Abstract
Aims
Physical activity has been shown to reduce mortality in a dose-response fashion. Current guidelines recommend 500–1000 metabolic equivalent task (MET)-min per week of regular physical activity. This study aimed to compare the impact of leisure-time physical activity on mortality in primary versus secondary cardiovascular prevention.
Methods and results
This study included a total of 131 558 and 310 240 subjects with and without cardiovascular disease (CVD), respectively, from a population-based cohort. Leisure-time physical activity was measured by self-report questionnaires. The study subjects were followed-up for a median of 5.9 years, and the main study outcome was all-cause mortality. There was an inverse relationship between the physical activity level and the mortality risk in both groups. The benefit in the secondary prevention group was shown to be greater than that in the primary prevention group: every 500 MET-min/week increase in physical activity resulted in a 14% and 7% risk reduction in mortality in the secondary and primary prevention groups, respectively (interaction P < 0.001). In addition, while individuals without CVD benefited the most between 1 and 500 MET-min/week of physical activity, the benefit in those with CVD continued above 500 − 1000 MET-min/week. The adjusted mortality risk of individuals with CVD who performed a high level of physical activity (≥1000 MET-min/week) was shown to be comparable to or lower than that of their counterparts without CVD.
Conclusion
Individuals with CVD may benefit from physical activity to a greater extent than do healthy subjects without CVD.
Keywords: Physical activity, Secondary prevention, Cohort study, Exercise, Risk reduction behaviour, Metabolic equivalent
Introduction
Strong evidence supports an inverse dose-response relationship between physical activity levels and mortality.1–3 Current guidelines recommend at least 500–1000 metabolic equivalent task (MET)-minutes per week of moderate-to-vigorous physical activity.4–6 Unfortunately, studies have shown that less than half of adults achieve this level of physical activity, while one-third do no physical activity at all.7,8
The existing evidence on the dose-response relationship is mostly based on healthy people.9–16 While individuals with cardiovascular disease (CVD) are at higher risk for mortality and morbidity, they also tend to have sedentary lifestyles and are less physically active than are those without CVD.17 Interventions with comprehensive cardiovascular rehabilitation programmes have been shown to reduce cardiac mortality in patients with coronary heart disease.18 While physical activity is generally recommended for secondary CVD prevention by contemporary guidelines, there is still a paucity of data regarding the relationship between physical activity and mortality, specifically among patients with pre-existing CVD.19–21 In addition, no previous studies have compared the beneficial effect of physical activity between primary vs. secondary CVD prevention.
We analysed a population-based cohort in this study to elucidate how the benefit of physical activity interacts with the presence of CVD. We specifically sought (i) to evaluate the levels of physical activity among participants with and without CVD, (ii) to identify the relationship between physical activity and mortality among patients with CVD, and (iii) to compare the associations between the primary and secondary prevention groups.
Methods
Data source and study subjects
A national health claims database, the National Health Insurance Services–Health Screening Cohort was used in this study. Details about the cohort have been described previously.22 Enrolees in the insurance system aged 40 years or older are entitled to undergo a general health screening programme every 2 years. The programme includes self-report questionnaires, anthropometric and blood pressure measurements, and laboratory tests using blood and urine samples. Standardized questionnaires are used to acquire information on previous medical history, family history, and lifestyle factors such as smoking, alcohol intake, and physical activity. This study was exempt from review by the Seoul National University, Bundang Hospital Institutional Review Board (I-2018-11316). It complied with the requirements of the Declaration of Helsinki, and the need for informed consent was waived.
A total of 441 798 individuals who underwent the screening programme between 2009 and 2015 and completed surveys on physical activity were extracted from the cohort. Each individual’s claims records were reviewed for a history of CVD from 2002 until the date of the health check-up. Study subjects with prior myocardial infarction (MI), other ischaemic heart diseases, prior stroke, or chronic heart failure (HF) were considered to have CVD (secondary prevention group). The primary prevention group included those without a history of CVD. Definitions of CVD are detailed in Supplementary material online, Table S1.
Physical activity
The level of leisure-time physical activity was collected with self-report structured questionnaires using a 7-day recall method. The survey included three questions that addressed the usual frequency (days per week) of (i) light-intensity activity for at least 30 min (e.g. walking at a slow or leisurely pace), (ii) moderate-intensity activity for at least 30 min (e.g. brisk walking, slow cycling, or tennis doubles), and (iii) vigorous-intensity activity for at least 20 min (e.g. jogging or running, bicycling >15 km/h, climbing briskly up a hill, or participating in an aerobics class). Ratings of 2.9, 4.0, and 7.0 METs were assigned for light-intensity, moderate-intensity, and vigorous-intensity activities, respectively. Physical activity-related energy expenditure (MET-min/week) was calculated by summing the product of frequency, intensity, and duration. The level of leisure-time physical activity was categorized into 0 (totally sedentary), <500, 500–999, 1000–1499, and ≥1500 MET-min/week.
Study outcomes
The primary study outcome measure was all-cause mortality. Study subjects were followed until the end of 2015. Vital status, date of death, and cause of death were certified by linking the records from the National Death Index using each individual’s identification code. Secondary outcomes included cardiovascular death and non-cardiovascular death.
