Abstract
Aims
The role of statin therapy in primary prevention of cardiovascular disease in persons older than 75 years remains a subject of debate with little evidence to support or exclude the benefit of this treatment. We assessed the effect of statin discontinuation on cardiovascular outcomes in previously adherent 75-year-olds treated for primary prevention.
Methods and results
A population-based cohort study using French national healthcare databases was performed, studying all subjects who turned 75 in 2012–14, with no history of cardiovascular disease and with a statin medication possession ratio ≥80% in each of the previous 2 years. Statin discontinuation was defined as three consecutive months without exposure. The outcome was hospital admission for cardiovascular event. The hazard ratio comparing statin discontinuation with continuation was estimated using a marginal structural model adjusting for both baseline and time-varying covariates (cardiovascular drug use, comorbidities, and frailty indicators). A total of 120 173 subjects were followed for an average of 2.4 years, of whom 17 204 (14.3%) discontinued statins and 5396 (4.5%) were admitted for a cardiovascular event. The adjusted hazard ratios for statin discontinuation were 1.33 [95% confidence interval (CI) 1.18–1.50] (any cardiovascular event), 1.46 (95% CI 1.21–1.75) (coronary event), 1.26 (95% CI 1.05–1.51) (cerebrovascular event), and 1.02 (95% CI 0.74–1.40) (other vascular event).
Conclusion
Statin discontinuation was associated with a 33% increased risk of admission for cardiovascular event in 75-year-old primary prevention patients. Future studies, including randomized studies, are needed to confirm these findings and support updating and clarification of guidelines on the use of statins for primary prevention in the elderly.
Keywords: Statins, Primary prevention, Cardiovascular disease, Elderly, Treatment discontinuation
Introduction
The use of statin therapy for secondary prevention has been clearly established in all age groups. However, the role for statin therapy in primary prevention in the elderly remains a subject of debate, with little evidence for or against its benefit.1–4
The available evidence is mainly derived from subgroup and post hoc analyses of the data of randomized trials.5–8 The results of these analyses in elderly individuals treated for primary prevention are inconsistent in terms of the effect on cardiovascular morbidity, while most studies reported a non-significant effect of statin therapy on all-cause mortality. Two relevant interventional studies have recently been launched, but their findings are expected only after 2020.9
Consequently, the European guidelines on the management of dyslipidaemias and on cardiovascular disease prevention in clinical practice, respectively, provide no recommendation for or against statin use for primary prevention in persons older than 75 years and the 2018 American College of Cardiology–American Heart Association (ACC-AHA) guidelines simply recommend a shared decision-making process between clinicians and these patients that targets individualized decisions, with regular reassessments over time.10–12 However, for older adults with a coronary artery calcium (CAC) score of 0, the likelihood of benefits from statin therapy does not outweigh the risks, according to the ACC-AHA guidelines. The three guidelines underline that in older patients lipid-lowering medication should be started at a lower dose than in younger subjects.
A particularly relevant practical question is whether existing statin therapy can be stopped in older people with no history of cardiovascular disease. This issue currently concerns a large proportion of the population over the age of 75 years as well as large numbers of people under the age of 75 years currently taking evidence-based treatment with statins and reaching ages for which only limited evidence of efficacy is available.13,14 None of the above mentioned guidelines provide specific recommendations for statin discontinuation for primary prevention in persons older than 75 years, except in older adults with severe age-related management complexities. To the best of our knowledge, no study has evaluated the impact of discontinuing primary prevention with statin therapy in older people.
We therefore conducted a study to assess the effect of statin discontinuation on cardiovascular outcomes in patients who turned 75 in 2012–14, previously adherent to statin therapy for at least 2 years with no history of cardiovascular disease, using the French healthcare databases.
Methods
Study design and data source
This retrospective cohort study was based on data derived from the French national health insurance claims database [Système National d’Information Inter-Régimes de l’Assurance Maladie (SNIIRAM)], which contains information on all health spending reimbursements and which is linked to the French hospital discharge database.15 Hospital discharge diagnoses and diagnoses related to specific health insurance benefits are recorded using the International Classification of Diseases, 10th revision (ICD-10). Clinical procedures are coded according to the French procedure classification [Classification Commune des Actes Médicaux (CCAM)] and drugs are coded according to the Anatomical Therapeutic Chemical (ATC) classification. Information on individual drug use is usually not available for patients admitted to hospital or a skilled nursing home with an internal pharmacy. Apart from these exceptions, comprehensive data are available for statin use, in particular, as statins are available by prescription only and all statin prescriptions are reimbursed. This study was approved by the French data protection agency [Commission Nationale de l'Informatique et des Libertés (CNIL)]. All databases used in this study only contain anonymous patient records.
