Abstract
Objective: To determine the utility of instillation negative pressure wound therapy (NPWT) in achieving eradication of infection and definitive wound closure in patients with infected left ventricular assist device (LVAD).
Approach: A retrospective review was performed in a series of patients with infected and exposed LVADs who were treated with instillation NPWT in conjunction with surgical debridement.
Results: Three consecutive patients were included who developed periprosthetic infection subsequent to LVAD implantation. In all cases, the utilization of a vacuum-assisted closure with instillation (VACi) along with surgical debridement and IV antibiotics eradicated infection resulting in successful retention of hardware. Cases 1 and 2 received definitive wound closure within 3 and 12 days of starting treatment, respectively. Case 3 initially deferred surgery in favor of local wound care. Eventually the patient elected for surgical treatment and underwent closure 164 days after initial presentation. All three patients healed completely without residual evidence of infection. Flap reconstruction with a pedicled rectus flap was used to achieve definitive closure in all patients. One patient subsequently required pump replacement secondary to thrombosis and mechanical pump failure.
Innovation: LVAD infections are met with high morbidity and mortality rates, and timely salvage is critical. In this initial series, VACi has proven a viable therapy option to help control and eradicate infection without LVAD removal.
Conclusion: This series illustrates the value of newer techniques such as VACi in combination with surgical debridement and antibiotic therapy in effectively salvaging LVADs that were infected.
Keywords: negative pressure, VAC with instillation, debridement, infection

Ian L. Valerio, MD
Introduction
Left ventricular assist devices (LVADs) are often the only treatment option for patients with severe heart failure, and/or they serve as a bridge to heart transplants for others; however, they are associated with severe complications, including stroke, bleeding, device malfunction, and infection.1 Infection is the most common complication of long-term LVAD use, with reports ranging from 25% to 80%.1,2 When LVAD hardware is involved, these infections can be particularly difficult to treat as hardware surfaces can promote biofilm formation, making bacteria less susceptible to antibiotics.3,4 Unlike other areas of the body where surgical removal of the infected implant can be performed, LVAD explantation is a last resort, often met with increased morbidity and mortality due to patient's heart function status.5–7 Most often, surgical treatment utilizes IV antibiotics, surgical debridement, and wound care such as negative pressure wound therapy (NPWT).4 Salvage procedures such as omental or muscle flaps are also employed for definitive closure and delivery of antibiotics. Despite these treatment options, morbidity and mortality due to infection or recurrent infection remain high, with >41% of all estimated LVAD deaths occurring because of infection.4
NPWT to combat infection is a technique first published by Fleischmann et al. in 1998 that utilizes a porous interface material combined with a vacuum to uniformly spread negative pressure across a wound.8,9 NPWT has been shown to encourage healing by increasing wound blood flow and granulation tissue formation resulting in decreased bacterial counts.10,11 It has also been found to aid in the removal of edema, bacterial debris, and excess inflammatory factors, and in the approximation of wound edges.12 Since 1998, this treatment has evolved to include an option for NPWT with instillation (NPWTi). NPWTi is a treatment that provides the combination of NPWT with cyclical instillation of a solution. This treatment has been described as having three distinct phases: an instillation phase wherein the solution is administered to the wound; a hold phase, in which the solution remains in contact with the wound at normal pressure; and then a vacuum phase, in which negative pressure is applied and the solution is removed through suction.13
NPWTi has had promising clinical results. In a retrospective study comparing NPWTi and NPWT alone, patients receiving NPWTi had statistically significant decreases in time of hospitalization, decreased number of operations, and decreased time to final surgical procedure.14 NPWTi has also shown promise in the management of implanted hardware infections. Lehner et al. reported that 86% of acute infections and 80% of chronic infections in orthopedic hardware had successful hardware retention at 4–6 months follow-up when treated with NPWTi.13 The authors have previously reported a case series utilizing vacuum-assisted closure with instillation (VACi), in which two patients with spinal hardware infections had successful infection eradication with hardware retention.15 To our knowledge, this treatment has not previously been reported in the management of infected LVADs. Given its successful use in other areas of hardware infection, it merits thorough investigation in the hope that debridement and a culture-guided topical solution delivery through VACi allow for more efficient and successful LVAD salvage.
Clinical Problem Addressed
Herein, we report the utilization of VACi therapy in conjunction with surgical debridement and IV antibiotics. All three cases describe treatment in the setting of acute postoperative infection to eradicate infection and enable retention of LVAD hardware.
Materials and Methods
We report a retrospective review of three consecutive cases treated at a single institution. Selection criteria included patients who presented with an infected and exposed LVAD where successful hardware salvage was critical to patient survival at the time and removal of the infected LVAD was not an option.