Statistical analysis
Summary statistics are reported as means ± standard deviations for numerical variables and as numbers (%) for categorical variables. Continuous variables were compared using the Student’s t-test or Mann–Whitney’s U test, as appropriate. Categorical variables were compared using the χ2 test. The incidence rate of mortality was calculated by dividing the number of deaths by the sum of the follow-up duration and presented as the rate per 1000 person-years. Kaplan–Meier survival curves were constructed and compared using the log-rank test. Cox proportional-hazard models were used to calculate hazard ratios (HRs) and 95% confidence intervals (CIs). Multivariable regression models were constructed with adjustment for (i) age and sex, and (ii) including age, sex, income level, residential area (urban or non-urban), body mass index, hypertension, diabetes mellitus, dyslipidaemia, smoking, renal disease, end-stage renal disease, liver disease, malignancy, fasting blood sugar, serum creatinine level, and the use of aspirin, statins, and antihypertensive medications. The interaction between physical activity and CVD was considered in the statistical models. Restricted cubic splines were fitted with four knots by treating the amount of physical activity as a continuous variable using the ‘rms’ package. Sensitivity analyses were done according to sex and baseline risk subgroups. The baseline cardiovascular risk of subjects without CVD was calculated using systematic coronary risk evaluation and a pooled cohort equation.23,24 All statistical analyses were performed with R programming version 3.3.3 (http://www.R-project.org; the R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
The baseline characteristics of a total of 441 798 study subjects are shown in Table 1. The median age of the participants was 59.5 years, 53.5% were men, 38.9% had hypertension, 12.6% had diabetes, and 16.9% were current smokers. The means of body mass index, blood pressure, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and fasting blood glucose were 24.0 kg/m2, 125.5/77.6 mmHg, 199.6 mg/dL, 55.0 mg/dL, 118.3 mg/dL, and 101.6 mg/dL, respectively.
Table 1.
Baseline characteristics of the study population
| Variables | Total (n = 441 798) | Cardiovascular disease (n = 131 558) | No cardiovascular disease (n = 310 240) |
|---|---|---|---|
| Age (years) | 59.5 ± 9.1 | 63.8 ± 9.4 | 57.8 ± 8.3 |
| Male sex | 236 517/441 798 (53.5%) | 66 030/131 558 (50.2%) | 170 487/310 240 (55.0%) |
| Income levels | |||
| Fifth quintile (highest) | 155 879/441 798 (35.3%) | 47 355/131 558 (36.0%) | 108 524/310 240 (35.0%) |
| Fourth quintile | 92 249/441 798 (20.9%) | 27 459/131 558 (20.9%) | 64 790/310 240 (21.0%) |
| Third quintile | 70 602/441 798 (16.0%) | 20 410/131 558 (15.5%) | 50 192/310 240 (16.2%) |
| Second quintile | 58 818/441 798 (13.3%) | 16 529/131 558 (12.6%) | 42 289/310 240 (13.6%) |
| First quintile (lowest) | 61 998/441 798 (14.0%) | 18 743/131 558 (14.2%) | 43 255/310 240 (13.9%) |
| Covered by medical aid | 2252/441 798 (0.5%) | 1062/131 558 (0.8%) | 1190/310 240 (0.4%) |
| Anthropometric measurements | |||
| Body mass index (kg/m2) | 24.0 ± 3.0 (441 570) | 24.4 ± 3.1 (131 444) | 23.9 ± 2.9 (310 126) |
| Systolic blood pressure (mmHg) | 125.5 ± 15.4 (441 680) | 127.5 ± 15.5 (131 501) | 124.7 ± 15.2 (310 179) |
| Diastolic blood pressure (mmHg) | 77.6 ± 10.0 (441 679) | 77.9 ± 10.0 (131 502) | 77.4 ± 10.0 (310 177) |
| Baseline risk factors | |||
| Hypertension | 171 658/441 798 (38.9%) | 90 139/131 558 (68.5%) | 81 519/310 240 (26.3%) |
| Diabetes mellitus | 55 748/441 798 (12.6%) | 28 027/131 558 (21.3%) | 27 721/310 240 (8.9%) |
| Dyslipidaemia | 182 133/441 798 (41.2%) | 86 661/131 558 (65.9%) | 95 472/310 240 (30.8%) |
| Current smoking | 74 605/441 798 (16.9%) | 17 137/131 558 (13.0%) | 57 468/310 240 (18.5%) |
| Renal disease | 6141/441 798 (1.4%) | 3993/131 558 (3.0%) | 2148/310 240 (0.7%) |
| End stage renal disease | 214/441 798 (0.05%) | 176/131 558 (0.13%) | 38/310 240 (0.01%) |
| Liver disease | 6675/441 798 (1.5%) | 3152/131 558 (2.4%) | 3523/310 240 (1.1%) |
| Any malignancy | 31 386/441 798 (7.1%) | 14 274/131 558 (10.8%) | 17 112/310 240 (5.5%) |
| Pre-existing cardiovascular disease | |||
| Ischaemic heart disease | 96 250/131 558 (73.2%) | ||
| Prior myocardial infarction | 13 445/131 558 (10.2%) | ||
| Prior stroke | 45 252/131 558 (34.4%) | ||
