Abstract
Objective: This systematic review and meta-analysis was conducted to collate the effects of curcumin on MDA and antioxidant markers in individuals with diseased conditions. In this study the research question was “does curcumin supplementation improves oxidative stress and antioxidant defense enzymes in human subjects compared to a group without curcumin supplementation?
Methods: This research included randomized controlled trials published in English in any year, in which intervention with curcumin was compared to either placebo, or standard of care or no intervention. Pubmed, Embase, Cochrane Central, Scopus and Google Scholar were searched. Meta-analysis was performed using RevMan (version 5.3), with standardized mean differences (SMD) and random-effects models.
Results: One hundred twenty-seven titles and abstracts were identified which 17 articles were included for final analysis. The number of participants ranged from 22 to 160 across the included studies. The duration of intervention, dose of curcumin and location of outcomes measurements varied across the studies. Curcumin significantly reduced MDA [SMD −0.46 (95% CI: −0.68 to −0.25)] and increased superoxide dismutase (SOD) [0.82 (0.27 to 1.38)], catalase [10.26 (0.92 to 19.61)], and glutathione peroxidase [8.90 (6.62 to 11.19)] when compared with control group. Subgroup analyses displayed that curcumin could significantly reduce MDA levels with or without use of piperine, however it could increase SOD level in presence of piperine.
Conclusions: These findings suggest that curcumin may be used as an adjunct therapy in individuals with oxidative stress. The administration of piperine with curcumin may enhance the efficacy of curcumin on antioxidant defense system.
Keywords: Curcumin, Curcuma longa, Curcuminoid, Malondialdehyde, Antioxidant, Oxidative stress, Randomized controlled trials, Turmeric
Abbreviations
- ARE
Antioxidant response element
- CAT
Catalase
- CMA
Comprehensive Meta-Analysis
- FOXO
Forkhead box O
- GC-MS
Gas chromatography mass spectrometry
- GSH
Glutathione
- GPX
Glutathione peroxidase
- GR
Glutathione reductase
- HO-1
Hemeoxygenase-1
- Keap1
Kelch ECH associating protein 1
- MDA
Malondialdehyde
- iNOS
Nitric oxide synthase
- NO
Nitrous oxide
- Nrf2
Nuclear factor erythroid 2-related factor 2
- NF-kB
Nuclear factor-kappaB
- PGC-1a
Peroxisome proliferator-activated receptor gamma coactivator 1-a
- RCT
Randomized controlled trial
- ROS
Reactive oxygen species
- GSH
Reduced glutathione
- SIRT
Sirtuin
- SOD
Superoxide dismutase
- TCF/LEF
T cell factor/lymphoid enhancer factor
- TBARS
Thiobarbituric acid-reactive substances
- TAC
Total antioxidant capacity.
1. Introduction
Oxidative stress is a condition in which reactive oxygen species (ROS) are produced in the cells of living beings. Under normal conditions, the concentration of ROS is regulated by internal defense mechanisms including enzymatic and non-enzymatic antioxidants. In living organisms, an increase in the concentration of oxidants compared to antioxidants is termed as oxidative stress, which can impair proteins, lipids, DNA and other components of the cells. Oxidative stress is involved in the initiation and progression of various pathological conditions including diabetes, cancer and neurological disorders [1, 2]. Production of malondialdehyde (MDA), a well-known end product of lipid peroxidation, is up-regulated in response to increased amount of free radicals. Therefore, MDA concentration is a marker of oxidative stress [3]. Increased levels of MDA contribute to the pathogenesis of several metabolic diseases including diabetes [4], cancer [5], cardiovascular events [6], obesity [7] and neurological diseases such as Alzheimer [8] and depression [9].
Antioxidants are molecules that inhibit the production of free radicals and are classified into two types; enzymatic [superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (CAT), etc.] and non-enzymatic [vitamin C, vitamin E, carotenoids, glutathione (GSH), etc.] [10]. In healthy conditions, the integrated antioxidant systems of organisms are able to scavenge ROS or suppress their detrimental effects. However, during illness, the antioxidant systems are unable to manage the increased amount of ROS. According to a large body of evidence, the concentration of endogenous antioxidants is decreased in various metabolic and neurological diseases [1, 11].
Curcumin is a potent exogenous non-enzymatic antioxidant with diverse pharmacological properties. It is a natural yellow polyphenol compound, derived from Curcuma Longa Rhizome, which possesses the capacity to scavenge free radicals, reduce the generation of ROS and act as strong inhibitor of lipid peroxidation and advanced glycation end products [12–15]. Despite various in vitro, animal models and human studies no significant toxicity of curcumin is reported and it is considered generally recognized as safe (GRAS) by United States Food and Drug Administration. Numerous clinical trials have indicated that curcumin inhibits oxidative stress in several chronic diseases [16]. However, it is not frequently used as a therapeutic approach in part due to the fact that information about its advantages have not been generally synthesized and disseminated. Recently, a meta-analysis study addressed curcumin and oxidative stress [17]. However, the antioxidant defense system has not been measured comprehensively and the effects of curcumin on several antioxidant variables such as GSH, CAT, TAC and GR have not been intensively reviewed. This study was therefore conducted to collate the effects of curcumin on oxidative and antioxidant markers, with particular emphasis on MDA, SOD, CAT, total antioxidant capacity (TAC), GSH, GPx and glutathione reductase (GR) in diseased individuals under conditions of oxidative stress.
2. Methods
2.1. Search strategy and selection criteria
This meta-analysis was based on the preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. The pre-defined inclusion criteria were as follows: published full text randomized controlled trials (RCTs) in English, published at any point until January 2019, and all of the studies comparing curcumin treatment (in any form) with a placebo intervention or with standard therapy. Studies assessing the effect of curcumin in combination with other plants were excluded. Markers of oxidative stress (MDA) and of the antioxidant defense system (SOD, CAT, GPx, GSH, TAC, GR) were the outcomes of interest. The databases searched were Pubmed, Embase, Cochrane Central, Scopus and Google Scholar. Keyword and MeSH searches for [curcumin or curcuminoid or curcuma longa or turmeric] [in title] and [oxidative stress or reactive oxygen species or free radicals or antioxidant defense or malondialdehyde or superoxide dismutase or oxidative damage or pro-oxidant] [in title / abstract] were carried out in each database. Google and Google Scholar cites were hand searched for additional studies and the reference lists of the relevant reviews were checked. The PICO (Patient/Population; Intervention; Comparator; Outcome) question was as follows: in humans with diseased conditions (P), does curcumin supplementation (I) compared to placebo or standard care (C), improves oxidative stress and antioxidant defense enzymes (O)? Description of the PICOS strategy is shown in Table 1.
Table 1.
Description of the PICOS strategy.
| Population | Patients under oxidative stress conditions |
| Intervention | Any form of curcumin supplementation |
| Comparator | Defined as placebo intervention or standard therapy |
| Outcome | MDA and antioxidant defense enzymes (SOD, CAT, TAC, GSH, GPx, and GR) |
| Setting | Serum, plasma and mucosa |
Superoxide dismutase (SOD); Total antioxidant capacity (TAC); Malondialdehyde (MDA); Reduced glutathione (GSH); Catalase (CAT); Glutathione reductase (GR); Glutathione peroxidase (GPX); Glutathione (GSH).
2.2. Data extraction and quality assessment
All relevant titles and abstracts were transferred to Endnote Web, sorted into separate duplicated references and then screened based on a critical analysis of title and article summary. Each article text was then individually reviewed for evaluation of the eligibility of the article and for data extraction. A third investigator was discussed when there was disagreement on an article, and the authors were emailed when insufficient information about the data was determined. Corel software [CorelDRAW Graphics Suite 2017 (64-Bit)] was used to extract the numeric values of data which had been presented as a figure. The quality of the studies included were evaluated using Review Manager, version 5.3 (Cochrane Collaboration), focusing on bias in selection, performance, detection, attrition, and reporting. In addition, bias in the matching of control and treatment groups at entry regarding of participants number; type and dose of medications, type of therapy, health status, age and sex was examined.
2.3. Meta-analysis
Of the studies included, data from after treatment (not baseline) from the curcumin and control groups were compared as mean ± standard deviation (SD) in the meta-analysis. SD = standard error of the mean × n (number of participants used for analyses) was used as the formula to estimate SD. The SI Conversion Calculator was used to obtain the desired units. MDA and TAC levels were collated in μmol/l; SOD, CAT, GPx and GR in U/ml; and GSH in μg/ml.