Case 1
A 68-year-old male presented with a past medical history, including type 2 diabetes mellitus, arrhythmia, coronary artery disease, hypertension, hyperlipidemia, obesity, chronic obstructive pulmonary disease (COPD), and chronic combined systolic and diastolic congestive heart failure. The patient had a HeartMate II LVAD (Thoratec Corporation, Pleasanton, CA) as destination therapy for heart failure for 2 years when he presented to us. During that time, he developed several driveline infections treated with irrigation and drainage, the most recent infection occurring 1 month before presentation.
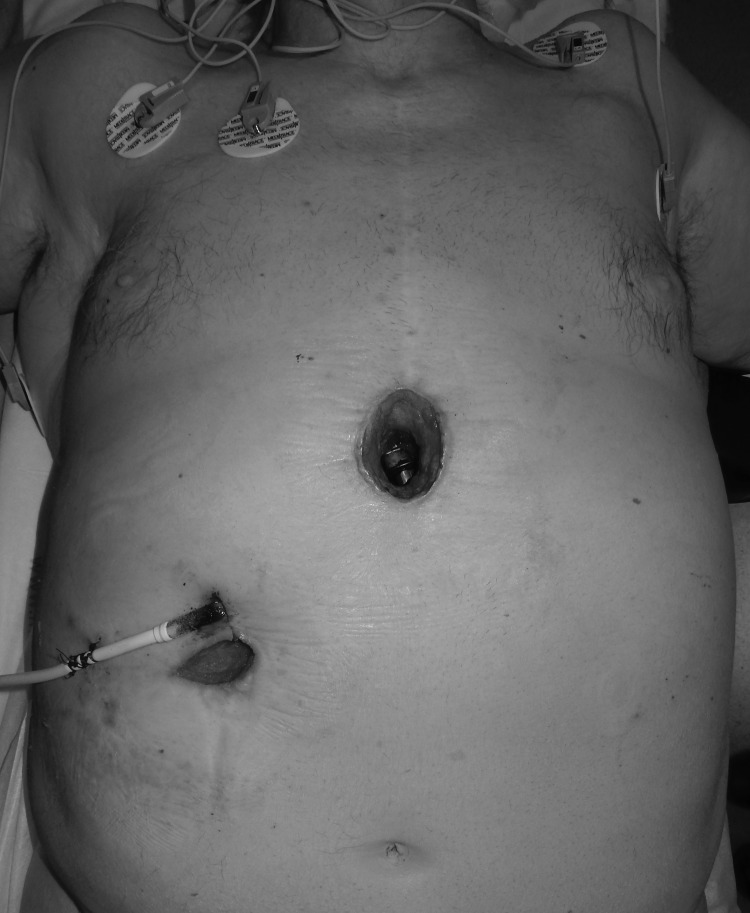
At the time of presentation, his chief complaint was sternal pain and redness along the subxiphoid region of his chest (Fig. 1). Computed tomography (CT) imaging revealed a fluid collection adjacent to the LVAD. The patient was taken for incision and drainage by cardiac surgery, which created a 6-cm subxiphoid midline incision through the subcutaneous tissues to drain the pocket of purulent fluid, resulting in LVAD exposure. Wound irrigation at the time of surgery included saline, hydrogen peroxide, betadine, and an antibiotic containing solution, with application of a standard NPWT system. Surgical site cultures grew methicillin-sensitive Staphylococcus aureus (MSSA). Infectious disease was consulted and recommended intravenous cefazolin twice daily for 6 weeks.
Figure 1.
Case 1 Preoperative photograph with exposed LVAD. LVAD, left ventricular assist device.
The plastic surgery team was consulted postoperatively, and on day 6 after initial presentation repeat wound irrigation and debridement were performed by the plastic surgery team. A VAC Veraflo dressing (KCI; an Acelity Company, San Antonio, TX) was placed intraoperatively and set to 125 mmHg continuous suction, and Dakin's 0.125% Solution was instilled for 10-min duration at 3.5-h intervals to decrease the bioburden and cleanse the wound (Fig. 2). Postdebridement wound cultures were negative, and definitive closure was performed utilizing a pedicled rectus muscle flap on day 9.
Figure 2.