| Heart failure | 36 308/131 558 (27.6%) | ||
| Pre-existing non-cardiovascular disease | |||
| Peptic ulcer disease | 237 844/441 798 (53.8%) | 89 243/131 558 (67.8%) | 148 601/310 240 (47.9%) |
| Rheumatic disease | 49 990/441 798 (11.3%) | 21 567/131 558 (16.4%) | 28 423/310 240 (9.2%) |
| Diabetes with chronic complication | 42 002/441 798 (9.5%) | 24 603/131 558 (18.7%) | 17 399/310 240 (5.6%) |
| Hemiplegia or paraplegia | 5465/441 798 (1.2%) | 4 586/131 558 (3.5%) | 879/310 240 (0.3%) |
| Renal disease | 6141/441 798 (1.4%) | 4 009/131 558 (3.0%) | 2 132/310 240 (0.7%) |
| Mild liver disease | 168 082/441 798 (38.0%) | 68 590/131 558 (52.1%) | 99 492/310 240 (32.1%) |
| Moderate or severe liver disease | 6675/441 798 (1.5%) | 3 167/131 558 (2.4%) | 3 508/310 240 (1.1%) |
| Any malignancy | 31 386/441 798 (7.1%) | 14 310/131 558 (10.9%) | 17 076/310 240 (5.5%) |
| Metastatic solid tumour | 4339/441 798 (1.0%) | 1 941/131 558 (1.5%) | 2 398/310 240 (0.8%) |
| Dementia | 10 181/441 798 (2.3%) | 7 680/131 558 (5.8%) | 2 501/310 240 (0.8%) |
| HIV/AIDS | 1/441 798 (0.0%) | 1/131 558 (0.0%) | 0/310 240 (0.0%) |
| Medication use | |||
| Aspirin | 52 788/441 798 (11.9%) | 35 399/131 558 (26.8%) | 17 389/310 240 (5.6%) |
| Antihypertensive agents | 119 687/441 798 (27.1%) | 64 615/131 558 (48.9%) | 55 072/310 240 (17.8%) |
| Statin | 38 698/441 798 (8.8%) | 24 954/131 558 (18.9%) | 13 744/310 240 (4.4%) |
| Laboratory findings | |||
| Total cholesterol (mg/dL) | 199.6 ± 38.0 (441 649) | 194.5 ± 39.5 (131 500) | 201.8 ± 37.2 (310 149) |
| Triglyceride (mg/dL) | 140.0 ± 90.9 (441 288) | 142.7 ± 89.1 (131 365) | 138.9 ± 91.7 (309 923) |
| HDL cholesterol (mg/dL) | 55.0 ± 28.8 (441 651) | 53.9 ± 29.5 (131 504) | 55.5 ± 28.5 (310 147) |
| LDL cholesterol (mg/dL) | 118.3 ± 37.8 (439 080) | 113.8 ± 38.6 (130 678) | 120.2 ± 37.2 (308 402) |
| Fasting blood glucose (mg/dL) | 101.6 ± 26.0 (441 694) | 104.4 ± 28.6 (131 517) | 100.4 ± 24.7 (310 177) |
| Serum creatinine (mg/dL) | 1.1 ± 1.3 (441 667) | 1.1 ± 1.2 (131 509) | 1.1 ± 1.3 (310 158) |
HDL, high-density lipoprotein; HIV/AIDS, human immunodeficiency virus infection and acquired immune deficiency syndrome; LDL, low-density lipoprotein; MET, metabolic equivalent of task.
Among the study population, 29.8% of participants had CVD (n = 131 558) and the other 70.2% (n = 310 240) had no history of CVD. Ischaemic heart disease accounted for the largest proportion of the participants with CVD, followed by prior stroke, chronic HF, and MI. Cardiovascular disease was associated with older age and a higher prevalence of comorbidities such as hypertension, diabetes, and hyperlipidaemia. The proportion of participants taking cardiovascular medications including aspirin, antihypertensive agents, and statins was higher in those with CVD.
Physical activity
The range of physical activity level of the study population was 0–2429 MET-min/week (Supplementary material online, Figure S1). Participants with CVD were less physically active than were those without CVD (Table 2 and Supplementary material online, Figure S1). The median physical activity level was 520 and 540 MET-min/week in the secondary and primary prevention groups, respectively. This difference was statistically significant (P < 0.001) although the effect size was small (Cohen’s d = 0.016). The proportion of subjects who were totally sedentary was higher in the secondary prevention group than in the primary prevention group (27.2% and 24.4%, respectively, P < 0.001), while 49.5% and 48.2% failed to achieve the recommended physical activity level of ≥500 MET-min/week in each group (P < 0.001).
Table 2.
Leisure-time physical activity of subjects with and without cardiovascular disease
| Physical activity | Total (n = 441 798) | Cardiovascular disease (n = 131 558) | No cardiovascular disease (n = 310 240) | P-value |
|---|---|---|---|---|
| Median (interquartile ranges) | 540 (0‒980) | 520 (0‒980) | 540 (87‒980) | <0.001 |
| Classification | <0.001 | |||
| ≥1500 MET-min/week | 8.8% (38 820) | 9.1% (11 938) | 8.7% (26 882) | |
| 1000‒1499 MET-min/week | 13.3% (58 977) | 12.4% (16 373) | 13.7% (42 604) | |
| 500‒999 MET-min/week | 29.3% (129 458) | 29.0% (38 091) | 29.5% (91 367) | |
| <500 MET-min/week | 23.3% (102 986) | 22.4% (29 434) | 23.7% (73 552) | |
| Totally sedentary | 25.3% (111 557) | 27.2% (35 722) | 24.4% (75 835) |
MET, metabolic equivalent of task.