If a study had more than one arm of curcumin treatment, each arm was considered as a single study. Review Manager (version Cochrane Collaboration) was used for meta-analysis, and effect size was calculated as the weighed mean difference with a 95% confidence interval. Funnel plots and Egger’s weighted regression test were used to explore potential publication bias using Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ). Meta-regression and subgroup analyses were conducted to investigate the role of factors possibly affecting heterogeneity, such as the dosage of curcumin supplements, the duration of treatment, the addition of absorption enhancers and the age of participants. Random-effects models were used due to the heterogeneity of participants. All of the outcomes were continuous measures. Multiple sensitivity analyses were carried out to identify if any of the findings were affected by the studies not included in the metaanalysis.
3. Results
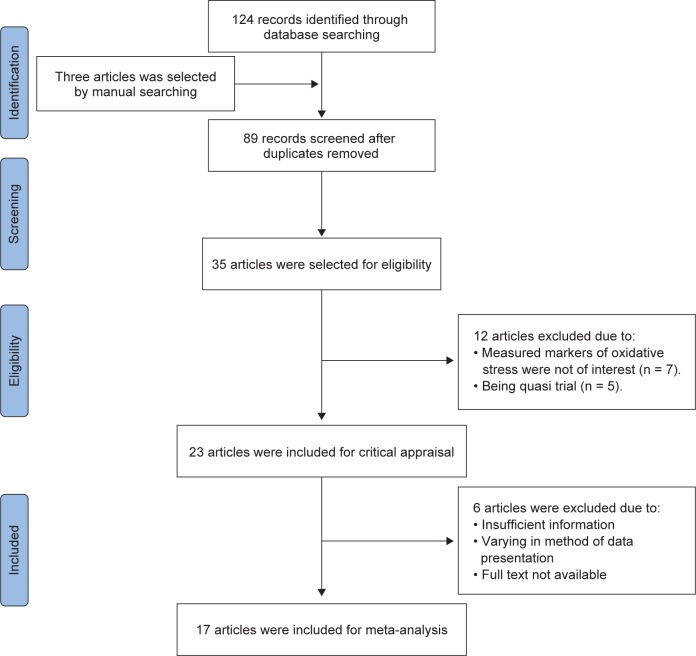
As shown in Fig. 1, one hundred and twenty-four records were found through database searches and three articles were identified by manual searching (one from Google, one from Google Scholar and one by reference searching). Eighty nine articles were screened after the duplicates were removed, of which 35 articles were eligible. Twelve articles were then excluded due to undesired markers of oxidative stress (n = 7) and being quasi trial (n = 5). Thus, 23 articles were remained for critical appraisal. At this phase, 6 articles were excluded due to lack of sufficient information, variation in method of data presentation and unavailability of the full text. At the end, 17 articles were included for meta-analysis. Of those, some articles were excluded for certain outcomes as follows: Judaki et al. [18] study for GPx; Pakfetrat et al. [19] study for GPx, CAT and GR; Panahi et al. [20] study for TAC; Sudheeran et al. [21] study for SOD, CAT and GSH outcomes since the data had been presented as g or mg protein.
Fig. 1.
Flow diagram of the study.
As shown in Fig. 2, reporting bias was not common among the studies. Allocation concealment and masking of outcome assessment was not stated by most of the studies. As shown in Fig. 3, the quality of the studies included differed and some of the studies did not contain sufficient details to evaluate all aspects of quality. The studies by Elavarasu et al. [22] and Takahashi et al. [23] did not get eligible criteria for inclusion. Publication bias of the studies included was shown by the Cochrane tool (Figures 2 and 3) and funnel plot (Fig. 4). According to Egger’s test there was no publication bias (p = 80) for MDA.
Fig. 2.
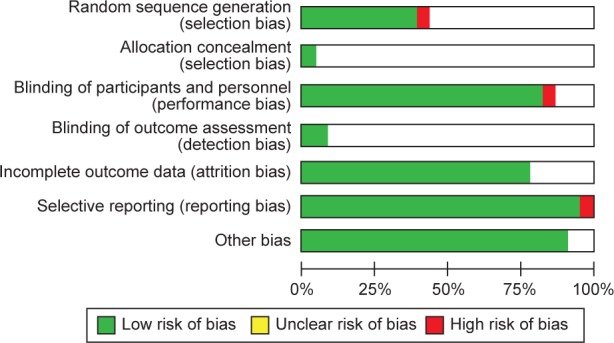
Risk of bias graph: review authors’ judgements about each risk of bias item presented as percentages across all included studies.
Fig. 3.
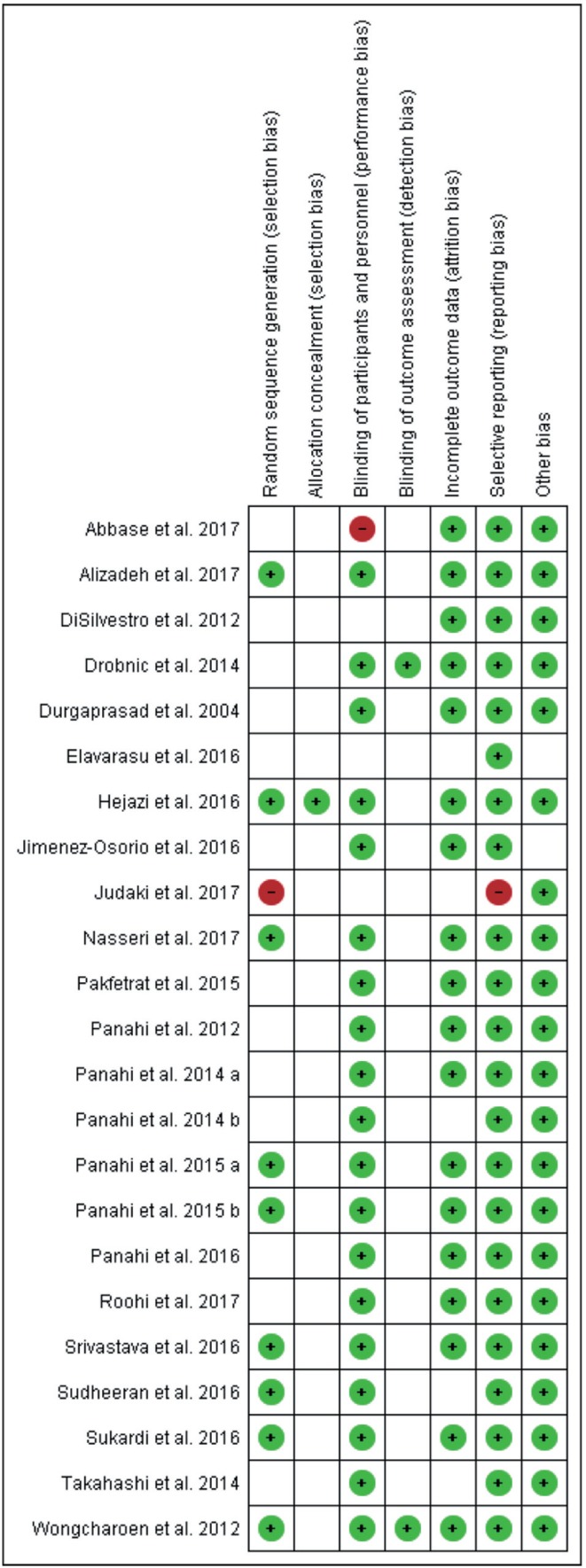
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study.
Fig. 4.
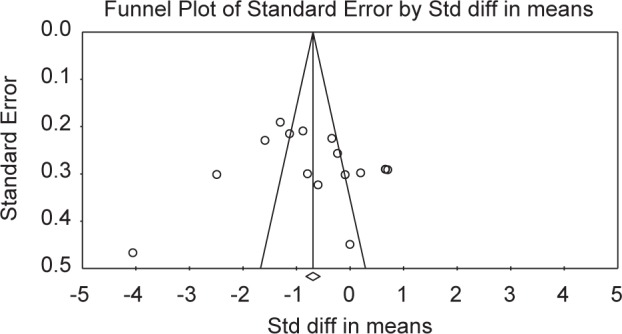
Funnel plot of the studies included.
The characteristics of the investigations included are summarized in Table 2. The included studies were published from 2012 to 2017, and the number of participants varied from 22 to 160. Further, the populations studied were with various health conditions (e.g. healthy, P-Thalassemia, chronic gastritis, etc.). The duration of treatment varied from 1 week to 4 months across the studies.
Table 2.