Study population
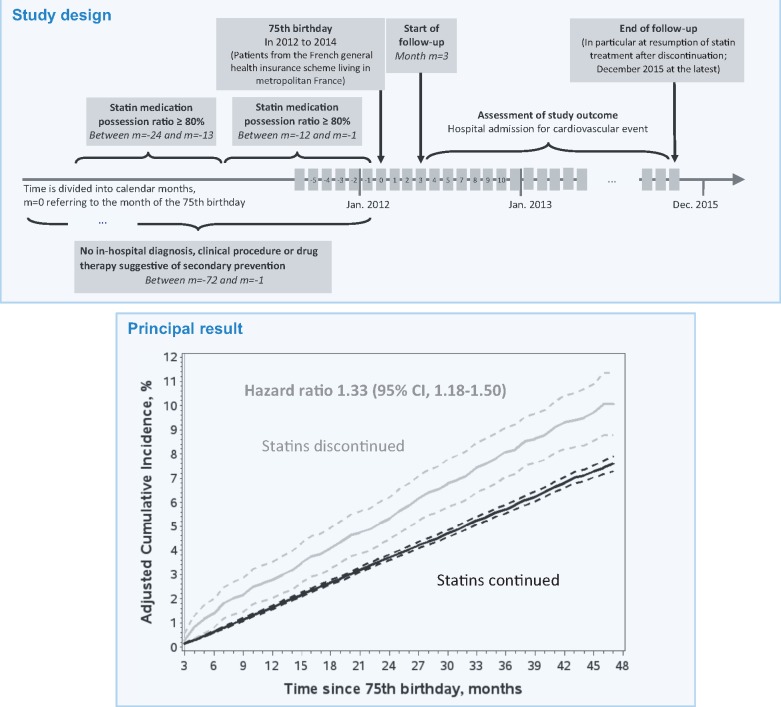
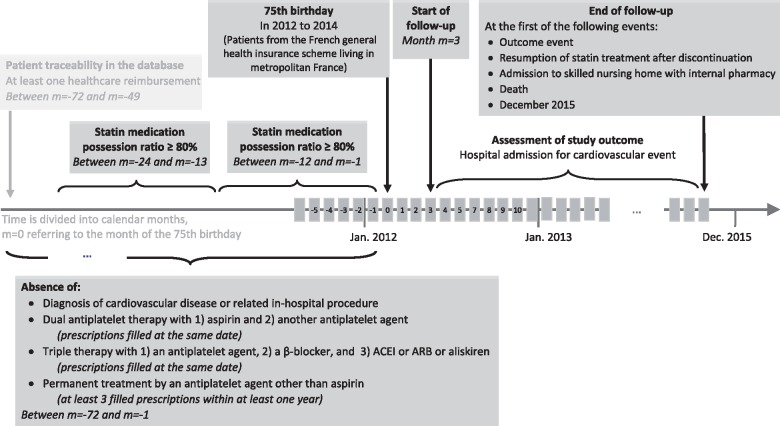
All patients who turned 75 in 2012–14 and with a statin medication possession ratio (MPR) of at least 80% in each of the previous 2 years were eligible (Figure 1, Take home figure). Patients with a diagnosis of cardiovascular disease or a related in-hospital procedure in the previous 2 years (codes in Supplementary material online, Table S1) and patients taking at least one of the following treatments during this period were excluded: (i) combined use of aspirin and another antiplatelet agent, (ii) combined use of antiplatelet agent, β-blocker, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin II receptor blocker (ARB) or aliskiren, and (iii) long-term treatment with an antiplatelet agent other than aspirin (at least three prescriptions filled over 1 year). Time was divided into calendar months, where month 0 refers to the month of the person’s 75th birthday.
Figure 1.
Diagram of the study design. Patients with an outcome or censoring event before the start of follow-up at month m = 3 were excluded. ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Take home figure.
In 75-year-old primary prevention patients previously adherent to statin therapy for at least 2 years, discontinuation of statins was associated with an increased risk of admission for a cardiovascular event. Cumulative incidence functions were adjusted for confounding due to baseline and time-varying covariates using inverse probability of treatment and censoring weighting. 95% confidence intervals are indicated by hatched curves. Under the assumptions required by this method (see Supplementary material online, Table S2), the cumulative incidence estimated for statin discontinuation represents the experience of the entire study population had all individuals discontinued statins right from the beginning of follow-up. The cumulative incidence estimated for statin continuation represents the experience of the entire study population had no individuals discontinued statins during follow-up.
Exposure
Statin exposure was modelled as a time-varying variable and was initially measured on a daily time-scale (days covered by the prescription) as described in detail in Supplementary material online, Table S2. Statin therapy was considered to be discontinued when the person presented three consecutive months without exposure.
Outcome variables and follow-up
The outcome was hospital admission for a cardiovascular event (ICD-10 and CCAM codes in Supplementary material online, Table S1, classified as coronary, cerebrovascular, and other vascular events).
Follow-up started at month 3 and continued until: (i) outcome, (ii) censoring for resumption of statin therapy after discontinuation, admission to a skilled nursing home with an internal pharmacy, or death, or (iii) December 2015, whichever occurred first (Figure 1).
Covariates
Baseline covariates included sex, deprivation index of the area of residence, residence in a skilled nursing home (without an internal pharmacy), cardiovascular drug use, comorbidities, frailty indicators, and hospital admission (total length of stay, elective, or emergency admission). Corresponding time-varying covariates were assessed monthly during follow-up. Detailed definitions are presented in Supplementary material online, Table S3.
Statistical analysis
A weighted Cox proportional hazard model (a so-called marginal structural Cox model) was used to estimate the hazard ratio (HR) of statin discontinuation vs. continuation, controlling for baseline and time-varying confounding.16–18 Each patient’s contribution to the risk set for a given month t was weighted by the inverse of the probability of the patient’s treatment history taking into account the patient’s covariate history. In order to also correct for potentially informative censoring at the time of treatment resumption, the patient’s initial weight was multiplied by the inverse probability of remaining uncensored up until month t, taking into account the patient’s covariate history. Details, including information on weight truncation, are provided in Supplementary material online, Figure S1 and Table S2. All baseline and time-varying covariates listed in Table 1 and Supplementary material online, Table S4, as well as the year of the 75th birthday, were used for weight calculation (except for the use of other lipid-lowering agents during follow-up, as it is strongly recommended to omit weak confounders that are strongly associated with treatment17).
Table 1.
