Key Points
This is the first report of successful treatment of therapy-resistant leptomeningeal T-PLL with intrathecal alemtuzumab.
Intrathecal alemtuzumab is a potentially safe and efficacious therapeutic alternative for treatment of leptomeningeal T-PLL.
Introduction
T-cell prolymphocytic leukemia (T-PLL) is a rare hematologic malignancy, accounting for 2% of all lymphocytic leukemias.1 Morphologically, the majority of cases of T-PLL are characterized by medium-sized lymphocytes with a single nucleolus together with a basophilic cytoplasm and cytoplasmic projections.2 Flow cytometry demonstrates a monotypic population of either CD4+ or CD4+/CD8+ postthymic T cells expressing CD2, CD3, and CD7 without expression of terminal deoxynucleotidyltransferase or CD1a.3 The most commonly affected sites of disease include blood, skin, bone marrow, lymph nodes, liver, and spleen, with occasional involvement of the central nervous system (CNS).4-6 To date, only 3 publications reference leptomeningeal or CNS involvement with T-PLL, and there has been no published experience on the successful treatment of leptomeningeal T-PLL.4-6 While the CD52-directed antibody alemtuzumab has become the standard of care for systemic disease, there are few data regarding the use of this agent for leptomeningeal disease, and CNS penetration of systemically delivered alemtuzumab is likely minimal.6 Here, we present a case of T-PLL with refractory leptomeningeal involvement that was successfully treated with intrathecal (IT) alemtuzumab.
Case description
A 56-year-old woman presented with an absolute lymphocytosis and mild splenomegaly over a 2-year period. She presented to our medical center in 2014 with a white blood cell count of 11.3 × 109/L (absolute lymphocyte count 7.8 × 109/L), hemoglobin of 13 g/dL, and platelet count of 136 × 109/L. Flow cytometry of the peripheral blood revealed a CD4+/CD8+ monotypic T-cell population suspicious for a T-cell lymphoproliferative disorder. A bone marrow aspiration and biopsy demonstrated an overall cellularity of 40% with notable lymphoid aggregates. Flow cytometry revealed a monotypic population of CD2+, CD3+, CD4+, CD7+, CD8+, and CD52+ T cells. Cytogenetics demonstrated an abnormal female karyotype: 44,XX,add(2)(q37),i(8)(q10),−11,-13,inv(14)(q11q32.1),add(17)(p11.2), −21,+mar1[11]/44,idem,del(12)(p11.2),+add(13)(q32), −add(17),add(18)(p11.2), −mar1,+mar2[2]/46,XX[7]. A computed tomography scan of the abdomen/pelvis revealed moderate splenomegaly (17 cm craniocaudal). Based on these features, she was diagnosed with T-PLL. Given her lack of significant clinical symptoms, she elected to pursue a watchful waiting approach.
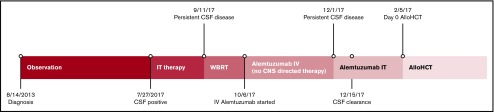
Three years after diagnosis (August 2017), she developed headaches with muffled hearing. Initial magnetic resonance imaging (MRI) of the head and neck demonstrated nonspecific lymphadenopathy of the neck, but a repeat MRI showed diffuse bilateral signal abnormalities. Following an emergency department visit for worsening headaches, a lumbar puncture revealed involvement of her cerebral spinal fluid (CSF) with T-PLL (Table 1; Figures 1 and 2). Together, the MRI findings and CSF findings were indicative of leptomeningeal T-PLL. She then received 1 dose of IT cytarabine, 2 doses of combined IT cytarabine and hydrocortisone, and 4 doses of combined IT methotrexate, cytarabine, and hydrocortisone, all given twice weekly, without resolution of symptoms or disease (Table 1; Figure 1). Given persistent disease, she received whole-brain radiation therapy throughout September 2017 (23.4 Gy in 1.8 fractions). Following radiation, IV alemtuzumab treatment was initiated three times weekly per Dearden et al.7 A bone marrow aspiration and biopsy performed 2 months into systemic therapy (December 2017) revealed no morphologic or immunophenotypic evidence of disease; however, her lumbar puncture revealed persistent T-PLL with progressive headaches and nausea (Figure 1). Given the paucity of literature regarding treatment of refractory leptomeningeal T-PLL, we initiated a consultation with our Office of Regulatory Affairs as well as the Campath distribution program regarding the IT administration of alemtuzumab under the Innovative Care Policy guidelines created at the University of Michigan.
Table 1.
