Abstract
Introduction
Ischemia-reperfusion injury (IRI) is a serious complication of hepatectomy and liver transplantation. The aim of this study was to evaluate the protective effects of salvianolic acid-A (Sal-A) against IRI-induced hepatocellular injury.
Material and methods
Forty rats were randomly divided into the following four groups: (1) sham group, (2) IR group, (3) Sal-A(10) group and (4) Sal-A(20) group. After 90 min of ischemia and 6 h of reperfusion, serum alanine aminotransferease (ALT) and apartate aminotransferase (AST) levels were measured; the amounts of malondialdehyde (MDA) and superoxide dismutase (SOD) in the liver tissue were determined; the expression of Bcl-2 and caspase-3 was detected and the severity of apoptosis, inflammation and pathological alterations were evaluated. Also apoptosis and mRNA and protein levels of TLR4 (toll-like receptor 4) were tested.
Results
The serum aminotransferases, hepatic MDA concentration, and apoptotic cells in the IR group were significantly higher than in the sham group (p < 0.01), whereas the Sal-A group values were lower than in the IR group (p < 0.05). Compared with the IR group, the Sal-A groups had significantly higher Bcl-2 expression and downregulated cleaved caspase-3 expression in liver tissue. Moreover, increased mRNA and protein levels of TLR4 in IR rats and Sal-A could improve the increased mRNA and protein levels of TLR4.
Conclusions
Sal-A had a synergistically protective effect on the liver tissue against IRI that might be due to decreased oxidative stress, inflammation, hepatocellular apoptosis and include, at least in part, the regulation of TLR4.
Keywords: ischemia-reperfusion, liver, salvianolic acid-A, inflammation, apoptosis, toll-like receptor 4
Introduction
Hepatic ischemia-reperfusion injury (IRI) is a common pathological process in many clinical situations, such as liver transplantation and liver surgery, as well as shock-induced liver failure [1]. More and more strategies have been studied to prevent IRI-related liver damage. Several studies suggest that a series of pathophysiological mechanisms, including the excessive production of reactive oxygen species (ROS) and inflammation, are involved in IRI [2, 3]. It has also been reported that apoptotic cell death participates in IRI-induced liver tissue damage [4]. Therefore, the anti-inflammatory and anti-apoptosis mechanisms involve the protective effects for hepatic IRI.
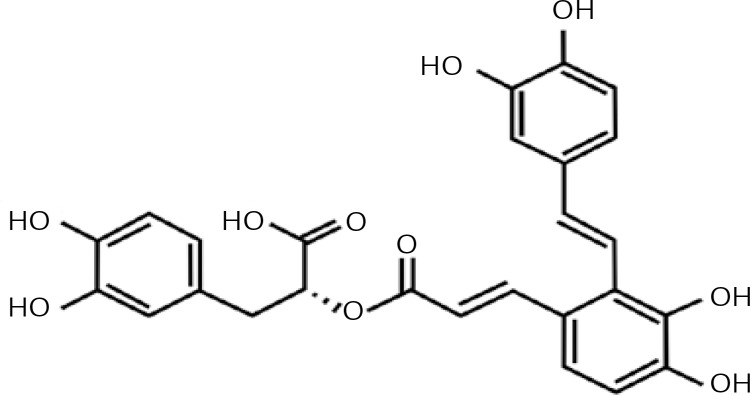
Salvianolic acid-A (Sal-A) is the most abundant and bioactive compound extracted from Salvia miltiorrhiza, which is a frequent component in traditional Chinese medications [5]. Its chemical structure is shown in Figure 1. Fan et al. reported that Sal-A has anti-oxidative and anti-apoptotic effects in myocardial IRI both in vivo and in vitro [6]. Pan et al. also confirmed that Sal-A can protect rat hearts and cardiomyocytes after IRI by reducing lipid peroxidation, scavenging reactive oxygen species and inflammation [7]. It has also been reported that Sal-A blocks inflammatory responses and preserves the integrity of the blood brain barrier; Sal-A can also enhance Bcl-2 expression levels for neuroprotection after cerebral ischemia in rats [8]. Presently, the majority of studies on Sal-A are focused on cardiomyocytes and cerebral IRI, while its protective effects against hepatic IRI have not been fully emphasized.
Figure 1.
