Abstract
Auditory function has been shown to be influenced by the circadian system. Increasing evidence point towards the regulation of inflammation and glucocorticoid actions by circadian rhythms in the cochlea. Yet, how these three systems (circadian, immune and endocrine) converge to control auditory function remains to be established. Here we review the knowledge on immune and glucocorticoid actions, and how they interact with the circadian and the auditory system, with a particular emphasis on cochlear responses to noise trauma. We propose a multimodal approach to understand the mechanisms of noise-induced hearing loss by integrating the circadian, immune and endocrine systems into the bearings of the cochlea. Considering the well-established positive impact of chronotherapeutic approaches in the treatment of cardiovascular, asthma and cancer, an increased knowledge on the mechanisms where circadian, immune and glucocorticoids meet in the cochlea may improve current treatments against hearing disorders.
Keywords: Circadian, Inflammation, Hearing loss, Glucocorticoids, Cochlea, Immune system
The Workings of Circadian Rhythms
The rotation of the Earth exposes all forms of life to a 24-h environmental cycle, which in turn has led to the evolution of the daily (circadian) rhythms with a periodicity of approximately 24 hours. Circadian rhythms are driven by the circadian clock machinery (biological clocks) to ensure that the organism anticipates and adapts to temporal changes in the environment (Scheiermann et al., 2018). The light/dark cycle has a strong influence on biological clocks, which is most obvious in the form of sleep-wake cycle. However, many other biological functions such as behavior, locomotor activity, metabolic function, cardiovascular, endocrine, digestive and immune systems are under the control of the circadian system. For instance, the circadian regulation of blood pressure ensures that it raises during the active phase (daytime for human and nighttime for rodents when the activity is high) but declines as the organism enter the inactive phase (nighttime for human and daytime for rodents when they are resting). Thus, having a tightly coupled circadian control over all bodily functions enhances the organismal fitness.
In mammals, the suprachiasmatic nucleus (SCN) of hypothalamus possess a central clock machinery that acts as circadian pacemaker to entrain the peripheral clocks (Reppert et al., 2002) found in nearly all cells of the body. The SCN is referred to as pacemaker clock as SCN neurons in vitro persistently generate a rhythmic expression of clock genes for more than a month, even in isolation from the body. In contrast, the rhythmic expression of the clock genes in peripheral tissue dampen over time in vitro because individual cells fail to maintain phase coherence (i.e. reach peak and trough at the same time). The SCN is unique in that it is the only clock that is directly reset by light received via the retinohypothalamic tract. Via the photic entrainment of the SCN, central and peripheral clocks are maintained in phase coherence (synchrony) with the environment. Temperature and feeding are other environmental factors that influence peripheral clocks (Albrecht et al., 2001; Roedel et al., 2006; Ruiter et al., 2003; Weinert et al., 1998). When the light/dark cycle is shifted, circadian rhythms are disrupted in nearly all bodily functions. After this shift, circadian clocks reset in order to synchronize themselves to the new light/dark cycle (e.g. jet lag). The SCN adjusts itself relatively rapidly but the peripheral tissues take a longer time to reset in a manner that is tissue-specific (Mohawk et al., 2012; Sellix et al., 2012). In SCN lesioned animals, circadian rhythms from peripheral clocks are found to be autonomous and self-sustained - yet their phase (coherence with central and peripheral clocks) is desynchronized in a tissue-specific manner highlighting their strong dependence on SCN-input (Yoo et al., 2004). To maintain the circadian synchrony in the peripheral tissue, the central clock communicates with the peripheral clocks through cues involving complex neuronal signaling (such as the sympathetic nervous system) (Scheiermann et al., 2012), hormonal signaling (such as glucocorticoids) (Oster et al., 2017) and metabolic cues (Thaiss et al., 2016). The phase coherence between the peripheral and central clocks enhances organismal fitness while disruption (circadian misalignments) caused by abnormal lighting or feeding schemes or mutations in the core clock genes results in pathological changes. In humans, these include cancer (Fu et al., 2003), metabolic diseases, cardiovascular and immune dysfunction (Evans et al., 2013) and neurological disorder (Johansson et al., 2016; Li et al., 2013). For instance chronic shift workers have a higher risk of developing cancer, metabolic diseases, cardiovascular and immune dysfunction (Scheiermann et al., 2018) as activity at night causes conflict with their circadian biology.
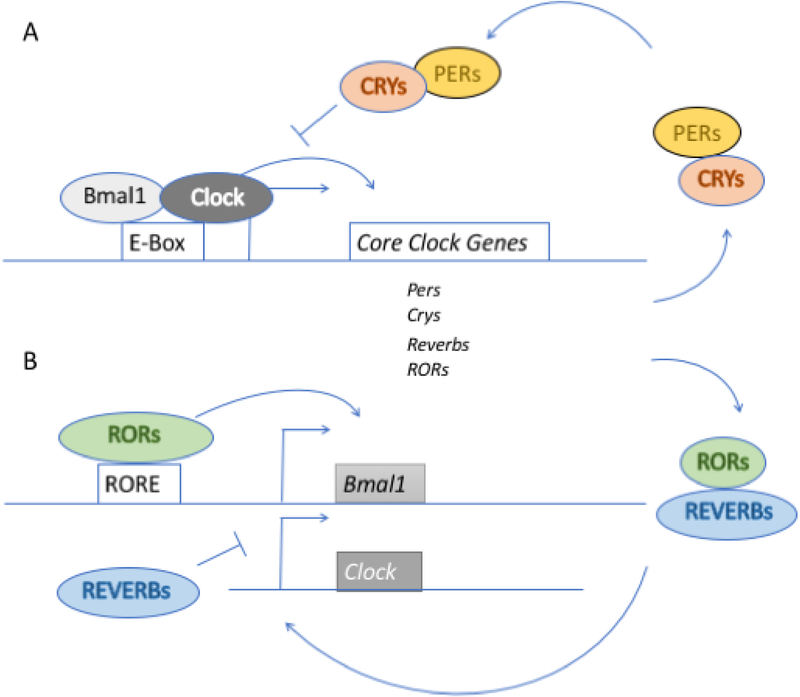
The circadian clock machinery at the core consists of transcription factors, CLOCK and BMAL1 (also known as ARNTL) (for review see (Basinou et al., 2017). Together CLOCK and BMAL1 form a heterodimer CLOCK-BMAL1 complex that binds to E-box elements on other clock genes to influence their transcription. These clock genes include Per1 and Per2, Cry1, Cry2, Rev-erbα (also known as Nr1d1) and Rev-erbβ (also known as Nr1d2). PER and CRY form a heterodimer and as PER/CRY reach a critical level, the PER/CRY heterodimer enters the nucleus and acts as corepressor to inhibit CLOCK/BMAL1 transactivation as well as their own (PER and CRY) expression. The decline in PER and CRY levels results in the release of CLOCK-BMAL1 complex inhibition which allow the starting a new cycle. This feedback loop results in oscillation of PER/CRY and CLOCK/BMAL1 complexes, which both follow a circadian pattern. There are two additional loops that cooperate in establishing a 24-hours rhythm. Firstly, the transcription factor retinoic orphan receptor (ROR) drives the expression of Bmal1, whereas REV-ERBs are repressor of Bmal1 transcriptions. The expression of REV-ERBs is activated by CLOCK/BMAL1 and transrepressed PER/CRY, which result in circadian oscillation (rhythmic) in the levels of REV-ERBs. Since RORα shares the same DNA binding site as REV-ERBs, a competitive repression by REV-ERBs leads to circadian oscillation in the levels of BMAL1. Consequently, Bmal1 transcription is typically in anti-phase (opposite) with that of Per, Cry and Rev-erb (Fig. 1). In other words, as the transcription of Per, Cry and Rev-erb increase, the transcription of Bmal1 decreases owing to the fact that PER and CRY are repressors of CLOCK/BMAL1 complex and REV-ERBs are inhibitors of Bmal1 transcription. Secondly, CLOCK-BMAL1 complex act on transcription factors such as Dbp, Hlf, and Tef, which in turn act on D-box element on target genes (genes harboring D box element). Thus, the interlocked loops act on the E-box, D-box and ROR elements to generate oscillations in the expression of the target genes (genes harboring E-, D-box and/or ROR elements) and give circadian temporal cues to the cellular processes.
