Management of Crohn’s-related intra-abdominal abscesses is challenging. In a retrospective study of patients undergoing non-operative intra-abdominal abscess management, radiographic factors including abscess size, pre-stenotic bowel dilation, and bowel wall thickening are associated with the need for future bowel resection.
Keywords: inflammatory bowel disease; Crohn disease; fibrosis; imaging, decision support; predictive modeling; CT enterography; MR enterography; abscess; IBD phenotype
Abstract
Background
Intraabdominal abscess management decisions in the treatment of Crohn disease (CD) can be challenging. Our aim was to determine the effect of clinical, medication use, and imaging disease characteristics on the need for future surgical management.
Methods
A retrospective chart review was performed in patients with CD hospitalized for abscess confirmed by imaging between 2008 and 2016. Selection criteria included nonoperative management with intravenous antibiotics at the index hospitalization and a minimum of 2 years of follow up. Demographic, disease, and medication history were extracted from electronic medical records. Radiographic disease features were assessed by an expert abdominal radiologist, blinded to clinical data. The primary outcome was resection of the bowel segment involving the abscess within 2 years of index hospitalization. Cox proportional hazards regression and statistical methods were performed using SAS 9.4.
Results
Of the 121 patients meeting the selection criteria, 36.4% avoided surgery after 2 years of follow up. On adjusted multivariable analysis, disease-activity factors including bowel wall thickness (HR 3.08, 95% CL 1.20–6.21), disease length (HR 2.67, 95% CL 1.40–6.20), bowel dilation (HR 2.19, 95% CL 1.02–4.68), and abscess size of greater than 6 cm (HR 2.47, 95%CL 1.17–5.21) were independent risk factors for future surgery in patients not undergoing immediate bowel resection for abscess management. Biologic use and percutaneous drainage were not risk factors for ultimate surgical management.
Conclusions
Radiographic CD features and abscess size over 6 cm are predictors of ultimately requiring bowel resection. Radiographic measures may help stratify patients to immediate surgery or conservative management for intraabdominal CD-related abscesses.
INTRODUCTION
Severe transmural damage caused by bowel wall inflammation results in the development of an intraabdominal abscess in approximately 20% of patients with Crohn disease (CD).1 Intraabdominal abscesses pose serious and potentially fatal dangers in this patient population because of the risk of bacteremia and sepsis, especially in patients using immunosuppressive medications to control CD-related inflammatory activity.2 Penetrating complications are precipitated by severe ulcerative erosion through the bowel wall, which is often accompanied by a high-pressure gradient generated from downstream fibrostenotic intestinal damage.3 Though nonoperative methods can manage an intraabdominal abscess, most patients with an abscess will eventually require surgical bowel resection, either for recurrent abscess development or for the underlying severe bowel damage contributing to abscess formation.
Several treatment options are available to manage intraabdominal abscesses. Surgical management, including drainage and resection of the perforated intestinal segment, remains a common approach.4 Image-guided percutaneous drainage using ultrasound or computed tomography (CT), developed in the late 1980s, has become an increasingly common means for abscess management because it offers the potential to spare a bowel resection.5 Antimicrobial treatment alone may be used in patients with small abscesses (less than 2 cm) or those found in technically challenging locations. Further, penetrating disease often co-occurs with fibrostenotic stricturing disease, which in itself may demand surgical management because of persistent obstructive symptoms, even when an abscess is durably controlled.
Choosing between nonsurgical and surgical management is difficult as the desire to avoid bowel resection is judged against the seeming inevitability of surgery in 70% of reported cases.6 Society guidelines recommend consideration of all treatment options; however, little evidence is available to guide initial management decision-making in patients presenting with a new intraabdominal abscess from CD.7, 8 As a result, providers vary extensively in their management, as demonstrated by a survey of 248 pediatric gastroenterologists showing inconsistency in decisions for surgery, percutaneous drainage, and immunosuppressant medication use in patients with intraabdominal abscesses.9 Cross-sectional imaging by CT or MR, especially enterography protocols, provides excellent detail of both inflammatory disease activity and deep bowel wall damage and structural changes not appreciated by ileocolonoscopy.10 We aimed to determine whether baseline clinical, therapeutic, and image-based abscess and disease features were associated with the avoidance of future surgery in patients with a nonoperatively managed intraabdominal abscess attributable to CD.
