Abstract
Introduction
Metabolic alterations have been recently associated with onset and progression of idiopathic pulmonary arterial hypertension (IPAH). We aimed to determine the prevalence and prognostic role of cardiovascular risk factors in patients with IPAH.
Material and methods
Between February 2009 and January 2015 we recruited consecutive IPAH patients. Clinical assessment included medical history, fasting glucose, lipid profile, N-terminal pro-brain natriuretic peptide concentration, 6-minute walk test distance, WHO functional class and hemodynamic evaluation. Patients’ risk was estimated based on the Swedish PAH Register grading system.
Results
The study group included 61 IPAH patients, and the control group included 2413 Polish residents. When compared to the general population, IPAH patients had lower low-density lipoprotein cholesterol (LDL-C) and a higher triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio. Female patients were characterized by elevated glucose level, higher prevalence of diabetes and lower HDL-C than controls. PAH severity grade correlated positively with age and TG/HDL-C ratio (R = 0.29, p = 0.02) and inversely with LDL-C (R = –0.28, p = 0.03) and HDL-C (R = –0.39, p = 0.02) concentrations. After a follow-up of 48 (23–79) months we recorded 28 deaths in the IPAH group. In the regression analysis lower LDL-C (p = 0.002) and HDL-C (p = 0.0002) levels, and higher TG/HDL-C ratio (p = 0.003) and glucose level (p = 0.003) were associated with all-cause mortality after adjustment for age, sex or PAH severity grade.
Conclusions
Patients with IPAH are characterized by an altered profile of lipid and glucose metabolism. Lowered levels of LDL-C and HDL-C and increased TG/HDL-C ratio correlate with disease severity and together with elevated plasma glucose level predict poor survival in IPAH.
Keywords: pulmonary hypertension, risk assessment, lipid metabolism
Introduction
Pulmonary arterial hypertension (PAH) is a progressive and incurable disease caused by uncontrolled hyperplasia of small pulmonary arteries. The European Society of Cardiology (ESC) proposed a set of prognostic factors including laboratory, clinical, imaging and hemodynamic parameters to stratify the risk of death in 1 year [1].
Recent experimental and clinical studies demonstrated the association between severity of pulmonary vascular disease and a spectrum of metabolic alterations [2–4]. Decreased levels of high-density lipoprotein cholesterol (HDL-C) were observed in subjects with PAH and were associated with worse clinical outcomes [5]. An increased triglyceride to HDL-C (TG/HDL-C) ratio was also found to be more prevalent in females with PAH than in healthy subjects and was proposed as a novel risk factor in this population [6, 7]. Patients with PAH are also more often characterized by glucose intolerance [8, 9]. The clinical significance of some other traditional cardiovascular disease (CVD) risk factors in patients with PAH may differ from the general population. In our recent study in a sample of 140 PAH males and females low-density lipoprotein cholesterol (LDL-C) level was lower than in age-matched controls and was associated with higher mortality [10]. What is more, in patients with pulmonary hypertension, similarly to other populations with severe chronic diseases, obesity was found to correlate with better survival [11].
Current knowledge about the significance of classical CVD risk factors in prognosis of patients with idiopathic PAH (IPAH) is however scarce and based only on single reports with a relatively short observation period. In most of them the PAH populations were heterogeneous and included patients with connective tissue disease, which itself may affect CVD risk status.
Therefore in the present study we aimed to determine the prevalence of traditional CVD risk factors in the population of patients with IPAH as compared to the general population and to assess their prognostic role. We also evaluated the relationship between CVD risk factors and severity of IPAH using a recently validated comprehensive risk assessment tool.
Material and methods
Patient population
We prospectively recruited consecutive IPAH patients from a single pulmonary hypertension reference center between February 2009 and January 2015. Eligible patients had pre-capillary pulmonary hypertension with pulmonary vascular resistance > 3 Wood units in the absence of other causes of pre-capillary pulmonary hypertension [1]. The only absolute contraindication was a lack of informed consent; the relative contraindications were active infection and hemodynamic instability. The control group was chosen from the NATPOL 2011 study designed to analyze the prevalence of CVD risk factors in the Polish population [12].
Follow-up
All-cause mortality of IPAH patients was assessed using reports of the Department of Nationals’ and Foreigners’ Affairs and telephone interviews. We observed the patients from the time of the first clinical assessment in our center until April 2018. The local ethics committee revised and approved the study protocol which conforms to the guidelines of the Declaration of Helsinki. Prior to the study enrollment, we collected informed consent from each participant.
Evaluation of IPAH patients
Clinical evaluation, laboratory tests and assessment of hemodynamic parameters were made during the first assessment of patients in our department. We collected demographic data and relevant medical history including use of lipid-lowering drugs such as statin, niacin, fibrate and estrogen replacement therapy. Diabetes, hypertension, overweight (body mass index; BMI ≥ 25 kg/m2) and obesity (BMI ≥ 30 kg/m2) were diagnosed based on current guidelines [13–15]. We measured arterial blood pressure (BP) and N-terminal pro-brain natriuretic peptide (NT-proBNP) concentration and assessed 6-minute walk test distance (6-MWD) and WHO functional class (WHO-FC). Active smoking was defined as smoking for one month or more in the previous year [16]. We collected venous blood from each participant after 12 h of fasting on the day of hemodynamic assessment. Complete lipid profile was assessed using the direct colorimetric method as described previously [10]. Right heart catheterization was performed using a Swan-Ganz catheter. We calculated cardiac output with the oxygen consumption method [17]. Methods of clinical and laboratory assessment of the controls from the NATPOL study were described previously [12].
