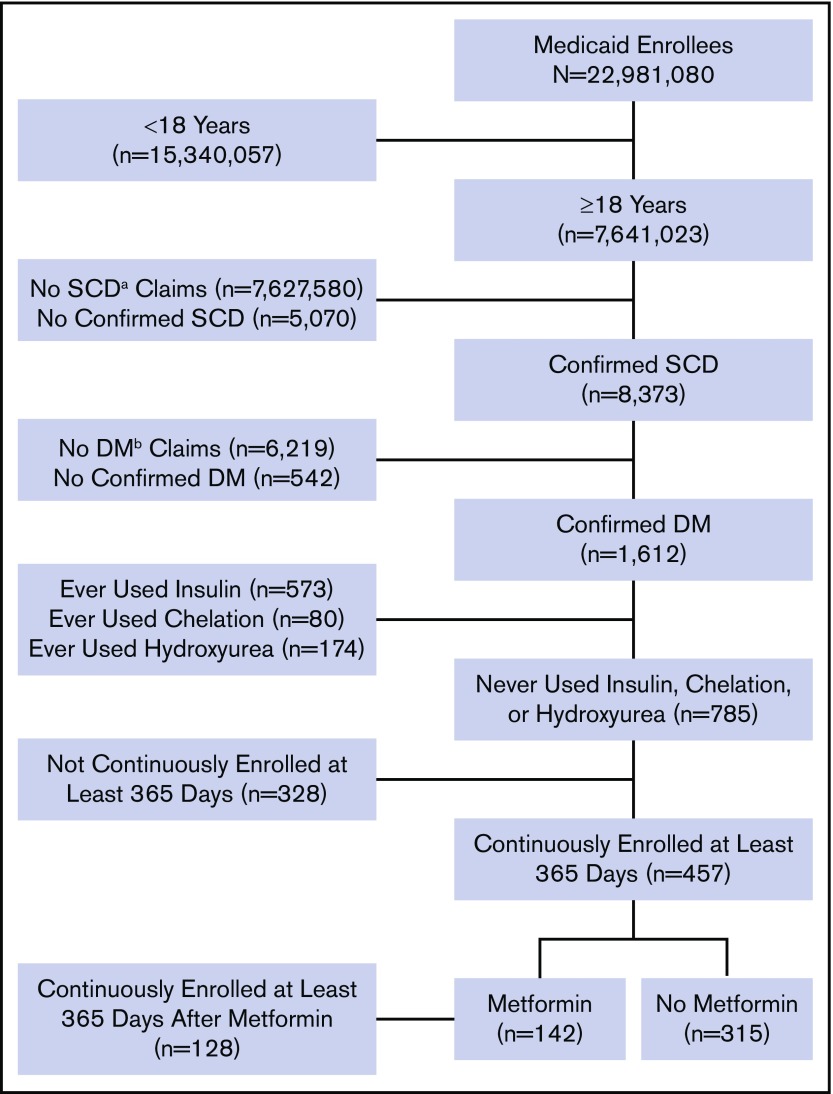
The data were extracted from the Truven Health Analytics MarketScan Medicaid Multi-State Database for the period from 1 January 2006 to 30 June 2016. The database pools claims from ∼23 million Medicaid enrollees from 8 to 12 states, varying by year. The database was accessed on 2 October 2017 via the Treatment Pathways 4.0 tool online analytic interface, and data were extracted to construct a cohort (Figure 1).
Figure 1.
Flow diagram of study participants and their eligibility for inclusion in the analysis. aSCD, sickle cell disease; bDM, diabetes mellitus.
Inclusion and exclusion criteria
Medicaid enrollees who were at least 18 years of age as of 1 January 2006 and were enrolled at any point in the study period were identified with SCD and DM based on medical claims coded using International Classification of Disease (ICD), ninth or tenth revision codes. Enrollees who met all of the following criteria were included in the analysis: (1) at least 2 outpatient SCD claims separated by at least 7 days during a 2-year period from the first encounter or at least 1 inpatient SCD claim; (2) at least 2 outpatient DM claims separated by at least 30 days during a 2-year period starting from the first encounter, 1 outpatient DM claim and 1 DM drug claim, or at least 1 inpatient diabetes claim; and (3) continuous enrollment at least 365 days after the first diabetes claim. These criteria of requiring 2 outpatient visits or 1 inpatient visit have been used to identify SCD and DM patients in other studies utilizing administrative data.33-36 A list of ICD-9 and ICD-10 codes used to identify SCD and diabetes claims are listed in supplemental Table 1.
We used additional exclusion criteria to eliminate potential sources of confounding. In particular, we excluded enrollees with drug claims for iron chelation drugs, insulin, or hydroxyurea. SCD patients receiving chronic blood transfusions to reduce the occurrence of SCD-related complications are at risk for iron overload–related endocrinopathies, including DM. We also excluded patients using insulin to avoid any potential interaction with metformin that could affect our interpretation of the study findings. Finally, hydroxyurea has known protective effects in patients with SCD, and we wanted to avoid any interaction or confounding effects related to its use along with metformin. Furthermore, a preliminary analysis indicated that inclusion of enrollees with drug claims for iron chelation drugs, insulin, or hydroxyurea altered the summary effect estimate by >10%, providing evidence of possible confounding.37
Metformin use
Among the enrollees identified with DM, metformin use was identified using drug claims data, and metformin users were contrasted with metformin nonusers. In addition, a secondary analysis was done of the association of metformin adherence with outcomes among metformin users. Adherence was assessed for those subjects enrolled at least 365 days after their first metformin claim based on the medication possession ratio (MPR).38 The MPR was calculated as the sum of days supplied with metformin, divided by the number of days of follow-up, less the number of days hospitalized.38-41 It was assumed that enrollees received a full supply of metformin and had 100% adherence during hospitalization. Adherence was categorized as high (MPR ≥ 80%), moderate (MPR < 80% to ≥ 40%), and/or low (MPR < 40%).