METHODS
Selection Criteria
In this retrospective study, we identified all adult patients hospitalized for a primary diagnosis of CD with an index presentation of an intraabdominal abscess between 2008 and 2016, at a large tertiary care center in the United States. We verified the diagnosis of CD using a combination of administrative codes, IBD-related medication use, and manual medical record review. Included patients had undergone cross-sectional imaging by CT or MR at or within 1 week of hospitalization. Selected patients underwent nonoperative intraabdominal abscess management with intravenous antibiotics; percutaneous drainage frequently occurred but was not required for inclusion. Included patients also were required to have a minimum of 2-years’ follow up with a university gastroenterologist to allow adequate interval observation of outcomes. Patients undergoing an operation during index hospitalization, or where the surgical consultants’ plan was for surgical management following medical abscess management, were labeled as having an immediate decision for surgery, and were not included in the primary analysis. Because our study pertained to intraabdominal abscesses, we excluded perianal, postoperative, and peristomal abscesses.
Outcome Definitions and Clinical Data Extraction
Selected patients underwent chart review of available electronic medical records from 2008–2018, using the EMERSE electronic record search system.11 We collected patient demographics, disease features, and medication use at the time of index hospitalization. Recorded interventions at index hospitalization included percutaneous drain placement, duration of drain placement, outpatient antibiotics, route of administration, and duration of antibiotic use. The primary outcome was the occurrence of surgical resection of the diseased bowel segment involving the abscess within 2 years of the index hospitalization. Indications for surgery included persistent or recurrent abscess, uncontrolled disease activity, or stricturing disease not amenable to medical therapy. Surgeries not directly involving the segment of bowel containing the abscess did not constitute a primary outcome event. Additionally, other abdominal surgeries (hernia repair, cholecystectomy, or fistulotomy) were not included in the primary outcome. The time (days) from admission to surgery was recorded for all patients who underwent surgery.
Measurement of Imaging Features of Abscess and Disease Activity
An expert abdominal radiologist (over 10-years’ experience) who was blinded to the clinical data reviewed all CT and MRI studies. We recorded the maximal dimensions of abscesses, defined as the greatest linear dimension inclusive of confluent loculations, and the presence of abscess-associated fistulas. A gas-predominant fistula was defined as 50% of the abscess volume being occupied by gas. The abscess rim was defined as the presence of a clearly demarcated and enhancing abscess perimeter, in contrast to poorly defined abscess borders, often clinically noted as a phlegmon. CD image–based activity measures, which are not dependent on imaging technique (between CT and MR), were collected. Disease length was the continuous length of the radiologically diseased bowel segment including the abscess; discontinuous segments not involving the abscess were not included in the measurement. “Mural enhancement” was defined as present or absent based on hyperenhancement relative to the normal bowel. “Bowel obstruction” was defined as bowel dilation of 30 mm or more diameter upstream of the diseased intestinal segment.
Statistical Analysis
All analyses considered a P-value of ≤ 0.05 as statistically significant, and were conducted using SAS 9.4 (Cary, NC). All study authors had access to study data, and all reviewed and approved the final manuscript. Demographic, clinical, and imaging features at the index hospitalization were compared between patients who avoided or underwent surgery within 2 years of hospitalization. Cox proportional hazards regression models were used in the univariate analysis, comparing each individual predictor to the time until surgery. In the multivariate Cox proportional hazards regression model building, we utilized a backward variable selection process with forced inclusion of age, gender, and other covariates deemed relevant a priori. Continuous variables were also explored as categorical variables (with and without ordinal features) to provide the best model fit. Cut-points for continuous variables (eg, abscess size) were chosen based on tertiles and area under the receiver operating curve (AUROC). Analysis of maximum likelihood estimates provided hazard ratios and confidence limits for each parameter within the model.