Figure 1.
The molecular clock machinery. (A) Core loop: CLOCK and BMAL1 together form CLOCK/BMAL1 complex that binds to E-box DNA motifs to induce the transcription of Per, Cry, and Reverbs. The resulting PER and CRY proteins form a corepressor complex, which repress the transcription of the target genes of CLOCK/BMAL1 complex including Per and Cry. Consequently, the levels of PER and CRY decline, which results in relief of CLOCK/BMAL1 inhibition allowing a new cycle of gene expression to start. This feedback loop results in oscillation of CLOCK/BMAL1 and PER/CRY which follow a circadian pattern. (B) Interlocking loop: RORα transcription factor acts on the RORE (retinoid-related orphan receptor response elements) and drives the transcription of BMAL1 in a feedforward loop whereas REV-ERBs by binding to the same DNA binding motif as RORα repress the transcription of BMAL1. REV-ERBs levels follow circadian oscillation (rhythmic) as a consequence of CLOCK/BMAL1 complex which activate transcription of REV-ERBs and PER/CRY resulting in transrepression of REV-ERBs. Thus, repression by REV-ERBs leads to circadian oscillation in BMAL1 transcription. Consequently, BMAL1 expression is in antiphase with that of PER, CRY and REV-ERBs such that when BMAL1 transcription is increasing the transcription of PER, CRY and REV-ERBs is declining.
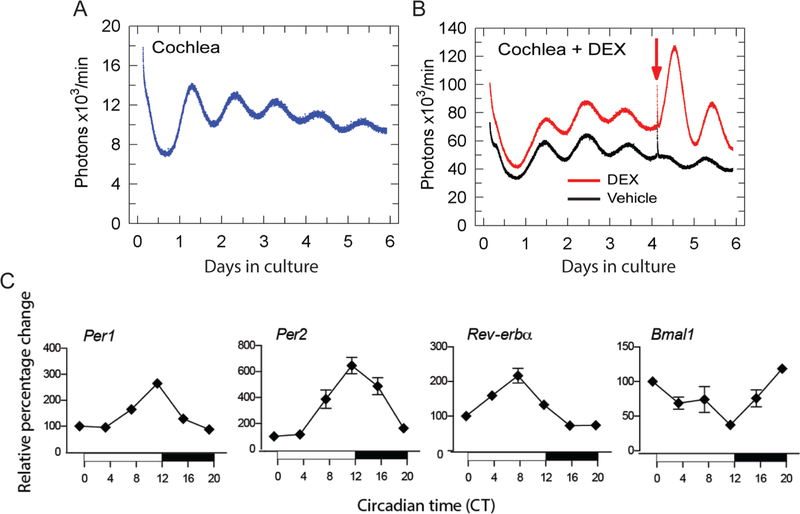
To assess if the cochlea demonstrates autonomous and self-sustained circadian oscillations, real-time bioluminescence of Period2 (PER2) in adult cochlear explants was recorded using PERIOD2::LUCIFERASE (PER2::LUC) mice. In these mice, the fusion of Luc gene in frame to the endogenous mouse Per2 gene results in the coupling of PER2 protein to luciferase, hence, allowing for the real-time tracking of bioluminescence in any organ expressing PER2 (Yoo et al., 2004). Isolated cochleae from young adults (4–8 weeks old) demonstrate a robust self-sustained rhythmic expression of PER2::LUC, which dampens over time as individual cells fail to maintain phase coherence (Fig. 2A). Indeed, individual cells rely on input from SCN to maintain phase coherence. The addition of glucocorticoid agonist dexamethasone (DEX), which acts as a synchronizing agent, prevented the dampening of rhythmic expression of PER2::LUC over time (Fig. 2B). In the mouse cochlea, the mRNA of the core clock genes, Per1, Per2, Bmal1, and Rev-erbα have been shown to have circadian oscillations (Fig. 2C) (Meltser et al., 2014). PER2 protein was found expressed mainly in inner and outer hair cells and in spiral ganglion neurons from the cochlea (Meltser et al., 2014). Furthermore, cochlear clocks have recently been evidenced at the cellular level using bioluminescence imaging showing a longitudinal distribution of PER2 rhythms along the cochlear tonotopic axis (Park et al., 2017). These multi-phased cellular clocks are arranged tonotopically along the length of the cochlea (4–8 weeks old) with oscillations initiating with high PER2 levels at the apex (low frequency region) and travelling towards the base (high frequency region) with phase differences of near 3 hours between cellular oscillators in the apical and middle regions. The implications of such tonotopic gradient in PER2 activity is unclear, but we speculate it may influence auditory sensitivity in a frequency-specific manner at different times of the day. Taken together, the cochlea has the molecular machinery for running a clock suggesting that circadian mechanisms may control a large number of auditory functions in the cochlea.