Patient characteristics and medication use at baseline and at selected time intervals during follow-up
| At baseline | During follow-upa |
||||
|---|---|---|---|---|---|
| Month m = 3 | Month m = 12 | Month m = 24 | Month m = 36 | ||
| (n = 120 173) | (n = 120 173) | (n = 115 314) | (n = 71 713) | (n = 33 394) | |
| Demographic characteristics | |||||
| Male sex | 49 055 (40.8) | 49 055 (40.8) | 46 760 (40.6) | 28 530 (39.8) | 12 889 (38.6) |
| Residence in skilled nursing home | 548 (0.5) | 598 (0.5) | 720 (0.6) | 585 (0.8) | 327 (1.0) |
| Cardiovascular drug useb | |||||
| Statins | 120 173 (100.0) | 119 035 (99.1) | 110 461 (95.8) | 66 033 (92.1) | 29 936 (89.6) |
| Antihypertensive agents | 94 882 (79.0) | 91 845 (76.4) | 88 602 (76.8) | 55 431 (77.3) | 26 084 (78.1) |
| Vasodilator agents | 3332 (2.8) | 2151 (1.8) | 1970 (1.7) | 1282 (1.8) | 574 (1.7) |
| Antiarrhythmic agents | 6872 (5.7) | 6349 (5.3) | 6231 (5.4) | 3949 (5.5) | 1859 (5.6) |
| Antiplatelet agents | 29 511 (24.6) | 27 968 (23.3) | 27 982 (24.3) | 17 997 (25.1) | 8602 (25.8) |
| Anticoagulants | 16 580 (13.8) | 12 555 (10.4) | 12 710 (11.0) | 8287 (11.6) | 4095 (12.3) |
| Antidiabetic agents | 31 912 (26.6) | 31 229 (26.0) | 30 108 (26.1) | 18 636 (26.0) | 8620 (25.8) |
| Other lipid-lowering agents | 2789 (2.3) | 2509 (2.1) | 2680 (2.3) | 1949 (2.7) | 903 (2.7) |
| Hospital admission in the 3 prior months | 5756 (4.8) | 6186 (5.1) | 6260 (5.4) | 4057 (5.7) | 1890 (5.7) |
| Comorbidities | |||||
| Cancer in preceding 2 years | 5128 (4.3) | 5330 (4.4) | 5249 (4.6) | 3347 (4.7) | 1546 (4.6) |
| Dementia | 2323 (1.9) | 2477 (2.1) | 2812 (2.4) | 2131 (3.0) | 1210 (3.6) |
| Chronic pulmonary disease | 21 902 (18.2) | 22 452 (18.7) | 22 756 (19.7) | 14 916 (20.8) | 7383 (22.1) |
| Connective tissue disease | 1832 (1.5) | 1884 (1.6) | 1954 (1.7) | 1321 (1.8) | 664 (2.0) |
| Liver disease | 1198 (1.0) | 1259 (1.0) | 1319 (1.1) | 885 (1.2) | 435 (1.3) |
| Diabetes (diagnosis) | 29 337 (24.4) | 29 805 (24.8) | 29 582 (25.7) | 18 676 (26.0) | 8811 (26.4) |
| Psychoses | 1461 (1.2) | 1485 (1.2) | 1485 (1.3) | 912 (1.3) | 442 (1.3) |
| Depression | 4751 (4.0) | 4930 (4.1) | 5177 (4.5) | 3557 (5.0) | 1759 (5.3) |
| Parkinson’s disease | 1064 (0.9) | 1118 (0.9) | 1245 (1.1) | 898 (1.3) | 488 (1.5) |
| Frailty indicators | |||||
| Malnutrition | 1245 (1.0) | 1467 (1.2) | 1970 (1.7) | 1565 (2.2) | 929 (2.8) |
| Tendency to fall | 430 (0.4) | 477 (0.4) | 628 (0.5) | 543 (0.8) | 315 (0.9) |
| Bed confinement status, home hospital bed, or anti-bedsore equipment | 2881 (2.4) | 3103 (2.6) | 3566 (3.1) | 2666 (3.7) | 1505 (4.5) |
| Wheelchair | 2166 (1.8) | 2305 (1.9) | 2554 (2.2) | 1871 (2.6) | 1018 (3.0) |
Data are expressed as number (column %). For cardiovascular drug use, the baseline values refer to the year preceding the 75th birthday and follow-up values refer to months m-3 to m-1. Baseline characteristics for comorbidities and the listed frailty indicators were identified during the 6 years preceding the 75th birthday, and once detected, a comorbidity or frailty indicator was assumed to persist until the end of follow-up, except for cancer. Details of certain characteristics, e.g. antihypertensive agents, cancer, and previous hospital admissions (total length of stay and emergency admission), as well as additional characteristics are given in Supplementary material online, Table S4.
Data refer to patients present in the study at the beginning of the month considered. Note that follow-up started at month 3 after the month of the 75th birthday and did not exceed month 47 and that follow-up stopped at the time of resumption of statin therapy (after discontinuation).
Statin exposure was determined as indicated in the text, while exposure to other drugs was identified by the presence of at least one filled prescription.
Predefined subgroup analyses, including tests for effect heterogeneity, were performed, as well as analyses separately considering hospital admission for coronary, cerebrovascular, and other vascular events, each with censoring at admission for one of the two other types of cardiovascular event.
Sensitivity analyses focused on the impact of weight truncation and the role of the various covariates in adjustment, as well as the impact of the definition of statin discontinuation and the sensitivity with respect to the use of other lipid-lowering agents during follow-up.
In complementary analyses, conventional multivariate Cox analyses were performed and hospital admission for renal colic (ICD-10 codes N20, N21, N23) was used as a negative control outcome to evaluate the healthy-adherer effect.
All calculations were performed using SAS, version 9.2 software (SAS Institute Inc.). P < 0.05 (two-tailed) was considered to be statistically significant.