PAH severity grade
Patients’ baseline risk was estimated based on the Swedish PAH Register (SPAHR) grading system [18]. The following variables were assigned 1, 2 or 3 points according to the cut-off values for low, intermediate and high risk, available in the risk assessment method of the ESC guidelines: WHO-FC, 6-MWD, NT-proBNP, area of the right atrium, right atrial pressure, presence of pericardial effusion, cardiac index, and mixed venous oxygen saturation [1]. A mean PAH severity grade for every patient was calculated by dividing the sum of all grades by the number of assessed variables and then was rounded off to the nearest integer to assign patients to specific risk groups [18].
Statistical analysis
We used mean ± standard deviation to present continuous variables and counts (%) for categorical variables. Comparison of continuous variables between groups was performed using Student’s t-test or the Mann-Whitney U-test, according to data distribution. Comparison of categorical variables was performed using the χ2 test. For the comparison between control and study groups, males and females were separately matched for age. To assess the relationship between CVD risk factors and all-cause mortality, we used Cox proportional hazards regression. Models were adjusted for age and/or sex and established markers of disease severity (PAH severity grade). The alpha level was set as 0.05. Statistical analysis was performed using Dell Inc. (2016), Dell Statistica (data analysis software system), version 13. software.dell.com and MedCalc Statistical Software version 18.6 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018).
Results
Study population
Between February 2009 and January 2015 we recruited 61 consecutive IPAH patients (all eligible subjects) of whom most (87%) were diagnosed for the first time and did not receive PAH-specific therapies. Other patients were treated with PAH-targeted drugs: 3 (5%) were receiving sildenafil in monotherapy, 3 (5%) were treated with bosentan, 1 patient was treated with a prostacyclin analogue and 1 with dual combination therapy (sildenafil and bosentan). In every patient PAH-specific treatment was initiated immediately after making a diagnosis during the first hospitalization of a patient in our center. Our study did not have any effect on the therapeutic decisions made in our patients. At the end of the observation period 26 (42.5%) patients were treated with monotherapy with PAH-specific drug (8 with sildenafil, 6 with bosentan, 6 with prostacyclin analogue and 6 with calcium channel blocker), 26 (42.5%) with dual and 9 (15%) with triple combination therapy with sildenafil, bosentan and prostacyclin analogue. A group of 34 (56%) patients were treated with parenteral prostacyclin analogues. Characteristics of study patients are shown in Table I. The control group included 1168 male and 1245 female Polish residents aged 42.9 ±24 and 44.8 ±27.1 years, respectively.
Table I.
Characteristics of the study group (IPAH patients, n = 61)
| Parameter | Value |
|---|---|
| Age [years] | 50.0 ±17.1 |
| Female sex, n (%) | 40 (66%) |
| WHO-FC, n (%): | |
| II | 5 (8) |
| III | 42 (69) |
| IV | 14 (23) |
| NT-proBNP [pg/ml] | 2405.0 ±3370.9 |
| 6-MWD [m] | 336.3 ±117 |
| CI [l/min/m2] | 1.86 ±0.59 |
| RAP [mm Hg] | 8.0 ±5.2 |
| mPAP [mm Hg] | 51.2 ±18.2 |
| PVR [WU] | 15.3 ±9.7 |
| SvO2 (%) | 57.8 ±11.4 |
6-MWD – 6-minute walk distance, CI – cardiac index, IPAH – idiopathic pulmonary arterial hypertension, NT-proBNP – N-terminal pro-brain natriuretic peptide, mPAP – mean pulmonary artery pressure, PVR – pulmonary vascular resistance, RAP – right atrial pressure, SvO2 – mixed venous oxygen saturation, WHO-FC – WHO functional class.
Prevalence of CVD risk factors in IPAH patients
We identified diabetes in 10 (17%) individuals, hypertension in 23 (38%), overweight in 21 (34%) and obesity in 8 (13%) patients. Three (5%) patients were classified as smokers. At enrollment 18 (30%) patients used statins; none of the patients was treated with niacin, fibrate or estrogen replacement therapy. Chronic kidney disease was diagnosed in 9 (15%) IPAH patients. Nine (15%) patients were previously diagnosed with coronary artery disease, 1 patient had myocardial infarction, 2 patients were diagnosed with peripheral artery disease and there were no cases of stroke in this group. Daily activities of study patients were significantly limited as the majority of them were in the WHO-FC III (42, 69%) or IV (14, 23%). At first evaluation all patients were recommended to avoid excessive physical activity that leads to distressing symptoms and were not advised to follow any specific dietary recommendations.
Comparison of the prevalence of CVD risk factors in IPAH males and females and age-matched controls
When compared to the general population, IPAH patients had lower LDL-C and a higher TG/HDL-C ratio. What is more, only female patients were characterized by elevated glucose level, higher prevalence of diabetes and lower HDL-C than controls. In our group 22 (36%) patients had diabetes or used statins. When only patients not using statins were analyzed (n = 43) they were still characterized by significantly lower LDL-C (2.79 ±0.8 in females and 2.31 ±0.94 mmol/l in males) than control patients who did not use statins (3.3 ±1.1 mmol/l, p = 0.02 for females, 3.3 ±1.2 mmol/l, p = 0.01 for males). Similarly, when females without diabetes (n = 32) were analyzed they were characterized by higher glucose (5.4 ±0.72 mmol/l) and TG/HDL-C (2.4 ±1.3) concentrations than female controls without diabetes (4.9 ±0.8 mmol/l, p < 0.001 and 2.0 ±1.7, p = 0.01 for glucose and TG/HDL, respectively). We found no differences in HDL-C concentration between females with and without diabetes (1.32 ±0.4 vs. 1.32 ±0.4 mmol/l, p = 0.9). There were no differences in BMI and TG concentration between patients and controls. Comparison of CVD risk factors between IPAH males and females and the age-matched control group is shown in Table II.