Outcome events
Outcome events following the first diabetes claim in the study period were identified using a method similar to Candrilli et al.42 ICD-10 codes were mapped to ICD-9 codes using the ICD-9 to ICD-10 Crosswalk Tool (http://www.icd10codesearch.com/). We identified inpatient encounters by searching for any claims in the inpatient setting. Inpatient encounters with SCD codes were identified by searching for any claims listing an SCD code (supplemental Table 1) in the inpatient setting. Similarly, ED encounters not resulting in an inpatient admission were identified by searching for any claims in the outpatient ED setting, and ED encounters with SCD codes were identified by searching for any claims listing an SCD code in the outpatient ED setting. We identified vaso-occlusive episodes (VOEs) by searching for the VOE-related claims (supplemental Table 2). Similarly, acute chest syndrome (ACS), AVN, and gallstone events were identified by searching for claims listing corresponding ICD-9 or ICD-10 codes for those outcomes (supplemental Table 2). We identified stroke events by searching for stroke-related claims within the inpatient setting (supplemental Table 2).
Statistical analysis
For the comparison of metformin users and nonusers, the numbers of events occurring after the first diabetes claim were calculated for each eligible enrollee. Rates of events were calculated as the number of events divided by the total number of enrollment days. The number of enrollees with at least 1 event was compared between groups using the χ2 test. The median rate of events was compared between groups using the nonparametric Mann-Whitney U test and adherence levels were compared using the Kruskal-Wallis test. Rate ratios (RRs) were calculated using negative binomial regression, with the natural logarithm of enrollment time as the offset.
We also conducted a subgroup analysis to examine the effect of adherence among metformin users. Data were limited to the 365 days after the first metformin claim in this subgroup analysis. The numbers of events occurring after the first metformin claim were calculated for each eligible enrollee. Rates of events were calculated as the number of events divided by 365, minus the number of inpatient days.
Adjusted RRs were produced using negative binomial regression, adjusting for age group (young adults [18 years ≤ age < 40 years] vs adults [age ≥ 40 years]),43,44 sex, and Charlson Comorbidity Index,45 which have been used in several prior SCD studies.42,46-49 The Charlson Comorbidity Index is a weighted index to predict risk of death within 1 year of hospitalization for patients with 19 specific comorbid conditions.45 The Charlson Comorbidity Index was calculated using claims data (see supplemental Table 3 for list of claims corresponding to each index item).45 Given the small sample size, we did not disaggregate results by sex. For the comparison of metformin users to nonusers, nonusers were considered the referent group. For the subgroup analysis of the associations of metformin adherence with events, nonadherent metformin users were considered the referent group. An additional subgroup analysis examined within-subject changes in rates of events before and after metformin initiation. Enrollees with 365 days of continuous enrollment before and after their first metformin claim were included. For each outcome, rates of events were calculated as the number of events in a year. Analyses were done using SAS 9.4 and GraphPad Prism 6. P < .05 was considered statistically significant.
Results
Study population
We identified 8373 patients with SCD and 1612 of them (19%) also had a diagnosis of DM. Our final cohort totaled 457 patients who met all of our predefined criteria (Figure 1). Of them, 142 were metformin users (31%) and 315 were nonusers (69%). Thirty-eight metformin users (27%) and 10 nonusers (3%) had claims for other oral glucose-lowering medications. The majority of patients were female (330; 72%). The median age [interquartile range] was 43 years [33-52 years] and the median Charlson Comorbidity Index score [interquartile range] was 1 [0-4] (Table 1). There were no statistically significant differences in patient characteristics according to metformin status or adherence level using the MPR percentage (Table 1). All clinical events in our cohort are summarized in Table 2, including all-cause inpatient encounters, inpatient encounters with SCD codes, all-cause ED encounters, ED encounters with SCD codes, VOE events, ACS episodes, and stroke, AVN, and gallstone events.
Table 1.
Characteristics of adults with SCD and DM enrolled in Medicaid for at least 365 days
| Characteristic | All patients, N = 457 | Metformin treatment | Adherence to metformin (MPR)* | |||
|---|---|---|---|---|---|---|
| No, n = 315 | Yes, n = 142 | MPR < 40%, n = 42 | MPR ≥ 40% to < 80%, n = 49 | MPR ≥ 80%, n = 37 | ||
| Females, n (%) | 330 (72) | 224 (71) | 106 (75) | 29 (69) | 36 (73) | 29 (78) |
| Age, mean (SD), y | 42.5 (11.4) | 42.1 (11.2) | 43.5 (11.9) | 41.2 (12.0) | 45.1 (12.5) | 45.3 (10.6) |
| Age, median (IQR), y | 43 (33-52) | 42 (33-50) | 44 (32-53) | 43 (28-51) | 46 (35-53) | 51 (38-53) |
| Age groups, n (%) | ||||||
| 18-39 y | 184 (40) | 130 (41) | 54 (38) | 18 (43) | 16 (33) | 11 (30) |
| ≥40 y | 273 (60) | 185 (59) | 88 (62) | 24 (57) | 33 (67) | 26 (70) |
| Comorbidity score, mean (SD)† | 2.6 (3.3) | 2.9 (3.5) | 1.9 (2.6) | 2.6 (2.8) | 1.3 (2.2) | 1.9 (2.7) |
| Comorbidity score, median (IQR)† | 1 (0-4) | 1 (0-5) | 1 (0-3) | 2 (0-4) | 0 (0-1) | 1 (0-2) |
IQR, interquartile range; SD, standard deviation.
MPR calculated by dividing the sum of days supply of metformin by the number of days enrolled, minus the days hospitalized.
Charlson Comorbidity Index score is calculated as a sum of 19 categories of comorbidity and predicts the 10-year mortality for a patient who may have a range of comorbid conditions.
Table 2.