Results
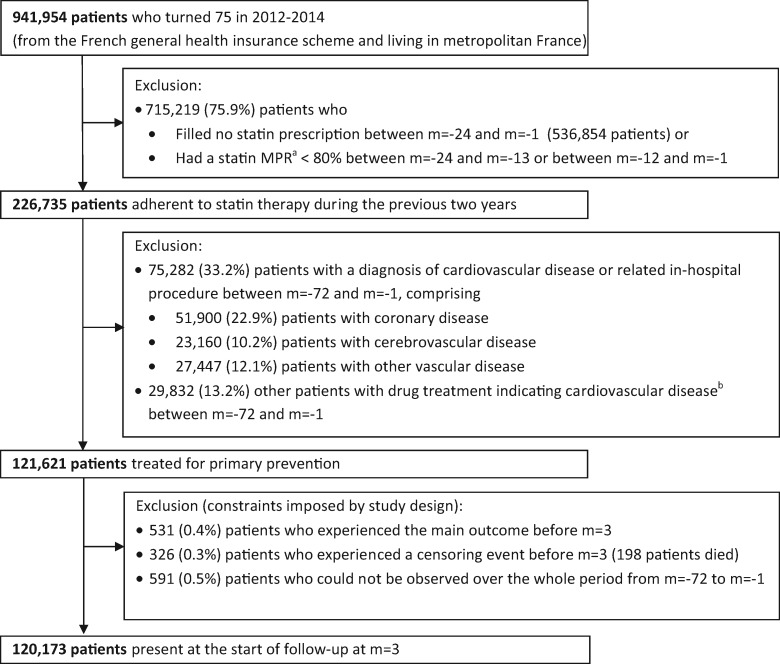
A total of 120 173 patients were included in the study (Figure 2). The mean duration of follow-up was 2.4 years (maximum 4 years). Baseline and time-varying characteristics are summarized in Table 1 and described in detail in Supplementary material online, Table S4. A total of 5396 patients were admitted for cardiovascular events, corresponding to a crude incidence rate of 2.1 per 100 patient-years. These patients comprised 2299 patients with a coronary event [including 1233 with ICD-10 diagnosis code I20 (Angina pectoris) and 542 with I21 (Acute myocardial infarction)], 2328 other patients with a cerebrovascular event [931 with ICD-10 code I63 (Cerebral infarction), 525 with G45 (Transient cerebral ischaemic attacks and related syndromes), and 521 with I65 (Occlusion and stenosis of precerebral arteries, not resulting in cerebral infarction)] and 769 other patients with another vascular event [324 with ICD-10 code I74 (Arterial embolism and thrombosis)]. A total of 3243 patients died during follow-up with no previous admission for cardiovascular events, including 1005 (31.0%) patients who died outside of hospital.
Figure 2.
Patient selection flow chart. aMedication possession ratio, calculated as the sum of the days with exposure to statins over the period considered divided by the total number of days in this period. Statin exposure was determined as indicated in the text. bAt least one of the following therapies: (i) combined use of aspirin and another antiplatelet agent (prescriptions filled on the same day), (ii) combined use of antiplatelet agent, β-blocker, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker or aliskiren (prescriptions filled on the same day), (iii) long-term treatment by an antiplatelet agent other than aspirin (at least three prescriptions filled during at least one of the 6 years before the 75th birthday). Time was divided into calendar months. Month m = 0 refers to the month of the 75th birthday.
Statin exposure and predictors of discontinuation and resumption
Total follow-up was 3 067 730 patient-months, including 176 373 (5.7%) patient-months after discontinuation of statin therapy.
The most important factors related to statin discontinuation were hospital admission during follow-up [adjusted odds ratio (aOR) up to 3.28], admission to a skilled nursing home (aOR 2.66), metastatic solid tumour (aOR 2.22), and initiation of enteral or oral feeding (aOR 2.13). Discontinuation of ACEIs, ARBs, or aliskiren during follow-up increased the probability of statin discontinuation (aOR 1.68), while initiation of these drugs during follow-up and continuation of baseline use both decreased this probability (aOR 0.89 and 0.75, respectively). A similar impact on statin discontinuation was also observed for most other cardiovascular drugs. Important factors related to subsequent resumption of statin therapy were use of antiplatelet agents other than aspirin (aOR 2.04), use of oral antidiabetic agents (aOR 1.54), use of insulin (aOR 1.52), metastatic solid tumour during the previous 2 years (aOR 0.42), and residence in a skilled nursing home (aOR 0.33). The estimated exposure model and the estimated model of treatment resumption are described in detail in Supplementary material online, Table S5. Note that these models determine the weights used to estimate the effect of statin discontinuation consequently allowing adjustment for all of the abovementioned factors.
Among the 17 204 patients who discontinued statin therapy, 7336 (42.6%) resumed statin therapy during follow-up.
Cardiovascular events after statin discontinuation
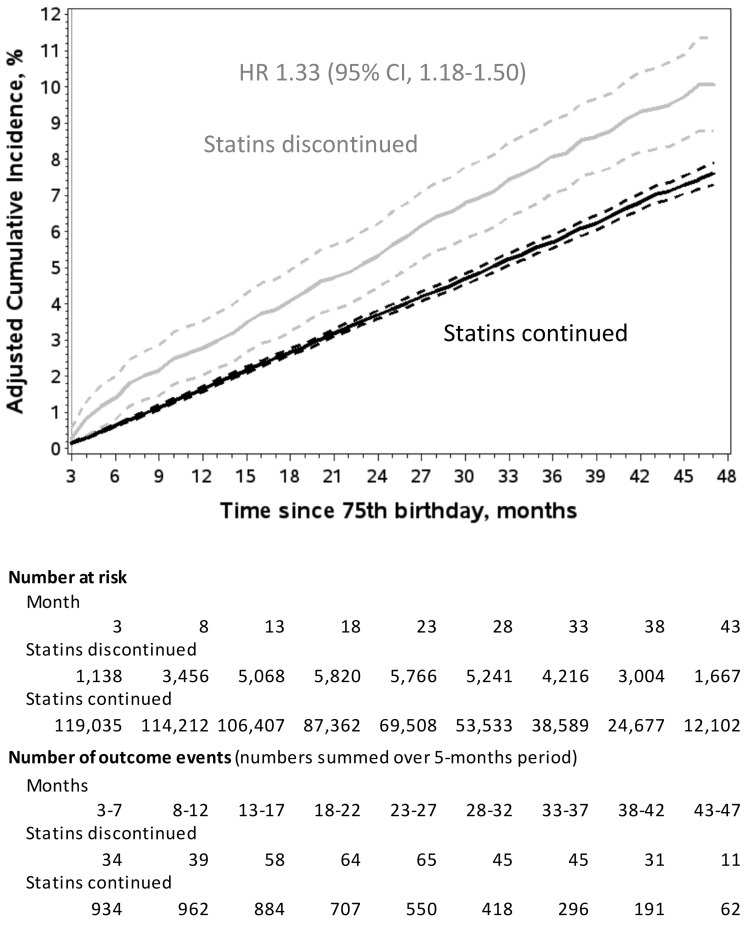
The crude HR for admission for a cardiovascular event, comparing statin discontinuation vs. continuation, was 1.26 (95% CI 1.14–1.40), while the adjusted HR was 1.33 (95% CI 1.18–1.50). Four years after the 75th birthday, the corresponding adjusted cumulative incidence rate was 10.1% (95% CI 8.8–11.3%) for statin discontinuation vs. 7.6% (95% CI 7.3–7.9%) for statin continuation (Figure 3, Take home figure).