RESULTS
Patient Characteristics
Of 157 identified patients with IBD-related nonperianal abscesses, 36 underwent immediate or near-term surgical management, leaving 121 patients with a plan of nonoperative management (Table 1). Of these patients managed with expectant, nonoperative care, 36.4% (n = 44/121) were able to avoid bowel resection for 2 years. Of those having future surgery, nearly half underwent a bowel resection within 6 months (48.1%, n = 37/77) of index hospitalization. Of those undergoing surgery, the indication was persistent abscess in 59.7% (n = 46/77) and refractory disease (fibrostenotic) in the remaining 40.3% (n = 31/77). Those undergoing future surgery as a group were younger than those who were able to avoid surgery (29.2 vs. 36.4 years, P = 0.019). Otherwise, there was no statistically significant difference in gender, disease duration, prior CD-related surgery, perianal disease, or medication use at index hospitalization between those having and those avoiding future surgery. Of those undergoing future surgery, over half (54.5%, n = 42/77) were rehospitalized for CD management within 1 year of index hospitalization; this excludes scheduled hospitalization for surgery planned in the outpatient setting. Those not undergoing surgery within 2 years were rehospitalized less frequently (11.4%, n = 5/44, P < 0.001). There were no significant differences in clinical characteristics between those undergoing immediate surgery and those undergoing future surgery after attempted conservative care alone (Supplemental Table 1). However, compared with those undergoing immediate surgery, surgery avoiders were significantly older (36.4 vs. 28.4 years, P = 0.023) and exhibited trends of less immunomodulator use (29.6% vs. 47.2%, P = 0.060) and less biologic use (25.0% vs. 41.7%, P = 0.028) at index presentation on univariate analysis.
TABLE 1.
Patient Characteristics
| Immediate Surgery | Nonoperative Management at Index Hospitalization |
P* |
||
|---|---|---|---|---|
| Future Surgery | No. Surgery at 2 Years | |||
| n (%) | 36 | 77 | 44 | |
| Age, years (SD) | 28.4 (11.7) | 29.2 (13.2) | 36.4 (18.5) | 0.019 |
| Sex, male | 16 (44.4) | 30 (38.9) | 19 (43.2) | 0.895 |
| Disease duration, years (SD) | 10.5 (9.1) | 11.2 (12.1) | 8.6 (10.3) | 0.255 |
| Prior bowel surgery | 12 (33.3) | 33 (33.0) | 18 (40.9) | 0.164 |
| Smoking history | 10 (27.8) | 16 (20.7) | 14 (31.8) | 0.092 |
| Perianal disease | 8 (22.2) | 18 (23.4) | 12 (27.2) | 0.163 |
| Medication use at index hospitalization | ||||
| Prednisone Use | 10 (27.8) | 18 (23.4) | 11 (25.5) | 0.700 |
| Pred dose, mean (SD) | 11.3 (19.3) | 7.6 (15.3) | 8.3 (15.4) | 0.808 |
| Immunomodulator, monotherapy | 17 (47.2) | 31 (40.3) | 13 (29.6) | 0.224 |
| Thiopurine | 14 (38.9) | 26 (33.8) | 11 (25.0) | 0.362 |
| Methotrexate | 3 (8.3) | 5 (6.5) | 2 (4.5) | 0.886 |
| Biologic use, monotherapy | 15 (41.7) | 23 (29.9) | 11 (25.0) | 0.500 |
| Combination therapy | 7 (19.4) | 14 (18.2) | 5 (11.4) | 0.238 |
| Interventions | ||||
| Outpatient Antibiotic type, IV | n/a | 23 (29.9) | 13 (29.5) | 0.880 |
| Drainage | ||||
| Percutaneous drain placed | 10 (27.8) | 43 (55.8) | 17 (38.6) | 0.109 |
| Duration drain | 9.2 (4.9) | 30.2 (3.0) | 34.3 (6.6) | 0.493 |
*P values shown only include patients with decision for nonoperative abscess management at the time of index hospitalization, comparing those undergoing future surgery to those avoiding surgery over at least 2 years of follow up.