Characteristics of the included studies.
| Author year (ref.) | Country | Number (sex) | Age | Type of disease | Duration of treatment | Control (number at entry) | Treatment group (number at entry) | Time of measures | Type of study |
|---|---|---|---|---|---|---|---|---|---|
| Abbase et al. 201724 | Iraq | 40 (M/F) | 42.62 ± 13.84 | Peptic ulcer disease | 14 days | Standard triple therapy (N = 19) | Curcumin (1500 mg/day) + standard triple therapy (N = 21) | Baseline and after 6 weeks | RCT |
| Alizadeh et al. 201725 | Iran | 60 (M) | 30.27 ± 3.99 | Infertile oligoasthenospermia | 10 weeks | Placebo (n = 30) | Curcumin nanomicelle 80 mg/day (n = 30) | Baseline and end of study | RCT |
| DiSilvestro et al. 201226 | USA | 38 (M/F) | 47.5 ± 10.5 | Healthy | 4 weeks | Placebo (n = 19) | Curcuma Longa root powder 400 mg/day (n = 19) | Before and after treatment | RCT |
| Hejazi et al. 201627 | Iran | 40 (M) | 70.71 ± 8.20 | Prostate cancer treated with radiotherapy | 3 months | Placebo (n = 23) | Curcuminoids 3 g/day (n = 22) | Baseline and after 3 months | RCT |
| Jimenez- Osorio et al. 201628 | Mexico | 101 (M/F) | 40.55 ± 3.05 | Non-diabetic or diabetic proteinuric chronic kidney disease | 8 weeks | Placebo groups: | Turmeric (320 mg curcumin /day: | Before and after treatment | RCT |
| Non-diabetic proteinuric CKD (n = 26) | Non-diabetic proteinuric CKD (n = 24) | ||||||||
| 55.6 ± 1.55 | Diabetic proteinuric CKD (n = 23) | Diabetic proteinuric CKD (n = 28) | |||||||
| Judaki et al. 201718 | Iran | 100 (M/F) | 54.15 ± 16.09 | Chronic gastritis | 4 weeks | Standard triple therapy (N = 50) | Standard triple therapy +Turmeric tablet (700 mg three times/day) (N = 50) | Before and after treatment | RCT |
| Nasseri et al. 201729 | Iran | 68 (M/F) | 26.79 ± 6.57 | P-Thalassemia | 12 weeks | Placebo (n = 34) | Curcumin 1000 mg/day (n = 34) | Baseline and after 12 weeks | RCT |
| Pakfetrat et al. 201519 | Iran | 50 (M) | 53.6 ± 14.7 | Hemodialysis | 8 weeks | Placebo (n = 25) | Turmeric 1500 mg/day (n = 25) | Before and after treatment | RCT |
| patients | |||||||||
| Panahi et al. 201230 | Iran | 96 (M) | 47.9 ± 9.6 | Veterans of the Iraq-Iran war with chronic pruritus | 4 weeks | Placebo (n = 50) | Curcuminoids 1 g/day (n = 46) + 5 mg bioperine | Before and after treatment | RCT |
| Panahi et al. 2014 a31 | Iran | 89 (M) | 50.97 ± 7.27 | Sulfur mustard Iraq-Iran war chronic pulmonary complications | 4 weeks | placebo (n = 44) | Curcuminoids 1500 mg/day + piperine 15 mg/day (n = 45) | Before and after treatment | RCT |
| Panahi et al. 2014 b32 | Iran | 80 (M/F) | 58.95 ± 15.31 | Solid cancer | 8 weeks | Placebo (n = 40) | Curcuminoids 900 mg/day (n = 40) | Before and after treatment | RCT |
| Panahi et al. 2015 a33 | Iran | 117 (M/F) | 44.13 ± 9.18 | Metabolic syndrome | 8 weeks | Placebo (n = 58) | Curcuminoids 1 g/day + 10 mg piperine (n = 59) | Before and after treatment | RCT |
| Panahi et al. 2015 b34 | Iran | 40 (M/F) | 57.44 ± 8.91 | Knee Osteoarthritis | 6 weeks | Placebo (n= 21) | Curcuminoid 1500 mg/day + 15 mg piperine (n= 19) | Before and after treatment | RCT |
| Panahi et al. 201620 | Iran | 118 (M/F) | 42 ± 7.5 | Type 2 diabetes | 8 weeks | Placebo | Curcuminoids 1000 mg/day + piperine 10 mg | Before and after treatment | RCT |
| Roohi et al. 201735 | Iran | 22 (M) | 24.85 ± 2.2 | Active healthy males | 1 week | Placebo (n = 11) | Curcuminoids 90 mg (n = 11) | Before and after treatment | RCT |
| Srivastava et al. 201636 | India | 160 (M/F) | 50.25 ± 8.35 | Osteoarthritis of knee | 4 months | Placebo (n = 82) | Curcuma longa extract 1000 mg/day (n = 78) | At day 0, 60, and 120 | RCT |
| Sudheeran et al. 201621 | India | 60 (M/F) | 33 ± 7 | Occupational stress- related anxiety and fatigue | 30 days | Placebo (n = 20) | Formulated curcuminoids 1000 mg/day (n = 20) | Before and after treatment | RCT |
| Standard curcuminoids (782 mg curcumin) (n = 20) |
Male (M); female (F); randomized controlled trial (RCT); Age was expressed as mean ± SD or range.
Curcumin had been used in various forms and the dose varied from 80 mg to 4 g/day among the studies. The comparators generally had either the same condition as the curcumin group but received a placebo or routine care. The duration and timings were mostly similar between the control and intervention groups. None of the studies reported any adverse effects for curcumin supplementation.
As shown in Table 3, the studies measured various indicators of oxidative stress and antioxidant defense markers; with measurements taken from areas including serum, plasma, gastric mucosa, gingival crevicular fluid to erythrocytes. MDA was measured mainly by using the thiobarbituric acid reactive substances (TBARS) method.
Table 3.