Figure 3.
Adjusted cumulative incidence of hospital admission for cardiovascular events according to statin use. Follow-up started at month 3 after the month of the 75th birthday. Cumulative incidence functions were adjusted for confounding due to all baseline and time-varying covariates listed in Table 1 and Supplementary material online, Table S4 (except for the use of other lipid-lowering agents during follow-up), plus the year of the 75th birthday, using inverse probability of treatment and censoring weighting. 95% confidence intervals are indicated by hatched curves. Under the assumptions required by this method (see Supplementary material online, Table S2), the cumulative incidence estimated for statin discontinuation represents the experience of the entire study population had all individuals discontinued statins right from the beginning of follow-up. The cumulative incidence estimated for statin continuation represents the experience of the entire study population had no individuals discontinued statins during follow-up. CI, confidence interval; HR, hazard ratio.
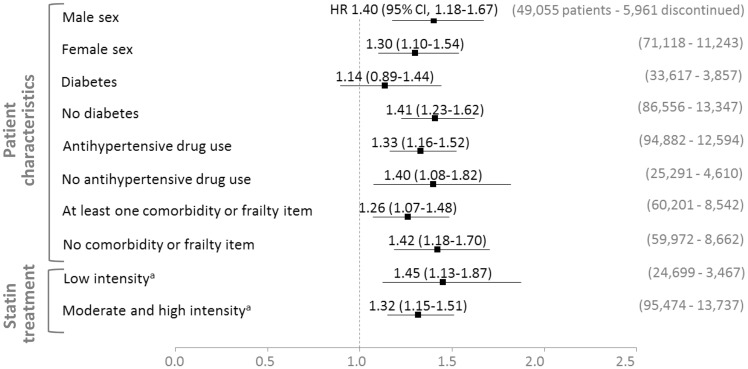
Subgroup analyses based on baseline characteristics showed no significant effect heterogeneity across sex, diabetes, antihypertensive drug use, presence of at least one of the comorbidities, and frailty indicators considered or intensity of statin therapy (Figure 4). In patients with diabetes at baseline, the estimated effect of statin discontinuation was not statistically significant.
Figure 4.
Estimated effect of statin discontinuation in patient subgroups. aLow intensity: fluvastatin 20–40 mg, pravastatin 10–20 mg, simvastatin 5–10 mg; moderate intensity: atorvastatin 10–20 mg, rosuvastatin 5–10 mg, fluvastatin 80 mg, pravastatin 40 mg, simvastatin 20–40 mg; high intensity: atorvastatin 40–80 mg, rosuvastatin 20 mg (rosuvastatin 40 mg and simvastatin 80 mg are not available in France). The outcome was hospital admission for cardiovascular event. All patient subgroups were defined at baseline. The estimated effects were adjusted for confounding due to all baseline and time-varying covariates listed in Table 1 and Supplementary material online, Table S4 (except for the use of other lipid-lowering agents during follow-up), plus the year of the 75th birthday. The result for the fluvastatin subgroup was omitted due to the small sample size of this subgroup (a total of 6345 patients with 11 and 277 events on statin discontinuation and continuation, respectively). CI confidence interval; HR, hazard ratio.
The adjusted HR for admissions for coronary, cerebrovascular, and other vascular events were 1.46 (95% CI 1.21–1.75), 1.26 (95% CI 1.05–1.51), and 1.02 (95% CI 0.74–1.40), respectively (Supplementary material online, Figure S2).
Sensitivity and complementary analyses
The results were not sensitive to the weight truncation levels used (Supplementary material online, Table S6). Cardiovascular drug use had the greatest impact on adjustment, followed by hospital admissions during follow-up, sex, and comorbidities (Supplementary material online, Table S7). When statin discontinuation was defined as 6 months without exposure to statins, the crude and adjusted HR for admission for a cardiovascular event were 1.23 (95% CI 1.09–1.38) and 1.34 (95% CI 1.18–1.53), respectively. For a 12-month gap, the crude and adjusted HR were 1.17 (95% CI 1.01–1.36) and 1.28 (95% CI 1.08–1.51), respectively. Sensitivity analyses concerning the use of other lipid-lowering agents during follow-up showed consistent results (Supplementary material online, Table S8).
The HR for admission for a cardiovascular event, estimated by a conventional Cox model using the same baseline and time-varying covariates, was 1.26 (95% CI 1.13–1.41) (Supplementary material online, Figure S3).
Finally, no significant association was observed between statin discontinuation and admission for renal colic: crude and adjusted hazard ratio (aHR) were 1.22 (95% CI 0.89–1.68) and 1.13 (95% CI 0.78–1.63), respectively.
Discussion
In this nationwide population-based study in 75-year-old primary prevention patients previously adherent to statin therapy for at least 2 years, discontinuation of statins was associated with an increased risk of admission for a cardiovascular event (+33%). This association was stronger for admissions for coronary events than for admissions for cerebrovascular events (+46% and +26% increased risk, respectively). Sensitivity analyses showed consistent results.