Chemical structure of Sal-A
Toll-like receptor 4 (TLR4), a member of the TLR family, exerts a critical role in organic IR injuries. Interestingly, TLR4 mutant mice have been recently demonstrated to be resistant to shock-induced gut injury [9]. TLR4 mutant significantly abated the intestinal and hepatic IRI at least in part by alleviating the inflammatory response and oxidative stress [10, 11]. However, to date, it is unclear whether Sal-A has an effect on hepatic IRI and the link between TLR4 and Sal-A. In our study, we investigated the roles of apoptosis, inflammation and oxidative stress in rats with hepatic IRI, and we proved that Sal-A can attenuate hepatic IRI by reducing hepatocellular apoptosis, oxidative stress, inflammation and mRNA and protein levels of TLR4 in rats.
Material and methods
Material
Forty healthy male Sprague-Dawley rats weighing 180–220 g were purchased from the Animal Center, the Dalian Medical University. Sal-A (purity > 98%) was purchased from Xiya Reagent Technology Co., Ltd. (Sichuang), China. Rabbit anti-rat Bcl-2 and caspase-3 polyclonal antibodies were purchased from Proteintech (Wuhan, China). Malondialdehyde (MDA) and adenine nucleotide assay kits were purchased from Nanjing Jiancheng Bioengineering Co. Ltd (Nanjing, China). A secondary antibody labeled with FITC was provided by Beijing Zhong Shan Biotechnology Co., Ltd. (Beijing, China). The terminal deoxynucleotidyl transferase (TdT) mediated dUTP nick-end labeling (TUNEL) in situ cell death detection kit was from Roche Co., Ltd.
Experimental groups
Forty rats were randomly divided into the following four groups: (1) sham group, (2) IR group, (3) Sal-A(10) group and (4) Sal-A(20) group. There were 10 rats in each group. The rats in each group received the following treatments: (1) sham group, anesthesia and a laparotomy; (2) IR group, the portal and arterial branches into the median and left lobes were blocked for 90 min; (3) Sal-A(10) group, 10 mg/kg injection of Sal-A intravenously 10 min prior to the sustained ischemia; and (4) Sal-A(20) group, 20 mg/kg injection of Sal-A intravenously 10 min prior to the sustained ischemia. The experimental protocol was approved by the Ethical and Research Committee of Dalian Medical University.
The rats were fasted overnight with free access to water before the surgical procedures. Anesthesia was achieved by an intraperitoneal injection of 1% pentobarbital sodium (40 mg/kg) before a midline incision was made through the abdomen to expose the left branches of the portal vein and hepatic artery. A model of 70% partial hepatic ischemia was established according to standardized procedures [12]. For IRI induction, the interruption went on for 90 min followed by reperfusion for 6 h. After these procedures, the abdominal cavity was closed with 3-0 silk suture. Liver samples were harvested for further examination after the respective treatments and before the blood was collected directly from the aorta.
Pathology examination
Hepatic samples were fixed in 4% paraformaldehyde for 48 h and then embedded in paraffin wax and cut into 4 μm sections. The sections were stained with hematoxylin and eosin (H&E). The degree of hepatic tissue damage was determined blindly by two experienced pathologists on ten separated microscopic fields and scored on a scale from 0 to 3. The severity of tissue damage was defined in a previous study [13].
Serum aminotransferase analysis
Blood samples from the abdominal aorta were centrifuged at 3,000 rpm for 5 min to collect the serum, which was then stored at –20°C until being assayed. The serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels were determined with an automatic biochemical analyzer (OLYMPUS AU5400, Tokyo, Japan).
Tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) in liver tissue by ELISA
The supernatant from the homogenate was collected, and the protein concentrations were determined with biochemical assays. The concentrations of TNF-α and IL-6 were evaluated by ELISA using TNF-α and IL-6 ELISA kits (Shanghai BlueGene Biotech Co., Ltd. China) according to the manufacturer’s instructions. The concentrations of TNF-α and IL-6 were determined by comparing the absorbance of the samples to the standard curves and normalized to the protein concentrations.
Biochemical assays
Frozen liver tissue (5 mg) was cut into pieces and homogenized with proteinase inhibitor containing pre-cooled phosphate buffered saline (PBS) solution. The supernatant from the homogenate was collected to evaluate the concentrations of malondialdehyde (MDA) and superoxide dismutase (SOD) using tissue MDA and SOD kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China) according to the manufacturer’s instructions. The protein concentrations were determined using a BCA Protein Assay kit (Transgen biotech, Beijing, China). The units for the concentration are expressed as nmol/mg protein and U/mg protein, respectively.
TUNEL staining in liver tissues
The liver tissues were post-fixed in 4% paraformaldehyde for 2 days. A sequential 20% to 30% sucrose treatment was performed for 1 day; then, the intestinal samples were cryosectioned (10 μm thickness), and the samples were sliced. Cell apoptosis in the intestines was evaluated by TUNEL staining using an In Situ Cell Death Detection Kit (Roche, Shanghai, China) according to the manufacturer’s protocol. A Leica DM 4000B microscope or Leica TCS SP5 microscope was used to examine staining via conventional or confocal imaging, respectively. TUNEL quantification was made by counting the TUNEL-positive cells using five random fields at 400× magnification.