Annual rates of clinical events in the study population, overall and stratified by metformin group
| Clinical events | All patients, N = 457 | Metformin treatment | ||
|---|---|---|---|---|
| No, n = 315 | Yes, n = 142 | P | ||
| Rate of all-cause inpatient encounters per year, median (IQR) | 0.9 (0.2-2.6) | 0.9 (0.3-3.2) | 0.6 (0.0-1.6) | <.01 |
| All-cause inpatient encounters ≥1, n (%) | 351 (77) | 247 (78) | 104 (73) | .23 |
| Rate of inpatient encounters with SCD codes per year, median (IQR) | 0.2 (0.0-0.7) | 0.2 (0.0-0.9) | 0.1 (0.0-0.5) | <.01 |
| Inpatient encounters with SCD codes ≥1, n (%) | 258 (56) | 185 (59) | 73 (51) | .14 |
| Rate of all-cause ED encounters per year, median (IQR) | 2.7 (1.0-6.2) | 2.9 (0.9-6.7) | 2.5 (1.0-5.3) | .24 |
| All-cause ED encounters ≥1, n (%) | 416 (91) | 282 (90) | 134 (94) | .09 |
| Rate of ED encounters with SCD codes per year, median (IQR) | 0.0 (0.0-0.8) | 0.0 (0.0-1.2) | 0.0 (0.0-0.5) | .01 |
| ED encounters with SCD codes ≥1, n (%) | 213 (47) | 155 (49) | 58 (41) | .10 |
| Rate of VOEs per year, median (IQR) | 0.0 (0.0-0.7) | 0.0 (0.0-0.9) | 0.0 (0.0-0.1) | <.01 |
| VOEs ≥1, n (%) | 168 (37) | 131 (42) | 37 (26) | <.01 |
| Rate of ACS episodes per year, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .01 |
| ACS episodes ≥1, n (%) | 30 (7) | 27 (9) | 3 (2) | <.01 |
| Rate of stroke events per year, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .29 |
| Stroke events ≥1, n (%) | 56 (12) | 42 (13) | 14 (10) | .29 |
| Rate of AVN events per year, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .15 |
| AVN events ≥1, n (%) | 53 (12) | 41 (13) | 12 (8) | .16 |
| Rate of gallstone events per year, median (IQR) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | 0.0 (0.0-0.0) | .73 |
| Gallstone events ≥1, n (%) | 69 (15) | 49 (16) | 20 (14) | .68 |
Clinical outcomes by metformin use
Overall, patients in the metformin group had clinically meaningful and statistically significantly less frequent clinical events compared with those in the nonmetformin group (Table 2). Patients in the metformin group had a significantly lower rate per year of all-cause inpatient encounters (P < .01), inpatient encounters with SCD codes (P < .01), ED encounters with SCD codes (P = .01), VOE events (P < .01), and ACS episodes (P = .01) (Table 2; supplemental Figure 1). Moreover, patients in the metformin group had significantly lower frequencies of 1 or more VOE (P < .01) and ACS episodes (P < .01) (Table 2).
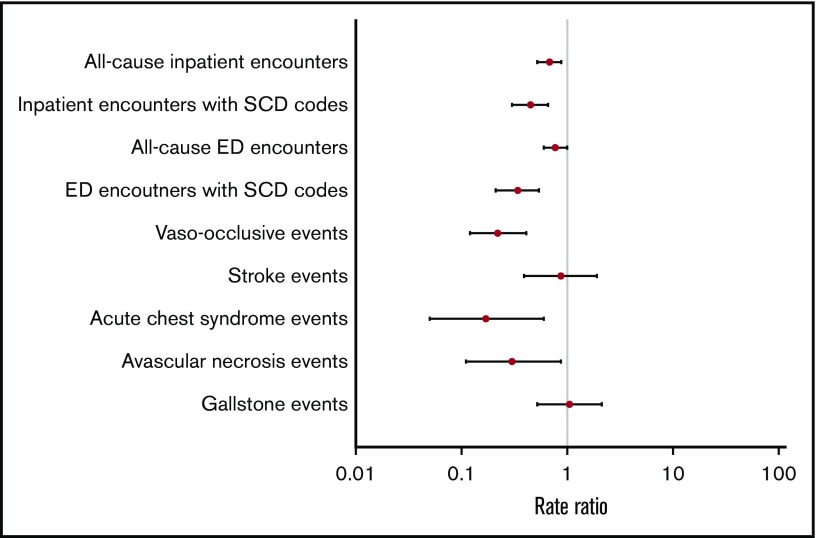
Overall, adjusted RRs of clinical events were lower in the metformin group compared with the nonmetformin group (Figure 2). Patients in the metformin group had significantly lower adjusted RRs of all-cause inpatient encounters (P < .01), inpatient encounters with SCD codes (P < .01), ED encounters with SCD codes (P < .01), VOE events (P < .01), ACS episodes (P = .01), and AVN events (P=.03) (Table 3). Similar trends of clinical events were also observed when we stratified patients by age groups as young adults (<40 years) vs adults (≥40 years) (Table 3).
Figure 2.
Adjusted RRs of clinical events in metformin users vs nonusers.
Table 3.
RRs of clinical events in metformin users vs nonusers, overall and stratified by age
| Clinical events | Overall, crude RR (95% CI)* | Overall, adjusted RR (95% CI)*† | Age groups, adjusted RR (95% CI)*‡ | |
|---|---|---|---|---|
| 18 to <40 y | ≥40 y | |||
| All-cause inpatient encounters | 0.53 (0.40-0.71) | 0.68 (0.52-0.88) | 0.61 (0.41-0.92) | 0.73 (0.52-1.02) |
| Inpatient encounters with SCD codes | 0.38 (0.26-0.57) | 0.45 (0.30-0.66) | 0.45 (0.25-0.82) | 0.45 (0.27-0.75) |
| All-cause ED encounters | 0.67 (0.51-0.87) | 0.77 (0.60-1.00) | 0.78 (0.52-1.17) | 0.76 (0.55-1.06) |
| ED encounters with SCD codes | 0.21 (0.13-0.35) | 0.34 (0.21-0.54) | 0.24 (0.12-0.50) | 0.42 (0.23-0.75) |
| VOEs | 0.17 (0.09-0.32) | 0.22 (0.12-0.41) | 0.22 (0.08-0.57) | 0.23 (0.10-0.50) |
| ACS episodes | 0.16 (0.05-0.56) | 0.17 (0.05-0.60) | 0.20 (0.04-1.02) | 0.12 (0.01-1.12) |
| Stroke events | 0.61 (0.28-1.33) | 0.87 (0.39-1.91) | 0.69 (0.19-2.55) | 0.99 (0.37-2.68) |
| AVN events | 0.38 (0.14-1.08) | 0.30 (0.11-0.87) | 0.53 (0.10-2.85) | 0.20 (0.05-0.79) |
| Gallstone events | 0.98 (0.48-2.02) | 1.05 (0.52-2.12) | 1.12 (0.36-3.45) | 1.00 (0.39-2.53) |
The reference group is the nonmetformin group.