Extracted data from the included trials.
| Author Year (ref.) | Measured outcomes | Placebo |
Curcumin |
unit | Method of measure | Data presented as: | |||
|---|---|---|---|---|---|---|---|---|---|
| before | after | before | after | ||||||
| Abbase et al. 20 1 724 | Serum | TAC | 50.7 ± 45 | 47.63 ± 25.27 | 37.06 ± 12.66 | 40.31 ± 13.24 | ng/ml | Elisa | Mean ± SD |
| Alizadeh et al. 20 1 725 | Serum | MDA | 1.05 ± 0.1 | 1.08 ± 0.1 | 0.96 ± 0.09 | 0.73 ± 0.07 | μmol/l | TBARS method | Mean ± SD |
| TAC | 1.36 ± 0.08 | 1.35 ± 0.09 | 1.24 ± 0.07 | 1.95 ± 0.05 | μmol/l | Colorimetry | Mean ± SD | ||
| DiSilvestro | CAT | 28 ± 4 | 27 ± 4 | 29 ± 2 | 50 ± 7 | nmol/ml | kit | Mean ± SD | |
| et al. 201226 | Plasma | SOD | 4658 ± 405 | 4748 ± 458 | 4798±401 | 4807 ± 445 | U/ml | Spectrophotometry | Mean ± SD |
| GPx | 14.6 ± 6.3 | 15.4 ± 6.2 | ELISA | Mean ± SD | |||||
| Hejazi et al. 20 1 627 | Plasma | TAC | 9.1 ± 2.3 | 10.6 ± 1.3 | 10.7 ± 2.0 | 12.8 ± 2.1 | U/mL | Assay kit | Mean ± SD |
| SOD | 195.2 ± 43.1 | 215.2 ± 83.5 | 226.1 ± 143.1 | 189.4 ± 115.4 | U/L | Mean ± SD | |||
| CAT | 134.4 ± 62.7 | 134.8± 60.2 | 139.7 ± 110.2 | 111.2 ± 80.9 | U/L | Mean ± SD | |||
| GPx | 125.9 ± 17.1 | 126.8± 12.0 | 123.4 ± 15.4 | 125.6 ± 13.4 | U/mL | Mean ± SD | |||
| Jimenez- Osorio et al. 20 1 628 | Plasma Diabetic | MDA | 3.66 ± 0.42 | 2.59 ± 0.26 | 3.44 ± 0.37 | 2.83 ± 0.24 | nM | Spectrophotometry | Mean ± SE |
| GSH | 3.49 ± 0.37 | 3.029 ± 0.24 | 2.70 ± 0.24 | 3.05 ± 0.24 | nM | Mean ± SE | |||
| GR | 0.107 ± 0.009 | 3.127± 0.01 | 0.116 ± 0.01 | 0.108 ± 0.008 | U/mL | Mean ± SE | |||
| GPX | 0.20 ± 0.01 | 0.20 ± 0.01 | 0.22 ± 0.01 | 0.18 ± 0.02 | U/mL | Mean ± SE | |||
| Jimenez- Osorio et al. 20 1 628 | Plasma Non-diabetic | MDA | 3.07 ± 0.39 | 3.05 ± 0.31 | 4.06 ± 0.37 | 2.94 ± 0.22 | nM | Spectrophotometry | Mean ± SE |
| GSH | 2.56 ± 0.28 | 3.03 ± 0.15 | 3.84 ± 0.42 | 3.71 ± 0.33 | nM | Mean ± SE | |||
| GR | 0.11 ± 0.008 | 0.118 ± 0.01 | 0.118 ± 0.007 | 0.126 ± 0.01 | U/mL | Mean ± SE | |||
| GPX | 0.22 ± 0.01 | 0.23 ± 0.04 | 0.23 ± 0.01 | 0.28 ± 0.06 | U/mL | Mean ± SE | |||
| Judaki et al. 201718 | Gastric mucosa | MDA | 2.63 ± 0.42 | 2.92 ± 0.24 | 2.47 ± 0.33 | 2.33 ± 0.70 | μmol/L | Mean ± SD | |
| TAC | 2.52 ± 0.37 | 2.50 ± 0.60 | 2.04 ± 0.47 | 1.94 ± 0.32 | μmol/L | Mean ± SD | |||
| Nasseri et al. 20 1 729 | Serum | MDA | 38.70 ± 12.24 | 39.90 ± 12.37 | 41.03 ± 14.96 | 37.12 ± 11.70 | μmol/L | Colorimetric assay | Mean ± SD |
| TAC | 191.46 ± 76.16 | 196.10 ± 72.57 | 187.09 ± 82.26 | 207.96 ± 91.72 | μmol/L | Mean ± SD | |||
| CAT | 6.20 ± 3.73 | 6.01 ± 3.87 | 5.68 ± 3.95 | 5.81 ± 3.76 | μ/mL | Mean ± SD | |||
| Pakfetrat et al. 201519 | Plasma | MDA | 8.6 ± 1.4 | 7.5 ± 1.1 | 7.5 ± 2.5 | 6.0 ± 2.4 | nmol/mL | Colorimetric | Mean ± SD |
| Panahi et al. 201230 | Serum | SOD | 58.01 ± 12.17 | 57.01 ± 8.84 | 57.68 ± 12.67 | 65.85 ± 13.84 | μKat/l | kits | Mean ± SD |
| GPX | 57.34 ± 9.67 | 56.34 ± 11.67 | 63.68 ± 9.34 | 74.68± 21.34 | μKat/l | Mean ± SD | |||
| CAT | 663.47 ± 66.35 | 656.63 ± 54.51 | 647.80 ± 87.68 | 832.33 ± 154.53 | μKat/l | Mean ± SD | |||
| Panahi et al. 2014 a31 | Serum | GSH | 8.91 ± 1.86 | 11.33 ± 4.32 | 10.90 ± 2.77 | 21.02 ± 4.24 | μg/mL | Spectrophotometry | Mean ± SD |
| MDA | 24.38 ± 3.21 | 22.47 ± 4.92 | 25.38 ± 3.61 | 11.84 ± 3.51 | nmol/mL | Mean ± SD | |||
| Panahi et al. 2014 b32 | Serum | SOD | 0.70 ± 0.19 | 0.80 ± 0.24 | 0.75 ± 0.33 | 2.43 ± 0.62 | U/mL | Spectrophotometry | Mean ± SD |
| CAT | 10.21 ± 1.76 | 9.57 ± 1.71 | 14.08 ± 3.13 | 27.93 ± 4.49 | U/mL | Mean ± SD | |||
| GSH | 8.85 ± 1.87 | 11.74 ± 4.25 | 10.69 ± 3.08 | 20.96 ± 3.40 | μg/mL | Mean ± SD | |||
| MDA | 24.38 ± 3.18 | 25.44 ± 4.75 | 25.53 ± 3.75 | 20.23 ± 21.32 | nmole/mL | Mean ± SD | |||
| Panahi et al. 2015 a33 | Serum | SOD | 1.70 ± 0.41 | 1.98 ± 0.29 | 1.47 ± 0.31 | 2.41 ± 0.37 | U/mL | Spectrophotometry | Mean ± SD |
| MDA | 19.56 ± 2.73 | 20.16 ± 3.11 | 19.38 ± 3.08 | 15.62 ± 2.59 | nmole/mL | Mean ± SD | |||
| Panahi et al. 2015 b34 | Serum | SOD | 4.13 ± 0.36 | 3.76 ± 0.44 | 4.02 ± 0.29 | 6.94 ± 0.91 | U/mL | Spectrophotometry | Mean ± SE |
| GSH | 3.43 ± 0.22 | 3.41 ± 0.43 | 3.67 ± 0.19 | 5.06 ± 0.62 | μg/mL | Mean ± SE | |||
| MDA | 23.11 ± 0.40 | 20.62 ± 0.99 | 23.00 ± 0.55 | 17.73 ± 1.19 | nmol/mL | Mean ± SE | |||
| Panahi et al. 20 1 620 | Serum | TAC | 3.17 (2.42-3.80) | 2.63 (2.24-3.20) | 3.17 (2.65-3.89) | 3.84 (2.43-4.30) | nmol/mg | Colorimetry | Median (IQR) |
| SOD | 3.39 ± 1.01 | 2.96 ± 0.78 | 3.46 ± 0.99 | 3.86 ± 0.76 | U/mL | Spectrophotometry | mean ± SD | ||
| MDA | 3.74 ± 1.32 | 3.88 ± 0.98 | 3.90 ± 1.06 | 3.05 ± 0.91 | nmol/mL | mean ± SD | |||
| Roohi et al. 201735 | Plasma | TAC | 207.21 ± 14.41 | 190.09 ± 13.51 | 190.99 ± 12.61 | 322.52 ± 21.62 | μmol/L | Spectrophotometer | Mean ± SE |