Outcomes in Patients with Percutaneous Drainage
The decision for percutaneous drainage at the index hospitalization was more common among those undergoing future surgery (55.8% vs. 38.6%, P = 0.029). Unsurprisingly, abscess size was larger among those undergoing percutaneous drainage at index hospitalization compared with those who were not drained (6.0 ± 3.2 cm vs. 3.5 ± 1.8 cm, P < 0.001). The smallest abscess undergoing percutaneous drainage was 1.9 cm at greatest dimension. The size range of abscesses not undergoing drainage was 0.9–6.2 cm, compared to 1.9–19.0 cm in drained abscesses. Of those with large abscesses (>6 cm), the majority underwent percutaneous drainage (86.7%, n = 26/30) but commonly underwent future surgery (80.1%, n = 21/26). Of the 4 patients with an abscess greater than 6 cm in size who did not have percutaneous drainage, all underwent eventual surgery.
Even though there was no difference in the use of intravenous (IV) versus oral antibiotics for abscess management following hospitalization, larger abscesses were more likely to be treated with IV antibiotics (5.4 vs. 4.1 cm, P = 0.019). A very small portion (14/121, 11.5%) of patients were continued on 20 mg or more of corticosteroids at discharge; steroid use was not associated with future surgery (P = 0.693). Anti-TNF medication use (resumed or new start) within 8, 16, and 24 weeks of discharge occurred in 26.2%, 35.5%, and 46.7% of patients, respectively. There was no difference in anti-TNF use following abscess detection between those undergoing and those avoiding future surgery (P = 0.427).
Abscess and Disease-Activity Characteristics by Imaging
Among all patients, the majority of the abscesses primarily involved the distal ileum (85.9%, n = 104). The portion of abscesses occurring at the site of an anastomosis were similar between those avoiding and those undergoing surgery (14.8% vs. 21.0%, P = 0.696). Radiologic abscess characteristics were also similar between those undergoing or those avoiding future surgery when assessing by group (Table 2). Abscess size was similar between groups and was not smaller in those avoiding surgery relative to those undergoing future surgery on univariate analysis (4.7 ± 3.1 cm vs. 4.8 ± 2.8 cm, P = 0.767). Radiologic visualization of a fistula associated with the abscess occurred in the majority of cases (87.6%, n = 106). Finally, the degree of abscess wall definition (well-defined vs. poorly defined or phlegmon) was similar between groups undergoing or avoiding future surgery (P = 0.769).
TABLE 2.
Radiologic Abscess and Disease Characteristics, Unadjusted
|
|
Immediate Surgery | Nonoperative Management at Index Hospitalization | P* | |
|---|---|---|---|---|
| Future Surgery | No. Surgery at 2 Years | |||
| Disease-activity features, n | 36 | 77 | 44 | |
| Disease length, cm | 12.6 (8.9) | 13.7 (7.2) | 9.8 (5.0) | 0.021 |
| Max bowel wall thickness, mm | 9.1 (4.2) | 9.5 (2.2) | 9.4 (3.2) | 0.625 |
| Mural enhancement | 33 (91.7) | 74 (96.1) | 41 (93.2) | 0.458 |
| Bowel obstruction | 6 (16.7) | 15 (19.5) | 4 (9.1) | 0.139 |
| Abscess characteristics | ||||
| Maximum diameter, cm | 5.2 (2.9) | 5.2 (2.9) | 4.8 (3.4) | 0.505 |
| Gas predominant | 7 (19.4) | 15 (15.0) | 6 (13.7) | 0.955 |
| Clear associated fistula | 29 (80.6) | 70 (91.0) | 36 (81.9) | 0.296 |
| Rim well-defined | 17 (47.2) | 40 (51.9) | 21 (47.7) | 0.561 |
*P values shown only include patients with decision for nonoperative abscess management at the time of index hospitalization, comparing those undergoing future surgery to those avoiding surgery over at least 2 years of follow up.