Table II.
Cardiovascular risk factors in males and females with idiopathic pulmonary arterial hypertension and age matched general population
| Parameter | IPAH (n = 61) | Controls (n = 2413) | P-value | |||
|---|---|---|---|---|---|---|
| Female (n = 40) | Male (n = 21) | Female (n = 1245) | Male (n = 1168) | Female | Male | |
| Age [years] | 49.0 ±15.9 | 51.9 ±19.2 | 44.8 ±27.1 | 42.9 ±24.0 | 0.3 | 0.09 |
| BMI [kg/m2] | 25.0 ±3.5 | 25.3 ±4.4 | 25.9 ±7.3 | 27.1 ±6.7 | 0.4 | 0.2 |
| SBP [mm Hg] | 111.5 ±13.2 | 107.8 ±17.6 | 130.1 ±30.0 | 136.7 ±26.9 | < 0.001 | < 0.001 |
| DBP [mm Hg] | 72.5 ±8.6 | 69.5 ±11.1 | 80.6 ±13.1 | 82.4 ±15.7 | < 0.001 | < 0.001 |
| LDL-C [mmol/l] | 2.7 ±0.8 | 2.1 ±0.8 | 3.2 ±1.1 | 3.2±1.2 | < 0.001 | < 0.001 |
| HDL-C [mmol/l] | 1.3 ±0.4 | 1.1 ±0.4 | 1.4 ±0.4 | 1.2 ±0.5 | 0.03 | 0.4 |
| TG [mmol/l] | 1.3 ±0.7 | 1.4±0.7 | 1.1 ±0.8 | 1.6 ±1.6 | 0.2 | 0.1 |
| TG/HDL-C | 2.5 ±1.7 | 3.6 ±3.5 | 2.1 ±2.0 | 3.4 ±4.0 | 0.002 | 0.03 |
| Glucose [mmol/l] | 6.0 ±3.0 | 5.9 ±2.3 | 5.0 ±1.0 | 5.3±1.6 | < 0.001 | 0.1 |
| Diabetes (%) | 20.0 | 9.5 | 5.9 | 4.6 | 0.001 | 0.6 |
| Statin use (%) | 25.0 | 38.1 | 11.9 | 11.2 | 0.03 | < 0.001 |
| Hypertension (%) | 30.0 | 52.4 | 25 | 28.2 | 0.6 | 0.03 |
| Active smoker (%) | 7.5 | 0 | 27.1 | 35.6 | < 0.001 | – |
BMI – body mass index, DBP – diastolic blood pressure, HDL-C – high-density lipoprotein cholesterol, IPAH – idiopathic pulmonary arterial hypertension, LDL-C – low-density lipoprotein cholesterol, SBP – systolic blood pressure, TG/HDL-C – triglyceride to high-density lipoprotein cholesterol ratio.
CVD risk factors in prediction of mortality in IPAH patients
After a median follow-up time of 48 (23–79) months we recorded 28 deaths in the IPAH group. In the univariable analysis we found that lower LDL-C and HDL-C levels, and higher TG/HDL-C ratio and glucose level were associated with all-cause mortality. All these metabolic parameters remained significant in prediction of mortality after adjusting for age, or age and sex or PAH severity grade. In Cox regression analysis statin use (p = 0.23) and presence of diabetes (p = 0.18) were not significant factors affecting patients’ prognosis. Details of Cox regression analysis are shown in Table III.
Table III.
Cox regression model for the association between cardiovascular risk factors, disease severity score and mortality (n = 61)
| Variable | Non adjusted | Age adjusted | PAH severity adjusted | Age and sex adjusted | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Age [years] | 1.02 | 1–1.04 | 0.13 | – | – | – | 0.99 | 0.97–1.02 | 0.69 | – | – | – |
| Sex (male) | 2.32 | 1.1–4.87 | 0.03 | 2.17 | 1.02–4.61 | 0.04 | 2.02 | 0.96–4.26 | 0.06 | – | – | – |
| PAH severity grade | 8.15 | 2.78–23.9 | 0.0001 | 9.13 | 2.66–31.2 | 0.0004 | – | – | – | 9.02 | 2.55–31.92 | 0.0006 |
| HDL-C [mmol/l] | 0.15 | 0.06–0.41 | 0.0002 | 0.15 | 0.06–0.40 | 0.0001 | 0.28 | 0.1–0.73 | 0.01 | 0.17 | 0.07–0.47 | 0.0005 |
| TG [mmol/l] | 1.18 | 0.78–1.79 | 0.43 | 1.19 | 0.79–1.8 | 0.41 | 1.1 | 0.68–1.8 | 0.7 | 1.28 | 0.84–1.94 | 0.25 |
| LDL-C [mmol/l] | 0.41 | 0.23–0.71 | 0.002 | 0.44 | 0.25–0.77 | 0.004 | 0.52 | 0.31–0.86 | 0.01 | 0.48 | 0.26–0.86 | 0.01 |
| TG/HDL-C | 1.55 | 1.16–2.07 | 0.003 | 1.58 | 1.18–2.11 | 0.002 | 1.39 | 1.01–1.91 | 0.04 | 1.57 | 1.17–2.11 | 0.003 |
| Glucose [mmol/l] | 1.13 | 1.04–1.23 | 0.003 | 1.12 | 1.02–1.22 | 0.01 | 1.12 | 1.02–1.24 | 0.02 | 1.14 | 1.04–1.25 | 0.006 |
| BMI [kg/m2] | 1.07 | 0.96–1.19 | 0.21 | 1.06 | 0.95–1.18 | 0.33 | 1.07 | 0.96–1.18 | 0.24 | 1.05 | 0.95–1.16 | 0.36 |
| SBP [mm Hg] | 0.98 | 0.95–1.0 | 0.055 | 0.97 | 0.95–0.99 | 0.03 | 0.99 | 0.96–1.01 | 0.28 | 0.98 | 0.96–1.0 | 0.08 |
| DBP [mm Hg] | 0.96 | 0.92–1.0 | 0.07 | 0.96 | 0.92–1.0 | 0.09 | 0.97 | 0.93–1.02 | 0.22 | 0.97 | 0.93–1.02 | 0.25 |
| Diabetes | 1.88 | 0.75–4.68 | 0.18 | 1.5 | 0.57–4.0 | 0.41 | 1.47 | 0.58–3.73 | 0.41 | 1.82 | 0.68–4.89 | 0.23 |
| Hypertension | 1.24 | 0.57–2.7 | 0.58 | 0.87 | 0.36–2.11 | 0.76 | 0.86 | 0.39–1.9 | 0.72 | 0.81 | 0.34–1.95 | 0.65 |
| Smoking | 0.97 | 0.5–1.88 | 0.92 | 0.96 | 0.48–1.9 | 0.9 | 0.89 | 0.44–1.82 | 0.76 | 0.82 | 0.39–1.74 | 0.61 |
| Statin use | 1.62 | 0.74–3.54 | 0.23 | 1.37 | 0.6–3.11 | 0.45 | 1.41 | 0.64–3.12 | 0.39 | 1.24 | 0.53–2.9 | 0.62 |
BMI – body mass index, DBP – diastolic blood pressure, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, PAH – pulmonary arterial hypertension, SBP – systolic blood pressure, TG – triglyceride, TG/HDL-C – triglyceride to high-density lipoprotein cholesterol ratio.