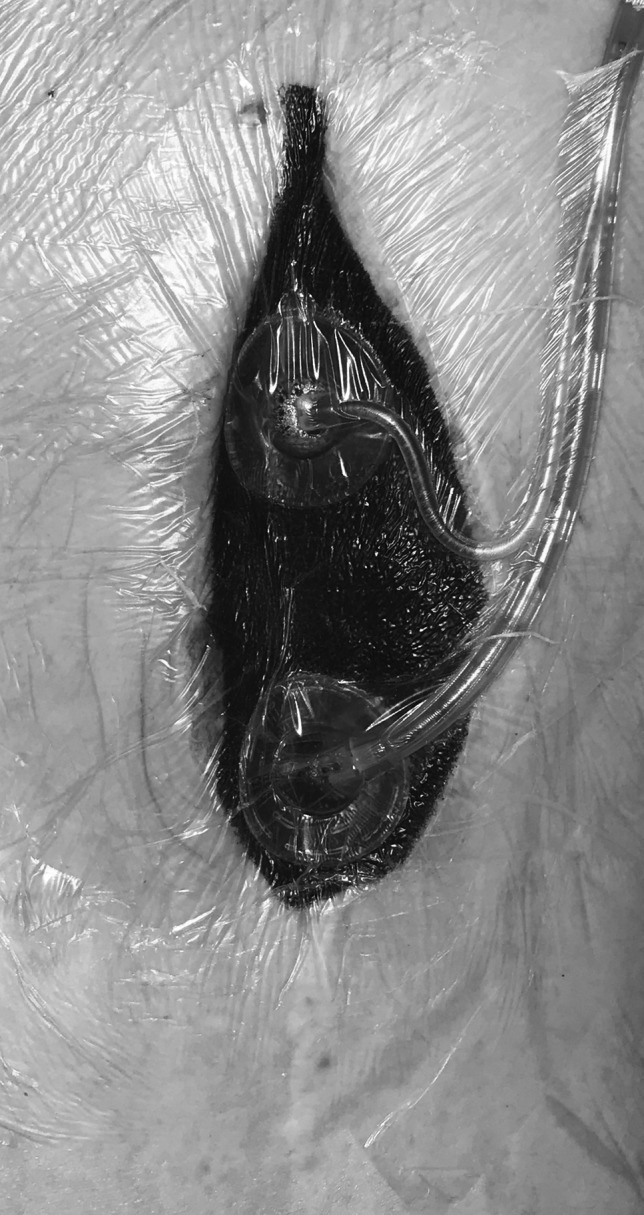
Example of VAC with instillation therapy. VAC, vacuum-assisted closure.
The patient initially did well after reconstruction without wound healing problems or infection. However, ∼1 month postoperatively he developed thrombosis and malfunction of the LVAD. He was taken back to the operating room for LVAD exchange by the cardiac surgery team requiring re-elevation of the muscle flap. The patient was last seen 4 months postoperatively after LVAD exchange with a healed incision and no clinical sign of infection.
Case 2
A 72-year-old male with past medical history of chronic renal failure and congestive heart failure presented with a purulent draining subxiphoid wound. The patient initially received a HeartMate 2 LVAD as destination therapy 3 years before presentation and had it replaced once due to pump thrombosis.
The patient was admitted for IV antibiotics and surgical wound debridement by the cardiac surgery team, which resulted in a 3-cm subxiphoid wound with exposed LVAD. A VAC Veraflo dressing (KCI; an Acelity Company) was placed intraoperatively and set to 125 mmHg continuous suction using Dakin's 0.125% Solution with a soak time of 10 min every 3.5 h. Wound cultures grew coagulase-negative Staphylococci. Infectious disease was consulted, and cefepime and vancomycin were prescribed for 6 weeks.