Western blotting analysis
The hepatic tissues were lysed in RIPA (Beyotime, China) and centrifuged at 10,000g for 10 min at 4°C. Homogenates containing 30 μg of protein per well were resolved on 12% polyacrylamide sodium dodecyl sulfate gels and electrophoretically transferred to polyvinylidene difluoride membranes. The membranes were incubated with primary antibodies after blocking with 5% bovine serum albumin and then with secondary antibody. Immunoreactive proteins were detected by enhanced chemiluminescence. β-actin was used as an internal control.
RNA extraction and qRT-PCR
Total RNA of liver was extracted using the TRIzol reagent (TaKaRa, Dalian, China). cDNA was synthesized with the TransScript All-in-One First-Strand cDNA Synthesis SuperMix for qPCR Kit (TransGen Biotech, Beijing, China). TLR4 mRNA levels were determined by a reverse transcription PCR analysis using the following primers: TIPE2, F: 5′-CTCACAACTTCAGTGGCTGGATTTA-3′; R: 5′-GTCTCCACA GCCACCAGATTCTC-3′; β-actin, F: 5′-AGCCATGTACGTAGCCATCC-3′; R: 5′-ACCCTCATAGATGGGCACAG- 3′. TLR4 and β-actin primers were provided by Invitrogen (Shanghai, China). Quantitative real-time PCR analyses were performed to detect mRNA level using a TransStart Top Green qPCR SuperMix kit (TransGen Biotech, Beijing, China). Samples were run in a 7500 Fast Real-time PCR System (Applied Biosystems), and β-actin was used as an internal control. Relative expression levels were calculated using the 2–ΔΔCt method.
Statistical analysis
Results were expressed as the mean ± standard error of the mean (SEM). Significant differences were analyzed by one-way ANOVA test. Statistical analysis was performed using SPSS 19.0 (SPSS, Inc., Chicago, IL, USA), and the difference was considered statistically significant when p < 0.05.
Results
Histopathological examination
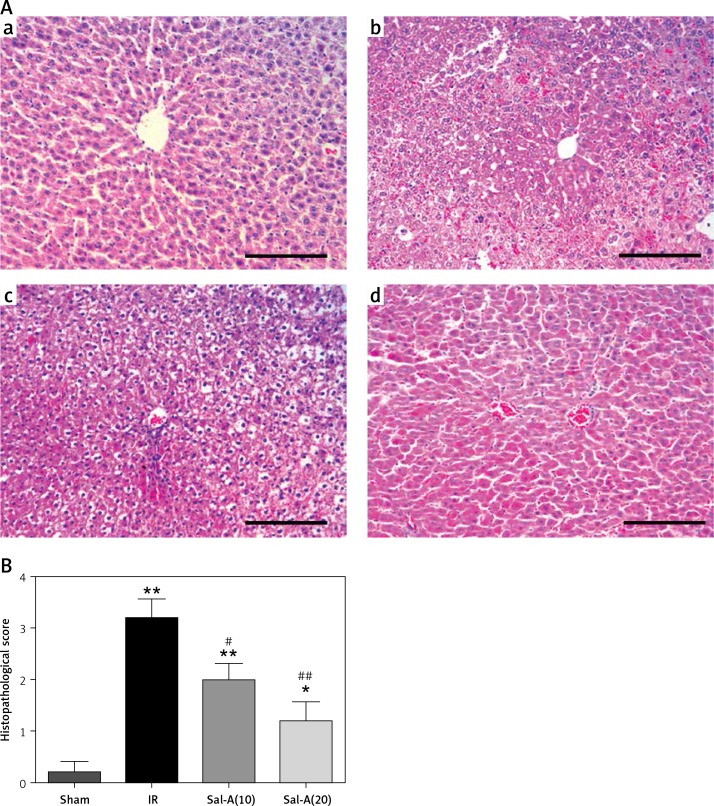
The histopathological changes and relevant scores for the sham, IR and Sal-A groups are shown in Figure 2. The sham group showed normal liver tissue. Samples from the IR group showed multiple areas of inflammatory infiltration and extensive nuclear pyknosis. In contrast, only mild necrosis and cytoplasmic vacuolation were observed in scattered areas in the liver from the rats in the Sal-A groups. Accordingly, the histopathological alterations in the IR group were scored higher than those in the sham group (p < 0.05), whereas the scores in the Sal-A groups were significantly lower than those in the IR group (p < 0.05).