RR and 95% CI adjusted for age group, sex, and Charlson Comorbidity Index score.
RR and 95% CI adjusted for sex and Charlson Comorbidity Index score.
Evaluating consistency of clinical outcomes with metformin use
To further evaluate the consistency of the association of metformin use with clinical outcomes and health care utilization, in a first subgroup analysis we assessed adherence levels in a subset of metformin users with at least 12 months of follow-up after their first metformin claim. We calculated adherence rates for most metformin users using the MPR (128 of 142; 90%). Adherence to metformin varied across different subgroups as follows (n, %): high (37, 29%), moderate (49, 38%), and low (42, 33%) adherence (Table 1). Overall, patients with higher adherence to metformin had less frequent clinical events, compared with those with lower adherence levels. In particular, patients with high and moderate adherence to metformin had significantly fewer inpatient encounters with SCD codes compared with those with low adherence (P < .01) (Table 4). Using negative binomial regression, adjusted for age, sex, and Charlson Comorbidity Index score, patients with high or moderate metformin adherence had less frequent all-cause inpatient encounters and inpatient encounters with SCD codes as well as all-cause ED encounters and ED encounters with SCD codes, compared with those with low adherence levels, although these differences were not statistically significant (Table 5; supplemental Figure 2).
Table 4.
Association between metformin adherence groups and the rate of clinical events in the study population
| Clinical events | Adherence to metformin (MPR) | |||
|---|---|---|---|---|
| MPR <40%, n = 42 | MPR ≥40% to <80%, n = 49 | MPR ≥80%, n = 37 | P | |
| Rate of all-cause inpatient encounters per year, median (IQR) | 1 (0-2) | 0 (0-1) | 0 (0-1) | .13 |
| All-cause inpatient encounters ≥1, n (%) | 22 (52) | 16 (33) | 16 (43) | .16 |
| Rate of inpatient encounters with SCD codes per year, median (IQR) | 0 (0-1) | 0 (0-0) | 0 (0-0) | <.01 |
| Inpatient encounters with SCD codes ≥1, n (%) | 15 (36) | 6 (12) | 4 (11) | <.01 |
| Rate of all-cause ED encounters per year, median (IQR) | 2 (1-7) | 1 (1-4) | 1 (1-4) | .17 |
| All-cause ED encounters ≥1, n (%) | 34 (81) | 37 (76) | 28 (76) | .79 |
| Rate of ED encounters with SCD codes per year, median (IQR) | 0 (0-1) | 0 (0-0) | 0 (0-0) | .19 |
| ED encounters with SCD codes ≥1, n (%) | 11 (26) | 10 (20) | 4 (11) | .22 |
Table 5.
Association between the rate of clinical events and the crude and adjusted RRs in the study population according to metformin adherence levels
| Clinical events | Crude RR (95% CI) | Adjusted RR*† (95% CI) | ||
|---|---|---|---|---|
| MPR ≥ 40% to < 80% | MPR ≥ 80% | MPR ≥ 40% to < 80% | MPR ≥ 80% | |
| All-cause inpatient encounters | 0.58 (0.27-1.22) | 0.33 (0.07-1.49) | 0.62 (0.29-1.31) | 0.38 (0.09-1.71) |
| Inpatient encounters with SCD codes | 0.64 (0.23-1.83) | 0.41 (0.05-3.34) | 0.53 (0.17-1.69) | 0.28 (0.03-2.84) |
| All-cause ED encounters | 0.94 (0.53-1.67) | 0.88 (0.28-2.79) | 0.68 (0.40-1.13) | 0.46 (0.16-1.28) |
| ED encounters with SCD codes | 0.58 (0.19-1.74) | 0.34 (0.04-3.04) | 0.74 (0.24-2.30) | 0.55 (0.06-5.31) |
The reference group is the patient group with MPR < 40%.
RR and 95% CI adjusted for age group, sex, and Charlson Comorbidity Index score.
A second subgroup analysis excluded 48 patients who took other oral glucose-lowering drugs; results were essentially unchanged (not reported). Another subgroup analysis conducted among 54 enrollees with 365 days of continuous enrollment before and after initiation of metformin did not indicate significant changes in rates of clinical events (Table 6; supplemental Figure 3).
Table 6.
Clinical events rates in metformin users 365 days before and after initiation of metformin
| Clinical events | Premetformin, median (IQR) | Postmetformin, median (IQR) | P |
|---|---|---|---|
| Rate of all-cause inpatient encounters per year, n = 31 | 0 (0-1) | 0 (0-0) | .68 |
| Rate of inpatient encounters with SCD codes per year, n = 14 | 0 (0-0) | 0 (0-0) | .61 |
| Rate of all-cause ED encounters per year, n = 50 | 2.5 (1-5) | 2 (1-5) | .73 |
| Rate of ED encounters with SCD codes per year, n = 16 | 0 (0-0) | 0 (0-0) | .71 |
Discussion
To our knowledge, this study is the first to examine clinical associations of metformin use with SCD-related complications in adults with SCD and DM covered by Medicaid. We found that metformin might have protective effects with less frequent hospital-treated clinical events. Among adults with SCD and DM, metformin users had significantly fewer all-cause inpatient encounters as well as inpatient and ED encounters with SCD codes. In particular, metformin users had significantly fewer VOEs, ACS episodes, and AVN events. A supplemental analysis among 90% of our original sample of metformin users suggested that patients with higher adherence to metformin had fewer clinical events than patients with low adherence.