Supplementary material online, Table S2 compares the differences in the baseline characteristics according to physical activity levels. Advanced age, male sex, and a high income were associated with a higher physical activity level. Comorbidities such as hypertension, diabetes, and dyslipidaemia were associated with a higher physical activity level, while current smoking was associated with a lower physical activity level. The pattern was similar when the study subjects were stratified according to the presence of CVD (Supplementary material online, Tables S3 and S4). Cardiovascular medication usage and adherence were higher among those with greater physical activity. Among the secondary prevention group, the presence of HF was associated with lower physical activity levels.
The amount of physical activity of the study population increased during the study duration (Supplementary material online, Figure S2). Moreover, a significant number of study subjects changed their amount of physical activity during the study duration and became either more active or less active (Supplementary material online, Figure S3). The use of medications also increased over time (Supplementary material online, Table S5). While the use of statins showed a remarkable increase over time, the use of aspirin was unchanged.
Impact of physical activity on mortality
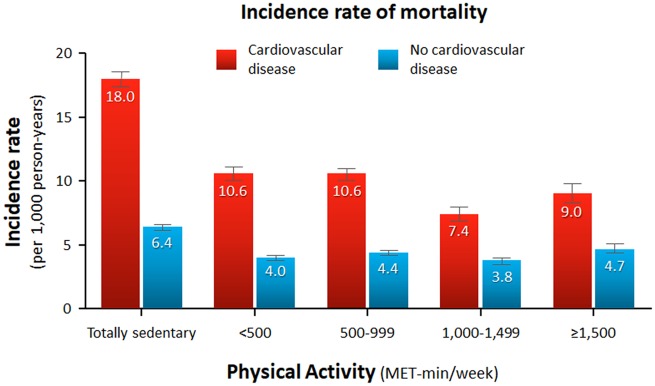
The median follow-up duration was 5.9 years. Figure 1shows the incidence rate of mortality per 1000 person-years stratified by the presence of CVD and the level of physical activity. Overall, CVD was associated with a significantly increased risk of mortality (HR, 2.57; 95% CI, 2.49–2.65; P < 0.001) (Supplementary material online, Figures S4 and S5). As also shown in Table 3, the unadjusted risk of all-cause mortality showed a J-shaped relationship with the amount of physical activity. Mortality risk was highest with a totally sedentary lifestyle and lowest with a physical activity level of 1000–1499 MET-min/week regardless of CVD. A higher level of physical activity (≥1500 MET-min/week) was associated with a significantly higher risk of mortality than the trough.
Figure 1.
Incidence rate of mortality per 1000 person-years according to physical activity level stratified by cardiovascular disease. Numbers in the box plot indicate incidence rates per 1000 person-years. Error bars indicate 95% confidence intervals.
Table 3.
Leisure-time physical activity and the risk of mortality stratified by the presence of cardiovascular disease
| Amount of leisure-time physical activity | 10-year event rate (%) | Unadjusted |
Age, sex adjusted |
Multivariable adjusteda |
|||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Cardiovascular disease | |||||||
| Totally sedentary | 18.0 | 3.81 (3.51–4.13) | <0.001 | 2.18 (2.01–2.36) | <0.001 | 1.87 (1.72–2.04) | <0.001 |
| <500 MET-min/week | 10.6 | 2.25 (2.06–2.46) | <0.001 | 1.60 (1.47–1.75) | <0.001 | 1.45 (1.32–1.58) | <0.001 |
| 500–999 MET-min/week | 10.6 | 2.25 (2.07–2.45) | <0.001 | 1.47 (1.35–1.60) | <0.001 | 1.37 (1.26–1.50) | <0.001 |
| 1000–1499 MET-min/week | 7.4 | 1.58 (1.42–1.76) | <0.001 | 1.19 (1.07–1.32) | 0.002 | 1.14 (1.02–1.27) | 0.018 |
| ≥1500 MET-min/week | 9.0 | 1.92 (1.72–2.14) | <0.001 | 1.19 (1.06–1.33) | 0.002 | 1.14 (1.02–1.28) | 0.019 |
| No cardiovascular disease | |||||||
| Totally sedentary | 6.4 | 1.35 (1.24–1.46) | <0.001 | 1.41 (1.30–1.53) | <0.001 | 1.27 (1.17–1.39) | <0.001 |
| <500 MET-min/week | 4.0 | 0.84 (0.77–0.91) | <0.001 | 1.13 (1.04–1.24) | 0.006 | 1.08 (0.99–1.18) | 0.099 |
| 500–999 MET-min/week | 4.4 | 0.93 (0.85–1.01) | 0.078 | 1.06 (0.98–1.16) | 0.155 | 1.02 (0.94–1.11) | 0.671 |
| 1000–1499 MET-min/week | 3.8 | 0.80 (0.72–0.88) | <0.001 | 1.03 (0.93–1.13) | 0.613 | 1.01 (0.91–1.11) | 0.913 |
| ≥1500 MET-min/week | 4.7 | Reference | — | Reference | — | Reference | — |
CI, confidence interval; HR, hazard ratio; MET, metabolic equivalent of task.
Multivariable-adjusted model was adjusted for age, sex, income levels, residential area (urban or non-urban), body mass index, hypertension, diabetes mellitus, dyslipidaemia, smoke, renal disease, end-stage renal disease, liver disease, malignancy, fasting blood sugar, creatinine, aspirin, statin use, and antihypertensive medication.