Results of IT and radiation therapy
| Date | WBC count, ×109/L | RBC count, ×109/L | Flow cytometry | Treatment |
|---|---|---|---|---|
| 11 August 2017 | 678 | 153 | (+) | Ara-C 100 mg |
| 16 August 2017 | 303 | 96 | (+) | Ara-C 100 mg , hydrocortisone 50 mg |
| 18 August 2017 | 472 | 87 | (+) | Ara-C 100 mg , hydrocortisone 50 mg1-4,12,15-17 |
| 22 August 2017 | 565 | 67 | (+) | Methotrexate 15 mg, ara-C 40 mg, hydrocortisone 50 mg |
| 25 August 2017 | 97 | 27 | (+) | Methotrexate 15 mg, ara-C 40 mg, hydrocortisone 50 mg |
| 28 August 2017 | 122 | 46 | (+) | Methotrexate 15 mg, ara-C 40 mg, hydrocortisone 50 mg |
| 31 August 2017 | 149 | 39 | (+) | Methotrexate 15 mg, ara-C 40 mg, hydrocortisone 50 mg → whole brain radiation 11-27 September 2017 |
| 1 December 2017 | 3 | 3 | (+) | Methotrexate 15 mg, ara-C 40 mg, hydrocortisone 50 mg |
| 11 December 2017 | 1 | 6 | (+) | Alemtuzumab 1 mg and hydrocortisone 50 mg |
| 15 December 2017 | 2 | 1550 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 18 December 2017 | 0 | 61 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 22 December 2017 | 0 | 87 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 26 December 2017 | 0 | 816 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 29 December 2017 | 2 | 3 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 5 January 2018 | 0 | 665 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 12 January 2018 | 0 | 7 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 19 January 2018 | 0 | 1 | (−) | Alemtuzumab 3 mg and hydrocortisone 50 mg |
| 11 July 2018 | 0 | 1 | (−) | Evaluation only |
RBC, red blood cell; WBC, white blood cell.
Figure 1.
Timeline of disease management. AlloHCT, allogeneic hematopoietic stem cell transplant; WBRT, whole-brain radiation therapy.
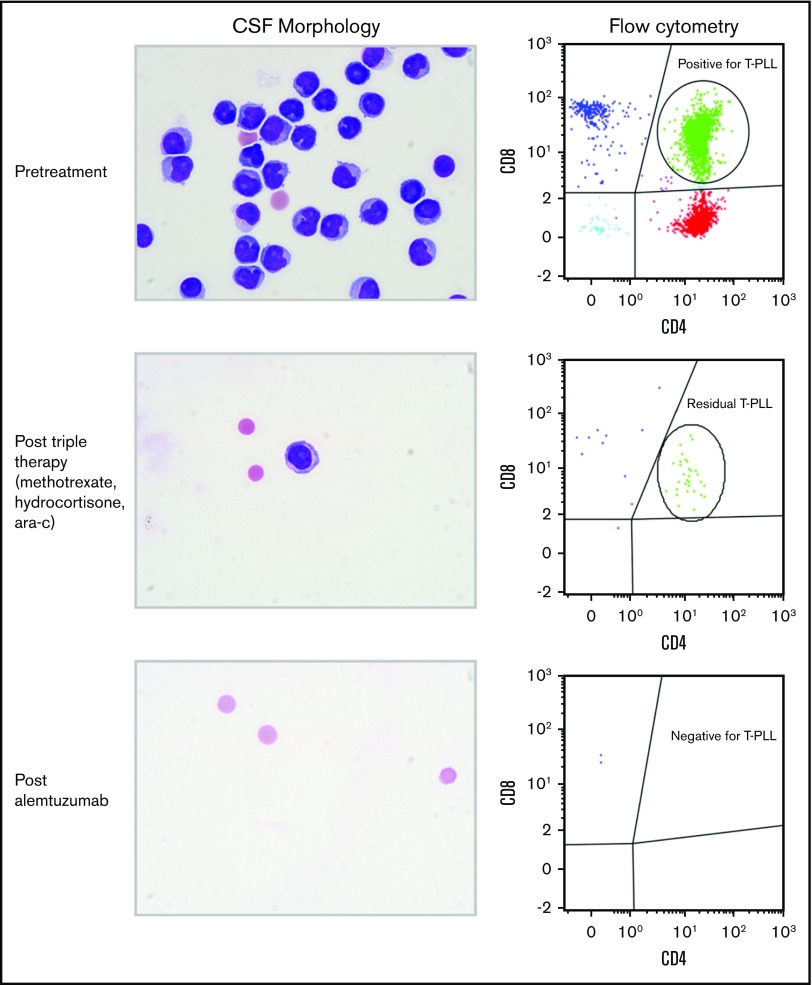
Figure 2.