Patients who avoided surgery had a shorter length of active intestinal disease compared to those who progressed to surgery within 2 years (9.8 ± 5.0 cm vs. 13.7 ± 7.2 cm, P = 0.021). The range of disease lengths for those avoiding and those undergoing surgery were 6.0–30.0 cm vs. 2.5–25.0 cm, respectively. Maximum bowel wall thickness (P = 0.625), the presence of mural enhancement (P = 0.458), and an associated bowel dilation (P = 0.139) were similar when comparing groups on unadjusted analysis. When comparing those undergoing immediate surgery to those treated nonoperatively at index presentation, with the exception of disease length, which had trend of being longer compared with those avoiding surgery (9.8 cm vs. 12.6 cm, P = 0.092), there were no significant differences observed in disease-activity features or abscess characteristics on univariate analysis (Supplemental Table 2).
Multivariable Model for Avoiding Surgery with Abscess
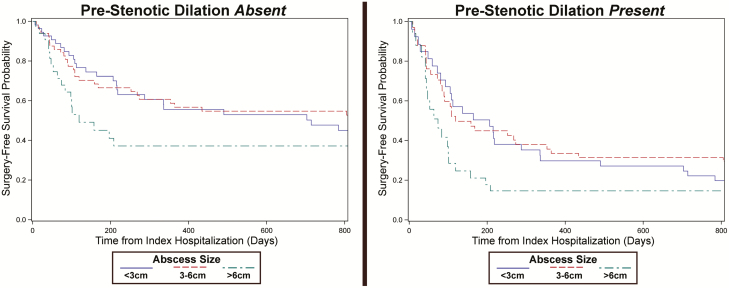
On adjusted analysis, Cox proportionate hazard regression identified several image-based factors, including increasing abscess size, bowel wall thickness, disease length, and an associated bowel obstruction as risk factors for future surgery in patients not undergoing immediate bowel resection for abscess management (Table 3). An abscess size of 6 cm was selected as the optimal cutoff (AUROC = 0.73) for association with future surgery compared with surgery avoidance. Projecting variables as dichotomous values, the hazard of future surgery was increased in patients with an abscess sized greater than 6 cm by a HR of 2.47 (95% CL 1.17–5.21), prestenotic bowel dilation of 30 mm or more by a HR of 2.19 (95% CI 1.02–4.68), bowel wall thickening greater than 6 mm by a HR of 3.08 (95% CL 1.20–6.21), and disease length greater than 15 cm by a HR of 2.67 (95% CL 1.40–6.20). When examining the combination of abscess size >6 cm in the presence of upstream bowel dilation, the hazard of future surgery was 4.19 (95% CI 1.40–8.43) compared to when neither feature was present. Projections of surgery-free estimated probability using the presented model demonstrate the increasing risk of eventual surgery in the setting of larger abscess size and bowel dilation (Fig. 1). Biologic medication use at the time of abscess identification, either as monotherapy or in combination with an immunomodulator, was not associated with risk for future surgery (HR 1.09, 95% CL 0.38–3.11). The decision to perform a percutaneous abscess drainage, adjusted for features noted in the multivariable model including abscess size, was not associated with avoidance of future surgery (HR 1.23, 95% CL 0.61–2.49).
TABLE 3.
Multivariable Cox Regression on Future Surgery Following Abscess
| Hazard Ratio | 95% Confidence Limits | P | ||
|---|---|---|---|---|
| Age, units per 10 years | 0.79 | 0.62 | 0.99 | 0.039 |
| Biologic monotherapy, at index | 1.05 | 0.36 | 3.10 | 0.927 |
| Combination therapy, at index | 2.28 | 0.69 | 7.49 | 0.176 |
| Bowel wall thickness, per 2 mm | 1.22 | 1.10 | 1.52 | 0.037 |
| Disease length, per cm | 1.10 | 1.02 | 1.29 | 0.019 |
| Abscess size, 6 cm or more | 2.47 | 1.17 | 5.21 | 0.018 |
| Prestenotic dilation >30 mm | 2.19 | 1.02 | 4.68 | 0.044 |
FIGURE 1.