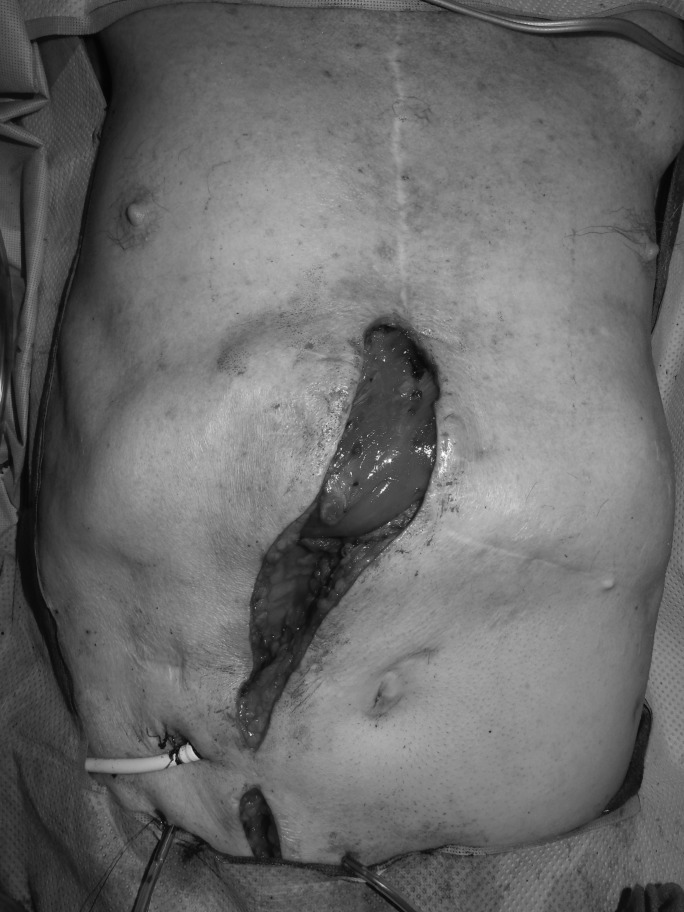
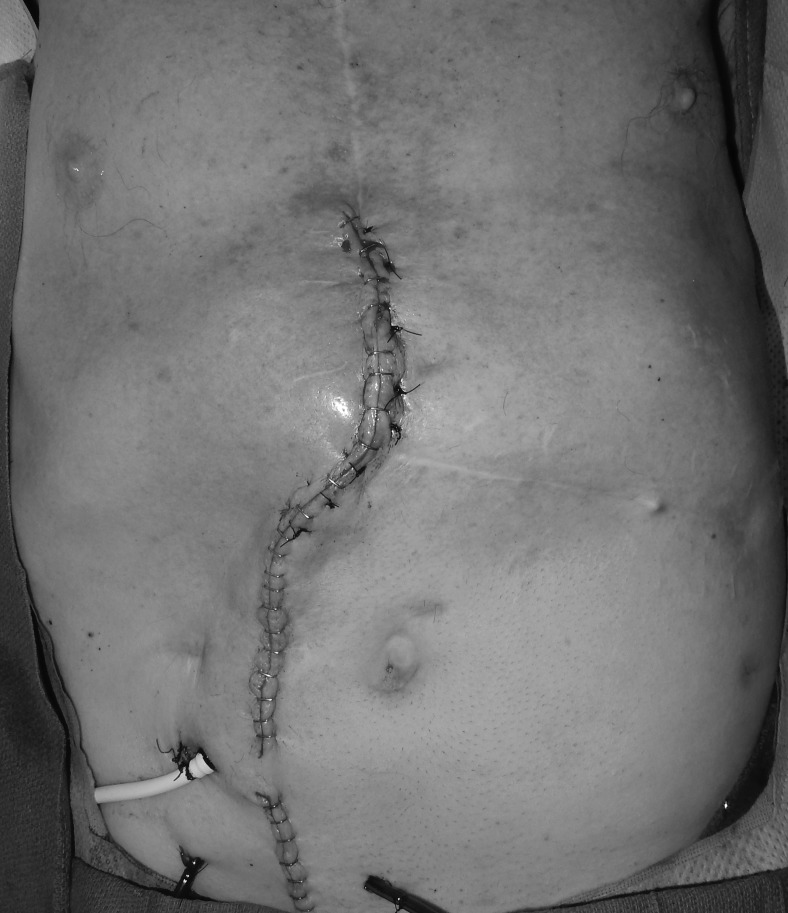
Due to the proximity to the heart and the hope to maintain as sterile an environment as possible, the VACi dressing was changed in the operating room, and the wound was irrigated with betadine, antibiotic solution, and saline on postoperative days 3 and 7. The VACi settings remained the same after each dressing change. On postoperative day 12, the wound received definitive closure with a pedicled rectus flap after consecutive wound cultures were negative (Figs. 3 and 4). The patient subsequently developed a hematoma in his chest, which required surgical exploration on day 13. Anticoagulation with coumadin was then started on day 14, which continued until a second hematoma was discovered on day 19. Anticoagulation was again held, so that surgical evacuation of the hematoma could be performed. Patient was discharged at day 29. He completed his IV antibiotics per infectious disease and is not on a suppressive antibiotic regimen. He remained well healed without evidence of infection when last seen at 6-month follow-up.
Figure 3.
Intraoperative muscle flap coverage of LVAD.
Figure 4.
Postclosure photograph.
Case 3
A 51-year-old female with coronary artery disease, COPD, and type 2 diabetes who had an LVAD placed as definitive treatment for ischemic cardiomyopathy 9 months before presentation presented with an open wound with exposed LVAD, leukocytosis, and persistent MSSA bacteremia (Fig. 5). CT scan revealed a fluid collection around her LVAD that was positive on a tagged white blood cell scan. She was taken to the operating room by the cardiac surgery team for irrigation and debridement at which time a VACi was placed and cultures were taken, which confirmed the presence of MSSA. The VAC Veraflo (KCI; an Acelity Company) was set to 125 mmHg continuous suction and was set to instill Dakin's 0.125% Solution with a 10-min soak time every 3.5 h (Fig. 6).