Figure 2 shows the multivariable-adjusted risk of mortality (fitted spline curve is shown in the Take home figure). After statistical adjustment, physical activity and mortality exhibited an inverse relationship (Table 3). The difference in the risk of mortality between a physical activity level of ≥1500 MET-min/week and 1000–1499 MET-min/week mostly disappeared. A 500 MET-min/week increase in physical activity was associated with a 14% and 7% risk reduction in mortality in the secondary and primary prevention groups, respectively (interaction P < 0.001).
Figure 2.
Distribution and adjusted risk of mortality according to physical activity levels stratified by cardiovascular disease. Cox regression analysis with physical activity classified as a categorical variable: 0 (totally sedentary), <500, 500 to 999, 1000 to 1499, and ≥1500 MET-min/week. The statistical models were adjusted for age, sex, income level, body mass index, hypertension, diabetes mellitus, dyslipidaemia, smoking, renal disease, end-stage renal disease, liver disease, any malignancy, fasting blood sugar, and serum creatinine levels. The red and blue lines indicate hazard ratios for subjects with and without CVD, respectively. Bar graph indicates the number of study subjects in each category.
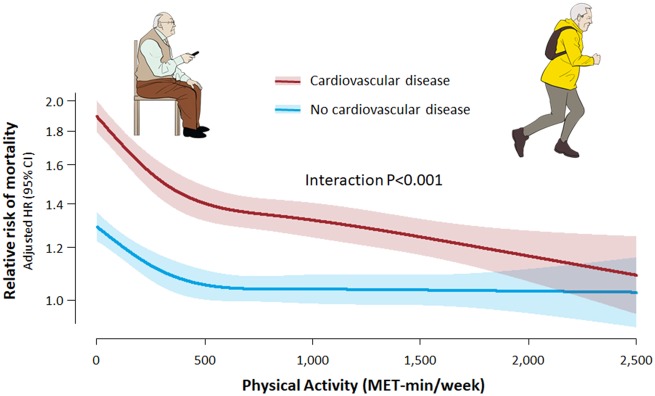
Take home figure.
Non-linear relationship between physical activity and mortality risk according to the presence of cardiovascular disease. Restricted cubic spline curves were constructed with regard to physical activity treated as a continuous variable. The red and blue lines and shades indicate adjusted hazard ratio and 95% confidence intervals for subjects with and without cardiovascular disease, respectively.
The relationship between physical activity and mortality showed different patterns between the primary and secondary prevention groups (Take home figure). Among the healthy subjects without CVD, the slope was the steepest between 0 and 499 MET-min/week, tended to flatten above 500 MET-min/week, and was essentially flat above 1000 MET-min/week. Although the mortality risk further reduced with a higher physical activity level, the benefit of increased physical activity was small beyond 500 MET-min/week. Among the participants with CVD, the benefit was also the greatest between 0 and 499 MET-min/week. However, the dose-response relationship extended beyond 500 to 1000 MET-min/week. The mortality risk of participants with CVD who performed physical activity ≥1000 MET-min/week (1000–1499 and ≥1500 MET-min/week) was significantly lower than that in participants who were free from CVD but were totally sedentary (P = 0.008 and 0.010, respectively).
Supplementary material online, Tables S6 and S7 show the unadjusted and adjusted risk of cardiac and non-cardiac death according to the level of physical activity. The overall pattern was similar to that of all-cause mortality except that CVD was associated with a significantly elevated risk of cardiac death despite a high level of physical activity. The benefit of physical activity in the secondary prevention group was consistent irrespective of the specific type of CVD (Supplementary material online, Table S8).
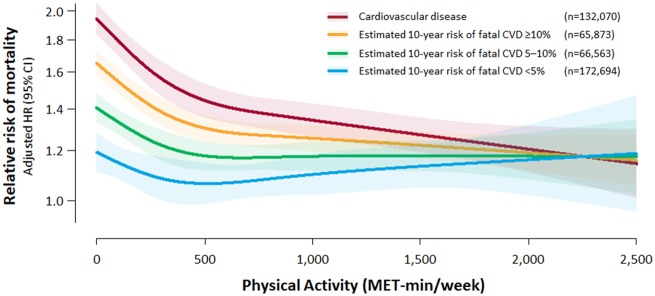
Sensitivity analysis found no significant interactions between sex and the amount of physical activity both in the CVD and in the no CVD groups (interaction P = 0.178 and 0.087, respectively) (Supplementary material online, Figures S6 and S7). Subjects without CVD were further divided according to the expected 10-year risk of fatal CVD (Figure 3). While the mortality risk was higher with increasing expected cardiovascular risk, the survival benefit of physical activity was also greater among those with higher expected cardiovascular risk. Analysis using pooled cohort equations showed similar patterns (Supplementary material online, Figure S8).
Figure 3.
Non-linear relationship between physical activity and mortality risk according to the presence of cardiovascular disease and expected 10-year risk of fatal cardiovascular disease. Restricted cubic spline curves were constructed with regard to physical activity treated as a continuous variable. Adjusted hazard ratio and 95% confidence intervals are illustrated. The red, orange, green, and blue lines and shades indicate subjects with cardiovascular disease and subjects without cardiovascular disease who have an estimated 10-year risk of fatal cardiovascular disease ≥10%, 5–10%, and <5%, respectively.