DM has not been commonly studied in patients with SCD, with published data mainly from case reports or case series17-23 and single-center studies.50-54 In our study, one-fifth of our cohort with a diagnosis of SCD also had a probable diagnosis of DM (19%). Our findings were comparable to the reported prevalence of DM in a recent study by Zhou et al (16.5%)24 and among African Americans, using data from the National Health and Nutrition Examination Survey (NHANES) (18.2%).55 In contrast, earlier studies suggested a much lower prevalence of DM among adults with different SCD genotypes, ranging from 3.5% to 7%.50-52,56 The relatively higher prevalence of DM in our cohort as well as others24,55 could be multifactorial. First, as SCD patients have an increasing life expectancy over the past 3 decades as a result of advances in therapy (ie, hydroxyurea and stem cell transplant),57,58 they will be at higher risk of developing chronic medical conditions, especially metabolic ones, such as DM, dyslipidemia, and hypertension.24,55 Second, a number of common risk factors have been associated with these metabolic conditions, including poor diet, sedentary lifestyle, and central obesity, all of which have been a growing problem in our American society.16,55,59-61 Third, the higher prevalence of DM in our study could be related to being an exclusively Medicaid population. Finally, confirming the diagnosis of DM in patients with SCD can be challenging. Because of the shortened lifespan of red blood cells in patients with SCD, glycosylated hemoglobin (HbA1c), a commonly used laboratory marker for uncontrolled hyperglycemia and DM, might be at falsely low levels.62 In support, other reports suggested that HbA1c is an unreliable screening test for hyperglycemia and DM in SCD patients as well as those with sickle cell trait.63,64 These findings suggest that previous studies may have underestimated the burden of these metabolic conditions, particularly DM, in SCD patients.
Hydroxyurea, approved by the US Food and Drug Administration, is a medication for SCD that has been associated with improvements in clinical outcomes.9,65-69 Previous studies have shown that patients with SCD who were on hydroxyurea had significantly lower rates of hospitalizations by 27% to 58%, ED visits by 43%, and VOEs by 36% to 44%.66,67,70 In our study, we found that metformin use was likewise associated with significantly lower rates of SCD-related clinical events compared with no metformin use. Metformin use was associated with significantly lower median rates of inpatient encounters with SCD codes by 55%, ED encounters with SCD codes by 66%, VOEs by 78%, ACS episodes by 83%, and AVN events by 70%. We also found significantly lower median rates of all-cause inpatient encounters but not ED encounters, which could be secondary to DM-related care in the ED setting (eg, diabetic foot wound or infection). In our analysis, we did not find significant differences in the Charlson Comorbidity Index scores between metformin users and nonusers, suggesting comparable health status in both groups. Furthermore, when we evaluated the effects of metformin on clinic outcomes in both groups, we adjusted for Charlson Comorbidity Index scores, which controls for non-SCD–specific comorbidity. We also found that patients with higher adherence to metformin had less frequent inpatient and ED encounters with SCD codes, albeit not statistically significant, which was consistent with a similar relationship with higher hydroxyurea adherence.42,71-73 Contraindications for metformin use include renal disease and hepatic disease. Although there were no statistically significant differences in Charlson Comorbidity Index scores between metformin users and nonusers, there were statistically significant differences in the proportion of users and nonusers with evidence of renal and/or hepatic disease in their administrative claims data. Fewer metformin users had evidence of renal and/or hepatic disease, likely reflecting the exclusion of these patients from using metformin due to contraindication. Nevertheless, a subanalysis excluding those with these contraindications produced similar results (data not shown).
Our study used a large, multistate database of public insurance claims data, which have been used in several published studies on SCD.74-78 Additionally, our study contained data across several years (2006-2016) with information from at least 300 hospitals each year that varied in size, teaching status, and geographical locations.
This study has limitations. The MarketScan data we used are composed of Medicaid data collected from contributing states. States contributing data may change year to year. For this reason, long-term follow-up of individual patients is limited. Thus, we chose to conduct a cross-sectional study to assess the association between metformin use and outcomes in persons with SCD and DM. Cross-sectional studies have limitations, including the inability to establish a temporal relationship between the exposure and the outcome. Furthermore, we lacked data to adjust for differences between metformin users and nonusers in underlying SCD severity. There is currently no validated SCD severity score that can be applied to administrative claims data; however, the Charlson Comorbidity Index has been used by other investigators to control for underlying comorbidity burden in SCD-related studies using administrative claims data.42,46-49 Therefore, although the study controlled for differences in non-SCD–specific comorbidity, we cannot exclude confounding by indication as an explanation for the findings. If patients who have a high risk of complications of SCD are less likely to be prescribed metformin, one would observe inverse associations between metformin use and the frequency of those complications even if metformin had no effect. Potential reasons for metformin prescription, in addition to DM treatment, include its antitumor, antiaging, cardiovascular-protective, and neuroprotective effects, and as a treatment of polycystic ovary syndrome.79 It is not obvious that prescription of metformin for any of these indications would be more common in patients with more severe SCD. Additionally, the MarketScan Multi-State Medicaid database is based on a convenience sample of participating states, and the enrollees in this database all have public insurance. This may limit the generalizability of our findings to other US populations, such as those who are uninsured or have private insurance, live in other states, or live outside of the United States. Furthermore, limiting inclusion to those with SCD and DM claims and to nonusers of hydroxyurea, insulin or iron chelation further limits generalizability. Another limitation is that, because claims data are collected for billing purposes, misclassification of clinical and pharmacy information can occur. The validity of claims-based algorithms for identifying individuals with probable diagnoses of SCD or DM has been shown to be acceptable, although for SCD, such data have been restricted to children.80,81 If misclassification bias is nondifferential, the associations with metformin will be biased toward the null. In addition, this database does not provide detailed information on other potentially important confounders. The identification of patients with probable SCD and DM on the basis of claims using SCD-specific and DM-specific ICD-9 codes may have excluded adults with milder SCD and DM and overrepresented those with more severe disease. On the other hand, by excluding SCD patients on hydroxyurea to avoid any interaction or confounding effects, we may have excluded SCD patients with more severe disease, who are more likely to use hydroxyurea. Lack of laboratory values for the study population, including HbF levels, is another inherent limitation in MarketScan data. Finally, our subgroup analysis finding that rates of hospital encounters with SCD codes were equivalent before and after metformin use was initiated is suggestive of the possibility of confounding by indication and contrasts with our main analysis findings. This second subgroup analysis was necessarily restricted to a select subgroup of 38% of our original metformin group who had continuous coverage 365 days before and after their initial metformin prescription. Thus, it is difficult to compare the findings with our main analysis, both because of the imprecision of the findings and the possibility that other non-SCD factors influenced health and care-seeking over time in this subgroup. Indeed, small sample size and lack of statistical power precluded us from examining the most specific SCD health events (VOEs, ACS episodes, and AVN events) in this subgroup. Furthermore, this subanalysis is itself subject to limitations and is inconsistent with another subgroup finding that higher adherence to metformin is associated with lower rates of hospital encounters with SCD codes. Thus, the findings from the current analyses do not provide sufficient information to assess to what extent confounding by indication has influenced the main study results. Nonetheless, the subgroup analyses findings highlight the need for a more rigorously designed trial in which patients with SCD are randomized to receive metformin before drawing conclusions about the causal effect of metformin use on SCD events. Furthermore, given the nature of our study, we cannot determine whether the observed benefits of metformin are a result of its effects on SCD pathophysiology, DM pathophysiology, or both.