Figure 2.
Histopathological examination of liver tissue. A – The liver histopathology changes (200×) in each group. a – Sham group, b – IR group, c – Sal-A(10) group, d – Sal-A(20) group. B – Liver histopathological scores for each group
*P < 0.05 vs. sham group, **p < 0.01 vs. sham group, #p < 0.05 vs. IR group, and ##p < 0.01 vs. IR group (n = 5).
Serum ALT and AST levels
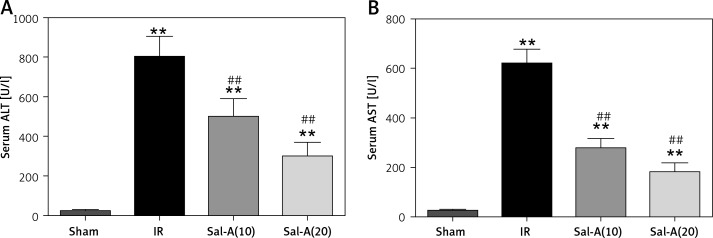
The serum ALT and AST values in each group are shown in Figure 3. Serum ALT and AST levels in the IR group were significantly higher than in the sham-operated animals after 90 min ischemia and 6 h reperfusion (p < 0.01), and the serum ALT and AST levels were significantly lower in the Sal-A groups than in the IR group (p < 0.01).
Figure 3.
Serum ALT and AST levels. A – Serum ALT levels for each group. B – Serum AST levels for each group
**P < 0.01 vs. sham group and ##p < 0.01 vs. IR group (n = 5).
Serum levels of inflammatory factors
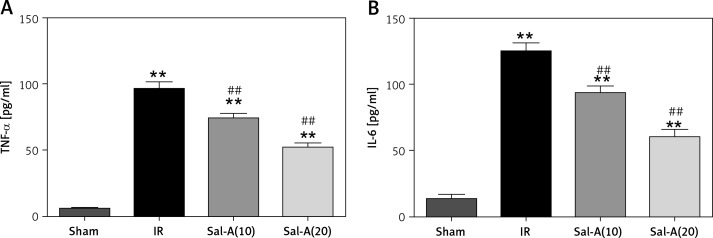
IRI resulted in a significant increase in serum levels of TNF-α and IL-6 compared to the sham group (p < 0.05). Sal-A could decrease the increased levels of TNF-α and IL-6 compared to the IR group (Figure 4).
Figure 4.
Serum levels of inflammatory factors. A – Serum TNF-α levels for each group. B – Serum IL-6 levels for each group
**P < 0.01 vs. sham group and ##p < 0.01 vs. IR group (n = 5).
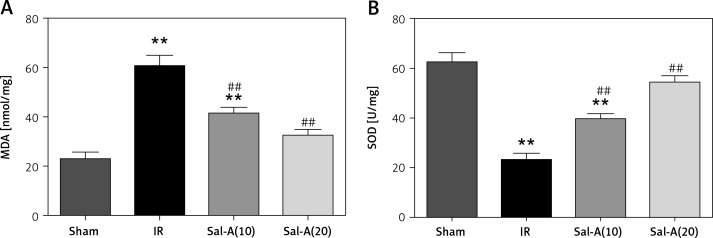
MDA and SOD in liver tissue
The liver tissue concentrations of MDA and SOD in each group are shown in Figure 5. The IR group displayed significantly increased MDA and decreased SOD compared with the IR group (p < 0.01). The MDA level was significantly lower, and SOD was higher in the Sal-A groups than in the IR group (p < 0.01).
Figure 5.
MDA and SOD in liver tissue. A – MDA concentrations for each group. B – SOD concentrations for each group