PAH severity grade
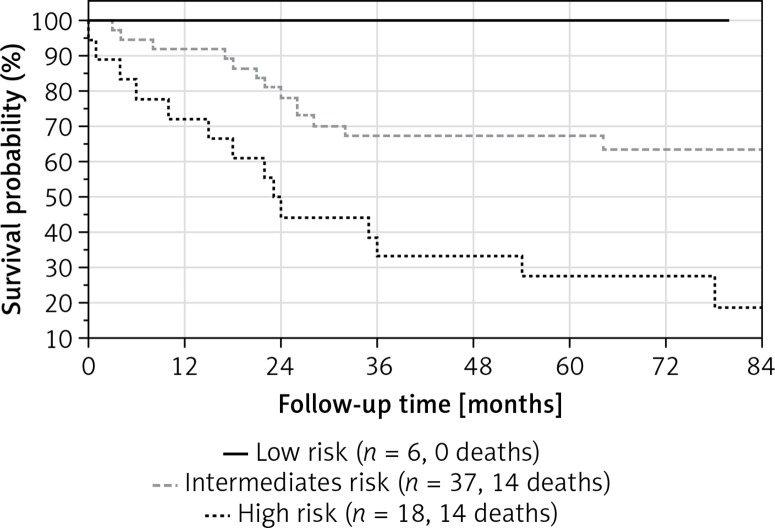
The median PAH severity grade in our patients was 2.3 (1.9–2.6). Most patients were at intermediate risk (n = 37, 61%), 6 (10%) were in the low risk and 18 (29%) in the high risk group. Mortality rates after 48 months of high, intermediate and low risk groups were calculated as 68%, 32% and 0%, respectively, as shown in Figure 1.
Figure 1.
Survival curves of patients in high, intermediate and low risk group
CVD risk factors and PAH severity
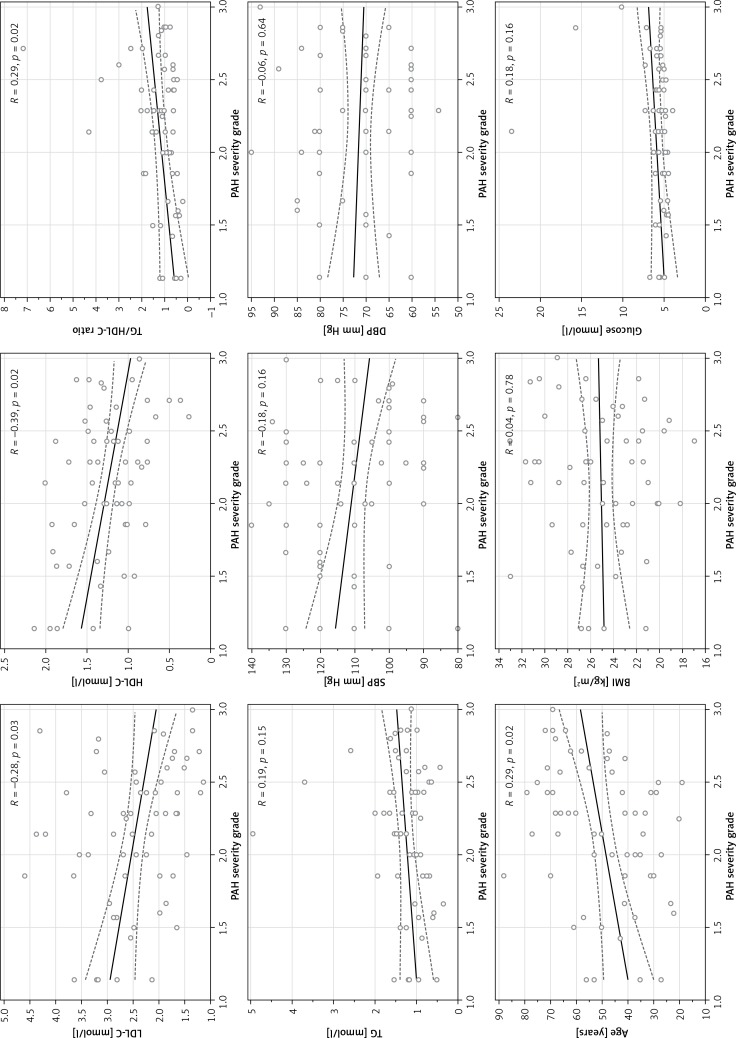
The PAH severity grade was found to correlate positively with age (R = 0.29, p = 0.02) and TG/HDL-C ratio (R = 0.29, p = 0.02) and inversely with LDL-C (R = –0.28, p = 0.03) and HDL-C (R = –0.39, p = 0.02) concentrations. We found no association between PAH severity grade and BMI, TG or glucose level (Figure 2).