Statin discontinuation rate
A low statin discontinuation rate was observed in this population with a mean follow-up of 2.8 years (14.3% of patients). Nevertheless, as indicated in Figure 2, the underlying general population of 75-year-old individuals was characterized by a high prevalence of statin use and poor adherence to statin therapy: 43% were treated with statins during the previous 2 years, but 44% of these patients had a statin MPR < 80% for at least 1 of the 2 years. These figures are similar to data from other studies. In the UK, prevalent statin use was observed in 49% of individuals 80 years and older (in 2011–15) and, in the US (in 2005–08), in 45% and 39% of men and women 75 years and older, respectively.13,14 A recent meta-analysis of data from over 40 countries reported a 40% non-adherence rate among statin users 65 years and older (MPR < 80% or similar criteria).19 The low discontinuation rate in our final study population can likely be explained by the selection of patients who had been previously adherent for at least 2 years. For patients previously adherent to statin therapy for at least 1 year, another French study reported a probability of statin discontinuation of 9–12% at 9-month follow-up (in which discontinuation was defined as two consecutive months without exposure).20 Finally, some of the predictors of statin discontinuation observed in our study, e.g. female gender, dementia, and cancer, have also been identified in another recent meta-analysis in older statin users.21
Cardiovascular events after discontinuation of statin therapy
Some studies have tried to specifically address the value of statins in the elderly population. The PROSPER (Prospective Study of Pravastatin in the Elderly at Risk) trial5 evaluated the effect of pravastatin 40 mg on the risk of cardiovascular events in people aged 70–82 years (mean age 75 years). In the primary prevention subgroup of 3239 patients, statin therapy did not significantly reduce the incidence of coronary heart disease and stroke vs. placebo.
Based on data from the JUPITER (Justification for Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin) trial, comparing Rosuvastatin 20 mg vs. placebo in patients with no history of cardiovascular disease and high C-reactive protein and low low-density lipoprotein (LDL) cholesterol levels, subgroup analysis in the 5695 participants aged 70 years or older (median age 74 years) showed a significant reduction of cardiovascular events (HR 0.61, 95% CI 0.46–0.82), but not all-cause mortality (HR 0.80, 95% CI 0.62–1.04).6
The meta-analysis of primary prevention by Savarese et al.,22 based on eight trials including 24 674 people over the age of 65 years with a mean age of 73 years concluded that statins vs. placebo significantly reduced the risk of myocardial infarction by 39.4% (95% CI 15.3–56.6%) and the risk of stroke by 23.8% (95% CI 7.4–37.4%), but statins did not provide any significant benefit on the risk of cardiovascular or all-cause mortality.
The recent meta-analysis by the Cholesterol Treatment Trialists’ Collaboration, based on 28 trials, included a subgroup of 6449 primary prevention patients older than 75 years.23 Within this subgroup, cardiovascular incidence rates of 2.7 and 2.8 per 100 patient-years were found for the patient groups ‘statin or more intensive treatment’ and ‘controls or less intensive treatment’, respectively, corresponding to a rate ratio per 1 mmol/L reduction in LDL cholesterol of 0.92 (95% CI 0.73–1.16).
In an observational study, Orkaby et al.24 considered 7213 men aged 70 years or older from the Physicians’ Health Study and found that baseline statin primary prevention was associated with an 18% lower risk of all-cause mortality (aHR 0.82, 95% CI 0.69–0.98) and a non-significantly lower risk of cardiovascular events (aHR 0.86, 95% CI 0.70–1.06). Ramos et al. studied the impact of initiation of primary prevention with statins at the age of 75 years or older using primary care data in Catalonia, Spain. For the subgroup of patients aged 75–84 years, they compared 5545 new statins users with 33 439 never users, and found that statin initiation was not associated with a reduction of coronary heart disease and/or stroke in patients without diabetes (aHR 0.94, 95% CI 0.86–1.04), but with a significant reduction in patients with diabetes (aHR 0.76, 95% CI 0.65–0.89).25
It is difficult to directly compare our results with those of these studies due to the marked differences in terms of methodology, particularly as none of the abovementioned studies compared statin discontinuation vs. continuation and most studies examined patients aged 65 or 70 years and older.
The elderly population is biologically heterogeneous in terms of frailty, comorbidities, and functional and cognitive capacities.1,26 The main analysis of the present study comprised adjustment for comorbidities and frailty indicators, but omitting these covariates from adjustment only slightly modified the estimated HR (Supplementary material online, Table S7).
In our subgroup analyses, the intensity of statin therapy at baseline had little influence on the estimated effect of statin discontinuation. Compared to patients treated with moderate- or high-intensity statin therapy, patients with low intensity statin therapy less often presented cardiovascular drug use at baseline (e.g. antiplatelet agents: 20.7% vs. 25.6%; antidiabetic agents: 21.1% vs. 28.0%) and a slight lower rate of comorbidities (e.g. chronic pulmonary disease: 17.1% vs. 18.5%). The most notable difference concerned the type of statin used at baseline (e.g. pravastatin: 73.2% vs. 7.1%). Our comparison between intensities of statin therapy could therefore be misleading in the presence of heterogeneity of effects according to the type of statin. Further research on the effect of discontinuation of individual statins in older primary prevention patients is warranted.
In our study, the adjusted HR for admission for a cardiovascular event, comparing statin discontinuation vs. continuation, was 1.14 (95% CI 0.89–1.44) and 1.41 (95% CI 1.23–1.62) depending on whether diabetes was present at baseline or not. The difference can be partly explained by the higher baseline risk in patients with diabetes. For statin continuation, the adjusted cumulative incidence rate of admission for a cardiovascular event 3 years after the 75th birthday was 7.6% in diabetic patients and 5.0% in non-diabetic patients. For statin discontinuation, these rates were 10.0% and 7.2%, respectively, which means that, compared to statin continuation, these rates were 2.4 and 2.2 percentage points higher in diabetic and non-diabetic patients, respectively. Therefore, although the rate differences comparing statin discontinuation to continuation were similar in diabetic patients and in non-diabetic patients, the relative rate increase was lower in diabetic patients than in non-diabetic patients. Compared to non-diabetic patients, diabetic patients had actually more often cardiovascular drug use at baseline (e.g. antihypertensive agents: 87.7% vs. 75.5%; antiplatelet agents: 35.1% vs. 20.5%), comorbidities (e.g. chronic pulmonary disease: 20.5% vs. 17.3%), and frailty indicators (e.g. wheelchair: 2.4% vs. 1.6%). However, further investigation of the effect of statin discontinuation in older primary prevention patients with diabetes is needed.