Discussion
Physical activity refers to any bodily movement produced by the skeletal muscles that requires energy expenditure.25 A previous study estimated that physical inactivity causes 9% of premature mortality worldwide, and that elimination of this unhealthy behaviour would lead to an increase in the life expectancy of the world’s population by 0.68 years.26 Contemporary guidelines emphasize that physical activity can make people feel better, function better, sleep better, and reduce the risk of many chronic diseases.6 The suggested public target range of physical activity is 500–1000 MET-minutes per week of aerobic physical activity, which is equivalent to 150–300 min of moderate-intensity or 75–150 min of vigorous-intensity physical activity per week. The recommendation is based on observations that the greatest survival benefit is provided by achieving 500–1000 MET-min/week of physical activity.2 The findings of the present study agree with these conclusions.
In this study, we found that approximately half of the study population did not reach the recommended level of leisure-time physical activity, and one-quarter had a totally sedentary lifestyle. Subjects with CVD had lower levels of physical activity than did those without CVD. There was an inverse relationship between the amount of physical activity and the risk of all-cause mortality. The main novel finding of this study was that the relationship between physical activity and mortality showed different patterns in the primary and secondary cardiovascular prevention settings. While individuals without CVD had reduced mortality with physical activity as has been previously documented, the expected survival benefit was even greater among patients with CVD. In addition, whereas the benefit in the primary prevention group was mostly confined to the range of 0–499 MET-min/week, the advantage in the secondary prevention group was still present above 500 MET-min/week. Interestingly, if an individual with CVD performed a high level of physical activity, the adjusted risk of mortality was shown to be similar to or even lower than that of an individual without CVD.
Previous studies that showed the survival benefits of physical activity primarily focused on healthy individuals and did not distinguish between primary and secondary CVD prevention.9–16 Peak exercise capacity has been shown to be the strongest predictor of survival in subjects with pre-existing CVD.27 Strong evidence supports exercise-based cardiac rehabilitation in patients with coronary heart disease and exercise training in patients with chronic HF.18,28,29 However, recommendations on the level of physical activity for secondary prevention have been less clear. The European Society of Cardiology guidelines on stable coronary artery disease encourage moderate-to-vigorous intensity aerobic exercise training ≥3 times a week for 30 min per session.19 Meanwhile, the guideline for stable ischaemic heart disease from the American College of Cardiology Foundation and American Heart Association recommends 30–60 min of moderate-intensity aerobic activity at least 5 days and preferably 7 days per week.30 The latter one states that the recommended level of physical activity was in line with that for healthy adults.31 In addition, guidelines for other CVDs such as prior MI, prior stroke, and chronic HF lack specific recommendations. This reflects the paucity of data for this specific group of patients.
Exercise has been proven to have a variety of cardiovascular benefits. An increase in physical activity has been shown to have beneficial effects on plasma lipoproteins such as decreased LDL and increased HDL.32 A meta-analysis showed that resistance training reduces systolic blood pressure by 6.19 mmHg and glycosylated haemoglobin by 0.48%.33 Studies have also proven a reduced production of atherogenic cytokine and an increase in atheroprotective cytokines,34 depression in platelet aggregation and adhesiveness,35 lower inflammatory markers,36 and reduced arrhythmogenicity with exercise.37 Evidence also indicates that exercise has a favourable impact on cardiac remodelling and, thus, improvements in cardiac performance.38
The present study provides a novel perspective into the preventive role of physical activity in patients with CVD. As shown in the present study, individuals with CVD tend to achieve a lower level of physical activity. Not only they are typically older with multiple comorbidities, but their cardiac condition also limits their physical capacity. However, clinicians should emphasize the importance of a physically active lifestyle for those patients. They should be encouraged to maintain as much physical activity as possible. They may even expect greater benefits compared to their counterparts without CVD while performing the same levels of physical activity. A level of 500 MET-min/week of physical activity should be considered the minimum requirement for patients with CVD. Although physical activity above 500–1000 MET-min/week would provide little advantage for healthy people without CVD, this would provide additive benefits for those with CVD.
Limitations
This study has several limitations. Recall bias is one of the major potential limitations. Information about physical activity relied on self-report questionnaires administered to the health check-up participants. These questionnaires surveyed lifestyle behaviours during the previous 1 week. Secondly, various types of physical activity occur throughout a day for diverse purposes.6 While only leisure-time physical activity was analysed in this study, occupation, transportation, and household physical activities also contribute to total daily physical activity. Third, although we performed rigorous statistical adjustment, we cannot exclude the presence of unadjusted confounding factors. For example, participants with a higher physical activity level may have had a lower disease burden, better cardiac function, and/or better cardiorespiratory fitness, which have been shown to predict a better prognosis.39 In addition, they may have favourable lifestyle behaviours such as a healthy diet, moderation in alcohol drinking, and better adherence to medications. Changes in baseline variables over time such as the amount of physical activity, medication use, and adherence to medications were not adjusted in the analyses. Fourth, several assumptions were made while calculating the amount of physical activity. As the questionnaires focused primarily on aerobic physical activity, information on muscle-strengthening and bone-strengthening exercises were limited. However, the large sample size in this study reduces the potential uncertainty and bias. Lastly, because the presence of CVD was determined using claims data, there is a possibility of misclassification bias.