Figure 2.
Associations between PAH severity grade and cardiovascular risk factors
BMI – body mass index, dBP – diastolic blood pressure, HDL-C – high-density lipoprotein cholesterol, LDL-C – low-density lipoprotein cholesterol, PAH – pulmonary arterial hypertension, sBP – systolic blood pressure, TG – triglyceride, TG/HDL-C – triglyceride to high-density lipoprotein cholesterol.
Discussion
In our study we observed that IPAH males and females when compared to age-matched controls were characterized by lower LDL-C levels and an increased TG/HDL-C ratio. Female IPAH patients were also characterized by elevated fasting glucose, higher prevalence of diabetes and a lower HDL-C level. We also found that abnormal lipid profile correlated with pulmonary hypertension severity and that decreased levels of LDL-C and HDL-C, increased TG/HDL ratio and increased plasma glucose were associated with poor prognosis. What is more, we implemented the recently proposed PAH severity grade in this group of patients.
The IPAH is a disease with a poor prognosis and accurate risk assessment is essential for appropriate management of patients [19–21]. Currently, the most commonly used approach is based on the ESC guidelines and includes assessment of several markers of PAH severity such as patients’ symptoms, signs of right heart failure, exercise capacity, laboratory, hemodynamic and imaging parameters[1]. Kylhammar et al. [18] recently applied a new guideline-based method for risk assessment and validated it in 530 patients with PAH from the SPAHR registry [22]. In our study this score was also shown to accurately predict survival of the IPAH group. However, recent data from large registries [18] underlined changing demographics among IPAH patients that also appeared to have a significant impact on patients’ prognosis. Increasing age of diagnosis and higher prevalence of co-morbidities resulted in diminished response to treatment and worse survival. As shown in the recent reports, assessment of parameters reflecting metabolic status alongside the conventional disease risk markers was able to provide additional information relevant in patients’ prognosis [5, 6, 10].
There is an expanding body of evidence that pulmonary hypertension is associated with alterations in numerous metabolic pathways contributing to the disease onset and progression [3]. Recent studies suggest a role of altered function of bone morphogenic protein receptor type 2 (BMPR2) in development of metabolic abnormalities, and severity of pulmonary vascular disease [23]. Mutation of BMPR2 is the most common cause of heritable PAH and its decreased signaling is also present in PAH of other etiologies [24]. Interestingly, altered BMPR2 function was also associated with obesity, dyslipidemia and metabolic syndrome [25–27]. Experimental studies also showed that metabolic alterations such as insulin resistance, low levels of plasma adiponectin as well as reduced expression of apolipoprotein E were able to induce PAH in an animal model. What is more, levels of circulating triglycerides and free fatty acids correlated with changes in pulmonary hemodynamics and degree of pulmonary artery remodeling in fat-fed animals, highlighting the role of metabolic alterations in PAH progression [28].
The latest clinical studies also suggest an important role of CVD risk factors including HDL-C and TG/HDL-C in prognosis of PAH patients [5, 29–32]. Heresi et al. observed that PAH patients were characterized by decreased HDL-C levels which were associated with higher mortality independently of disease severity, age and sex of patients [5]. The vasoprotective function of HDL-C in the pulmonary circulation was further explained by its abilities to mitigate inflammation and enhance prostacyclin half-life. Both decreased HDL-C and elevated TG in the general population have also been associated with features of metabolic syndrome and insulin resistance [33–35]. The TG/HDL-C ratio was previously established as a simple parameter able to identify insulin resistant individuals with better predictive value than other single or combined lipid fractions.
Numerous studies have confirmed that inflammatory cytokines can both increase TG and decrease HDL-C levels and in consequence elevate the TG/HDL-C ratio [36]. We have also shown that interleukin 1β, interleukin 6 and high-sensitivity C-reactive protein correlate with the level of HDL-C [37]. What is more, TG/HDL-C was recently proposed as a novel risk factor in PAH patients [6]. Our results, similarly to observations in the PAH population, indicate that TG/HDL-C ratio was able to predict worse prognosis and increased mortality.
In the present study we demonstrated that patients with IPAH are characterized by lower LDL-C, which is associated with increased mortality. Recent studies also underlined the association between LDL-C level and prognosis in PAH [10]. Elevated LDL-C is a well-established CVD risk factor in the general population, but in some chronic diseases including rheumatoid arthritis, heart failure and chronic kidney disease low LDL-C levels predicted poor prognosis [38]. This manifestation of reverse epidemiology was assigned to abnormal cytokine and neurohormonal secretion, resulting in some groups of patients in cardiac cachexia and increased mortality [11]. We recently demonstrated that patients with PAH and chronic thromboembolic pulmonary hypertension (CTEPH) have lower LDL-C levels compared to healthy controls and its concentration showed a strong linear relationship with risk of all-cause mortality [10]. Interestingly, improvement of hemodynamic parameters after treatment of CTEPH led to reversal of decreased levels of LDL-C. The observed findings were explained by the fact that lipids and lipoproteins can modulate inflammation, which has been associated with progression of pulmonary vascular disease, although the exact mechanism of observed changes still remains unknown [39–41].