Figure 2. Circadian oscillations in the cochlea:
(A) Representative bioluminescence records of circadian PER2::LUC expression in cultured adult cochleae explants. (B) Addition of Dexamethasone (DEX) as depicted by the arrow causes a rapid synchronization of PER2::LUC rhythms compared to vehicle. (C) Temporal expression of Per1, Per2, Rev-erbα and Bmal1 mRNAs in the cochlea assessed by Nanostring. The vertical axis shows normalized mean values ± SEM (n = 3–4). The horizontal axis shows the sampling Circadian Time (CT) across 24 hours at which the animals were sacrificed and samples collected. CT is defined by the fact that animals are placed three days in darkness prior to collecting cochlea samples (free running conditions). The shaded bar illustrates the dark phase of the day from CT 12 to CT 0, whereas the white bar illustrates the light phase from CT 0 to CT 12. All conditions were plotted as relative percentage change using CT 0 as baseline value. CT corresponds to the time based on the free-running period, meaning in absence of light entrainment. CT12 corresponds to the onset of activity (6 pm, when lights are turned off). Animals were kept three days in darkness before tissue collection.
Day and Night responses to noise exposure
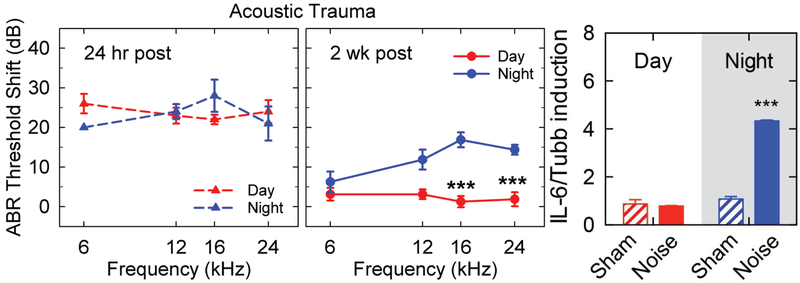
Testing the vulnerability to noise trauma during daytime and nighttime, diurnal differences in the physiological response were found (Meltser et al., 2014). When mice are exposed to a noise exposure at two different times of the day, in the morning (9 a.m., corresponding to Zeitgeber Time ZT3) and in the evening (9 p.m., corresponding to ZT15), differences in hearing recovery were observed using auditory brainstem responses (ABR). These two time points were selected because they correspond to the peak and trough levels of cochlear PER2 protein expression, slightly delayed from peak (ZT12) and trough (ZT0) Per2 mRNA levels (Fig. 2C). CBA mice exposed to noise trauma in the morning displayed complete recovery of hearing thresholds after two weeks whereas those exposed in the evening still exhibited threshold elevation (Fig. 3A). We previously reported this noise paradigm to trigger a temporary threshold shift (TTS) with no loss of auditory hair cells when delivered during the day (Meltser et al., 2010). The unexpected permanent threshold shift (PTS) observed after nighttime noise exposure indicates that the auditory system is more vulnerable at night. Also, it has been shown that this diurnal sensitivity to noise trauma is independent of behavior since the animals displayed similar locomotor activity during day or night noise exposure (Park et al., 2016). The underlying mechanism for this differential sensitivity to noise is not known but could include immune and hormonal responses, which are involved in the response to noise trauma and known to be under circadian regulation. Our data show that the induction of IL-6 mRNA, a pro-inflammatory cytokine, is only triggered after noise exposure at nighttime (9 p.m.), but not after noise exposure in daytime (9 a.m.) (Fig. 3B). These findings suggest that a greater inflammatory response occurs after nighttime exposure and could be a contributing factor to the persisting ABR loss. Below, we review the circadian involvement in glucocorticoid and inflammation processes and discuss their role in the auditory system.
Figure 3. Diurnal variation in sensitivity to noise:
(A) Auditory brainstem response (ABR) of CBA/Sca mice exposed to noise in the morning (ZT 3–5, red triangles) or at night (ZT 14–16, blue triangles) show similar levels of hearing damage when measured 24 h post exposure (left panel). However, 2 weeks later the morning group shows complete recovery, whereas the night group continue to show a threshold shift. Modified with permission from Cell Press (Meltser et. al., 2014). Noise exposure corresponds to a 6–12 kHz narrow band noise at 100 dB SPL for 1 hour. Filtered sine-waves were used to evoke ABR. (B) IL-6 mRNA expression analysis from cochleae (in absence of outer bony shell of the cochlea and the vascular tissue) isolated 2 hours after day or night noise exposure in comparison to sham-exposed groups. Taqman qRT-PCR (probe: Mm00446190_m1) was performed using the same material and protocols reported by Meltser et al., 2014, normalization was performed against Tubb. Results are mean values ± SEM (n = 3). ZT0 corresponds to the onset of the resting phase (6 am, when lights – the Zeitgeber - are turned on).
Circadian Regulation of the Glucocorticoid secretion
Glucocorticoids (cortisol in humans and corticosterone in rodents) are released from the adrenal glands in a circadian manner. Their peak concentration is found just prior to the onset of the active phase in humans and rodents (daytime for human and nighttime for rodents when the activity is high) and reaches a minimum when in the inactive state. Secretion of glucocorticoids becomes arrhythmic in the absence of the suprachiasmatic nucleus (Radziuk, 2013; Stephan et al., 1972). This demonstrates that variations in the daily secretion are regulated in a versatile way by the SCN via the neuroendocrine axis. Glucocorticoid receptors (GR) are widely distributed in the CNS and other organs (Androutsellis-Theotokis et al., 2013; Herman et al., 2003) and have been detected in the inner ear of animals and humans both in the cochlear and vestibular system (Erichsen et al., 1996; Furuta et al., 1994; Rarey et al., 1993). In the cochlear tissues, GR have been identified in the hair cells, supporting cells, spiral ligament and stria vascularis indicating a possible role in the regulation of both sensory and non-sensory tissues. Hence, the SCN uses hormone signalling to apply a temporal control over several physiological processes and behaviours in glucocorticoid-sensitive tissues, possibly including the cochlea. Furthermore, glucocorticoid signalling can be modulated by a variety of stimuli, including stressors or drugs that can alter the circadian pattern of glucocorticoid secretion.