**P < 0.01 vs. sham group and ##p < 0.01 vs. IR group (n = 3).
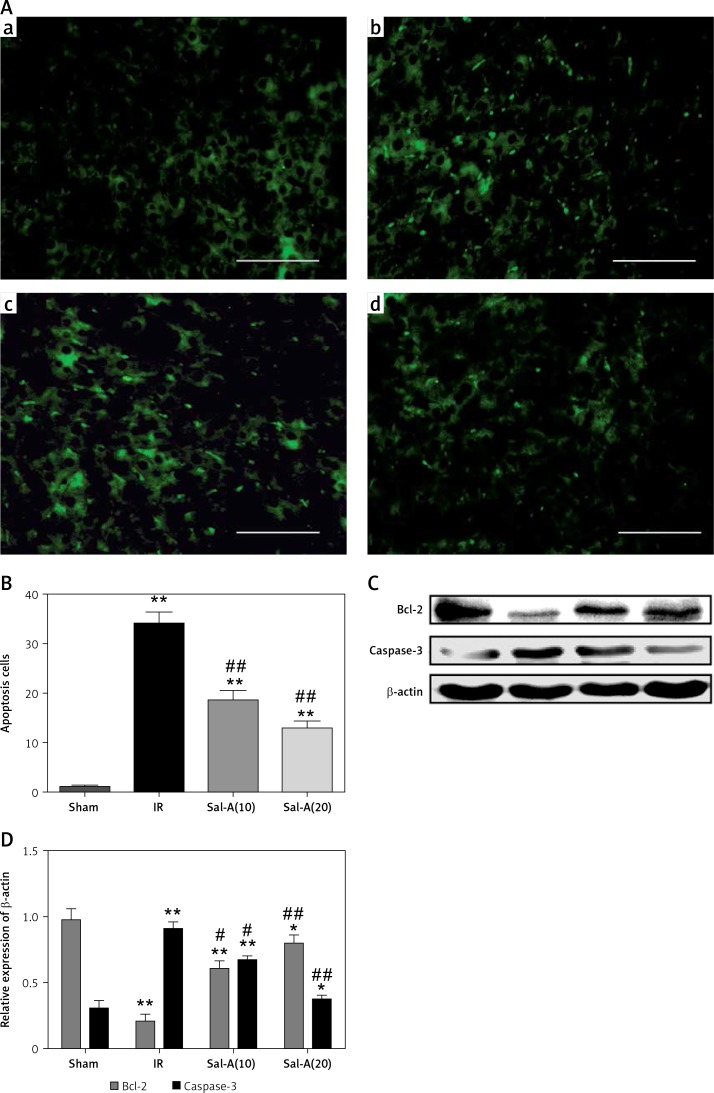
Apoptosis evaluation in liver tissue
The TUNEL staining and apoptosis index are shown in Figures 6 A and B, respectively. The apoptosis indexes in the livers from the IR group were significantly higher than in the sham-operated animals after 90 min ischemia and 6 h reperfusion (p < 0.01). In contrast, treatment with Sal-A (Sal-A groups) significantly decreased the apoptotic index compared with the IR group (p < 0.01).
Figure 6.
Apoptosis evaluation in liver tissue. A – TUNEL staining for each group (400×). a – Sham group, b – IR group, c – Sal-A(10) group, d – Sal-A(20) group. B – Apoptosis index for each group (n = 5). C, D – Protein expression levels for Bcl-2 and caspase-3 index in each group (n = 3)
*P < 0.05 vs. sham group, **p < 0.01 vs. sham group, #p < 0.05 vs. IR group and ##p < 0.01 vs. IR group.
We also detected the expression of apoptosis-related proteins (Figures 6 C, D). We found that Bcl-2 was weakly expressed in the IR group, with Bcl-2 levels being higher in the Sal-A groups than the IR group. In contrast, the reverse pattern was observed for caspase-3, with the strongest expression occurring in the IR group and the weakest expression in the Sal-A groups.
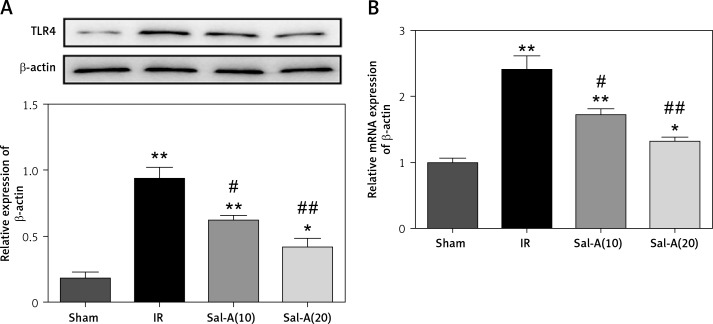
Sal-A down-regulated the protein and mRNA levels of TLR4
Recent studies have reported that TLR4 mutant significantly abated the hepatic IRI at least in part by alleviating the inflammatory response and oxidative stress. In addition, our study detected the protein and mRNA levels of TLR4 in liver by western blot and qRT-PCR. The results revealed significantly increased TLR4 in the IR group compared with the control groups. The mRNA and protein levels of TLR4 were significantly decreased in Sal-A(10) and Sal-A(20) groups compared with the IR group (Figures 7 A, B).
Figure 7.