We found that a significant number of study patients were using statins when they were first evaluated in our center. This could have resulted from the presence of comorbid conditions or previous misdiagnosis. Recent studies have shown that diagnosis of PAH may be challenging. Strange et al. demonstrated that correct PAH diagnosis takes 47 ±34 months. Patients report 5.3 ±3.8 general physician visits and 3.0 ±2.1 specialist reviews before being seen at a pulmonary hypertension center. Before the final diagnosis of PAH they have approximately two false diagnoses such as ischemic heart disease, heart failure or pulmonary disease [42]. These misdiagnoses might have contributed at least partly to the high number of patients using statins during first assessment in our center. What is more, according to data from large PAH registries [43], comorbid conditions in this population are becoming more frequent in recent years. Prevalence of coronary artery disease in PAH patients increased from 7% to 16% between the years 2001 and 2009. Similarly, in our study 15% of patients (and 29% of male patients) had a previously established diagnosis of coronary artery disease. This may explain the high frequency of statin treatment, especially in male subjects.
In the current study we also demonstrated that changes in glucose metabolism may affect prognosis in IPAH patients. Diabetes and elevated fasting glucose were more common in IPAH females than in their counterparts in the general population. Similarly, recent clinical studies confirmed that patients with pulmonary hypertension are characterized by glucose intolerance, pancreatic β-cell dysfunction and elevated hemoglobin A1c [9]. The exact pathophysiological link between changed metabolism of glucose and prognosis in IPAH is unknown; however, both clinical and experimental data indicate that chronic inflammation plays an important role in glucose homeostasis and severity of pulmonary vascular disease [2]. Higher prevalence of diabetes in women with IPAH than in their age-matched counterparts from the general population is a novel finding but corresponds with the results of another study which showed an increased prevalence of insulin resistance in PAH females [6]. The association between female sex and abnormalities of glucose metabolism in PAH has not been well established but experimental data suggest a role of estrogen metabolism [44].
The alterations in lipid and glucose metabolism are often ascribed to obesity. In our study we however did not find differences in BMI between IPAH patients and age-matched controls. What is more, BMI was not associated with disease severity or increased mortality in the IPAH population. The association between obesity and prognosis in pulmonary hypertension is still not clear. Data from the REVEAL registry showed that PAH patients are characterized by similar BMI as the control population. Obese patients were older, and had higher prevalence of CVD risk factors including diabetes and hypertension. Interestingly, this group was also characterized by more severe disease with higher WHO-FC, right atrial pressure, pulmonary vascular resistance and lower cardiac index [45]. Other studies suggest that in PAH patients, similarly to other populations with severe chronic diseases, obesity is associated with improved survival [11]. This finding was further explained by the phenomenon known as the “obesity paradox,” indicating a prognostic role of abnormal cytokine secretion and cardiac cachexia. This may suggest that the association between obesity and prognosis in pulmonary hypertension may be more complex and dependent on chronic inflammation and other obesity-related risk factors [46, 47].
Strengths and limitations
Our study has several strengths. First, the findings are novel since the prognostic values of CVD risk factors in IPAH patients are hardly known. In contrast to most previous studies we analyzed a homogeneous group of patients with IPAH. This is a specific group of patients in whom secondary causes of pulmonary hypertension including congenital heart disease, connective tissue disease, HIV infection and hepatic cirrhosis have been excluded. All of the diseases can significantly influence the prevalence of CVD and their clinical relevance. Second, the follow-up time in our study was longer than that used in most similar studies. Third, our study has important clinical implications. It describes a metabolic profile of patients with IPAH which is different than in the general population and may have a different clinical impact. It also indicates new prognostic factors that may be helpful when assessing the risk of death in individual patients with IPAH. Finally, our study implemented for the first time the recently proposed method of risk assessment in Polish IPAH patients.
We acknowledge that our study also has limitations, due to the single-center design and limited sample size, and the number of events we needed to limit adjustment of our regression models only for selected variables (age or age and sex or PAH severity grade). We were however able to include in the modeling a score of PAH severity which integrates several aspects of PAH risk markers. Further multicenter studies may be needed to confirm our findings in different populations. Second, an important number of IPAH patients were taking statins and/or had diabetes, which may influence the LDL-C level and TG/HDL-C ratio, respectively. However, when we excluded patients who used statins or patients who had diabetes, still IPAH patients had a lower LDL-C level or increased TG/HDL-C ratio than observed in the general population. Additionally, we did not find evidence for an association between statin use or the diagnosis of diabetes and mortality in our group. Third, we only assessed the level of HDL-C without investigating its structure and function. It is well known that inflammation can also alter the structure and function of HDL-C particles, enabling progression of vascular disease. During conditions associated with oxidative stress, inflammation and hyperglycemia, HDL-C may undergo post-transitional changes that affect their vasoprotective properties. This altered HDL-C can then exacerbate the inflammatory response and is often referred to as dysfunctional HDL-C [32]. Finally, in the present study we cannot determine exact mechanisms of the observed findings. We were however able to propose potential mechanisms based on similar observations in different populations and in an experimental setting.