The influence of glucocorticoids on auditory function was first reported in the late 1970s when a substantial improvement of hearing was obtained after therapy with two synthetic analogues of the glucocorticoid hormone (cyclophosphamide and DEX) in patients with autoimmune hearing loss (McCabe, 2004). GR was further identified in the inner ear in humans and animals (Rarey et al., 1993; Shimazaki et al., 2002; Zuo et al., 1995), and several studies have shown that acoustic trauma or restraint stress can modulate their expression (Rarey et al., 1995; Tahera et al., 2006a; Tahera et al., 2006b; Terunuma et al., 2001). Through binding to GR, glucocorticoid analogues (i.e. DEX) have been shown to activate signalling pathways in the cochlea, including NFκB and MAPK, and protect auditory function and morphology against acoustic trauma (Meltser et al., 2009; Tahera et al., 2006a; Tahera et al., 2006b). Glucocorticoids have been shown to downregulate inflammatory responses through multiple mechanisms. Through the binding to the positive glucocorticoid-response element (GRE), glucocorticoids directly upregulate the levels of anti-inflammatory cytokines such as IL-10 and downregulate proinflammatory cytokines such as IL-1, IL-6 and TNF-α (Ronchetti et al., 2018). Glucocorticoids also block the activity of transcription factors and MAP kinases, important for inflammation. Glucocorticoid-GR complexes tether several transcription factors such as NFκB and AP-1 and block their activity (Ray et al., 1994; Ronchetti et al., 2018). For instance, proinflammatory cytokines such as IL-6, TNF-α and IL-1 harbour a NFκB binding motif in the promoter region that activates these genes. The glucocorticoid-GR complex inhibits the transcription of these genes by preventing the binding of NFκB to its motif. Another mechanism by which glucocorticoids antagonize the activity of NFκB and AP-1 is through upregulation of glucocorticoid-induced leucine zipper (GILZ), an important mediator of glucocorticoid anti-inflammatory effects (Ronchetti et al., 2015). GILZ also inhibits the activation of JNK and p38 kinases, which control the production of inflammatory mediators such as IL-6, TNF-α, prostaglandins and nitric oxide (Cheng et al., 2013). Glucocorticoids can also control JNK and p38 kinases mediated inflammatory responses by increasing the expression of MAP kinase phosphatase-1 (MKP-1). MKP-1 inhibits JNK and p38, thereby blocking their entire signaling pathways (Keranen et al., 2017). In addition to cytokines, glucocorticoid-dependent supression of chemokine expression such as Cxcl1, Cxcl2, and IL-8 inhibits the recruitment of inflammatory cells to the site of inflammation (Mukaida et al., 1994; Ronchetti et al., 2018). It is thus likely that the mechanisms involving the protection of auditory function by DEX are in part via anti-inflammatory mechanisms, however this remains to be demonstrated.
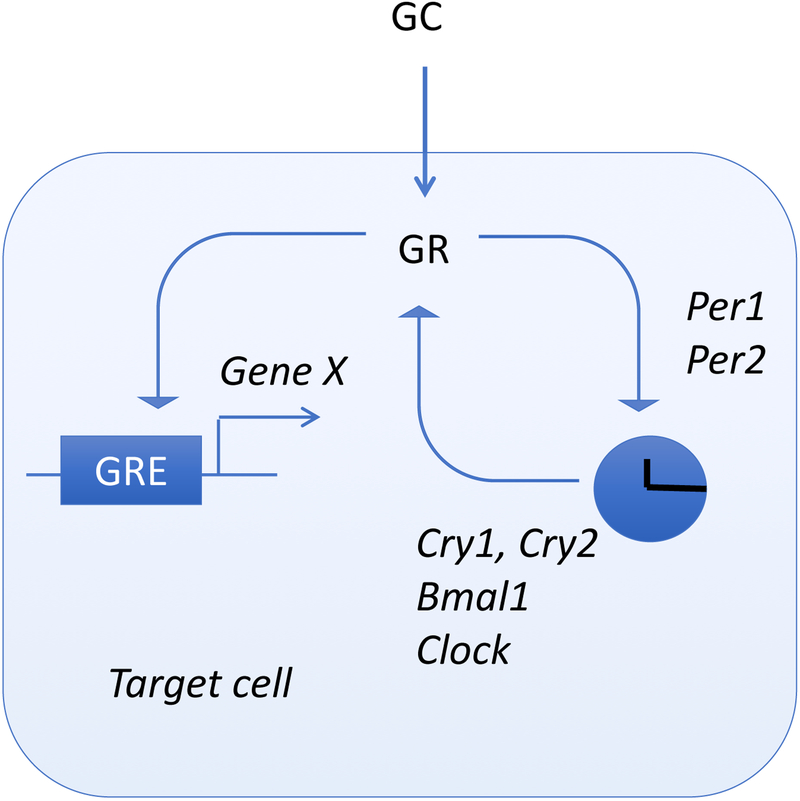
The first evidence of glucocorticoid actions on the clock system was provided by Balsalobre et al. who exposed rat fibroblasts to DEX and found a robust circadian induction of Per gene expression (Balsalobre et al., 2000). Administration in vivo during the descending phase of CORT secretion caused a phase shift in Per1 liver expression (Balsalobre et al., 2000). The regulation of Per1 expression triggered by restraint stress involves the direct binding of GR to the distal and proximal GRE of Per1 in peripheral tissues (Yamamoto et al., 2005). This regulation of Per1 expression via the GRE does not require Bmal1, unlike for Per2 (Cheon et al., 2013). Conversely, alterations in the clock system also impact glucocorticoid homeostasis. Mice with a mutation in the circadian genes Bmal1 or Clock suffer from hypercortisolism at the onset of night (Leliavski et al., 2014; Turek et al., 2005). Moreover, Per2 mutants no longer demonstrate a circadian release of glucocorticoids (Yang et al., 2009). Per2 deletion also leads to increased immobilization, stress-induced grooming, and nociceptive behaviors associated with increased corticotropin-releasing hormone (CRH) expression (Zhang et al., 2011). Overall, it appears that glucocorticoid actions are tightly coupled to clock genes (Fig. 4). It can thus be speculated that the differential sensitivity of the auditory system to noise trauma is related to glucocorticoids.
Figure 4. Molecular scheme for glucocorticoid actions on clock genes.
Glucocorticoids (GC) interact with the glucocorticoid receptor (GR) that binds to the positive GRE and regulate gene expression (Gene X). GR can also directly regulate the expression of Per1 and Per2, which in turn will control the expression of additional clock genes (Cry1, Cry2, Bmal1 and Clock).
Circadian Regulation of the Immune System
The immune system consists of innate and adaptive arms of immunity that provide defense against noxious stimuli such as pathogens or intrinsic insults and promote tissue repair. Emerging evidence suggest that inflammatory responses are involved in the loss of sensory hair cells or spiral ganglion neurons. Under physiological conditions, cochlear tissue resident macrophages act as sentinels and promote phagocytic clearance of cellular debri and provide trophic support to ensure tissue repair. However, upon insult (e.g. noise damage, ototoxic drugs), macrophages become activated and migrate to the sensory epithelium where they actively ingest hair cell debris (Hirose et al., 2017). Cytokines and chemokines, known to be released after noise damage (Fujioka et al., 2006; Keithley et al., 2008; Satoh et al., 2002), may recruit other leukocytes to the site of inflammation (Hirose et al., 2017). Although several studies have investigated the role of cochlear tissue resident macrophages, the role of other leukocytes involved in innate (granulocytes, NK cells) or adaptive immunity (B and T lymphocytes) remains to be uncovered.