Conclusion
In this study, we found that physical activity provides survival benefits both in primary and secondary CVD prevention. While individuals with pre-existing CVD were less likely to be physically active, their expected benefit from physical activity was greater than that of individuals without CVD. In addition, the advantage of physical activity in secondary prevention extended above 500–1000 MET-min/week. Patients with CVD should be encouraged to avoid a sedentary lifestyle and to maintain physically active behaviours for secondary prevention.
Supplementary Material
Acknowledgements
We thank Moon Ju Kim for performing data collection and statistical analyses.
Funding
This work was supported by the Basic Science Research Programme through the National Research Foundation of Korea [2019R1C1C1006611].
Conflict of interest: none declared.
See page 3556 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz697)
References
- 1. Moore SC, Patel AV, Matthews CE, Berrington de Gonzalez A, Park Y, Katki HA, Linet MS, Weiderpass E, Visvanathan K, Helzlsouer KJ, Thun M, Gapstur SM, Hartge P, Lee IM.. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med 2012;9:e1001335.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arem H, Moore SC, Patel A, Hartge P, Berrington de Gonzalez A, Visvanathan K, Campbell PT, Freedman M, Weiderpass E, Adami HO, Linet MS, Lee IM, Matthews CE.. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175:959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu Y, Shu XO, Wen W, Saito E, Rahman MS, Tsugane S, Tamakoshi A, Xiang YB, Yuan JM, Gao YT, Tsuji I, Kanemura S, Nagata C, Shin MH, Pan WH, Koh WP, Sawada N, Cai H, Li HL, Tomata Y, Sugawara Y, Wada K, Ahn YO, Yoo KY, Ashan H, Chia KS, Boffetta P, Inoue M, Kang D, Potter JD, Zheng W.. Association of leisure-time physical activity with total and cause-specific mortality: a pooled analysis of nearly a half million adults in the Asia Cohort Consortium. Int J Epidemiol 2018;47:771–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckel RH, Jakicic JM, Ard JD, de Jesus JM, Houston Miller N, Hubbard VS, Lee IM, Lichtenstein AH, Loria CM, Millen BE, Nonas CA, Sacks FM, Smith SC Jr, Svetkey LP, Wadden TA, Yanovski SZ; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;63:2960–2984. [DOI] [PubMed] [Google Scholar]
- 5. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corra U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Lochen ML, Lollgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.2018 Physical Activity Guidelines Advisory Committee. 2018 Physical Activity Guidelines Advisory Committee Scientific Report. Washington, DC: US Department of Health and Human Services; 2018. [Google Scholar]
- 7. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Roger VL, Thom T, Wasserthiel-Smoller S, Wong ND, Wylie-Rosett J; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation 2010;121:e46–e215. [DOI] [PubMed] [Google Scholar]
- 8. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD.. The physical activity guidelines for Americans. JAMA 2018;320:2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Koster A, Harris TB, Moore SC, Schatzkin A, Hollenbeck AR, van Eijk JT, Leitzmann MF.. Joint associations of adiposity and physical activity with mortality: the National Institutes of Health-AARP Diet and Health Study. Am J Epidemiol 2009;169:1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Calle EE, Rodriguez C, Jacobs EJ, Almon ML, Chao A, McCullough ML, Feigelson HS, Thun MJ.. The American Cancer Society Cancer Prevention Study II Nutrition Cohort: rationale, study design, and baseline characteristics. Cancer 2002;94:2490–2501. [DOI] [PubMed] [Google Scholar]
- 11. Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ.. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol 2004;160:1223–1233. [DOI] [PubMed] [Google Scholar]
- 12. Howard RA, Leitzmann MF, Linet MS, Freedman DM.. Physical activity and breast cancer risk among pre- and postmenopausal women in the U.S. Radiologic Technologists cohort. Cancer Causes Control 2009;20:323–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lee IM, Cook NR, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE.. Vitamin E in the primary prevention of cardiovascular disease and cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:56–65. [DOI] [PubMed] [Google Scholar]
- 14. Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE.. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA 2005;294:47–55. [DOI] [PubMed] [Google Scholar]
- 15. Margolis KL, Mucci L, Braaten T, Kumle M, Trolle Lagerros Y, Adami HO, Lund E, Weiderpass E.. Physical activity in different periods of life and the risk of breast cancer: the Norwegian-Swedish Women's Lifestyle and Health cohort study. Cancer Epidemiol Biomarkers Prev 2005;14:27–32. [PubMed] [Google Scholar]
- 16. Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, Hennekens CH, Buring JE.. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med 2005;352:1293–1304. [DOI] [PubMed] [Google Scholar]
- 17. Vasankari V, Husu P, Vähä-Ypyä H, Suni JH, Tokola K, Borodulin K, Wennman H, Halonen J, Hartikainen J, Sievänen H, Vasankari T.. Subjects with cardiovascular disease or high disease risk are more sedentary and less active than their healthy peers. BMJ Open Sport Exerc Med 2018;4:e000363.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anderson L, Oldridge N, Thompson DR, Zwisler AD, Rees K, Martin N, Taylor RS.. Exercise-based cardiac rehabilitation for coronary heart disease: cochrane systematic review and meta-analysis. J Am Coll Cardiol 2016;67:1–12. [DOI] [PubMed] [Google Scholar]