Sal-A down-regulated the protein and mRNA levels of TLR4. A – Protein levels of TLR4 in the liver were detected via western blot. B – mRNA levels of TLR4 in the liver were detected via qRT-PCR
*P < 0.05 vs. sham group, **p < 0.01 vs. sham group, #p < 0.05 vs. IR group and ##p < 0.01 vs. IR group.
Discussion
Hepatic injury caused by ischemia/reperfusion can be a limiting factor for hepatectomy and transplantation in clinical settings. In these conditions, reperfusion induces further hepatocellular damage due to the accumulation of inflammatory cells and mediators, reactive oxygen species, and subsequent biochemical derangements in intracellular homeostasis [14]. Hepatic IRI may lead to delayed graft function and a higher incidence of chronic rejection in the case of transplant recipients or may increase the complications and length of hospital stay for patients experiencing hepatectomy or shock [15]. In the current study, we aimed to investigate whether Sal-A could significantly decrease ALT and AST serum levels and markedly attenuate hepatic histopathological deterioration, inflammation and apoptosis induced by IRI. This study provides data that can be used in further investigations and that hold great potential for possible clinical applications.
The antioxidant effects of Salvia miltiorrhiza have been recently identified in many studies [16, 17]. Oxidative stress is a powerful inducer of cell death in IRI. Several studies have indicated that Sal-A, a natural and potent antioxidant, can scavenge OFRs generated in IRI. MDA and SOD are regarded as indirect indexes that reflect the levels of OFRs and the degree of lipid peroxidation; these concentrations are believed to represent levels of lipid peroxidation [18]. The present study documented a significant reduction in MDA levels and increased SOD levels in liver tissues from the Sal-A groups compared with those from the IR group, which confirms that the effect of Sal-A can be attributed to suppressing oxidative stress.
It has been demonstrated that hepatic IR injury can be characterized as an inflammatory response that includes the activation of Kupffer cells, release of proinflammatory cytokines and chemokines, increased expression of adhesion molecules, and leukocyte infiltration [19]. These acute inflammatory responses ultimately cause hepatocellular damage and organ dysfunction [20]. In recent studies, Sal-A exhibited strong anti-inflammatory activities [21]. Sal-A could inhibit granulocyte adherence to vascular endothelial cells by decreasing the expression of intercellular cell adhesion molecule-1 while treating ischemic stroke [22]. Further investigation showed that Sal-A exerted an anti-inflammatory effect by modulating lipopolysaccharide-induced nuclear factor-κB pathway activation [23]. The activity of TNF-α-induced CCL-20 production appeared to be significantly suppressed by Sal-A [24]. Consistent with the results from our study, we found that IRI resulted in a significant increase in serum TNF-α and IL-6 levels and that Sal-A could decrease these increased levels compared to the IR group.
Apoptosis is a key pathway that induces cell death during hepatic IRI [25]. Caspase-3 is an executor caspase in the apoptosis pathway [26]; once it is activated, the process of apoptosis occurs irreversibly [27]. Bcl-2, a classic anti-apoptotic protein, suppresses apoptosis and plays a key role in preventing apoptotic cell death in IRI [28]. In several studies, Sal-A reduced hepatic cell apoptosis by modulating the Bax/Bcl-2 ratio and inhibiting caspase-3 activation [29, 30]. In the present study, Sal-A significantly up-regulated the expression of Bcl-2 and down-regulated the expression of caspase-3 compared with the IR group. Moreover, Sal-A could reverse the expression levels of Bcl-2 and caspase-3. Apoptosis, which is consistent with Bcl-2 and caspase-3, was significantly lower in the Sal-A group than in the IR group. These results indicate that Sal-A probably exerts its protective effect against IRI by inhibiting apoptosis in hepatocytes; however, the exact mechanism of these effects needs to be investigated further.
A previous study showed that liver subjected to ischemia reperfusion has an inflammatory injury response that possibly depends on TLR4 [31]. TLR4 signaling is necessary for the hepatic I/R response, and this response is in part mediated by HMGB1 [32]. He et al. reported that the TLR4/NF-κB signaling pathway is involved in hepatocyte apoptosis and production of TNF-α and IL-1β in serum caused by hepatic IRI stimulation [33]. Based on our results, the mRNA and protein levels of TLR4 were up-regulated in the liver of IR group. Sal-A attenuated oxidative stress and apoptosis and inhibited the mRNA and protein levels of TLR4. The protective effects of Sal-A on liver injury induced by ischemia/reperfusion may occur through inhibiting TLR4 which is induced by hepatic IRI.
In conclusion, our study provides evidence that Sal-A can protect rat livers from IRI. These protective effects are mediated by the potent anti-oxidation and anti-apoptosis properties of Sal-A, which result in a decrease in histopathological changes, oxidation, inflammation and apoptosis after hepatic IRI. The mechanisms by which Sal-A protects against hepatic IRI in rats include, at least in part, the regulation of mRNA and protein levels of TLR4.