In the blood, the total number of leukocytes (hematopoietic stem cells (HSCs) and mature leukocytes, with the exception of CD8 T cells, oscillate and peak at the rest phase (night in humans and day in rodents) and decrease in the active phase (daytime humans, nighttime rodents) (Haus et al., 1999; Scheiermann et al., 2013; Scheiermann et al., 2012). In contrast, the migration of leukocytes into tissues occurs in the active phase. Both deletion of Bmal1 (Scheiermann et al., 2012) or chronic SCN arrhythmia (Prendergast et al., 2013) diminish the oscillatory responses evidencing that the immune system is under circadian control. The rhythmic oscillations appear in many aspects of immunity such as immune cell trafficking, phagocytosis, antigen presentation, release of cytokines and chemokines, endotoxin signaling pathways and susceptibility to pathogens. Thus, both cell intrinsic (autonomous to immune cells) and cell extrinsic (non-autonomous to immune cells) factors regulate the oscillatory responses of the immune system.
In the majority of immune cells, their intrinsic functions are regulated by molecular clocks (Table 1). These intrinsic clocks control the temporal gating of specific processes. The temporal gating could be for instance histamine release by mast cells during an allergic reaction or the effector function of NK cells. In support of this temporal regulation, the levels of IFN-γ and cytolytic factors are elevated in rat splenic NK cells at night rendering them more efficient (Arjona et al., 2006). Similarly, BMAL1, which regulates the diurnal oscillation of inflammatory Ly6Chigh monocytes, enhances the organismal fitness against infection at ZT8 compared to ZT0. It has been revealed that, at this time point, an elevated number of Ly6Chigh monocytes is found in all monocyte reservoirs including liver, spleen and peritoneum, when compared to ZT0 when Ly6Chigh monocytes are found instead in the circulation (Nguyen et al., 2013). Thus, clock genes influence the time-dependent responsiveness of the immune system. Approximately 8% of the macrophage transcriptome is circadian, which also establishes a circadian phase during which endotoxin signaling is most effective (Keller et al., 2009). This could explain the time-dependent changes in the immune responsiveness that have an important impact on the outcome of infections. For instance, the colon of mice inoculated with Salmonella Typhimurium shows higher bacterial load and inflammation when mice are infected during the early rest period (ZT4) compared to the active period at night (ZT16) (Bellet et al., 2013). Similar time-dependent changes in immune responsiveness are also observed in humans where the levels of proinflammatory cytokines are found significantly higher when LPS is administered during the rest phase (at night) compared to the day (Alamili et al., 2014). Immune responsiveness appears to be influenced by the presence of the effector cells at the time of insult (Scheiermann et al., 2012). Indeed, mice challenged with a lethal dose of LPS had a higher mortality rate when the LPS was administered at night, which coincides with an increased recruitment of neutrophils (Scheiermann et al., 2012).
Table 1.
Examples of circadian responses in immune cells
| Immunity arm | Cell type | Clock knockout driven by | Effect | Ref |
|---|---|---|---|---|
| Innate | Macrophage | BMAL1−/− Lyz2-Cre |
Loss of temporal gating of endotoxin-induced cytokines such as IL6, CCL5, IL12(P40) | (Gibbs et al., 2012) |
| Monocytes | BMAL1−/− Lyz2-Cre |
Loss of diurnal variation in Ly6C high monocyte numbers in spleen, blood and bone marrow. Increased susceptibility to infections and metabolic disorders. |
(Nguyen et al., 2013) | |
| NK cells | Per1−/− | Supressed NK effector activity by altered rhythmicity of IFNγ, perforin and granzyme B. | (Logan et al., 2013; Logan et al., 2012) | |
| Adaptive | ||||
| T cells | BMAL1−/− Cd4-Cre Lck-Cre |
Ablated homing of T cells to lymph nodes by reducing expression of promigratory factors Ccr7 and S1pr1. T lymphocyte differentiation and function unaffected. Loss of circadian gating in susceptibility to EAE. |
(Druzd et al., 2017; Hemmers et al., 2015) | |
| B cells | BMAL1−/− Cd19-Cre Mb1-Cre |
Ablated homing of B cells to lymph nodes. B lymphocyte differentiation and function unaffected. |
(Druzd et al., 2017; Hemmers et al., 2015) |
Emerging evidence suggest that the effector function of immune cells is directly coupled to the clock genes. For instance, REV-ERBα directly suppresses Ccl2 expression and hence reduce macrophage trafficking (Sato et al., 2014). Likewise, Bmal1 deficiency in mice hampers the maturation of B-cells leading to a reduction in the blood and the spleen. Adoptive transfer experiments revealed that the expression of Bmal1 in the microenvironment of the bone marrow rather than B cells is sufficient to promote B cell maturation (Sun et al., 2006). Similarly, Per1 is important for the efficient function of splenic NK cells. In the absence of Per1, NK cells remained rhythmic but the rhythmicity of IFN-γ, granzyme B and perforin were altered (Logan et al., 2013).
Although the intrinsic clock machinery holds a circadian control over the cell autonomous functions of the immune cells such as phagocytosis, the circadian regulation of cell extrinsic (environmental cues) mechanisms largely influences their trafficking and recruitment. For example, the rhythmic release of CXCL12 (environmental cue) from bone marrow stromal cells regulates the HSC oscillations in the blood. In this case, the central clock drives the rhythmic discharge of noradrenaline through the sympathetic nervous system (SNS), which subsequently regulates the rhythmic release of CXCL12 chemokine by the stromal cells (Mendez-Ferrer et al., 2008). Since CXCL12 is a major retention factor for HSCs in the bone marrow, its circadian increase leads to homing of HSCs to the bone marrow (Mendez-Ferrer et al., 2008). Likewise, in the early active phase (ZT13), the SNS drives the expression of Ccl2 and Icam-1 in the muscle cells and those of P- and E-selectins, and Vcam1 on the endothelial cells in bone marrow (Scheiermann et al., 2012). As a consequence, the trafficking of leukocytes to the respective organ increases. These examples illustrate that the environmental cues under circadian mechanisms act on tissue-specific cells to generate a rhythmic release of chemokines and adhesion control, which have an impact on leukocyte trafficking or recruitment to specific tissues. However, the clock machinery also governs a cell-autonomous control of leukocyte trafficking. Deletion of Bmal1 in B or T lymphocytes inhibits their homing to the lymph nodes without perturbing the function or differentiation of these cells (Druzd et al., 2017; Hemmers et al., 2015). The deficiency in homing is due to the loss of rhythmic expression CCR7 and S1P1, which are involved in the lymphocytes homing to and egress from the lymph nodes (Druzd et al., 2017).