Figure 5.
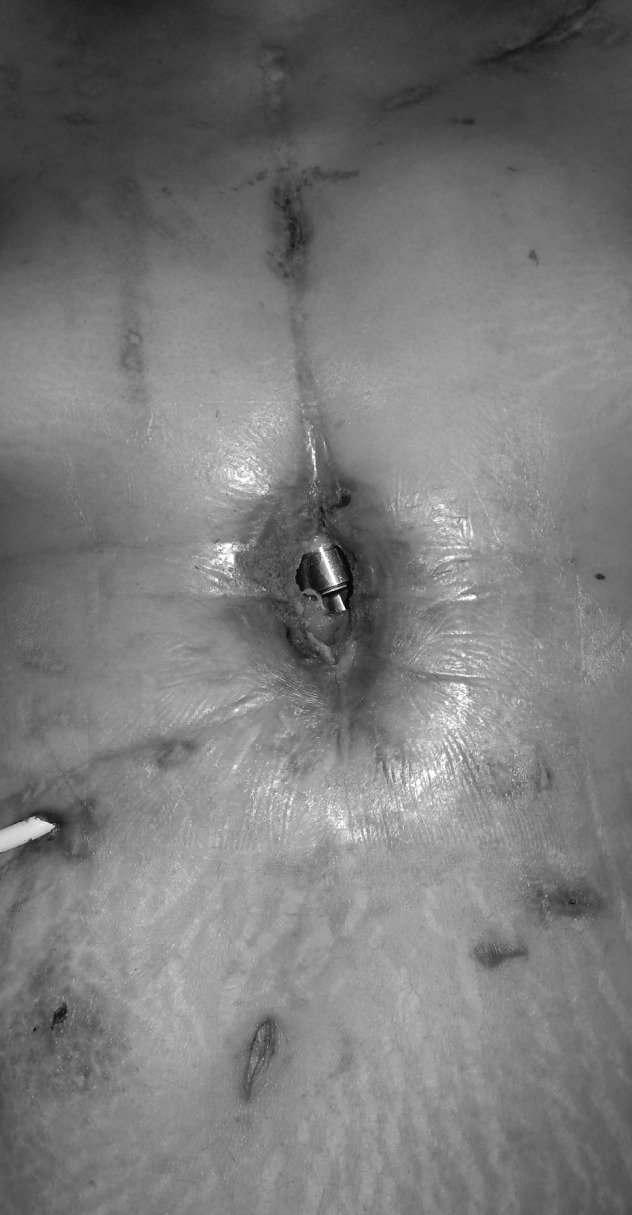
Preoperative photograph with exposed LVAD.
Figure 6.

Case 3 VAC with instillation.
On postoperative day 3, the wound was again irrigated and VACi was replaced. The plastic surgery team was consulted and recommended flap reconstruction for definitive wound closure. The patient opted for local wound care instead of using the VACi, in the hope that the wound would close without surgery. She underwent biweekly VACi changes. The VACi settings remained the same after each dressing change. After 5 months, the wound had not healed, and the patient opted for surgical closure.
Her wound cultures at the time were positive for Serratia marcescens, so irrigation and serial debridement continued for another month until two consecutive wound cultures came back negative (Fig. 7). At that point, 164 days after initial presentation, the wound was definitively closed with a vertical rectus flap (Fig. 8). At last follow-up in clinic, 2 months after definitive closure, the patient appeared well healed and without any signs of infection.
Figure 7.