In conclusion, the findings from this study are in agreement with the hypothesis that metformin might have protective clinical effects in adults with SCD. Moreover, the findings are in line with a previous laboratory biology study documenting positive effects of metformin on HbF induction. Nonetheless, given the inherent limitations of claims data and the conflicting results from 1 subgroup analysis, we cannot rule out the possibility of confounding by indication as an explanation for the findings presented here. Thus, these findings are best considered hypothesis-generating. Given the known safety profile of metformin and the emerging evidence of the possibility that metformin might have beneficial effects in patients with SCD, future prospective studies are warranted to evaluate its efficacy and cost-effectiveness in relation to clinical outcomes, quality of life, mortality, and health care utilization in adults with SCD.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank Scott D. Grosse, at the National Center on Birth Defects and Developmental Disabilities, Centers for Disease Control and Prevention, for his support and guidance during data analysis.
This work was supported by grant number K12HS023011 (S.M.B.) from the Agency for Healthcare Research and Quality.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Data-sharing requests may be e-mailed to the corresponding author.
Authorship
Contribution: S.M.B. and A.B.P. designed the research study and interpreted the data; A.B.P. analyzed the data and critically revised the paper; S.M.B. drafted the paper; and both authors approved the submitted final version of the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sherif M. Badawy, Ann & Robert H. Lurie Children’s Hospital of Chicago, 225 E Chicago Ave, Box #30, Chicago, IL 60611; e-mail: sbadawy@luriechildrens.org.
References
- 1.Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. 2010;38(suppl 4):S512-S521. [DOI] [PubMed] [Google Scholar]
- 2.Piel FB, Patil AP, Howes RE, et al. . Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. 2017;376(16):1561-1573. [DOI] [PubMed] [Google Scholar]
- 4.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376(9757):2018-2031. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Data & statistics on sickle cell disease. Available at: www.cdc.gov/ncbddd/sicklecell/data.html. Accessed 10 March 2019.
- 6.Raphael JL, Mei M, Mueller BU, Giordano T. High resource hospitalizations among children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2012;58(4):584-590. [DOI] [PubMed] [Google Scholar]
- 7.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303(13):1288-1294. [DOI] [PubMed] [Google Scholar]
- 8.Panepinto JA, Brousseau DC, Hillery CA, Scott JP. Variation in hospitalizations and hospital length of stay in children with vaso-occlusive crises in sickle cell disease. Pediatr Blood Cancer. 2005;44(2):182-186. [DOI] [PubMed] [Google Scholar]
- 9.Badawy SM, Thompson AA, Holl JL, Penedo FJ, Liem RI. Healthcare utilization and hydroxyurea adherence in youth with sickle cell disease. Pediatr Hematol Oncol. 2018;35(5-6):297-308. [DOI] [PubMed] [Google Scholar]
- 10.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. . Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033-1048. [DOI] [PubMed] [Google Scholar]
- 11.Nevitt SJ, Jones AP, Howard J. Hydroxyurea (hydroxycarbamide) for sickle cell disease. Cochrane Database Syst Rev. 2017;4:CD002202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loiselle K, Lee JL, Szulczewski L, Drake S, Crosby LE, Pai AL. Systematic and meta-analytic review: medication adherence among pediatric patients with sickle cell disease. J Pediatr Psychol. 2016;41(4):406-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walsh KE, Cutrona SL, Kavanagh PL, et al. . Medication adherence among pediatric patients with sickle cell disease: a systematic review. Pediatrics. 2014;134(6):1175-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Badawy SM, Thompson AA, Penedo FJ, Lai JS, Rychlik K, Liem RI. Barriers to hydroxyurea adherence and health-related quality of life in adolescents and young adults with sickle cell disease. Eur J Haematol. 2017;98(6):608-614. [DOI] [PubMed] [Google Scholar]
- 15.Badawy SM, Thompson AA, Liem RI. Beliefs about hydroxyurea in youth with sickle cell disease. Hematol Oncol Stem Cell Ther. 2018;11(3):142-148. [DOI] [PubMed] [Google Scholar]
- 16.NIH National Institute of Diabetes and Digestive and Kidney Diseases Diabetes statistics. Available at: www.niddk.nih.gov/health-information/health-statistics/diabetes-statistics. Accessed 10 March 2019.