CSF morphologic and flow cytometry findings during the treatment course. Pretreatment CSF showed definitive morphologic evidence of abnormal lymphocytes, with flow cytometry showing a prominent CD4 and CD8 double-positive population (green), consistent with involvement by T-PLL. Following triple therapy, the burden of disease was reduced but with persistent morphologic and flow cytometric evidence of disease. Following alemtuzumab therapy, the CSF was entirely cleared, with no morphologic or flow cytometric evidence of T-PLL (Wright Giemsa stain; original magnification ×1000).
With approval of all groups, we began the IT administration of 1 mg alemtuzumab with resolution of disease and symptoms after the first 2 injections (Table 1; Figure 2). We continued twice-weekly IT therapy with 3 mg alemtuzumab for 6 total treatments and then transitioned to weekly IT therapy together with 30 mg IV alemtuzumab 3 times weekly. She subsequently underwent a matched-unrelated donor allogeneic hematopoietic stem cell transplant (Figure 1). All subsequent CSF analyses remain negative for T-PLL, and she remains without CNS symptoms to date, 18 months following initiation of IT alemtuzumab.
Methods
CSF samples were stained using Wright-Giemsa stain. Flow cytometric immunophenotyping was performed using CSF samples prepared on the Prep Plus II instrument and subsequently run on a Gallios 10-color calibrated flow cytometer (Beckman Coulter, Miami, FL). Analysis was performed using Kaluza version 2.1 (Beckman Coulter).
Results and discussion
Since its initial clinical description in 1988, alemtuzumab has been an off-label therapeutic option for patients with lymphoproliferative neoplasms.6,8 Phase 2 clinical trials in T-PLL have demonstrated systemic responses in both treatment-naive and previously treated patients, although the agent was never approved by the US Food and Drug administration for T-PLL and must now be obtained through the Campath distribution program.9-11
Given that leptomeningeal disease appears to occur in <10% of all cases of T-PLL, there is no standard approach to this complication. Current literature suggests IT treatment with either triple therapy (methotrexate, cytarabine, and hydrocortisone) or high-dose systemic methotrexate if there is concurrent systemic disease.1 Systemic administration of purine analogs thought to achieve CNS penetration, including nelarabine and fludarabine, could be considered, but neither has demonstrated successful treatment of CNS T-PLL.12 To our knowledge, there is no published report on the successful treatment of leptomeningeal T-PLL. In this case, the IT administration of single-agent cytotoxic therapy and triple therapy failed to resolve the disease or symptoms. Moreover, salvage whole-brain radiation therapy temporized the patient’s symptoms but did not clear her disease.
CD52-directed antibodies were originally developed in 1979, and several lytic antibodies recognizing CD52 were subsequently identified, including immunoglobulin M (IgM; YTH66.9, Campath-1M) and IgG2a (YTH34.5, Campath-1G), although these antibodies do not cross the blood-brain barrier.6,13 In 1989, Dyer et al reported the use of IT Campath-1G in a patient with B-cell chronic lymphocytic leukemia with leptomeningeal involvement.6 In the aforementioned case, IT administration of ≤10 mg Campath-1G was well tolerated but provided no improvement in the patient’s leptomeningeal disease or symptoms. The development of human anti-rat neutralizing antibodies or the inefficient activation of complement or antibody-dependent cellular cytotoxicity may have limited the efficacy of this treatment.
In 1986, researchers began work to fuse the complementary determining region of Campath-1G to various human Fc frameworks. Ultimately, a panel of chimeric humanized antibodies were created and confirmed that an IgG1 isotype (alemtuzumab, Campath-1H) was the most potent for both complement activation and antibody-dependent cellular cytotoxicity. This antibody was then clinically developed for use, receiving accelerated approval in 2001, and was subsequently granted full approval in 2007.
We postulated that given the excellent systemic control of the patient’s T-PLL, IT alemtuzumab would allow for leptomeningeal control of her disease. After consultation with our Office of Regulatory Affairs and the distribution program for alemtuzumab, informed consent was obtained from the patient, allowing the initiation of IT administration of alemtuzumab under our institutional Innovative Care Policy guidelines, which mirror the published guidelines at Stanford University.14 We chose to begin with 1 mg, similar to the initial dose of Campath-1G administered by Dyer et al.6 After the safe initial dose, we escalated the dose to 3 mg 3 days later. Given the morphologic and immunophenotypic disappearance of her leptomeningeal disease, the 3 mg dosage was maintained.