Acknowledgments
Long Yang and Lu Jiang have contributed equally to this work.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Guan LY, Fu PY, Li PD, et al. Mechanisms of hepatic ischemia- reperfusion injury and protective effects of nitric oxide. World J Gastrointest Surg. 2014;6:122–8. doi: 10.4240/wjgs.v6.i7.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, et al. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–9. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 4.Kupiec-Weglinski JW, Busuttil RW. Ischemia and reperfusion injury in liver transplantation. Transplant Proc. 2005;37:1653–6. doi: 10.1016/j.transproceed.2005.03.134. [DOI] [PubMed] [Google Scholar]
- 5.Lu L, Zhang H, Qian Y, Yuan Y. Isolation of salvianolic acid A, a minor phenolic carboxylic acid of Salvia miltiorrhiza. Nat Prod Commun. 2010;5:805–8. [PubMed] [Google Scholar]
- 6.Fan H, Yang L, Fu F, et al. Cardioprotective effects of salvianolic acid a on myocardial ischemia-reperfusion injury in vivo and in vitro. Evid Based Complement Alternat Med. 2012;2012:508938. doi: 10.1155/2012/508938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan H, Li D, Fang F, et al. Salvianolic acid A demonstrates cardioprotective effects in rat hearts and cardiomyocytes after ischemia/reperfusion injury. J Cardiovasc Pharmacol. 2011;58:535–42. doi: 10.1097/FJC.0b013e31822de355. [DOI] [PubMed] [Google Scholar]
- 8.Chien MY, Chuang CH, Chern CM, et al. Salvianolic acid A alleviates ischemic brain injury through the inhibition of inflammation and apoptosis and the promotion of neurogenesis in mice. Free Radic Biol Med. 2016;5:508–19. doi: 10.1016/j.freeradbiomed.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Reino DC, Palange D, Feketeova E, et al. Activation of toll-like receptor 4 is necessary for trauma hemorrhagic shock-induced gut injury and polymorphonuclear neutrophil priming. Shock. 2012;38:107–14. doi: 10.1097/SHK.0b013e318257123a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu Q, He G, Wang J, et al. Down-regulation of toll-like receptor 4 alleviates intestinal ischemia reperfusion injury and acute lung injury in mice. Oncotarget. 2017;8:13678–89. doi: 10.18632/oncotarget.14624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang W, Liu G, Tang W. MicroRNA-182-5p ameliorates liver ischemia- reperfusion injury by suppressing toll-like receptor 4. Transplant Proc. 2016;48:2809–14. doi: 10.1016/j.transproceed.2016.06.043. [DOI] [PubMed] [Google Scholar]
- 12.Kong R, Gao Y, Sun B, et al. The strategy of combined ischemia preconditioning and salvianolic acid-B pretreatment to prevent hepatic ischemia-reperfusion injury in rats. Dig Dis Sci. 2009;54:2568–76. doi: 10.1007/s10620-008-0681-4. [DOI] [PubMed] [Google Scholar]
- 13.Cavalieri B, Perrelli MG, Aragno M, et al. Ischemic preconditioning attenuates the oxidant-dependent mechanisms of reperfusion cell damage and death in rat liver. Liver Transpl. 2002;8:990–9. doi: 10.1053/jlts.2002.35549. [DOI] [PubMed] [Google Scholar]
- 14.Montalvo-Jave EE, Escalante-Tattersfield T, Ortega-Salgado JA, et al. Factors in the pathophysiology of the liver ischemia-reperfusion injury. J Surg Res. 2008;147:153–9. doi: 10.1016/j.jss.2007.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Howard TK, Klintmalm GB, Cofer JB, et al. The influence of preservation injury on rejection in the hepatic transplant recipient. Transplantation. 1990;49:103–7. doi: 10.1097/00007890-199001000-00023. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Chen L, Wu H, et al. The mixture of salvianolic acids from salvia miltiorrhiza and total flavonoids from Anemarrhena asphodeloides attenuate sulfur mustard-induced injury. Int J Mol Sci. 2015;16:24555–73. doi: 10.3390/ijms161024555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gao LN, Yan K, Cui YL, et al. Protective effect of Salvia miltiorrhiza and Carthamus tinctorius extract against lipopolysaccharide-induced liver injury. World J Gastroenterol. 2015;21:9079–92. doi: 10.3748/wjg.v21.i30.9079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drewa G, Krzyzyńska-Malinowska E, Woźniak A, et al. Activity of superoxide dismutase and catalase and the level of lipid peroxidation products reactive with TBA in patients with psoriasis. Med Sci Monit. 2002;8:338–43. [PubMed] [Google Scholar]