Circadian integration of inflammation and glucocorticoid actions
Glucocorticoids have been shown to interact with the circadian machinery to regulate innate and adaptive immune responses. Oscillations in the adaptive arm of the immune system (T and B cells) depend on glucocorticoids and runs in anti-phase with corticosterone in mice (Kawate et al., 1981; Man et al., 2016). Central regulators of the circadian clock machinery such as CRY1 and CRY2, have been shown to physically interact with GR and that this association is enhanced by GR agonists such as DEX (Lamia et al., 2011). Long-term exposure to DEX suppresses corticosterone production in wild-type mice but not as effectively in Cry1/2 knock-outs (Lamia et al., 2011). CRY1 blocks GR-mediated transcription induced by DEX demonstrating the direct role of Cry proteins in regulating GR function, ultimately modulating the production of glucocorticoids (Gibbs et al., 2014). In LPS-induced pulmonary inflammation, signaling through glucocorticoid was necessary for the time-dependent variation in antibacterial responses and neutrophil recruitment to the lung (Gibbs et al., 2014). Glucocorticoids drive the rhythmic expression of the chemokine Cxcl5 in bronchial epithelial cells via Bmal1. The peak in glucocorticoids at nighttime lead to the recruitment of neutrophils to the lung, which is no longer observed in adrenalectomized mice (Gibbs et al., 2014). Moreover, local bronchiole-specific ablation of Bmal1 leads to an increased expression of Cxcl5 despite normal corticosteroid secretion and a loss of DEX response after LPS-induced neutrophilia (Gibbs et al., 2014). Thus, non-immune cells influence the outcome of inflammatory responses by expressing clock genes. These findings on glucocorticoids are of interest for the cochlea since it has been shown that the auditory function is modulated by these hormonal cues. These results further corroborate the tight connection between the circadian-regulated immune and glucocorticoid mechanisms since the anti-inflammatory efficacy of glucocorticoids depends on an intact clock machinery. Therefore, human diseases that have disturbed circadian rhythms may be accompanied by impaired response to the anti-inflammatory actions of glucocorticoid treatment.
Bridging the circadian, inflammation and glucocorticoid action on auditory function
At present, there is no direct evidence for the involvement of these three disciplines, acting in concert to regulate auditory function. However, in isolation, each show a connection with the auditory system: i) the cochlea is known to possess a circadian machinery (Meltser et al., 2014) and the clock system is known to regulate inflammation and glucocorticoid influences clock function; ii) glucocorticoids are known to act on the cochlea to protect against noise trauma. It has been shown in numerous tissues that glucocorticoids have immunosuppressive actions and also interacts with the clock system (Balsalobre et al., 2000; Yang et al., 2009); and iii) inflammation occurs in response to noise damage and has been shown in other system to be under the control of the circadian system and glucocorticoids. There are several gaps in knowledge for bridging the circadian, inflammation and glucocorticoid action on the cochlea. There is no direct evidence that glucocorticoid actions in the cochlea affect immune cells or cytokine secretion after noise trauma or other challenges. In addition, such glucocorticoid actions might differ depending on the time of the day when the challenge is delivered. For instance, the greater IL-6 mRNA induction after night noise trauma goes against a protective role of circulating glucocorticoids, which peak at nighttime in rodents. However, there is a body of literature indicating that it is not the absolute levels of corticosterone that play a role, but instead it is the rise and fall that matters. In this regard, Lightman and Conway-Campbell elegantly review the function of the HPA axis and show that the HPA-response to noise exposure (as a stressor), which increases circulating corticosterone, depends on whether the exposure occurs on the rising slope (facilitated HPA response) or the descending slope (suppressed HPA response), rather than the absolute levels of corticosterone (Flynn et al., 2018). Thus, the contribution of glucocorticoids on auditory function is likely a matter of dose, time, and duration. Arguably, potential reasons for an increased vulnerability to night noise trauma could stem from variations in the inflammation in the cochlea that may vary over the day. How inflammation and glucocorticoids contribute to the circadian regulation of noise-induced hearing loss remains to be investigated.
Perspectives and conclusion
Interventions aiming at modulating inflammation and glucocorticoids at particular times of the day will increase the knowledge on factors contributing to the permanent damage occurring after night noise trauma. As the majority of research is performed on rodents during daytime (their inactive period), results from those experiments may not provide meaningful data on how glucocorticoids, inflammation and the clock system interact to regulate auditory function. Experiments performed around the clock on rodents will provide a better understanding of the underlying mechanisms in noise-induced hearing loss and other auditory disorders and will open avenues for therapeutic approaches based on chronopharmacology.
Acknowledgements
Research in the Canlon lab is funded by Communication Disorders of the National Institutes of Health R21DC013172 and 1R56DC016415–01, the Swedish Medical Research Council K2014-99X-22478-01-3, Knut and Alice Wallenberg Foundation (B.C. #KAW2008), Karolinska Institutet, Tysta Skolan, Hörselforskningsfonden, Magnus Bergvalls, and the EU (H2020-MSCA-ITN, ESIT, C.R.C - project # 722046). B.C. and C.R.C. also received funding from the Office of the Assistant Secretary of Defense for Health Affairs, through the Neurosensory and Rehabilitation under Award No. W81XWH-16-1-0032. Opinions, interpretations, conclusions and recommendations are those of the authors and are not necessarily endorsed by the Department of Defense.