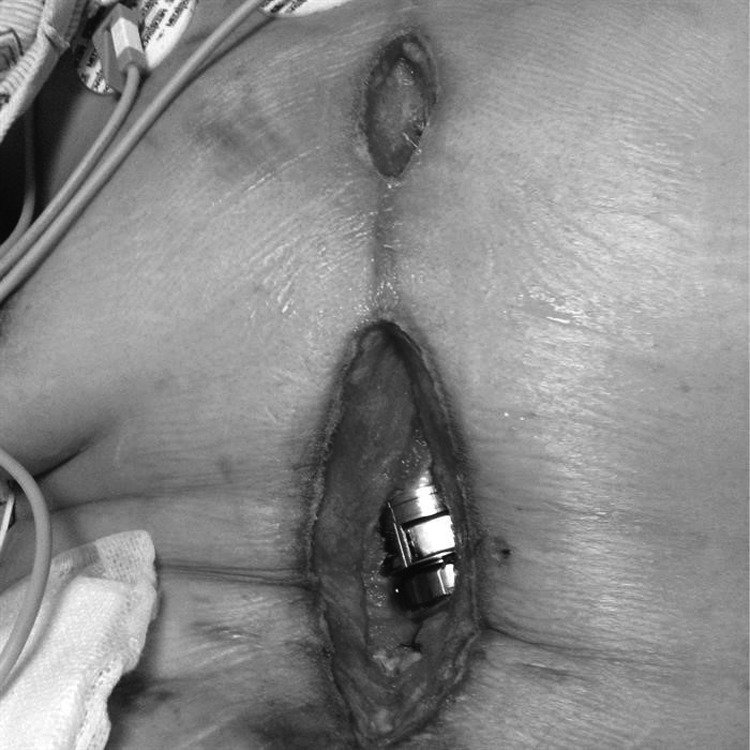
Chest wound after 6 weeks of VACi therapy. VACi, VAC with instillation.
Figure 8.
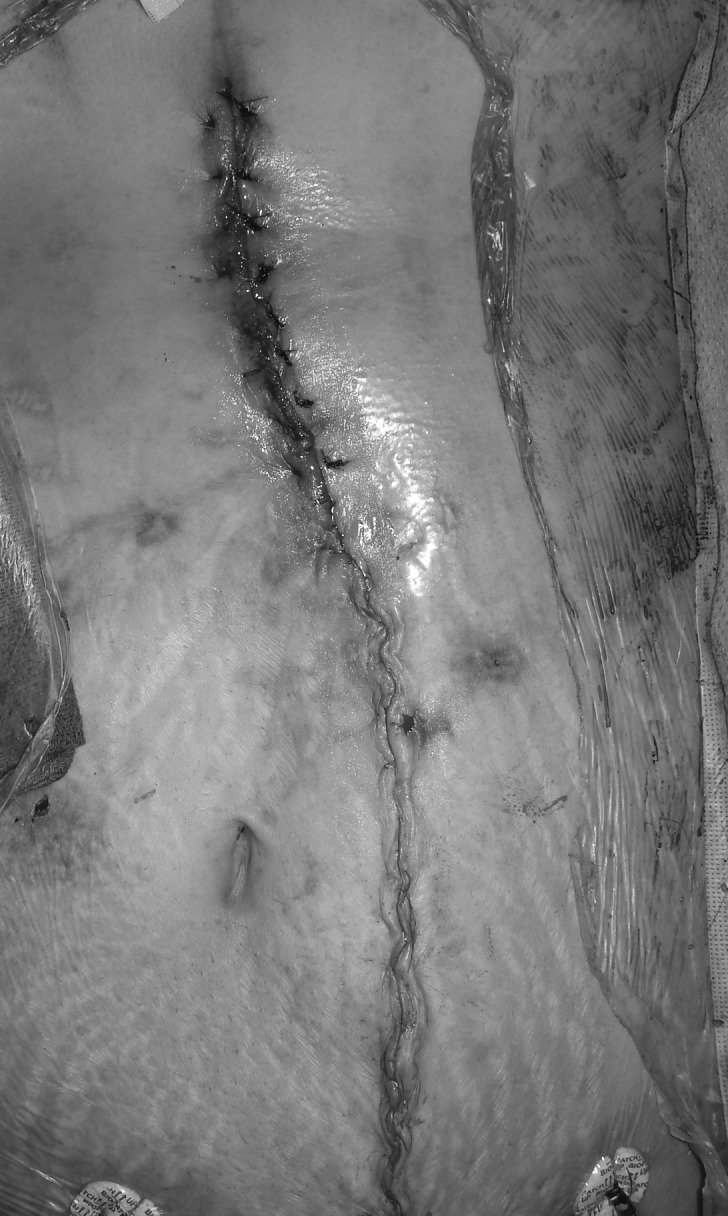
Postclosure photograph.
Results
Three patients were included in our series, and all achieved successful wound healing with hardware retention and resolution of infection. In all cases, VACi was utilized in conjunction with surgical debridement and IV antibiotics. This allowed for definitive wound closure using a pedicled rectus muscle flap to provide vascularized soft tissue coverage of the exposed device. Cases 1 and 2 achieved closure within 3 and 12 days, respectively, of initiating VACi treatment. Case 2 had an LVAD malfunction requiring delayed exchange due to thrombosis. Case 3 deferred surgery initially, before opting for definitive surgical closure at 164 days after beginning VACi therapy. Excluding the patient who deferred surgery from initial presentation, patients were treated and closed within an average of 7.5 days after initiating treatment and healed completely without residual evidence of infection.