Our results are consistent with the reported relationship between LDL levels and cardiovascular risk. According to the consensus statement from the European Atherosclerosis Society Consensus Panel, there is strong and consistent evidence from genetic studies, prospective epidemiologic cohort studies, Mendelian randomization studies, and randomized intervention trials (including statin, PCSK9 inhibitor, and ezetimibe trials) that LDL is not merely a biomarker of increased risk, but a causal factor in the pathophysiology of atherosclerotic cardiovascular disease.27 Observational studies and a Mendelian randomization study demonstrated that the LDL cholesterol level remained related to cardiovascular risk and mortality even at an advanced age.28,29 However, multiple comorbidities, often present in older patients, could modify the LDL-lowering effect of statins or amplify their side effects.
Strengths and limitations
This study, based on a population of 120 173 individuals aged 75 years at study entry derived from the comprehensive French healthcare databases, focused on patients of a given age in order to address a practical clinical issue: whether or not to stop statin primary prevention at the age of 75.
Observational studies with time-varying exposure are particularly challenging. One of the strengths of the present study is the use of marginal structural models designed to appropriately correct for time-varying confounders affected by previous exposure and for informative censoring. These models are increasingly used in pharmacoepidemiologic studies, including studies on statins.30 In this study, they allowed, in particular, adjustment for discontinuation of other cardiovascular drug therapies and other time-varying factors that contributed to statin discontinuation, such as hospital admission, admission to a skilled nursing home, metastatic solid tumour, and initiation of enteral or oral feeding. All recommendations on study design to avoid time-related bias were applied and both prevalent user bias and potential bias related to competing risks were limited, as indicated in Supplementary material online, Table S2.
This real-life study presents the fundamental limitations common to all observational studies using healthcare databases. Firstly, statin exposure was defined by prescriptions filled, but very few patients are likely to have not taken the dispensed pills, as all patients regularly filled their prescriptions. Secondly, detailed medical and socioeconomic characterization of patients was limited and lifestyle factors were not available. Certain cardiovascular risk factors, such as baseline LDL cholesterol level, tobacco use, obesity, and frailty markers, could therefore not be taken into account. Thirdly, and related to the second limitation, the exact reasons for statin discontinuation were unknown. However, the presence of major cardiovascular risk factors, indicated by cardiovascular drug use, comorbidities, and frailty indicators, was investigated both at baseline and continuously during follow-up and their association with treatment discontinuation was taken into account by the analytical method adopted. In particular, discontinuation of other cardiovascular drug therapies was corrected for. Discontinuation for statin-related adverse events was also likely less frequent in patients who had already tolerated treatment for at least 2 years. Moreover, analysis of hospital admissions for renal colic, as a negative control outcome, did not indicate the presence of a healthy-adherer bias.
Only a very small proportion of patients in the study population may have actually been treated in the context of secondary prevention, as such patients were excluded by the corresponding in-hospital diagnoses and clinical procedures, as well as drug therapy suggestive of secondary prevention. In fact, 28.4% of the identified secondary prevention patients were detected only on the basis of drug therapy. Discontinuation of statin therapy was defined as at least three consecutive months without exposure to statins and a potential short-term negative impact of statin discontinuation could therefore not be measured.
Unfortunately, the impact of statin discontinuation on cardiovascular mortality could not be studied, as causes of death were not yet included in the available databases.
Finally, the generalizability of our findings to a broader geographical context may also be limited because of the differences among countries with respect to the healthcare system, statin market, lifestyle, environment, and genetics.14
Conclusion
The results of this study suggest potential cardiovascular risk reduction associated with continuing statin therapy after the age of 75 years in persons already taking these drugs for primary prevention. However, due to the observational nature of this study, residual confounding cannot be excluded. Future studies, including interventional randomized studies, are needed to confirm these findings and support updating and clarification of guidelines on the use of statins for primary prevention in the elderly.
Supplementary Material
Acknowledgements
The authors wish to thank Drs J.M. Race and G. Even of French National Agency for Medicines and Health Products Safety for fruitful discussions during this research.
Conflict of interest: none declared.
See page 3526 for the editorial comment on this article (doi: 10.1093/eurheartj/ehz629)
References
- 1. Strandberg TE, Kolehmainen L, Vuorio A.. Evaluation and treatment of older patients with hypercholesterolemia: a clinical review. JAMA 2014;312:1136–1144. [DOI] [PubMed] [Google Scholar]
- 2. Petersen LK, Christensen K, Kragstrup J.. Lipid-lowering treatment to the end? A review of observational studies and RCTs on cholesterol and mortality in 80+-year olds. Age Ageing 2010;39:674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gurwitz JH, Go AS, Fortmann SP.. Statins for primary prevention in older adults: uncertainty and the need for more evidence. JAMA 2016;316:1971–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pedro-Botet J, Climent E, Chillarón JJ, Toro R, Benaiges D, Roux Ja F-L.. Statins for primary cardiovascular prevention in the elderly. J Geriatr Cardiol 2015;12:431–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey G, Westendorp RG; PROSPER study group; PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002;360:1623–1630. [DOI] [PubMed] [Google Scholar]