- 19.Task Force Members, Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, Ferreira JR, Gersh BJ, Gitt AK, Hulot JS, Marx N, Opie LH, Pfisterer M, Prescott E, Ruschitzka F, Sabate M, Senior R, Taggart DP, van der Wall EE, Vrints CJESC Committee for Practice GuidelinesZamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, Knuuti J, Valgimigli M, Bueno H, Claeys MJ, Donner-Banzhoff N, Erol C, Frank H, Funck-Brentano C, Gaemperli O, Gonzalez-Juanatey JR, Hamilos M, Hasdai D, Husted S, James SK, Kervinen K, Kolh P, Kristensen SD, Lancellotti P, Maggioni AP, Piepoli MF, Pries AR, Romeo F, Ryden L, Simoons ML, Sirnes PA, Steg PG, Timmis A, Wijns W, Windecker S, Yildirir A, Zamorano JL.. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J 2013;34:2949–3003. [DOI] [PubMed] [Google Scholar]
- 20. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S; ESC Scientific Document Group. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 21. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P; ESC Scientific Document Group. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 22. Seong SC, Kim YY, Park SK, Khang YH, Kim HC, Park JH, Kang HJ, Do CH, Song JS, Lee EJ, Ha S, Shin SA, Jeong SL.. Cohort profile: the National Health Insurance Service-National Health Screening Cohort (NHIS-HEALS) in Korea. BMJ Open 2017;7:e016640.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goff David C, Lloyd-Jones Donald M, Bennett G, Coady S, D’Agostino Ralph B, Gibbons R, Greenland P, Lackland Daniel T, Levy D, O’Donnell Christopher J, Robinson Jennifer G, Schwartz JS, Shero Susan T, Smith Sidney C, Sorlie P, Stone Neil J, Wilson Peter WF.. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. Circulation 2014;129(25_suppl_2):S49–S73. [DOI] [PubMed] [Google Scholar]
- 24. Conroy RM, Pyörälä K, Fitzgerald AP, Sans S, Menotti A, De Backer G, De Bacquer D, Ducimetière P, Jousilahti P, Keil U, Njølstad I, Oganov RG, Thomsen T, Tunstall-Pedoe H, Tverdal A, Wedel H, Whincup P, Wilhelmsen L, Graham IM; SCORE project group. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003;24:987–1003. [DOI] [PubMed] [Google Scholar]
- 25.WHO Guidelines Approved by the Guidelines Review Committee. Global Recommendations on Physical Activity for Health. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 26. Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT.. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 2012;380:219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE.. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med 2002;346:793–801. [DOI] [PubMed] [Google Scholar]
- 28. Piepoli MF, Davos C, Francis DP, Coats AJ, ExTra MC.. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004;328:711–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, Leifer ES, Kraus WE, Kitzman DW, Blumenthal JA, Rendall DS, Miller NH, Fleg JL, Schulman KA, McKelvie RS, Zannad F, Pina IL.. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA 2009;301:1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB 3rd, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV.. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60:2564–2603. [DOI] [PubMed] [Google Scholar]
- 31. Haskell WL, Lee IM, Pate RR, Powell KE, Blair SN, Franklin BA, Macera CA, Heath GW, Thompson PD, Bauman A.. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Med Sci Sports Exerc 2007;39:1423–1434. [DOI] [PubMed] [Google Scholar]
- 32. Kraus WE, Houmard JA, Duscha BD, Knetzger KJ, Wharton MB, McCartney JS, Bales CW, Henes S, Samsa GP, Otvos JD, Kulkarni KR, Slentz CA.. Effects of the amount and intensity of exercise on plasma lipoproteins. N Engl J Med 2002;347:1483–1492. [DOI] [PubMed] [Google Scholar]
- 33. Strasser B, Siebert U, Schobersberger W.. Resistance training in the treatment of the metabolic syndrome: a systematic review and meta-analysis of the effect of resistance training on metabolic clustering in patients with abnormal glucose metabolism. Sports Med 2010;40:397–415. [DOI] [PubMed] [Google Scholar]
- 34. Smith JK, Dykes R, Douglas JE, Krishnaswamy G, Berk S.. Long-term exercise and atherogenic activity of blood mononuclear cells in persons at risk of developing ischemic heart disease. JAMA 1999;281:1722–1727. [DOI] [PubMed] [Google Scholar]
- 35. Wang JS, Jen CJ, Chen HI.. Effects of exercise training and deconditioning on platelet function in men. Arterioscler Thromb Vasc Biol 1995;15:1668–1674. [DOI] [PubMed] [Google Scholar]
- 36. Gielen S, Adams V, Mobius-Winkler S, Linke A, Erbs S, Yu J, Kempf W, Schubert A, Schuler G, Hambrecht R.. Anti-inflammatory effects of exercise training in the skeletal muscle of patients with chronic heart failure. J Am Coll Cardiol 2003;42:861–868. [DOI] [PubMed] [Google Scholar]
- 37. Mozaffarian D, Furberg CD, Psaty BM, Siscovick D.. Physical activity and incidence of atrial fibrillation in older adults: the cardiovascular health study. Circulation 2008;118:800–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wilson MG, Ellison GM, Cable NT.. Basic science behind the cardiovascular benefits of exercise. Br J Sports Med 2016;50:93–99. [DOI] [PubMed] [Google Scholar]
- 39. Imboden MT, Harber MP, Whaley MH, Finch WH, Bishop DL, Kaminsky LA.. Cardiorespiratory fitness and mortality in healthy men and women. J Am Coll Cardiol 2018;72:2283–2292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.