REFERENCES
- Alamili M, Bendtzen K, Lykkesfeldt J, Rosenberg J, Gogenur I, 2014. Pronounced inflammatory response to endotoxaemia during nighttime: a randomised cross-over trial. PloS one 9, e87413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Oster H, 2001. The circadian clock and behavior. Behavioural brain research 125, 89–91. [DOI] [PubMed] [Google Scholar]
- Androutsellis-Theotokis A, Chrousos GP, McKay RD, DeCherney AH, Kino T, 2013. Expression profiles of the nuclear receptors and their transcriptional coregulators during differentiation of neural stem cells. Hormone and metabolic research = Hormon- und Stoffwechselforschung = Hormones et metabolisme 45, 159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, Sarkar DK, 2006. Evidence supporting a circadian control of natural killer cell function. Brain, behavior, and immunity 20, 469–76. [DOI] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, Schutz G, Schibler U, 2000. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–7. [DOI] [PubMed] [Google Scholar]
- Basinou V, Park JS, Cederroth CR, Canlon B, 2017. Circadian regulation of auditory function. Hearing research 347, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Deriu E, Liu JZ, Grimaldi B, Blaschitz C, Zeller M, Edwards RA, Sahar S, Dandekar S, Baldi P, George MD, Raffatellu M, Sassone-Corsi P, 2013. Circadian clock regulates the host response to Salmonella. Proceedings of the National Academy of Sciences of the United States of America 110, 9897–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Q, Fan H, Ngo D, Beaulieu E, Leung P, Lo CY, Burgess R, van der Zwan YG, White SJ, Khachigian LM, Hickey MJ, Morand EF, 2013. GILZ overexpression inhibits endothelial cell adhesive function through regulation of NF-kappaB and MAPK activity. Journal of immunology 191, 424–33. [DOI] [PubMed] [Google Scholar]
- Cheon S, Park N, Cho S, Kim K, 2013. Glucocorticoid-mediated Period2 induction delays the phase of circadian rhythm. Nucleic acids research 41, 6161–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druzd D, Matveeva O, Ince L, Harrison U, He W, Schmal C, Herzel H, Tsang AH, Kawakami N, Leliavski A, Uhl O, Yao L, Sander LE, Chen CS, Kraus K, de Juan A, Hergenhan SM, Ehlers M, Koletzko B, Haas R, Solbach W, Oster H, Scheiermann C, 2017. Lymphocyte Circadian Clocks Control Lymph Node Trafficking and Adaptive Immune Responses. Immunity 46, 120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erichsen S, Bagger-Sjoback D, Curtis L, Zuo J, Rarey K, Hultcrantz M, 1996. Appearance of glucocorticoid receptors in the inner ear of the mouse during development. Acta oto-laryngologica 116, 721–5. [DOI] [PubMed] [Google Scholar]
- Evans JA, Davidson AJ, 2013. Health consequences of circadian disruption in humans and animal models. Prog Mol Biol Transl Sci 119, 283–323. [DOI] [PubMed] [Google Scholar]
- Flynn BP, Conway-Campbell BL, Lightman SL, 2018. The emerging importance of ultradian glucocorticoid rhythms within metabolic pathology. Annales d’endocrinologie 79, 112–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu L, Lee CC, 2003. The circadian clock: pacemaker and tumour suppressor. Nature reviews. Cancer 3, 350–61. [DOI] [PubMed] [Google Scholar]
- Fujioka M, Kanzaki S, Okano HJ, Masuda M, Ogawa K, Okano H, 2006. Proinflammatory cytokines expression in noise-induced damaged cochlea. J Neurosci Res 83, 575–83. [DOI] [PubMed] [Google Scholar]
- Furuta H, Mori N, Sato C, Hoshikawa H, Sakai S, Iwakura S, Doi K, 1994. Mineralocorticoid type I receptor in the rat cochlea: mRNA identification by polymerase chain reaction (PCR) and in situ hybridization. Hearing research 78, 175–80. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Ince L, Matthews L, Mei J, Bell T, Yang N, Saer B, Begley N, Poolman T, Pariollaud M, Farrow S, DeMayo F, Hussell T, Worthen GS, Ray D, Loudon A, 2014. An epithelial circadian clock controls pulmonary inflammation and glucocorticoid action. Nature medicine 20, 919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haus E, Smolensky MH, 1999. Biologic rhythms in the immune system. Chronobiology international 16, 581–622. [DOI] [PubMed] [Google Scholar]
- Hemmers S, Rudensky AY, 2015. The Cell-Intrinsic Circadian Clock Is Dispensable for Lymphocyte Differentiation and Function. Cell Rep 11, 1339–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE, 2003. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Frontiers in neuroendocrinology 24, 151–80. [DOI] [PubMed] [Google Scholar]
- Hirose K, Rutherford MA, Warchol ME, 2017. Two cell populations participate in clearance of damaged hair cells from the sensory epithelia of the inner ear. Hearing research 352, 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson AS, Owe-Larsson B, Hetta J, Lundkvist GB, 2016. Altered circadian clock gene expression in patients with schizophrenia. Schizophrenia research 174, 17–23. [DOI] [PubMed] [Google Scholar]
- Kawate T, Abo T, Hinuma S, Kumagai K, 1981. Studies of the bioperiodicity of the immune response. II. Co-variations of murine T and B cells and a role of corticosteroid. Journal of immunology 126, 1364–7. [PubMed] [Google Scholar]
- Keithley EM, Wang X, Barkdull GC, 2008. Tumor necrosis factor alpha can induce recruitment of inflammatory cells to the cochlea. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology 29, 854–9. [DOI] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B, 2009. A circadian clock in macrophages controls inflammatory immune responses. Proceedings of the National Academy of Sciences of the United States of America 106, 21407–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keranen T, Moilanen E, Korhonen R, 2017. Suppression of cytokine production by glucocorticoids is mediated by MKP-1 in human lung epithelial cells. Inflammation research : official journal of the European Histamine Research Society … [et al. ] 66, 441–449. [DOI] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, Downes M, Evans RM, 2011. Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature 480, 552–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliavski A, Shostak A, Husse J, Oster H, 2014. Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology 155, 133–42. [DOI] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, Evans SJ, Choudary PV, Cartagena P, Barchas JD, Schatzberg AF, Jones EG, Myers RM, Watson SJ Jr., Akil H, Bunney WE, 2013. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America 110, 9950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Wynne O, Levitt D, Price D, Sarkar DK, 2013. Altered circadian expression of cytokines and cytolytic factors in splenic natural killer cells of Per1(−/−) mutant mice. J Interferon Cytokine Res 33, 108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man K, Loudon A, Chawla A, 2016. Immunity around the clock. Science 354, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe BF, 2004. Autoimmune sensorineural hearing loss. 1979. The Annals of otology, rhinology, and laryngology 113, 526–30. [DOI] [PubMed] [Google Scholar]
- Meltser I, Canlon B, 2010. The expression of mitogen-activated protein kinases and brain-derived neurotrophic factor in inferior colliculi after acoustic trauma. Neurobiology of disease 40, 325–30. [DOI] [PubMed] [Google Scholar]
- Meltser I, Tahera Y, Canlon B, 2009. Glucocorticoid receptor and mitogen-activated protein kinase activity after restraint stress and acoustic trauma. Journal of neurotrauma 26, 1835–45. [DOI] [PubMed] [Google Scholar]
- Meltser I, Cederroth CR, Basinou V, Savelyev S, Lundkvist GS, Canlon B, 2014. TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Current biology : CB 24, 658–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS, 2008. Haematopoietic stem cell release is regulated by circadian oscillations. Nature 452, 442–7. [DOI] [PubMed] [Google Scholar]