- 6. Glynn RJ, Koenig W, Nordestgaard BG, Shepherd J, Ridker PM.. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: exploratory analysis of a randomized trial. Ann Intern Med 2010;152:488–496, W174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yusuf S, Bosch J, Dagenais G, Zhu J, Xavier D, Liu L, Pais P, López-Jaramillo P, Leiter LA, Dans A, Avezum A, Piegas LS, Parkhomenko A, Keltai K, Keltai M, Sliwa K, Peters RJG, Held C, Chazova I, Yusoff K, Lewis BS, Jansky P, Khunti K, Toff WD, Reid CM, Varigos J, Sanchez-Vallejo G, McKelvie R, Pogue J, Jung H, Gao P, Diaz R, Lonn E; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 8. Han BH, Sutin D, Williamson JD, Davis BR, Piller LB, Pervin H, Pressel SL, Blaum CS; ALLHAT Collaborative Research Group. Effect of statin treatment vs usual care on primary cardiovascular prevention among older adults: the ALLHAT-LLT randomized clinical trial. JAMA Intern Med 2017;177:955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonnet F, Poulizac P, Joseph J-P.. Safety and efficacy of statins. Lancet 2017;389:1097–1098. [DOI] [PubMed] [Google Scholar]
- 10. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen M-R, Tokgozoglu L, Verschuren WMM, Vlachopoulos C, Wood DA, Zamorano JL; ESC Scientific Document Group. 2016 ESC/EAS Guidelines for the management of dyslipidaemias. Eur Heart J 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 11. Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney M-T, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen M-L, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der WH, van DI, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J 2016;37:2315–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, S de F, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC, Sperling L, Virani SS, Yeboah J.. AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the management of blood cholesterol. Circulation 2018;2018:CIR0000000000000625. [Google Scholar]
- 13. Gulliford M, Ravindrarajah R, Hamada S, Jackson S, Charlton J.. Inception and deprescribing of statins in people aged over 80 years: cohort study. Age Ageing 2017;46:1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laslett LJ, Alagona P, Clark BA, Drozda JP, Saldivar F, Wilson SR, Poe C, Hart M.. The worldwide environment of cardiovascular disease: prevalence, diagnosis, therapy, and policy issues: a report from the American College of Cardiology. J Am Coll Cardiol 2012;60:S1–S49. [DOI] [PubMed] [Google Scholar]
- 15. Tuppin P, Rudant J, Constantinou P, Gastaldi-Ménager C, Rachas A, Roquefeuil L. D, Maura G, Caillol H, Tajahmady A, Coste J, Gissot C, Weill A, Fagot-Campagna A.. Value of a national administrative database to guide public decisions: from the système national d’information interrégimes de l’Assurance Maladie (SNIIRAM) to the système national des données de santé (SNDS) in France. Rev Epidemiol Sante Publique 2017;65 Suppl 4:S149–S167. [DOI] [PubMed] [Google Scholar]
- 16. Hernán MA, Brumback B, Robins JM.. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 17. Cole SR, Hernán MA.. Constructing inverse probability weights for marginal structural models. Am J Epidemiol 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Westreich D, Cole SR, Tien PC, Chmiel JS, Kingsley L, Funk MJ, Anastos K, Jacobson LP.. Time scale and adjusted survival curves for marginal structural cox models. Am J Epidemiol 2010;171:691–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ofori-Asenso R, Jakhu A, Zomer E, Curtis AJ, Korhonen MJ, Nelson M, Gambhir M, Tonkin A, Liew D, Zoungas S.. Adherence and persistence among statin users aged 65 years and over: a systematic review and meta-analysis. J Gerontol A Biol Sci Med Sci 2018;73:813–819. [DOI] [PubMed] [Google Scholar]
- 20. Bezin J, Francis F, Nguyen NV, Robinson P, Blin P, Fourrier-Réglat A, Pariente A, Moore N.. Impact of a public media event on the use of statins in the French population. Arch Cardiovasc Dis 2017;110:91–98. [DOI] [PubMed] [Google Scholar]
- 21. Ofori-Asenso R, Jakhu A, Curtis AJ, Zomer E, Gambhir M, Jaana Korhonen M, Nelson M, Tonkin A, Liew D, Zoungas S.. A systematic review and meta-analysis of the factors associated with nonadherence and discontinuation of statins among people aged ≥65 years. J Gerontol A Biol Sci Med Sci 2018;73:798–805. [DOI] [PubMed] [Google Scholar]
- 22. Savarese G, Gotto AM, Paolillo S, D'Amore C, Losco T, Musella F, Scala O, Marciano C, Ruggiero D, Marsico F, De Luca G, Trimarco B, Perrone-Filardi P.. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol 2013;62:2090–2099. [DOI] [PubMed] [Google Scholar]
- 23.Cholesterol Treatment Trialists’ Collaboration. Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet 2019;393:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Orkaby AR, Gaziano JM, Djousse L, Driver JA.. Statins for primary prevention of cardiovascular events and mortality in older men. J Am Geriatr Soc 2017;65:2362–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ramos R, Comas-Cufí M, Martí-Lluch R, Balló E, Ponjoan A, Alves-Cabratosa L, Blanch J, Marrugat J, Elosua R, Grau M, Elosua-Bayes M, García-Ortiz L, Garcia-Gil M.. Statins for primary prevention of cardiovascular events and mortality in old and very old adults with and without type 2 diabetes: retrospective cohort study. BMJ 2018;362:k3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Singh M, Stewart R, White H.. Importance of frailty in patients with cardiovascular disease. Eur Heart J 2014;35:1726–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ference BA, Ginsberg HN, Graham I, Ray KK, Packard CJ, Bruckert E, Hegele RA, Krauss RM, Raal FJ, Schunkert H, Watts GF, Borén J, Fazio S, Horton JD, Masana L, Nicholls SJ, Nordestgaard BG, van de Sluis B, Taskinen M-R, Tokgözoğlu L, Landmesser U, Laufs U, Wiklund O, Stock JK, Chapman MJ, Catapano AL.. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur Heart J 2017;38:2459–2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lind L, Sundström J, Ärnlöv J, Lampa E.. Impact of aging on the strength of cardiovascular risk factors: a longitudinal study over 40 years. J Am Heart Assoc 2018;7:e007061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Postmus I, Deelen J, Sedaghat S, Trompet S, Craen A. D, Heijmans BT, Franco OH, Hofman A, Dehghan A, Slagboom PE, Westendorp RGJ, Jukema JW.. LDL cholesterol still a problem in old age? A Mendelian randomization study. Int J Epidemiol 2015;44:604–612. [DOI] [PubMed] [Google Scholar]
- 30. Yang S, Eaton CB, Lu J, Lapane KL.. Application of marginal structural models in pharmacoepidemiologic studies: a systematic review. Pharmacoepidemiol Drug Saf 2014;23:560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.