- 19.Shen XD, Gao F, Ke B, et al. Inflammatory responses in a new mouse model of prolonged hepatic cold ischemia followed by arterialized orthotopic liver transplantation. Liver Transpl. 2005;10:1273–81. doi: 10.1002/lt.20489. [DOI] [PubMed] [Google Scholar]
- 20.Selzner N, Rudiger H, Graf R, et al. Protective strategies against ischemic injury of the liver. Gastroenterology. 2003;125:917–36. doi: 10.1016/s0016-5085(03)01048-5. [DOI] [PubMed] [Google Scholar]
- 21.Ding M, Ye TX, Zhao GR, et al. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int Immunopharmacol. 2005;5:1641–51. doi: 10.1016/j.intimp.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 22.Yang XY, Qiang GF, Zhang L, et al. Salvianolic acid A protects against vascular endothelial dysfunction in high-fat diet fed and streptozotocin-induced diabetic rats. J Asian Nat Prod Res. 2011;13:884–94. doi: 10.1080/10286020.2011.598457. [DOI] [PubMed] [Google Scholar]
- 23.Oh KS, Oh BK, Mun J, et al. Salvianolic acid A suppress lipopolysaccharide- induced NF-kappaB signaling pathway by targeting IKK beta. Int Immunopharmacol. 2011;11:1901–6. doi: 10.1016/j.intimp.2011.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XC, Chen JQ, Li B. Salvianolic acid A suppresses CCL-20 expression in TNF-alpha-treated macrophages and ApoE-deficient mice. J Cardiovasc Pharmacol. 2014;64:318–25. doi: 10.1097/FJC.0000000000000117. [DOI] [PubMed] [Google Scholar]
- 25.Kohli V, Selzner M, Madden JF, et al. Endothelial cell and hepatocyte deaths occur by apoptosis after ischemia-reperfusion injury in the rat liver. Transplantation. 1999;67:1099–105. doi: 10.1097/00007890-199904270-00003. [DOI] [PubMed] [Google Scholar]
- 26.Kam PC, Ferch NI. Apoptosis: mechanisms and clinical implications. Anaesthesia. 2000;55:1081–93. doi: 10.1046/j.1365-2044.2000.01554.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–33. [PubMed] [Google Scholar]
- 28.Zhao H, Yenari MA, Cheng D, et al. Bcl-2 overexpression protects against neuron loss within the ischemic margin following experimental stroke and inhibits cytochrome c translocation and caspase-3 activity. J Neurochem. 2003;85:1026–36. doi: 10.1046/j.1471-4159.2003.01756.x. [DOI] [PubMed] [Google Scholar]
- 29.Yan X, Jiang Z, Bi L, et al. Salvianolic acid A attenuates TNF-alpha- and D-GalN-induced ER stress-mediated and mitochondrial-dependent apoptosis by modulating Bax/Bcl-2 ratio and calcium release in hepatocyte LO2 cells. Naunyn Schmiedebergs Arch Pharmacol. 2015;388:817–30. doi: 10.1007/s00210-015-1116-3. [DOI] [PubMed] [Google Scholar]
- 30.Lin YL, Lee TF, Huang YJ, et al. Antiproliferative effect of salvianolic acid A on rat hepatic stellate cells. J Pharm Pharmacol. 2006;58:933–9. doi: 10.1211/jpp.58.7.0008. [DOI] [PubMed] [Google Scholar]
- 31.McDonald KA, Huang H, Tohme S, et al. Toll-like receptor 4 (TLR4) antagonist eritoran tetrasodium attenuates liver ischemia and reperfusion injury through inhibition of high-mobility group box protein B1 (HMGB1) signaling. Mol Med. 2014;20:639–48. doi: 10.2119/molmed.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tsung A, Klune JR, Zhang X, et al. HMGB1 release induced by liver ischemia involves Toll-like receptor 4 dependent reactive oxygen species production and calcium-mediated signaling. J Exp Med. 2007;204:2913–23. doi: 10.1084/jem.20070247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He D, Guo Z, Pu JL, et al. Resveratrol preconditioning protects hepatocytes against hepatic ischemia reperfusion injury via Toll-like receptor 4/nuclear factor-kappaB signaling pathway in vitro and in vivo. Int Immunopharmacol. 2016;35:201–9. doi: 10.1016/j.intimp.2016.03.032. [DOI] [PubMed] [Google Scholar]