- Mohawk JA, Green CB, Takahashi JS, 2012. Central and peripheral circadian clocks in mammals. Annual review of neuroscience 35, 445–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukaida N, Morita M, Ishikawa Y, Rice N, Okamoto S, Kasahara T, Matsushima K, 1994. Novel mechanism of glucocorticoid-mediated gene repression. Nuclear factor-kappa B is target for glucocorticoid-mediated interleukin 8 gene repression. The Journal of biological chemistry 269, 13289–95. [PubMed] [Google Scholar]
- Nguyen KD, Fentress SJ, Qiu Y, Yun K, Cox JS, Chawla A, 2013. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science 341, 1483–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, Lightman S, Vgontzas A, Van Cauter E, 2017. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr Rev 38, 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Cederroth CR, Basinou V, Meltser I, Lundkvist G, Canlon B, 2016. Identification of a Circadian Clock in the Inferior Colliculus and Its Dysregulation by Noise Exposure. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 5509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Cederroth CR, Basinou V, Sweetapple L, Buijink R, Lundkvist GB, Michel S, Canlon B, 2017. Differential Phase Arrangement of Cellular Clocks along the Tonotopic Axis of the Mouse Cochlea Ex Vivo. Current biology : CB 27, 2623–2629 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Cable EJ, Patel PN, Pyter LM, Onishi KG, Stevenson TJ, Ruby NF, Bradley SP, 2013. Impaired leukocyte trafficking and skin inflammatory responses in hamsters lacking a functional circadian system. Brain, behavior, and immunity 32, 94–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radziuk JM, 2013. The suprachiasmatic nucleus, circadian clocks, and the liver. Diabetes 62, 1017–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rarey KE, Curtis LM, ten Cate WJ, 1993. Tissue specific levels of glucocorticoid receptor within the rat inner ear. Hearing research 64, 205–10. [DOI] [PubMed] [Google Scholar]
- Rarey KE, Gerhardt KJ, Curtis LM, ten Cate WJ, 1995. Effect of stress on cochlear glucocorticoid protein: acoustic stress. Hearing research 82, 135–8. [DOI] [PubMed] [Google Scholar]
- Ray A, Prefontaine KE, 1994. Physical association and functional antagonism between the p65 subunit of transcription factor NF-kappa B and the glucocorticoid receptor. Proceedings of the National Academy of Sciences of the United States of America 91, 752–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert SM, Weaver DR, 2002. Coordination of circadian timing in mammals. Nature 418, 935–41. [DOI] [PubMed] [Google Scholar]
- Roedel A, Storch C, Holsboer F, Ohl F, 2006. Effects of light or dark phase testing on behavioural and cognitive performance in DBA mice. Laboratory animals 40, 371–81. [DOI] [PubMed] [Google Scholar]
- Ronchetti S, Migliorati G, Riccardi C, 2015. GILZ as a Mediator of the Anti-Inflammatory Effects of Glucocorticoids. Frontiers in endocrinology 6, 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronchetti S, Migliorati G, Bruscoli S, Riccardi C, 2018. Defining the role of glucocorticoids in inflammation. Clin Sci (Lond) 132, 1529–1543. [DOI] [PubMed] [Google Scholar]
- Ruiter M, La Fleur SE, van Heijningen C, van der Vliet J, Kalsbeek A, Buijs RM, 2003. The daily rhythm in plasma glucagon concentrations in the rat is modulated by the biological clock and by feeding behavior. Diabetes 52, 1709–15. [DOI] [PubMed] [Google Scholar]
- Sato S, Sakurai T, Ogasawara J, Takahashi M, Izawa T, Imaizumi K, Taniguchi N, Ohno H, Kizaki T, 2014. A circadian clock gene, Rev-erbalpha, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. Journal of immunology 192, 407–17. [DOI] [PubMed] [Google Scholar]
- Satoh H, Firestein GS, Billings PB, Harris JP, Keithley EM, 2002. Tumor necrosis factor-alpha, an initiator, and etanercept, an inhibitor of cochlear inflammation. The Laryngoscope 112, 1627–34. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Frenette PS, 2013. Circadian control of the immune system. Nature reviews. Immunology 13, 190–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Gibbs J, Ince L, Loudon A, 2018. Clocking in to immunity. Nature reviews. Immunology 18, 423–437. [DOI] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS, 2012. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 37, 290–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellix MT, Evans JA, Leise TL, Castanon-Cervantes O, Hill DD, DeLisser P, Block GD, Menaker M, Davidson AJ, 2012. Aging differentially affects the re-entrainment response of central and peripheral circadian oscillators. The Journal of neuroscience : the official journal of the Society for Neuroscience 32, 16193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki T, Ichimiya I, Suzuki M, Mogi G, 2002. Localization of glucocorticoid receptors in the murine inner ear. The Annals of otology, rhinology, and laryngology 111, 1133–8. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I, 1972. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the United States of America 69, 1583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Yang Z, Niu Z, Peng J, Li Q, Xiong W, Langnas AN, Ma MY, Zhao Y, 2006. MOP3, a component of the molecular clock, regulates the development of B cells. Immunology 119, 451–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Hansson AC, Canlon B, 2006a. Glucocorticoid receptor and nuclear factor-kappa B interactions in restraint stress-mediated protection against acoustic trauma. Endocrinology 147, 4430–7. [DOI] [PubMed] [Google Scholar]
- Tahera Y, Meltser I, Johansson P, Bian Z, Stierna P, Hansson AC, Canlon B, 2006b. NF-kappaB mediated glucocorticoid response in the inner ear after acoustic trauma. Journal of neuroscience research 83, 1066–76. [DOI] [PubMed] [Google Scholar]
- Terunuma T, Hara A, Senarita M, Motohashi H, Kusakari J, 2001. Effect of acoustic overstimulation on regulation of glucocorticoid receptor mRNA in the cochlea of the guinea pig. Hearing research 151, 121–124. [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, Jaitin DA, David E, Winter DR, Gury-BenAri M, Tatirovsky E, Tuganbaev T, Federici S, Zmora N, Zeevi D, Dori-Bachash M, Pevsner-Fischer M, Kartvelishvily E, Brandis A, Harmelin A, Shibolet O, Halpern Z, Honda K, Amit I, Segal E, Elinav E, 2016. Microbiota Diurnal Rhythmicity Programs Host Transcriptome Oscillations. Cell 167, 1495–1510 e12. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, Eckel RH, Takahashi JS, Bass J, 2005. Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinert D, Waterhouse J, 1998. Diurnally changing effects of locomotor activity on body temperature in laboratory mice. Physiology & behavior 63, 837–43. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, Yasuda A, Mamine T, Takumi T, 2005. Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. The Journal of biological chemistry 280, 42036–43. [DOI] [PubMed] [Google Scholar]
- Yang S, Liu A, Weidenhammer A, Cooksey RC, McClain D, Kim MK, Aguilera G, Abel ED, Chung JH, 2009. The role of mPer2 clock gene in glucocorticoid and feeding rhythms. Endocrinology 150, 2153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SH, Yamazaki S, Lowrey PL, Shimomura K, Ko CH, Buhr ED, Siepka SM, Hong HK, Oh WJ, Yoo OJ, Menaker M, Takahashi JS, 2004. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proceedings of the National Academy of Sciences of the United States of America 101, 5339–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Wu Z, Zhou L, Li H, Teng H, Dai W, Wang Y, Sun ZS, 2011. Deficiency of antinociception and excessive grooming induced by acute immobilization stress in Per1 mutant mice. PloS one 6, e16212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Curtis LM, Yao X, ten Cate WJ, Bagger-Sjoback D, Hultcrantz M, Rarey KE, 1995. Glucocorticoid receptor expression in the postnatal rat cochlea. Hearing research 87, 220–7. [DOI] [PubMed] [Google Scholar]