In conclusion, patients with IPAH are characterized by an altered profile of lipid and glucose metabolism. Lowered levels of LDL-C and HDL-C and increased TG/HDL-C ratio correlate with disease severity and together with elevated plasma glucose level predict poor survival in IPAH.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Heart J. 2016;37:67–119. doi: 10.1093/eurheartj/ehv317. [DOI] [PubMed] [Google Scholar]
- 2.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115:165–75. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paulin R, Michelakis ED. The metabolic theory of pulmonary arterial hypertension. Circ Res. 2014;115:148–64. doi: 10.1161/CIRCRESAHA.115.301130. [DOI] [PubMed] [Google Scholar]
- 4.Assad TR, Hemnes AR. Metabolic dysfunction in pulmonary arterial hypertension. Curr Hyper Rep. 2015;17:20. doi: 10.1007/s11906-014-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heresi GA, Aytekin M, Newman J, DiDonato J, Dweik RA. Plasma levels of high-density lipoprotein cholesterol and outcomes in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:661–8. doi: 10.1164/rccm.201001-0007OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zamanian RT, Hansmann G, Snook S, et al. Insulin resistance in pulmonary arterial hypertension. Eur Respir J. 2009;33:318–24. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Naderi N, Boobejame P, Bakhshandeh H, Amin A, Taghavi S, Maleki M. Insulin resistance in pulmonary arterial hypertension, is it a novel disease modifier? Res Cardiovasc Med. 2014;3:e19710. doi: 10.5812/cardiovascmed.19710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugh ME, Robbins IM, Rice TW, West J, Newman JH, Hemnes AR. Unrecognized glucose intolerance is common in pulmonary arterial hypertension. J Heart Lung Transplant. 2011;30:904–11. doi: 10.1016/j.healun.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heresi GA, Malin SK, Barnes JW, Tian L, Kirwan JP, Dweik RA. Abnormal glucose metabolism and high-energy expenditure in idiopathic pulmonary arterial hypertension. Ann Am Thorac Soc. 2017;14:190–9. doi: 10.1513/AnnalsATS.201608-605OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kopec G, Waligora M, Tyrka A, et al. Low-density lipoprotein cholesterol and survival in pulmonary arterial hypertension. Sci Rep. 2017;7:41650. doi: 10.1038/srep41650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zafrir B, Adir Y, Shehadeh W, Shteinberg M, Salman N, Amir O. The association between obesity, mortality and filling pressures in pulmonary hypertension patients; the “obesity paradox”. Respir Med. 2013;107:139–46. doi: 10.1016/j.rmed.2012.10.019. [DOI] [PubMed] [Google Scholar]
- 12.Zdrojewski T, Rutkowski M, Bandosz P, et al. Prevalence and control of cardiovascular risk factors in Poland. Assumptions and objectives of the NATPOL 2011 Survey. Kardiol Pol. 2013;71:381–92. doi: 10.5603/KP.2013.0066. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes A 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;(39 Suppl 1):S13–22. doi: 10.2337/dc16-S005. [DOI] [PubMed] [Google Scholar]
- 14.Zahorska-Markiewicz B, Podolec P, Kopec G, et al. Polish Forum for Prevention Guidelines on overweight and obesity. Kardiol Pol. 2008;66:594–6. [PubMed] [Google Scholar]
- 15.Kopec G, Podolec P, Podolec J, Rubis P, Zmudka K, Tracz W. Atherosclerosis progression affects the relationship between endothelial function and aortic stiffness. Atherosclerosis. 2009;204:250–4. doi: 10.1016/j.atherosclerosis.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Kopeć G, Moertl D, Jankowski P, Tyrka A, Sobień B, Podolec P. Pulmonary artery pulse wave velocity in idiopathic pulmonary arterial hypertension. Can J Cardiol. 2013;29:683–90. doi: 10.1016/j.cjca.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Kurzyna M, Araszkiewicz A, Blaszczak P, et al. Summary of recommendations for the haemodynamic and angiographic assessment of the pulmonary circulation. Joint statement of the Polish Cardiac Society’s Working Group on Pulmonary Circulation and Association of Cardiovascular Interventions. Kardiol Pol. 2015;73:63–8. doi: 10.5603/KP.2015.0011. [DOI] [PubMed] [Google Scholar]
- 18.Kylhammar D, Kjellstrom B, Hjalmarsson C, et al. A comprehensive risk stratification at early follow-up determines prognosis in pulmonary arterial hypertension. Eur Heart J. 2017 doi: 10.1093/eurheartj/ehx257. [DOI] [PubMed] [Google Scholar]
- 19.Kopec G, Waligora M, Stepniewski J, Podolec P. Indwelling central venous catheter occlusion during chronic epoprostenol infusion. J Heart Lung Transplant. 2018;37:938–940. doi: 10.1016/j.healun.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Kopec G, Waligora M, Tyrka A, Komar M, Herman N, Podolec P. Clinical response to calcium channel blockers in a hemodynamically unstable patient with reactive idiopathic pulmonary arterial hypertension. Arch Med Sci. 2017;13:504–6. doi: 10.5114/aoms.2017.65230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Al-Naamani N, Palevsky HI, Lederer DJ, et al. Prognostic significance of biomarkers in pulmonary arterial hypertension. Ann Am Thorac Soc. 2016;13:25–30. doi: 10.1513/AnnalsATS.201508-543OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hjalmarsson C, Radegran G, Kylhammar D, et al. Impact of age and comorbidity on risk stratification in idiopathic pulmonary arterial hypertension. Eur Respir J. 2018;51 doi: 10.1183/13993003.02310-2017. [DOI] [PubMed] [Google Scholar]