| GSH | 1.36 ± 0.05 | 1.51 ± 0.10 | 1.26 ± 0.029 | 1.90 ± 0.17 | μmol/L | Mean ± SE | |||
| MDA | 0.96 ± 0.04 | 0.97 ± 0.01 | 0.99 ± 0.026 | 0.97 ± 0.005 | μmol/L | TBARS method | Mean ± SE | ||
| Srivastava et al. 201636 | Serum | MDA | 5.15 ± 0.14 | 4.91 ± 0.11 | 5.03 ± 0.16 | 3.69 ± 0.12 | nmol/ml | TBARS method | Mean ± SE |
| Sudheeran et al. 201621 (Standard curcumin) | Plasma | SOD | 0 | 0 | 0 | 0 | U/mL | – | Mean ± SD |
| GPx | 25.97 ± 3.29 | 28.60 ± 2.96 | 33.04 ± 2.17 | 39.13 ± 1.30 | U/mL | Mean ± SD | |||
| Sudheeran et al. 20 1 621 (Formulated curcumin) | Plasma | SOD | 0 | 0 | 0 | 0 | U/mL | Mean ± SD | |
| GPx | 25.97 ± 3.29 | 28.60 ± 2.96 | 41.85 ± 4.36 | 67.57 ± 2.61 | U/mL | Mean ± SD | |||
Superoxide dismutase (SOD); Total antioxidant capacity (TAC); Malondialdehyde (MDA); Reduced glutathione (GSH); Catalase (CAT); Glutathione reductase (GR); Glutathione peroxidase (GPX); Glutathione (GSH); Thiobarbituric acid-reactive substances (TBARS); Gas chromatography mass spectrometry (GC-MS)
Note: Data of one article was emailed by author. (DiSilvestro et al. 2012)
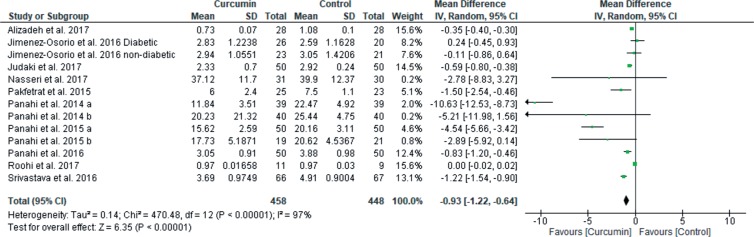
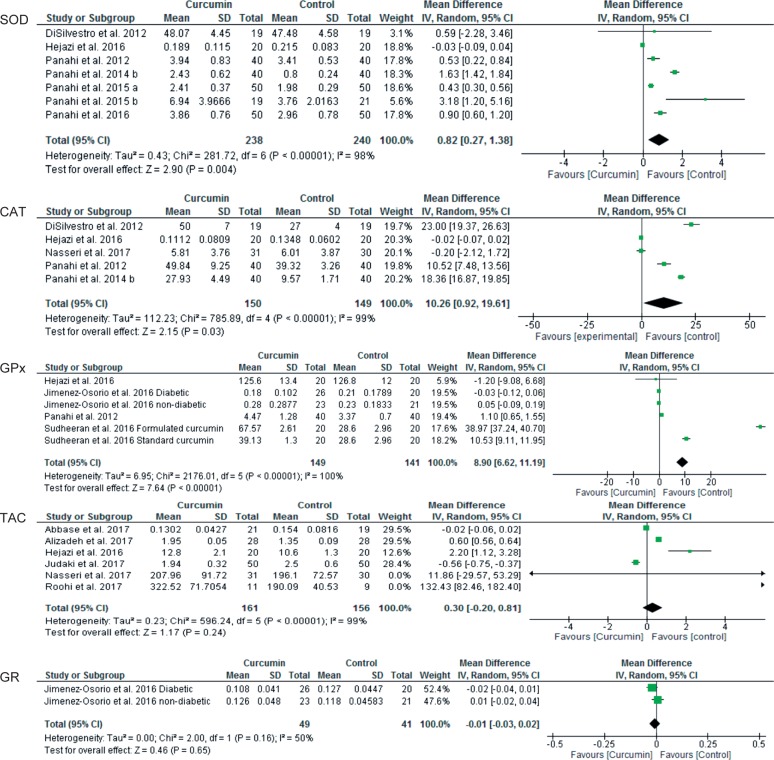
When after intervention levels of MDA and antioxidant enzymes were compared between curcumin and control groups, curcumin treatment significantly reduced MDA levels [13 studies, total 95% CI: −0.93 (−1.22 to −0.64), overall effect: Z = 6.35, p < 0.00001, I2 = 97%] (Fig. 5); but increased levels of SOD [7 studies, total 95% CI: 0.82 (0.27 to 1.38), overall effect: Z = 2.90, p = 0.004, I2 = 98%] (Fig. 6); CAT [5 studies, total 95% CI: 10.26 (0.92 to 19.61), overall effect: Z = 2.15, p = 0.03, I2 = 99%] (Fig. 6); GPx [6 studies, total 95% CI: 8.90 (6.62 to 11.19), overall effect: Z = 7.64, p < 0.00001, I2 = 100%] (Fig. 6). However, curcumin treatment did not significantly impact on levels of TAC [6 studies, total 95% CI: 0.30 (−0.20 to 0.81), overall effect: Z = 1.17, p = 0.24, I2 = 99%] (Fig. 6) and GR [2 studies, total 95% CI: −0.01 (−0.03 to 0.02), overall effect: Z = 0.46, p = 0.65, I2 = 50%] (Fig. 6) compared to the control group.
Fig. 5.
Forest plot of MDA outcome.
Fig. 6.
Forest plots of antioxidants markers.
In another analysis, before and after intervention levels of MDA and antioxidant enzymes were compared only in curcumin treated groups. Curcumin treatment significantly reduced MDA levels [13 studies, total 95% CI: −1.41 (−1.76 to −1.06), overall effect: Z = 7.82, p < 0.00001; I2 = 97%]; but significantly increased SOD [7 studies, total 95% CI: 0.83 (0.19 to 1.46), overall effect: Z = 2.56, p = 0.01, I2 = 98%]; CAT [5 studies, total 95% CI: 9.11 (1.58 to 16.64), overall effect: Z = 2.37, p = 0.02, I2 = 99%]; and GPx [7 studies, total 95% CI: 4.04 (3.02 to 5.06), overall effect: Z = 7.78, p < 0.00001, I2 = 99%]. However, curcumin treatment did not significantly affect TAC [7 studies, total 95% CI: 0.42 (−0.03 to 0.88), overall effect: Z = 1.81, p = 0.07; I2 = 100%]; and GR [2 studies, total 95% CI: 0.00 (−0.02 to 0.02), overall effect: Z = 0.04, p = 0.96, I2 = 0%] levels as compared to before intervention values (Data not shown).
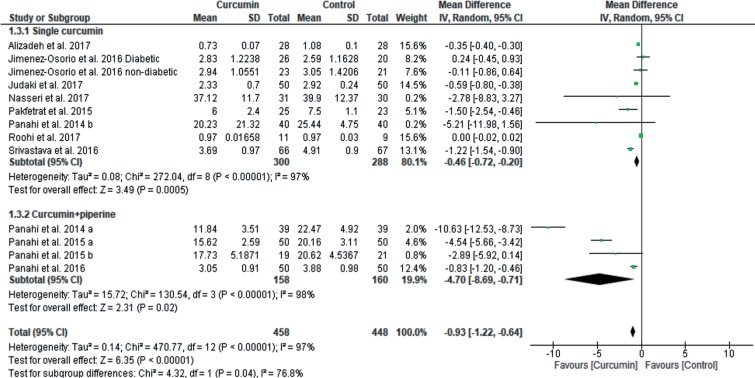
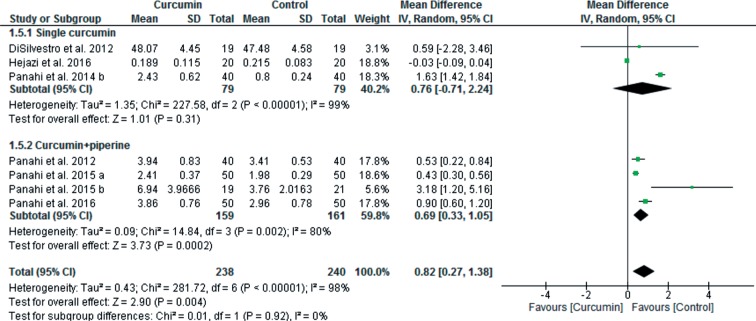
Heterogeneity was high for all of the outcomes. The findings of the meta-regression showed that the dose of supplemented curcumin was a factor that significantly influenced heterogeneity (slope: −0.0003; 95% CI: −0.0006 to −0.00007; p = 0.01) for MDA outcome, but age (slope: 0.003; 95% CI: −0.01 to 0.02; p = 0.67) and duration of curcumin supplementation (slope: −0.009; 95% CI: −0.04 to 0.02; p = 0.61) did not impact. Furthermore, according to the poor bioavailability of curcuminoids, some of the studies supplemented curcumin with small doses of pipeline to overcome to this problem. Therefore, subgroup analysis was done to clarify the effect of curcumin supplementation alone compared to curcumin plus piperine supplementation on MDA and SOD levels. As shown in Fig. 7, curcumin significantly reduced MDA levels both without pipeline [9 studies, total 95% CI: −0.46 (−0.72 to −0.20), overall effect: Z = 3.49, p = 0.0005, I2 = 97%] and with piperine [4 studies, total 95% CI: −4.70 (−8.69 to −0.71), overall effect: Z = 2.31, p = 0.02, I2 = 98%]. As shown in Fig. 8, supplementation of curcumin along with piperine significantly increased SOD levels [4 studies; total 95% CI: 0.69 (0.33 to 1.05), overall effect: Z = 3.73, p = 0.0002; I2 = 80%], but without piperine did not significantly increase SOD levels [3 studies, total 95% CI: 0.76 (−0.71 to 2.24); overall effect: Z = 1.01, p = 0.31; I2 = 99%].
Fig. 7.
Forest plots of MDA subgroup analysis. (Single curcumin vs. Curcumin + piperine)
Fig. 8.