- 17.Reid HL, Ene MD, Photiades DP, Famodu AA. Insulin-dependent diabetes mellitus in homozygous sickle-cell anaemia. Trop Geogr Med. 1990;42(2):172-173. [PubMed] [Google Scholar]
- 18.Jarrett OO, Olorundare EI. Type 1 diabetes mellitus in a known sickle cell anaemia patient: a rare combination in Nigeria. Afr J Med Med Sci. 2014;43(2):177-181. [PubMed] [Google Scholar]
- 19.Mohapatra MK. Type 1 diabetes mellitus in homozygous sickle cell anaemia. J Assoc Physicians India. 2005;53:895-896. [PubMed] [Google Scholar]
- 20.Shoar Z, Rezvani G, De Luca F. Type 1 diabetes mellitus in a patient with homozygous sickle cell anemia. J Pediatr Endocrinol Metab. 2013;26(11-12):1205-1207. [DOI] [PubMed] [Google Scholar]
- 21.Ngombe LK, Mulangu AM, Kasole TL, Numbi OL. Sickle cell disease and diabetes: a rare combination in a black teenager in Lubumbashi, Democratic Republic of Congo [in French]. Pan Afr Med J. 2014;18:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abraham EC, Rao KR. Glycosylated hemoglobins in a diabetic patient with sickle cell anemia. Clin Physiol Biochem. 1987;5(6):343-349. [PubMed] [Google Scholar]
- 23.Miodovnik M, Hurd WW, Lobel JS, Siddiqi TA. Pregnancy associated with both insulin-dependent diabetes mellitus and sickle cell disease. A report of two cases. J Reprod Med. 1987;32(4):317-319. [PubMed] [Google Scholar]
- 24.Zhou J, Han J, Nutescu EA, et al. . Similar burden of type 2 diabetes among adult patients with sickle cell disease relative to African Americans in the U.S. population: a six-year population-based cohort analysis. Br J Haematol. 2019;185(1):116-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inzucchi SE, Bergenstal RM, Buse JB, et al. . Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38(1):140-149. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Rangel E, Inzucchi SE. Metformin: clinical use in type 2 diabetes. Diabetologia. 2017;60(9):1586-1593. [DOI] [PubMed] [Google Scholar]
- 27.Marques P, Limbert C, Oliveira L, Santos MI, Lopes L. Metformin effectiveness and safety in the management of overweight/obese nondiabetic children and adolescents: metabolic benefits of the continuous exposure to metformin at 12 and 24 months. Int J Adolesc Med Health. 2016;29(5): [DOI] [PubMed] [Google Scholar]
- 28.Seifarth C, Schehler B, Schneider HJ. Effectiveness of metformin on weight loss in non-diabetic individuals with obesity. Exp Clin Endocrinol Diabetes. 2013;121(1):27-31. [DOI] [PubMed] [Google Scholar]
- 29.Umek N, Ugwoke CK. Potential benefits of metformin use in sickle cell anemia. J Hematol (Brossard). 2016;5(2):41-48. [Google Scholar]
- 30.Zhang Y, Paikari A, Sumazin P, et al. . Metformin induces FOXO3-dependent fetal hemoglobin production in human primary erythroid cells. Blood. 2018;132(3):321-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han J, Saraf SL, Molokie RE, Gordeuk VR. Use of metformin in patients with sickle cell disease. Am J Hematol. 2019;94(1):E13-E15. [DOI] [PubMed] [Google Scholar]
- 32.Boulassel MR, El-Hussain AI, Hassan MM, et al. . Stability of fetal hemoglobin levels in patients receiving metformin therapy. Haematologica. 2018;103(10):e440-e442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebert PL, Geiss LS, Tierney EF, Engelgau MM, Yawn BP, McBean AM. Identifying persons with diabetes using Medicare claims data. Am J Med Qual. 1999;14(6):270-277. [DOI] [PubMed] [Google Scholar]
- 34.Shankar SM, Arbogast PG, Mitchel E, Cooper WO, Wang WC, Griffin MR. Medical care utilization and mortality in sickle cell disease: a population-based study. Am J Hematol. 2005;80(4):262-270. [DOI] [PubMed] [Google Scholar]
- 35.Shrestha SS, Zhang P, Thompson TJ, Gregg EW, Albright A, Imperatore G. Medical expenditures associated with diabetes among youth with Medicaid coverage. Med Care. 2017;55(7):646-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sox CM, Cooper WO, Koepsell TD, DiGiuseppe DL, Christakis DA. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA. 2003;290(8):1057-1061. [DOI] [PubMed] [Google Scholar]
- 37.Grayson DA. Confounding confounding. Am J Epidemiol. 1987;126(3):546-553. [DOI] [PubMed] [Google Scholar]
- 38.Vekeman F, Sasane M, Cheng WY, et al. . Adherence to iron chelation therapy and associated healthcare resource utilization and costs in Medicaid patients with sickle cell disease and thalassemia. J Med Econ. 2016;19(3):292-303. [DOI] [PubMed] [Google Scholar]
- 39.Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7-8):1280-1288. [DOI] [PubMed] [Google Scholar]
- 40.Sclar DA, Chin A, Skaer TL, Okamoto MP, Nakahiro RK, Gill MA. Effect of health education in promoting prescription refill compliance among patients with hypertension. Clin Ther. 1991;13(4):489-495. [PubMed] [Google Scholar]
- 41.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11(7):449-457. [PubMed] [Google Scholar]
- 42.Candrilli SD, O’Brien SH, Ware RE, Nahata MC, Seiber EE, Balkrishnan R. Hydroxyurea adherence and associated outcomes among Medicaid enrollees with sickle cell disease. Am J Hematol. 2011;86(3):273-277. [DOI] [PubMed] [Google Scholar]
- 43.Geiger AM, Castellino SM. Delineating the age ranges used to define adolescents and young adults. J Clin Oncol. 2011;29(16):e492-e493. [DOI] [PubMed] [Google Scholar]
- 44.Thomas DM, Albritton KH, Ferrari A. Adolescent and young adult oncology: an emerging field. J Clin Oncol. 2010;28(32):4781-4782. [DOI] [PubMed] [Google Scholar]
- 45.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. [DOI] [PubMed] [Google Scholar]
- 46.Aljuburi G, Laverty AA, Green SA, Phekoo KJ, Bell D, Majeed A. Socio-economic deprivation and risk of emergency readmission and inpatient mortality in people with sickle cell disease in England: observational study. J Public Health (Oxf). 2013;35(4):510-517. [DOI] [PubMed] [Google Scholar]
- 47.Linton E, Langer AL, Glassberg J. Hospital admissions, mortality and comorbidities among New York state sickle cell patients, 2005-2013. J Natl Med Assoc. 2018;110(2):149-156. [DOI] [PubMed] [Google Scholar]
- 48.Roghmann F, Becker A, Sammon JD, et al. . Incidence of priapism in emergency departments in the United States. J Urol. 2013;190(4):1275-1280. [DOI] [PubMed] [Google Scholar]
- 49.Swede H, Sarwar A, Magge A, et al. . Mortality risk from comorbidities independent of triple-negative breast cancer status: NCI-SEER-based cohort analysis. Cancer Causes Control. 2016;27(5):627-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koduri PR, Patel AR, Bernstein HA. Concurrent sickle cell hemoglobin C disease and diabetes mellitus: no added risk of proliferative retinopathy? J Natl Med Assoc. 1994;86(9):682-685. [PMC free article] [PubMed] [Google Scholar]