We report this successful use of IT alemtuzumab (Campath) to note that it may provide an alternative to the use of cytotoxic therapy or radiation therapy, especially for patients with resistant or relapsed leptomeningeal T-PLL. More data are needed to establish the safety and efficacy of IT alemtuzumab and compare the efficacy of IT alemtuzumab to traditional approaches for leptomeningeal disease. Nonetheless, this report demonstrates the promise of IT alemtuzumab as a viable alternative to traditional treatments of leptomeningeal disease.
Acknowledgments
The authors thank the Office of Regulatory Affairs for assisting in reviewing the clinical case and outlining the requirements for the delivery of clinical care under the Innovative Care Policy. The authors also thank the Campath distribution program for reviewing the case and providing approval for the delivery of IT care as well as alemtuzumab.
Authorship
Contribution: D.B., B.M., A.P., P.B., and K.P. reviewed the clinical case and generated the concept of the care delivered; A.S. assisted in the application for the acquisition of alemtuzumab; D.B. and H.F. delivered the clinical care; F.A., L.B., and D.B. wrote the manuscript; S.C. created the figure; and all authors approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dale Bixby, Division of Hematology and Medical Oncology, Department of Internal Medicine, Michigan Medicine, F4811A UH South, 1500 E Medical Center Dr, Ann Arbor, MI 48109; e-mail: dbixby@med.umich.edu.
References
- 1.Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon, France: IARC Press; 2008:. [Google Scholar]
- 2.Khot A, Dearden C. T-cell prolymphocytic leukemia. Expert Rev Anticancer Ther. 2009;9(3):365-371. [DOI] [PubMed] [Google Scholar]
- 3.Dearden C. Management of prolymphocytic leukemia. Hematology Am Soc Hematol Educ Program. 2015;2015:361-367. [DOI] [PubMed] [Google Scholar]
- 4.Malkan UY, Gunes G, Yayar O, Demiroglu H, Yesilirmak A, Uner A. A T-cell prolymphocytic leukemia case with central nervous system involvement. Int J Clin Exp Med. 2015;8(8):14207-14209. [PMC free article] [PubMed] [Google Scholar]
- 5.Göçmen S, Kutlay M, Erikçi A, Atabey C, Sayan O, Haholu A. Central nervous system involvement of T-cell prolymphocytic leukemia diagnosed with stereotactic brain biopsy: case report. Turk J Haematol. 2014;31(1):75-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dyer MJ, Hale G, Hayhoe FG, Waldmann H. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood. 1989;73(6):1431-1439. [PubMed] [Google Scholar]
- 9.Dyer MJ, Hale G, Hayhoe FG, Waldmann H. Effects of CAMPATH-1 antibodies in vivo in patients with lymphoid malignancies: influence of antibody isotype. Blood. 1989;73(6):1431-1439. [PubMed] [Google Scholar]
- 7.Dearden CE, Matutes E, Cazin B, et al. . High remission rate in T-cell prolymphocytic leukemia with CAMPATH-1H. Blood. 2001;98(6):1721-1726. [DOI] [PubMed] [Google Scholar]
- 8.Hale G, Dyer MJ, Clark MR, et al. . Remission induction in non-Hodgkin lymphoma with reshaped human monoclonal antibody CAMPATH-1H. Lancet. 1988;2(8625):1394-1399. [DOI] [PubMed] [Google Scholar]
- 10.Pawson R, Dyer MJ, Barge R, et al. . Treatment of T-cell prolymphocytic leukemia with human CD52 antibody. J Clin Oncol. 1997;15(7):2667-2672. [DOI] [PubMed] [Google Scholar]
- 11.Keating MJ, Cazin B, Coutré S, et al. . Campath-1H treatment of T-cell prolymphocytic leukemia in patients for whom at least one prior chemotherapy regimen has failed. J Clin Oncol. 2002;20(1):205-213. [DOI] [PubMed] [Google Scholar]
- 12.Dearden CE, Khot A, Else M, et al. . Alemtuzumab therapy in T-cell prolymphocytic leukemia: comparing efficacy in a series treated intravenously and a study piloting the subcutaneous route. Blood. 2011;118(22):5799-5802. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi V, Tam C, O’Brien S, et al. . Phase I trial of nelarabine in indolent leukemias. J Clin Oncol. 2008;26(7):1098-1105. [DOI] [PubMed] [Google Scholar]
- 16.Hale G, Bright S, Chumbley G, et al. . Removal of T cells from bone marrow for transplantation: a monoclonal antilymphocyte antibody that fixes human complement. Blood. 1983;62(4):873-882. [PubMed] [Google Scholar]
- 17.Innovative Care Guidelines Stanford University Medical Center. Available at: http://med.stanford.edu/shs/update/archives/JULY2011/Innovative%20Care%20Guidelines.pdf. Accessed 20 February 2018.