Forest plots of SOD subgroup analysis. (Single curcumin vs. Curcumin + piperine)
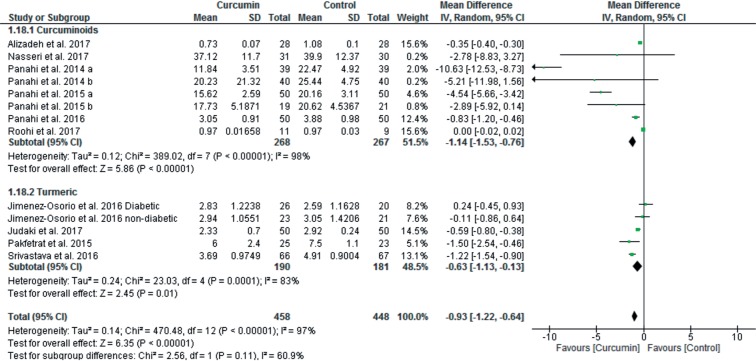
Fourteen studies used curcumin with or without other curcuminoids as supplement in intervention group, which we put all in curcuminoids group, whereas, three studies used turmeric powder. To separate effects of curcuminoids from turmeric powder on variables studied, we did subgroup analysis for MDA. As shown in Fig. 9, either curcuminoids [total 95% CI: −1.19 (−1.53 to −0.76); overall effect: Z = 5.86, p < 0.00001; I2 = 98%] or turmeric powder [total 95% CI: −0.63 (−1.13 to −0.13); overall effect: Z = 2.45, p = 0.01; I2 = 83%] significantly reduced MDA level. However, for antioxidant variables, number of studies with turmeric supplementation were too few, for that we did sensitivity test to isolate its effect on the results. Exclusion of studies with turmeric supplementation increased significance of the results for SOD [6 studies, total 95% CI: 0.83 (0.27 to 1.40); overall effect: Z = 2.88, p = 0.004; I2 = 98%)] and TAC [5 studies, total 95% CI: 0.63 (0.05 to 1.21); overall effect: Z = 2.14, p = 0.03; I2 = 99%)], but reduced for CAT [4 studies, total 95% CI: 7.15 (−2.62 to 16.92); overall effect: Z = 1.43, p = 0.15; I2 = 100%)] and GPx [4 studies, total 95% CI: 12.50 (−4.92 to 29.93); overall effect: Z = 1.41, p = 0.16; I2 = 100%].
Fig. 9.
Forest plots of MDA subgroup analysis (Curcuminoids vs. Turmeric powder)
4. Discussion
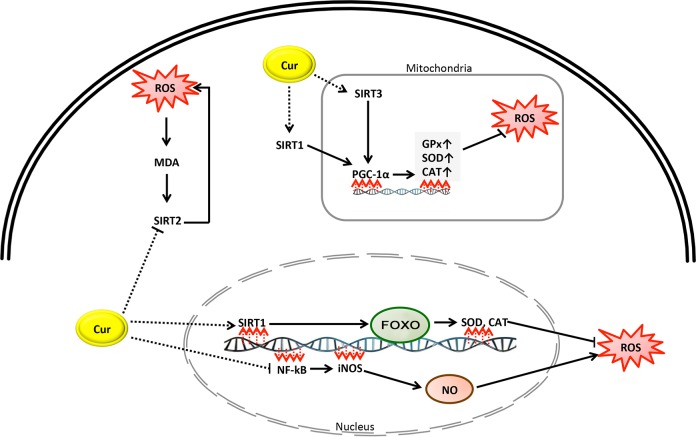
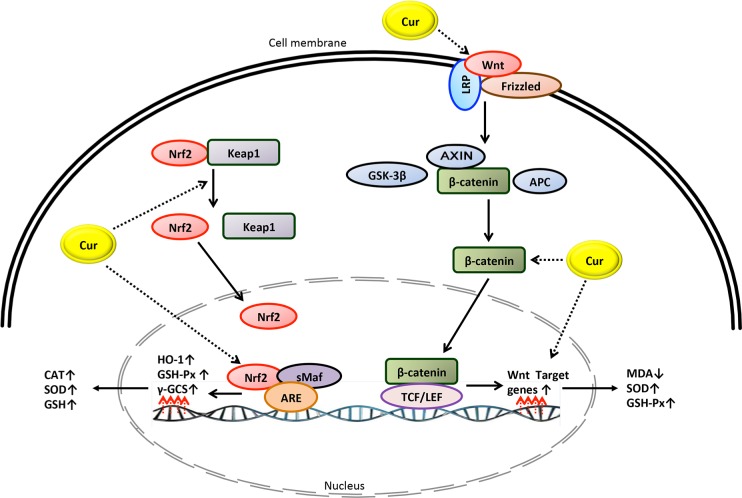
This study showed that curcumin was effective in reducing MDA and in increasing levels of antioxidants. A large amount of in vivo, experimental and human evidence has suggested that curcumin can act as a free radical scavenger and an inhibitor of MDA production. However, the exact mechanism by which curcumin inhibits oxidative stress is unclear. As shown in Fig. 10 and Fig. 11, it is speculated that curcumin prevents oxidative stress through various pathways:
Fig. 10.
A simplified mechanistic model of curcumin in prevention of oxidative stress through activation or inhibition of sirtuin proteins and NF-KB pathways.
Fig. 11.
A simplified mechanistic model of curcumin in prevention of oxidative stress through activation of Keap1-Nrf2-ARE and Wnt/β-Catenin signaling pathways.
4.1. Sirtuins 1, 2 and 3 pathways
The sirtuins (SIRT) are a group of proteins which act as intracellular regulatory proteins, and are involved in multiple cellular processes including aging, resistance to stress, metabolic regulation and transcription. As shown in Fig. 10, the activation or inhibition of sirtuins of 1, 2 and 3 by curcumin may be involved in reducing malondialdehyde and increasing the levels of antioxidants. Various studies suggest that SIRT1 and SIRT3 inhibit oxidative stress in cells [37], whereas SIRT 2 triggers it [38]. Curcumin has been suggested to act as an activator of SIRT1 and SIRT3, but as an inhibitor of SIRT2.
4.1.1. SIRT1
SIRT1 is mostly located in the nucleus and functions by reducing the acetylation of Forkhead box O (FOXO) 3a protein, increasing the binding of FOXO to DNA and activating the FOXO transcription factors that regulate antioxidant genes including SOD and CAT in order to reduce cellular levels of ROS [39–41]. In a review article by Zhang et al. [42] it was concluded that SIRT1 inhibits cellular oxidative stress. Further, Miao et al. [37] reported that curcumin increased SIRT1 expression. Furthermore, peroxisome proliferator-activated receptor gamma coactivator a (PGC-1a) is a transcriptional modulator which regulates the expression of genes contributing to mitochondrial metabolism, biogenesis and oxidative stress [43]. SIRT1 activates PGC-1a, which enhances mitochondrial expression of antioxidant genes including GPx, CAT, and SOD [41, 44]. SIRT1 may reduce the cellular ROS load via preventing the expression and production of inducible nitric oxide synthase (iNOS) and nitrous oxide (NO) through the deacetylation of p65, leading to the suppression of the nuclear factor-kappaB (NF-kB) signaling pathway [45].
4.1.2. SIRT2
SIRT2 is located in the cytoplasm. Nie et al. [38] reported that oxidative stress augments SIRT2 levels in cells and reduction of SIRT2 results in a lower production of H2O2-induced ROS. Wang et al. [39] reported that oxidative stress upregulates SIRT2 expression in cells. Keskin-Aktan et al. [46] found in an animal model study that curcumin treatment significantly reduced MDA and SIRT2 expression in the hippocampus of rats, and that SIRT2 expression was positively associated with MDA. Taken together, the protective effect of curcumin against oxidative stress might be attributed to its capacity to increase levels of SIRT1 and reduce levels of SIRT2 [39].
4.1.3. SIRT3/PGC-1a signaling pathway
SIRT3 is another member of the sirtuins family, which is mainly localized in the mitochondrial matrix and controls mitochondrial fatty-acid oxidation [47]. Overexpression of SIRT3 augments the expression of PGC-1a and lowers the production of ROS (Fig. 10). Zhang et al. [48] showed that curcumin reduced oxidative stress by decreasing MDA and increasing SOD, GPx and CAT levels in skeletal muscle mitochondria in a rat model of COPD. Curcumin also upregulated mRNA and protein expression of PGC-1a and SIRT3. The authors concluded that curcumin may possibly attenuate oxidative stress by augmenting the PGC-1a/SIRT3 signaling pathway [48].
4.1.4. Keap1-Nri2-ARE signaling pathway
The Keap1-Nrf2-ARE pathway is known to be the main regulator of oxidative and electrophilic stress responses. In this pathway, the nuclear factor erythroid 2-related factor 2 (Nrf2), a transcription factor, binds to the antioxidant response element (ARE) in the regulatory regions of target genes along with small Maf proteins, allowing Kelch ECH associating protein 1 (Keapl) to bind to Nrf2 and repress it [49]. According to the current evidence, activation of the Keap1-Nrf2- ARE signaling pathway by curcumin may possibly diminish oxidative stress. He et al. [50] reported that curcumin treatment in mice fed with a high fat diet diminished the expected increase in muscular MDA and ROS and reversed the reduced level of nuclear factor erythroid-related factor-2 (Nrf2) and hemeoxygenase-1 (HO-1, a stress-response protein). Shi et al. [51] reported that activation of the Keap1-Nrf2-ARE pathway decreased oxidative stress. Xie et al. [52] showed that curcumin treatment in diabetic rats inhibited oxidative stress through elevated expression of CAT, GSH-Px, HO-1 and norvegicus NAD(P)H quinone dehydrogenase 1, while reducing the expression of SOD1. Moreover, curcumin treatment enhanced expression of the Keap1 protein and increased the nuclear accumulation of Nrf2. The authors concluded that oxidative stress may be diminished by curcumin via activating the Keap1-Nrf2-ARE signaling pathway [52]. Wicha et al. [53], in an animal model study, demonstrated that treating rats with hexa-hydrocurcumin significantly lowered oxidative stress, MDA and NO levels and also enhanced Nrf2 and HO-1 expression, antioxidative enzyme and SOD activity.