- 23.Austin ED, Phillips JA, Cogan JD, et al. Truncating and missense BMPR2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respir Res. 2009;10:87. doi: 10.1186/1465-9921-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aldred MA, Comhair SA, Varella-Garcia M, et al. Somatic chromosome abnormalities in the lungs of patients with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2010;182:1153–60. doi: 10.1164/rccm.201003-0491OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schleinitz D, Klöting N, Böttcher Y, et al. Genetic and evolutionary analyses of the human bone morphogenetic protein receptor 2 (BMPR2) in the pathophysiology of obesity. PLoS One. 2011;6:e16155. doi: 10.1371/journal.pone.0016155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hansmann G, de Jesus Perez VA, Alastalo TP, et al. An antiproliferative BMP-2/PPARgamma/apoE axis in human and murine SMCs and its role in pulmonary hypertension. J Clin Invest. 2008;118:1846–57. doi: 10.1172/JCI32503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.West J, Niswender KD, Johnson JA, et al. A potential role for insulin resistance in experimental pulmonary hypertension. Eur Respir J. 2013;41:861–71. doi: 10.1183/09031936.00030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansmann G, Wagner RA, Schellong S, et al. Pulmonary arterial hypertension is linked to insulin resistance and reversed by peroxisome proliferator-activated receptor-gamma activation. Circulation. 2007;115:1275–84. doi: 10.1161/CIRCULATIONAHA.106.663120. [DOI] [PubMed] [Google Scholar]
- 29.Larsen CM, McCully RB, Murphy JG, Kushwaha SS, Frantz RP, Kane GC. Usefulness of high-density lipoprotein cholesterol to predict survival in pulmonary arterial hypertension. Am J Cardiol. 2016;118:292–7. doi: 10.1016/j.amjcard.2016.04.028. [DOI] [PubMed] [Google Scholar]
- 30.Cracowski JL, Labarere J, Renversez JC, Degano B, Chabot F, Humbert M. Plasma levels of high-density lipoprotein cholesterol are not associated with survival in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;186:107. doi: 10.1164/ajrccm.186.1.107. [DOI] [PubMed] [Google Scholar]
- 31.Zhao QH, Peng FH, Wei H, et al. Serum high-density lipoprotein cholesterol levels as a prognostic indicator in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. 2012;110:433–9. doi: 10.1016/j.amjcard.2012.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Otocka-Kmiecik A, Mikhailidis DP, Nicholls SJ, Davidson M, Rysz J, Banach M. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog Lipid Res. 2012;51:314–24. doi: 10.1016/j.plipres.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 33.Tresaco B, Moreno LA, Ruiz JR, et al. Truncal and abdominal fat as determinants of high triglycerides and low HDL-cholesterol in adolescents. Obesity. 2009;17:1086–91. doi: 10.1038/oby.2008.626. [DOI] [PubMed] [Google Scholar]
- 34.Ghomari-Boukhatem H, Bouchouicha A, Mekki K, Chenni K, Belhadj M, Bouchenak M. Blood pressure, dyslipidemia and inflammatory factors are related to body mass index in scholar adolescents. Arch Med Sci. 2017;13:46–52. doi: 10.5114/aoms.2017.64713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Bartolo BA, Schwarz N, Andrews J, Nicholls SJ. Infusional high-density lipoproteins therapies as a novel strategy for treating atherosclerosis. Arch Med Sci. 2017;13:210–4. doi: 10.5114/aoms.2016.60941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–96. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 37.Jonas K, Magon W, Waligora M, Seweryn M, Podolec P, Kopec G. High-density lipoprotein cholesterol level and pulmonary artery vasoreactivity in patients with idiopathic pulmonary arterial hypertension. Pol Arch Intern Med. 2018;128:440–6. doi: 10.20452/pamw.4306. [DOI] [PubMed] [Google Scholar]
- 38.Charach G, George J, Roth A, et al. Baseline low-density lipoprotein cholesterol levels and outcome in patients with heart failure. Am J Cardiol. 2010;105:100–4. doi: 10.1016/j.amjcard.2009.08.660. [DOI] [PubMed] [Google Scholar]
- 39.Rysz-Gorzynska M, Gluba-Brzozka A, Sahebkar A, et al. Efficacy of statin therapy in pulmonary arterial hypertension: a systematic review and meta-analysis. Sci Rep. 2016;6:30060. doi: 10.1038/srep30060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehra P, Mehta V, Sukhija R, et al. Pulmonary hypertension in left heart disease. Arch Med Sci. 2017 doi: 10.5114/aoms.2017.68938.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jasinska-Stroschein M, Owczarek J, Soltysiak U, Orszulak-Michalak D. Rosuvastatin intensifies the beneficial effects of rho-kinase inhibitor in reversal of monocrotaline-induced pulmonary hypertension. Arch Med Sci. 2016;12:898–905. doi: 10.5114/aoms.2015.49740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strange G, Gabbay E, Kermeen F, et al. Time from symptoms to definitive diagnosis of idiopathic pulmonary arterial hypertension: the delay study. Pulm Circ. 2013;3:89–94. doi: 10.4103/2045-8932.109919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ling Y, Johnson MK, Kiely DG, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;186:790–6. doi: 10.1164/rccm.201203-0383OC. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Austin ED, Talati M, et al. Oestrogen inhibition reverses pulmonary arterial hypertension and associated metabolic defects. Eur Respir J. 2017;50 doi: 10.1183/13993003.02337-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burger CD, Foreman AJ, Miller DP, Safford RE, McGoon MD, Badesch DB. Comparison of body habitus in patients with pulmonary arterial hypertension enrolled in the Registry to Evaluate Early and Long-term PAH Disease Management with normative values from the National Health and Nutrition Examination Survey. Mayo Clin Proc. 2011;86:105–12. doi: 10.4065/mcp.2010.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis CE, McTigue KM, Burke LE, et al. Mortality, health outcomes, and body mass index in the overweight range: a science advisory from the American Heart Association. Circulation. 2009;119:3263–71. doi: 10.1161/CIRCULATIONAHA.109.192574. [DOI] [PubMed] [Google Scholar]
- 47.Liu B, Dong X, Xiao Y, et al. Variability of metabolic risk factors associated with prehypertension in males and females: a cross-sectional study in China. Arch Med Sci. 2018;14:766–72. doi: 10.5114/aoms.2018.76066. [DOI] [PMC free article] [PubMed] [Google Scholar]