Predicted probability of surgery-free survival in common clinical scenarios. The multivariable model was used to estimate the probability of surgery-free survival given an abscess size of less than 3 cm, 3–6 cm, or greater than 6 cm. The left and right panels display the probability of surgery-free survival in the absence or presence, respectively, of prestenotic bowel dilation greater than 3.0 cm.
DISCUSSION
In this study, we identified large abscess size, bowel wall thickening, disease length, and associated bowel obstruction to be imaging features associated with future surgery. Though the majority of patients required eventual surgery, 36.4% with initial nonoperative abscess management were able to avoid bowel resection for at least 2 years. Together, this suggests radiologic findings aid decisions between attempting conservative abscess management alone or instead optimizing the patient and planning for near-term surgery. Additionally, similar trends of clinical and radiologic features between those undergoing surgery at first presentation and those undergoing future surgery suggests that the presented risk factors can inform overall surgical decision-making. Percutaneous abscess drainage, despite adjustment for abscess size, did not change the risk of future surgery.
Percutaneous drainage is appealing as a means to mitigate the acute threats of an intraabdominal abscess and to provide an opportunity for antimicrobial and disease modifying therapies to heal bowel wall damage. A meta-analysis of percutaneous drainage outcomes reported that in 184 patients where percutaneous drainage was performed, 30% were able to avoid surgery.6 Additionally, Nguyen and colleagues found that percutaneous abscess drainage and immediate surgical management did not differ in the recurrence of abscess (31.2% vs. 20.3%, P = 0.250).12 Unlike the Clancy et al. meta-analysis, we did not find percutaneous drainage to be associated with a reduced risk of future surgery, likely because of adjustment for abscess size and disease-activity characteristics. Further, a retrospective study in pediatric patients with CD and an intraabdominal abscess who did not undergo immediate surgery found no difference in the need for future surgery, compared to medical treatment alone, among patients with immediate percutaneous drainage.13
Even if the abscess resolves without an operation, the abscess occurrence itself indicates severe disease activity potentially demanding bowel resection. High-pressure transition zones within the intestine result in bowel obstruction, which when prolonged are believed to contribute to penetrating disease.14 In some cases, immunosuppressive or biologic medical therapy use can contribute to sufficient healing to avoid further obstruction and related penetrating complications. On multivariate analysis, the need for future surgery was not modified by the use of anti-TNF therapy after hospital discharge. Nguyen and colleagues reported that anti-TNF therapy was associated with an increase in future bowel resection for abscess, although this is likely confounded by disease severity and an aggressive phenotype.12 Further, Samimi and colleagues’ retrospective study of patients with stricturing and penetrating phenotypes who were initially managed medically found that a similar portion (64% of the cohort) ultimately required surgical management.15 We hypothesize that the degree of fibrostenotic damage is associated with luminal pressurization and refractory penetrating complications. The association of increased bowel wall thickening with future surgery supports this hypothesis, but it is not conclusive and further study is needed.
This study was subject to several limitations requiring consideration when interpreting the findings. First, treatment decision-making (including the decisions to treat patients with drain placement or antimicrobial therapy alone, or with surgery after hospital discharge, and the timing of immunosuppressive medication use after hospital discharge) was uncontrolled. Considering that over 30 supervising providers were involved in the inpatient care of included patients, the study sample size was insufficient to meaningfully examine provider bias. The collection of radiologic features was principally performed to help address some of these biases and explain decision-making at the index hospitalization. Second, although we had interest in factors associated with decisions for immediate resection of bowel associated with an abscess, we were unable to capture symptom-level data at index hospitalization which influenced management. We suspect that symptom features including pain, ability to tolerate a diet, and subjective improvement with conservative medical management all influenced decision-making for immediate surgery or the the timing of eventual surgery. Addressing these selection biases will require a prospectively performed interventional study with randomized treatment allocation.