4.1.5. Wnt/p-Catenin Signaling Pathway
The Wnt/p-catenin pathway is activated by connecting a Wnt-protein ligand to a Frizzled family receptor and a lipoprotein receptor related protein 6/5 (LRP6 or LRP5), which leads to the accumulation of P-catenin in the nucleus that forms complexes with DNA-bound T cell [54]. Lima et al. [55] have shown the Wnt signaling pathway participates in the reduction of oxidative stress and the enhancement of antioxidant activity. Wang et al. [56] demonstrated that curcumin treatment leads to higher mRNA and protein expressions of Wnt3a and P-catenin and mRNA expressions of c-myc and cyclinD1 as well as elevated SOD and GSH-Px levels, while lowered level of MDA in rat models of Parkinson disease. The authors concluded that the protective effect of curcumin against oxidative stress is related to the activation of the Wnt/p-catenin signaling pathway.
This study had some limitations. There was heterogeneity in the studies included with regards to population characteristics; the form, dose and time of the curcumin supplementation; and the measurement method of the variables studied. Most of the studies potentially eligible for this review had to be excluded due to inadequate information.
5. Conclusion
The findings indicate that supplementation with curcumin was effective in decreasing MDA and improving levels of antioxidants in diseased individuals under conditions of oxidative stress. The reduction of oxidative stress by curcumin supplementation was dependent on the dose of curcumin and the duration of treatment. The administration of piperine with curcumin may enhance the efficacy of curcumin on antioxidant defense system. The findings suggest the use of curcumin as a cheap and safe adjunct therapy in individuals with oxidative-associated metabolic or neurological diseases.
Authors’ contributions
Both of the authors were involved in the searching and selection of the articles, data extraction, meta-analysis and participated in manuscript writing. Both of the authors read and approved the final manuscript.
Conflicts of interest statement
The authors have declared that no conflict of interest exists.
Funding support
There was no financial support for this study.
References
- 1. Moneim AE. Oxidant/Antioxidant imbalance and the risk of Alzheimer’s disease. Curr Alzheimer Res. 2015; 12: 335–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang WJ, Zhang X, Chen WW. Role of oxidative stress in Alzheimer’s disease. Biomed Rep. 2016; 4: 519–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gawel S, Wardas M, Niedworok E, Wardas P. Malondialdehyde (MDA) as a lipid peroxidation marker. Wiad Lek. 2004; 57: 453–5. [PubMed] [Google Scholar]
- 4. Kaefer M, De Carvalho JA, Piva SJ, da Silva DB, Becker AM, Sangoi MB, et al. Plasma malondialdehyde levels and risk factors for the development of chronic complications in type 2 diabetic patients on insulin therapy. Clin Lab. 2012; 58: 973–8. [PubMed] [Google Scholar]
- 5. Bitla AR, Reddy EP, Sambasivaih K, Suchitra MM, Reddy VS, Srinivasa Rao PVLN. Evaluation of plasma malondialdehyde as a biomarker in patients with carcinoma of stomach. Biomed Res. 2011; 22: 63–8. [Google Scholar]
- 6. Boaz M, Matas Z, Biro A, Katzir Z, Green M, Fainaru M, et al. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999; 56: 1078–83. [DOI] [PubMed] [Google Scholar]
- 7. Sankhla M, Sharma TK, Mathur K, Rathor JS, Butolia V, Gadhok AK, et al. Relationship of oxidative stress with obesity and its role in obesity induced metabolic syndrome. Clin Lab. 2012; 58: 385–92. [PubMed] [Google Scholar]
- 8. López-Riquelme N, Alom-Poveda J, Viciano-Morote N, Llinares-Ibor I, Tormo-Díaz C. Apolipoprotein E ε4 allele and malondialdehyde level are independent risk factors for Alzheimer’s disease. SAGE Open Med. 2016; 4: 2050312115626731. doi: 10.1177/2050312115626731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bajpai A, Verma AK, Srivastava M, Srivastava R. Oxidative stress and major depression. J Clin Diagn Res. 2014; 8: CC04–7. doi: 10.7860/JCDR/2014/10258.5292. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5: 9–19. doi: 10.1097/W0X.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ganjifrockwala FA, Joseph JT, George G. Decreased total antioxidant levels and increased oxidative stress in South African type 2 diabetes mellitus patients. JEMDSA. 2017; 22: 21–25. [Google Scholar]
- 12. Alizadeh M, Kheirouri S. Curcumin against advanced glycation end products (AGEs) and AGEs-induced detrimental agents. Crit Rev Food Sci Nutr. 2017; 29: 1–9. [DOI] [PubMed] [Google Scholar]
- 13. Sreejayan Rao MN. Curcuminoids as potent inhibitors of lipid peroxidation. J Pharm Pharmacol. 1994; 46: 1013–6. [DOI] [PubMed] [Google Scholar]
- 14. Cekmen M, Ilbey YO, Ozbek E, Simsek A, Somay A, Ersoz C. Curcumin prevents oxidative renal damage induced by acetaminophen in rats. Food Chem Toxicol. 2009; 47: 1480–4. [DOI] [PubMed] [Google Scholar]
- 15. El-Demerdash FM, Yousef MI, Radwan FM. Ameliorating effect of curcumin on sodium arsenite-induced oxidative damage and lipid peroxidation in different rat organs. Food Chem Toxicol. 2009; 47: 249–54. [DOI] [PubMed] [Google Scholar]
- 16. Zhao WC, Zhang B, Liao MJ, Zhang WX, He WY, Wang HB, et al. Curcumin ameliorated diabetic neuropathy partially by inhibition of NADPH oxidase mediating oxidative stress in the spinal cord. Neurosci Lett. 2014; 560: 81–5. [DOI] [PubMed] [Google Scholar]
- 17. Qin S, Huang L, Gong J, Shen S, Huang J, Tang Y, et al. Metaanalysis of randomized controlled trials of 4 weeks or longer suggest that curcumin may afford some protection against oxidative stress. Nutr Res. 2018; 60: 1–12. doi: 10.1016/j.nutres.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 18. Judaki A, Rahmani A, Feizi J, Asadollahi K, Hafezi Ahmadi MR. Curcumin in combination with triple therapy regimes ameliorates oxidative stress and histopathologic changes in chronic gastritis-associated helicobacter pylori infection. Arq Gastroenterol. 2017; 54: 177–82. [DOI] [PubMed] [Google Scholar]
- 19. Pakfetrat M, Akmali M, Malekmakan L, Dabaghimanesh M, Khorsand M. Role of turmeric in oxidative modulation in end-stage renal disease patients. Hemodial Int. 2015; 19: 124–31. [DOI] [PubMed] [Google Scholar]
- 20. Panahi Y, Khalili N, Sahebi E, Namazi S, Karimian MS, Majeed M, et al. Antioxidant effects of curcuminoids in patients with type 2 diabetes mellitus: a randomized controlled trial. Inflammopharmacology. 2017; 25: 25–31. [DOI] [PubMed] [Google Scholar]
- 21. Sudheeran SP, Jacob D, Natinga Mulakal J, Gopinathan Nair G, Maliakel A, Maliakel B, et al. , Safety, Tolerance, and Enhanced Efficacy of a Bioavailable Formulation of Curcumin With Fenugreek Dietary Fiber on Occupational Stress: A Randomized, Double-Blind, Placebo- Controlled Pilot Study. J Clin Psychopharmacol. 2016; 36: 236–43. [DOI] [PubMed] [Google Scholar]
- 22. Elavarasu S, Suthanthiran T, Thangavelu A, Alex S, Palanisamy VK, Kumar TS. Evaluation of superoxide dismutase levels in local drug delivery system containing 0.2% curcumin strip as an adjunct to scaling and root planing in chronic periodontitis: A clinical and biochemical study. J Pharm Bioallied Sci. 2016; 8: S48–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Takahashi M, Suzuki K, Kim HK, Otsuka Y, Imaizumi A, Miyashita M, et al. Effects of curcumin supplementation on exercise-induced oxidative stress in humans. Int J Sports Med. 2014; 35: 469–75. [DOI] [PubMed] [Google Scholar]
- 24. Abbas SH, Abdulridha MK, Najeb AA. Potential benefit of curcumin adjuvant therapy to the standard Helicobacter pylori eradication therapy in patients with peptic ulcer disease. Asian J Pharm Clin Res. 2017; 10: 313–317. [Google Scholar]