- 51.Sandhu MK, Cohen A. Aging in sickle cell disease: co-morbidities and new issues in management. Hemoglobin. 2015;39(4):221-224. [DOI] [PubMed] [Google Scholar]
- 52.Mohamed AA, Al-Qurashi F, Whitford DL. Does sickle cell disease protect against diabetes mellitus? Cross-sectional study. Sultan Qaboos Univ Med J. 2015;15(1):e116-e119. [PMC free article] [PubMed] [Google Scholar]
- 53.Morrison JC, Schneider JM, Kraus AP, Kitabchi AE. The prevalence of diabetes mellitus in sickle cell hemoglobinopathies. J Clin Endocrinol Metab. 1979;48(2):192-195. [DOI] [PubMed] [Google Scholar]
- 54.Reid HL, Photiades DP, Oli JM, Kaine W. Concurrent sickle cell disease and diabetes mellitus. Trop Geogr Med. 1988;40(3):201-204. [PubMed] [Google Scholar]
- 55.Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029. [DOI] [PubMed] [Google Scholar]
- 56.Zhang X, Zhang W, Saraf SL, et al. . Genetic polymorphism of APOB is associated with diabetes mellitus in sickle cell disease. Hum Genet. 2015;134(8):895-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lanzkron S, Carroll CP, Haywood C Jr. Mortality rates and age at death from sickle cell disease: U.S., 1979-2005. Public Health Rep. 2013;128(2):110-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanni E, Grosse SD, Yang Q, Olney RS. Trends in pediatric sickle cell disease-related mortality in the United States, 1983-2002. J Pediatr. 2009;154(4):541-545. [DOI] [PubMed] [Google Scholar]
- 59.Centers for Disease Control and Prevention (CDC). National diabetes statistics report, 2017: estimates of diabetes and its burden in the United States. Atlanta, GA: Centers for Disease Control and Prevention; 2017. [Google Scholar]
- 60.Geiss LS, Wang J, Cheng YJ, et al. . Prevalence and incidence trends for diagnosed diabetes among adults aged 20 to 79 years, United States, 1980-2012. JAMA. 2014;312(12):1218-1226. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention (CDC) CDC grand rounds: newborn screening and improved outcomes. MMWR Morb Mortal Wkly Rep. 2012;61(21):390-393. [PubMed] [Google Scholar]
- 62.Smiley D, Dagogo-Jack S, Umpierrez G. Therapy insight: metabolic and endocrine disorders in sickle cell disease. Nat Clin Pract Endocrinol Metab. 2008;4(2):102-109. [DOI] [PubMed] [Google Scholar]
- 63.Reid HL, Famodu AA, Photiades DP, Osamo ON. Glycosylated haemoglobin HbA1c and HbS1c in non-diabetic Nigerians. Trop Geogr Med. 1992;44(1-2):126-130. [PubMed] [Google Scholar]
- 64.Schnedl WJ, Trinker M, Lipp RW. HbA1c determination in patients with hemoglobinopathies. Diabetes Care. 1999;22(2):368-369. [DOI] [PubMed] [Google Scholar]
- 65.Wang WC, Ware RE, Miller ST, et al. ; BABY HUG Investigators . Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG). Lancet. 2011;377(9778):1663-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thornburg CD, Files BA, Luo Z, et al. ; BABY HUG Investigators. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22):4304-4310, quiz 4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Charache S, Terrin ML, Moore RD, et al. ; Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia . Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med. 1995;332(20):1317-1322. [DOI] [PubMed] [Google Scholar]
- 68.Steinberg MH, Barton F, Castro O, et al. . Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA. 2003;289(13):1645-1651. [DOI] [PubMed] [Google Scholar]
- 69.Badawy SM, Thompson AA, Lai JS, Penedo FJ, Rychlik K, Liem RI. Health-related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatr Blood Cancer. 2017;64(6): [DOI] [PubMed] [Google Scholar]
- 70.Quarmyne MO, Dong W, Theodore R, et al. . Hydroxyurea effectiveness in children and adolescents with sickle cell anemia: a large retrospective, population-based cohort. Am J Hematol. 2017;92(1):77-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phillips K, Healy L, Smith L, Keenan R. Hydroxyurea therapy in UK children with sickle cell anaemia: a single-centre experience. Pediatr Blood Cancer. 2018;65(2):e26833. [DOI] [PubMed] [Google Scholar]
- 72.Estepp JH, Smeltzer MP, Kang G, et al. . A clinically meaningful fetal hemoglobin threshold for children with sickle cell anemia during hydroxyurea therapy. Am J Hematol. 2017;92(12):1333-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Anders DG, Tang F, Ledneva T, et al. . Hydroxyurea use in young children with sickle cell anemia in New York state. Am J Prev Med. 2016;51(suppl 1):S31-S38. [DOI] [PubMed] [Google Scholar]
- 74.Alonso A, MacLehose RF, Lutsey PL, Konety S, Chen LY. Association of amiodarone use with acute pancreatitis in patients with atrial fibrillation: a nested case-control study. JAMA Intern Med. 2015;175(3):449-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Baggs J, Fridkin SK, Pollack LA, Srinivasan A, Jernigan JA. Estimating national trends in inpatient antibiotic use among US hospitals from 2006 to 2012. JAMA Intern Med. 2016;176(11):1639-1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kirby JS, Miller JJ, Adams DR, Leslie D. Health care utilization patterns and costs for patients with hidradenitis suppurativa. JAMA Dermatol. 2014;150(9):937-944. [DOI] [PubMed] [Google Scholar]
- 77.Neprash HT, Chernew ME, Hicks AL, Gibson T, McWilliams JM. Association of financial integration between physicians and hospitals with commercial health care prices. JAMA Intern Med. 2015;175(12):1932-1939. [DOI] [PubMed] [Google Scholar]
- 78.Neugut AI, Zhong X, Wright JD, Accordino M, Yang J, Hershman DL. Nonadherence to medications for chronic conditions and nonadherence to adjuvant hormonal therapy in women with breast cancer. JAMA Oncol. 2016;2(10):1326-1332. [DOI] [PubMed] [Google Scholar]
- 79.Wang YW, He SJ, Feng X, et al. . Metformin: a review of its potential indications. Drug Des Devel Ther. 2017;11:2421-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Reeves S, Garcia E, Kleyn M, et al. . Identifying sickle cell disease cases using administrative claims. Acad Pediatr. 2014;14(suppl 5):S61-S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rhodes ET, Laffel LM, Gonzalez TV, Ludwig DS. Accuracy of administrative coding for type 2 diabetes in children, adolescents, and young adults. Diabetes Care. 2007;30(1):141-143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.