Discussion
VACi has been shown to be superior to NPWT alone in decreasing hospital length of stay, number of operations, and time to final surgical procedure.14 If a wound is amenable to NPWT for treatment, it can potentially benefit from VACi. VACi has been shown to be effective in conjunction with surgical debridement and culture-directed antibiotics in treating osteomyelitis, closing complex wounds, and salvaging infected orthopedic and spinal hardware.13,15–22 Topical delivery of solutions that are tailored based on patients wound cultures and delivered consistently allows for a low-risk adjunct therapy. This method allows for cyclical topical delivery of a solution, which helps reduce biofilm thickness and biomass present on the hardware.3,11 Furthermore, the fluid may be capable of penetrating into irregular or hard-to-reach spaces in wounds where a traditional dressing would not, decreasing diffusion distances for the penetration of antimicrobials through biofilms.3 Less frequent dressing changes may also provide less risk of outside contamination and increase patient comfort.
In our case series, LVAD placement was the definitive treatment for all patients; however, many patients have LVADs placed as a precursor to heart transplantation. In patients on the transplant list active infection with an open wound is a contraindication to receiving a transplant. The effective and expeditious LVAD salvage demonstrated in our case series could prove to be a crucial solution to a patient awaiting a heart transplant who presents with an infected LVAD.
Several limitations should be noted in this study. One obvious limitation is the small number of patients observed and the retrospective nature of this case series. The LVAD patient population is a small subset of the overall patient population, but one that continues to increase as more patients have LVADs placed as a bridge to heart transplant or used as destination therapy. Another challenge when evaluating the effectiveness of any protocol serving the LVAD patient population is the number of comorbid conditions that exist, which act as confounding variables when evaluating outcomes. Further study is necessary to evaluate the use and effectiveness of VACi in LVAD salvage, and to follow eventual successful heart transplantation and overall life expectancy after infection. The long-term outcomes of the role VACi plays in salvage of implant or hardware infections, specifically LVAD salvage, should be followed in future studies.
Innovation
VACi was successfully utilized in conjunction with surgical debridement and antibiotics to eliminate infection and prepare wounds for closure without the removal of infected LVAD hardware. Our cases emphasize that in situations where infected hardware cannot be removed, creative new protocols and treatments are necessary to improve infection outcomes. Our series is a first step, suggesting that VACi can be an effective tool in the salvage of LVAD hardware.
Key Findings.
VACi can be used in conjunction with surgical debridement and antibiotic therapy to eradicate infection in LVAD hardware, allowing for surgical closure with LVAD retention.
Further research is needed to evaluate the efficacy of VACi in clearing infections for hardware salvage in other areas of the body.
Acknowledgments and Funding Sources
No funding was given or requested for this article.
Abbreviations and Acronyms
- CT
computed tomography
- LVAD
left ventricular assist device
- MSSA
methicillin-sensitive Staphylococcus aureus
- NPWT
negative pressure wound therapy
- NPWTi
NPWT with instillation
- VAC
vacuum-assisted closure
- VACi
VAC with instillation
Author Disclosure and Ghostwriting
No competing financial interests applicable to this series. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Trevor Hodson, BS, is a medical student at The Ohio State University College of Medicine. He has interest in medical innovations and surgery. Julie M. West, MS, PA-C is a physician assistant in plastic and reconstructive surgery at The Ohio State University Wexner Medical Center who has extensive experience with wound care and clinical trials. Stephen J. Poteet, MD, is a plastic and reconstructive surgeon at Ohio State specializing in both reconstructive and aesthetic procedures. He is currently developing plastic surgery opportunities among underserved populations throughout the world. Peter H. Lee, MD, PhD, MPH, is an accomplished cardiac surgeon at Ohio State. His practice focuses on adult cardiac surgery and heart and lung transplantation. Ian L. Valerio, MD, is an accomplished surgeon who served 4 years as a reconstructive surgeon in the U.S. Navy at trauma centers across the world before joining The Ohio State University Wexner Medical Center. His research interests include reconstructing and salvaging parts of the human body after trauma or disease, advancing stem cell therapies, and improving prosthetics to improve people's lives.