- 25. Alizadeh F, Javadi M, Karami AA, Gholaminejad F, Kavianpour M, Haghighian HK. Curcumin nanomicelle improves semen parameters, oxidative stress, inflammatory biomarkers, and reproductive hormones in infertile men: A randomized clinical trial. Phytother Res. 2018; 32(3): 514–21. doi: 10.1002/ptr.5998. [DOI] [PubMed] [Google Scholar]
- 26. DiSilvestro RA, Joseph E, Zhao S, Bomser J. Diverse effects of a low dose supplement of lipidated curcumin in healthy middle aged people. Nutr J. 2012; 11: 79. doi: 10.1186/1475-2891-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hejazi J, Rastmanesh R, Taleban FA, Molana SH, Hejazi E, Ehtejab G, et al. Effect of Curcumin supplementation during radiotherapy on oxidative status of patients with prostate cancer: A double blinded, randomized, placebo-controlled study. Nutr Cancer. 2016; 68: 77–85. [DOI] [PubMed] [Google Scholar]
- 28. Jiménez-Osorio AS, García-Niño WR, González-Reyes S, Álvarez-Mejía AE, Guerra-León S, Salazar-Segovia J, et al. The effect of dietary supplementation with curcumin on redox status and Nrf2 activation in patients with nondiabetic or diabetic proteinuric chronic kidney disease: A pilot study. J Ren Nutr. 2016; 26: 237–244. [DOI] [PubMed] [Google Scholar]
- 29. Nasseri E, Mohammadi E, Tamaddoni A, Qujeq D, Zayeri F, Zand H. Benefits of curcumin supplementation on antioxidant status in β-Thalassemia major patients: A double-blind randomized controlled clinical trial. Ann Nutr Metab. 2017; 71: 136–44. [DOI] [PubMed] [Google Scholar]
- 30. Panahi Y, Sahebkar A, Amiri M, Davoudi SM, Beiraghdar F, Hoseininejad SL, et al. Improvement of sulphur mustard-induced chronic pruritus, quality of life and antioxidant status by curcumin: results of a randomised, double-blind, placebo-controlled trial. Br J Nutr. 2012;108:1272–9. [DOI] [PubMed] [Google Scholar]
- 31. Panahi Y, Ghanei M, Hajhashemi A, Sahebkar A. Effects of curcuminoids-piperine combination on systemic oxidative stress, clinical symptoms and quality of life in subjects with chronic pulmonary complications due to sulfur mustard: A randomized controlled trial. J Diet Suppl. 2016; 13: 93–105. [DOI] [PubMed] [Google Scholar]
- 32. Panahi Y, Saadat A, Beiraghdar F, Hosseini-Nouzari SM, Jalalian HR, Sahebkar A. Antioxidant effects of bioavailability-enhanced curcuminoids in patients with solid tumors: A randomized double-blind placebo-controlled trial. J Funct Foods. 2014; 6: 615–22. [Google Scholar]
- 33. Panahi Y, Hosseini MS, Khalili N, Naimi E, Majeed M, Sahebkar A. Antioxidant and anti-inflammatory effects of curcuminoid-piperine combination in subjects with metabolic syndrome: A randomized controlled trial and an updated meta-analysis. Clin Nutr. 2015; 34: 1101–8. [DOI] [PubMed] [Google Scholar]
- 34. Panahi Y, Alishiri GH, Parvin S, Sahebkar A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: results of a randomized controlled trial. J Diet Suppl. 2016; 13: 209–20. [DOI] [PubMed] [Google Scholar]
- 35. Roohi BN, Moradlou AN, Bolboli L. Influence of curcumin supplementation on exercise-induced oxidative stress. Asian J Sports Med. 2017; 8: e35776. [Google Scholar]
- 36. Srivastava S, Saksena AK, Khattri S, Kumar S, Dagur RS. Curcuma longa extract reduces inflammatory and oxidative stress biomarkers in osteoarthritis of knee: a four-month, double-blind, randomized, placebo-controlled trial. Inflammopharmacology. 2016; 24: 377–88. [DOI] [PubMed] [Google Scholar]
- 37. Miao Y, Zhao S, Gao Y, Wang R, Wu Q, Wu H, et al. Curcumin pretreatment attenuates inflammation and mitochondrial dysfunction in experimental stroke: The possible role of Sirt1 signaling. Brain Res Bull. 2016; 121: 9–15. [DOI] [PubMed] [Google Scholar]
- 38. Nie H, Hong Y, Lu X, Zhang J, Chen H, Li Y, et al. SIRT2 mediates oxidative stress-induced apoptosis of differentiated PC12 cells. Neuroreport. 2014; 25: 838–42. doi: 10.1097/WNR.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 39. Wang F, Nguyen M, Qin FX, Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007; 6: 505–14. [DOI] [PubMed] [Google Scholar]
- 40. Lai L, Yan L, Gao S, Hu CL, Ge H, Davidow A, et al. Type 5 adenylyl cyclase increases oxidative stress by transcriptional regulation of manganese superoxide dismutase via the SIRT1/FoxO3a pathway. Circulation. 2013; 127: 1692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Merksamer PI, Liu Y, He W, Hirschey MD, Chen D, Verdin E. The sirtuins, oxidative stress and aging: an emerging link. Aging (Albany NY). 2013; 5: 144–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhang W, Huang Q, Zeng Z, Wu J, Zhang Y, Chen Z. Sirt1 inhibits oxidative stress in vascular endothelial cells. Oxid Med Cell Longev. 2017; 2017: 7543973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Houten SM, Auwerx J. PGC-1alpha: turbocharging mitochondria. Cell. 2004; 119: 5–7. [DOI] [PubMed] [Google Scholar]
- 44. St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jäger S, et al. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006; 127: 397–408. [DOI] [PubMed] [Google Scholar]
- 45. Lee JH, Song MY, Song EK, Kim EK, Moon WS, Han MK, et al. Overexpression of SIRT1 protects pancreatic beta-cells against cytokine toxicity by suppressing the nuclear factor- kappaB signaling pathway. Diabetes. 2009; 58: 344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Keskin-Aktan A, Akbulut KG, Yazici-Mutlu Q, Sonugur G, Ocal M, Akbulut H. The effects of melatonin and curcumin on the expression of SIRT2, Bcl-2 and Bax in the hippocampus of adult rats. Brain Res Bull. 2018; 137: 306–10. [DOI] [PubMed] [Google Scholar]
- 47. Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010; 464: 121–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhang M, Tang J, Li Y, Xie Y, Shan H, Chen M, et al. Curcumin attenuates skeletal muscle mitochondrial impairment in COPD rats: PGC-1a/SIRT3 pathway involved. Chem Biol Interact. 2017; 277: 168–75. [DOI] [PubMed] [Google Scholar]
- 49. Kansanen E, Kuosmanen SM, Leinonen H, Levonen AL. The Keap1-Nrf2 pathway: Mechanisms of activation and dysregulation in cancer. Redox Biol. 2013; 1: 45–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes. 2012; 3: 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shi L, Wu L, Chen Z, Yang J, Chen X, Yu F, et al. MiR-141 activates Nrf2-dependent antioxidant pathway via down-regulating the expression of keap1 conferring the resistance of hepatocellular carcinoma cells to 5-fluorouracil. Cell Physiol Biochem. 2015; 35: 2333–48. [DOI] [PubMed] [Google Scholar]
- 52. Xie Z, Wu B, Shen G, Li X, Wu Q. Curcumin alleviates liver oxidative stress in type 1 diabetic rats. Mol Med Rep. 2018; 17: 103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wicha P, Tocharus J, Janyou A, Jittiwat J, Changtam C, Suksamrarn A, et al. Hexahydrocurcumin protects against cerebral ischemia/reperfusion injury, attenuates inflammation, and improves antioxidant defenses in a rat stroke model. PLoS One. 2017; 12: e0189211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009; 17: 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lima MDR, Lopes AP, Martins C, Brito GAC, Carneiro VC, Goes P. The effect of calendula officinalis on oxidative stress and bone loss in experimental periodontitis. Front Physiol. 2017; 8: 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wang YL, Ju B, Zhang YZ, Yin HL, Liu YJ, Wang SS, et al. Protective effect of curcumin against oxidative stress-induced injury in rats with parkinson’s disease through the Wnt/ β-catenin signaling pathway. Cell Physiol Biochem. 2017; 43: 2226–41. [DOI] [PubMed] [Google Scholar]