References
- 1. Robertson J, Long B, Koyfman A. The emergency management of ventricular assist devices. Am J Emerg Med 2016;34:1294–1301 [DOI] [PubMed] [Google Scholar]
- 2. Nienaber J, Kusne S, Riaz T, et al. . Clinical manifestations and management of left ventricular assist device—associated infections. Clin Infect Dis 2013;57:1438–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tahir S, Malone M, Hu H, Deva A, Vickery K. The effect of negative pressure wound therapy with and without instillation on mature biofilms in vitro. Materials 2018;11:811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hieda M, Sata M, Nakatani T. The importance of the management of infectious complications for patients with left ventricular assist device. Healthcare (Basel) 2015;3:750–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sekiguchi Y. Conservative therapy for the management of cardiac implantable electronic device infection. J Arrhythm 2016;32:293–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kuzyk P, Dhotar H, Sternheim A, Gross A, Safir O, Backstein D. Two-stage revision arthroplasty for management of chronic periprosthetic hip and knee infection. J Am Acad Orthop Surg 2014;22:153–164 [DOI] [PubMed] [Google Scholar]
- 7. Califano S, Pagani F, Malani P. Left ventricular assist device—associated infections. Infect Dis Clin North Am 2012;26:77–87 [DOI] [PubMed] [Google Scholar]
- 8. Fleischmann W, Russ M, Westhauser A, Stampehl M. Vacuum sealing as carrier system for controlled local drug administration in wound infection [in German]. Unfallchirurg 1998;101:649–654 [DOI] [PubMed] [Google Scholar]
- 9. Kim P, Attinger C, Crist B, et al. . Negative pressure wound therapy with instillation: review of evidence and recommendations. Wounds 2015;27:S2–S19 [PubMed] [Google Scholar]
- 10. Meaike JD, Kaufman MG, Izaddoost SA. Orthopedic prosthetic infections: plastic surgery management. Semin Plast Surg 2016;30:73–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ngo Q, Vickery K, Deva A. The effect of topical negative pressure on wound biofilms using an in vitro wound model. Wound Repair Regen 2011;20:83–90 [DOI] [PubMed] [Google Scholar]
- 12. Orgill D, Bayer L. Negative pressure wound therapy: past, present and future. Int Wound J 2013;10(Suppl 1):15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lehner B, Fleischmann W, Becker R, Jukema G. First experiences with negative pressure wound therapy and instillation in the treatment of infected orthopaedic implants: a clinical observational study. Int Orthop 2011;35:1415–1420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim PJ, Attinger CE, Steinberg JS, et al. . The impact of negative-pressure wound therapy with instillation compared with standard negative-pressure wound therapy: a retrospective, historical, cohort, controlled study. Plast Reconstr Surg 2014;133:709–716 [DOI] [PubMed] [Google Scholar]
- 15. West J, Jordan S, Mendel E, Khan S, Chandawarkar R, Valerio I. Instillation negative pressure wound therapy: an effective tool for complex spine wounds. Adv Wound Care (New Rochelle) 2018;7:333–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kirr R, Wiberg J, Hertlein H. [Clinical experience and results of using the V.A.C. instill therapy in infected hip- and knee prosthetics]. Zentralbl Chir 2006;131 suppl 1:S79–S82 [DOI] [PubMed] [Google Scholar]
- 17. Lehner B, Bernd L. [V.A.C.-instill therapy in periprosthetic infection of hip and knee arthroplasty]. Zentralbl Chir 2006;131 Suppl 1:S160–S164 [DOI] [PubMed] [Google Scholar]
- 18. Timmers MS, Graafland N, Bernards AT, et al. . Negative pressure wound treatment with polyvinyl alcohol foam and polyhexanide antiseptic solution instillation in posttraumatic osteomyelitis. Wound Repair Regen 2009;17:278–286 [DOI] [PubMed] [Google Scholar]
- 19. Karaaslan F, Erdem Ş, Mermerkaya MU. Wound management with vacuum-assisted closure in postoperative infections after surgery for spinal stenosis. Int Med Case Rep J 2015;8:7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wen G, Wang C, Chai Y, Cheng L, Chen M, Yi-Min L. Distally based saphenous neurocutaneous perforator flap combined with vac therapy for soft tissue reconstruction and hardware salvage in the lower extremities. Microsurgery 2013;33:625–630 [DOI] [PubMed] [Google Scholar]
- 21. Zwolak P, König MA, Osterhoff G, Wilzeck V, Simmen H-P, Jukema GN. Therapy of acute and delayed spinal infections after spinal surgery treated with negative pressure wound therapy in adult patients. Orthop Rev 2013;5:e30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Söylemez MS, Özkan K, Kılıç B, Erinç S. Intermittent negative pressure wound therapy with instillation for the treatment of persistent periprosthetic hip infections: a report of two cases. Ther Clin Risk Manag 2016;12:161–166 [DOI] [PMC free article] [PubMed] [Google Scholar]