In summary, we found that over one-third of patients undergoing nonoperative intraabdominal abscess management were able to avoid future surgery for either recurrent abscess or medically nonresponsive CD over at least a 2-year period. Risk factors for failure of nonoperative management at index hospitalization included large abscess size (>6 cm), the increasing bowel wall thickness, and the presence of bowel dilation as detected by CT or MRI. Patients with these features may be better served by surgical management, either immediately or after optimization with antimicrobial therapy and drainage where appropriate. After adjustment for covariates (including abscess size), percutaneous drainage was not associated with increased success of nonoperative management. Future work examining detailed image-based assessments of disease activity, including modern assessments of bowel wall characteristics to assess the degree of fibrostenotic features within the bowel wall, may improve the ability to predict the success of conservative abscess management outcomes or the inevitability of a surgical resection.
Supplementary Material
Author contributions: D.P., data acquisition, drafting of the manuscript, data interpretation, and critical review of the manuscript; A.K.W., data analysis, data interpretation, and critical review of the manuscript; P.D.R.H., data analysis, data interpretation, and critical review of the manuscript; S.B., data analysis, data interpretation, and critical review of the manuscript; A.P.W., study design, data acquisition, drafting of the manuscript, data interpretation, and critical review of the manuscript; R.W.S., study design, data analysis, data acquisition, drafting of the manuscript, data interpretation, data analysis, critical review of the manuscript, and study supervision.
Authors’ declaration of personal interests: none (D.P., A.K.W., S.B., A.W.).
P.D.R.H. has served as a consultant for or has received research grants from Abbvie, Janssen, Merck, Takeda, and Buhlmann Labs.
R.W.S. has served as a consultant for Abbvie, Janssen, and Merck.
Declaration of funding interests: none (D.P., A.K.W., P.D.R.H., S.B., A.W.).
R.W.S. declares that this work was funded through the following awards: NIH-K23-DK101687.
Supported by: National Institutes of Health K23-DK101687 (Stidham).
Disclosures: No potential conflicts of interest relevant to this manuscript are present.
Writing assistance: No writing assistance was provided.
REFERENCES
- 1. Thia KT, Sandborn WJ, Harmsen WS, et al. Risk factors associated with progression to intestinal complications of Crohn’s disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agrawal A, Durrani S, Leiper K, et al. Effect of systemic corticosteroid therapy on risk for intra-abdominal or pelvic abscess in non-operated Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:1215–1220. [DOI] [PubMed] [Google Scholar]
- 3. Bermejo F, Garrido E, Chaparro M, et al. Efficacy of different therapeutic options for spontaneous abdominal abscesses in Crohn’s disease: are antibiotics enough? Inflamm Bowel Dis. 2012;18:1509–1514. [DOI] [PubMed] [Google Scholar]
- 4. de Groof EJ, Carbonnel F, Buskens CJ, et al. Abdominal abscess in Crohn’s disease: multidisciplinary management. Dig Dis. 2014;32(Suppl 1):103–109. [DOI] [PubMed] [Google Scholar]
- 5. Gervais DA, Hahn PF, O’Neill MJ, et al. Percutaneous abscess drainage in Crohn disease: technical success and short- and long-term outcomes during 14 years. Radiology. 2002;222:645–651. [DOI] [PubMed] [Google Scholar]
- 6. Clancy C, Boland T, Deasy J, et al. A meta-analysis of percutaneous drainage versus surgery as the initial treatment of Crohn’s disease-related intra-abdominal abscess. J Crohns Colitis. 2016;10:202–208. [DOI] [PubMed] [Google Scholar]
- 7. Dignass A, Van Assche G, Lindsay JO, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: current management. J Crohns Colitis. 2010;4:28–62. [DOI] [PubMed] [Google Scholar]
- 8. Lichtenstein GR, Hanauer SB, Sandborn WJ; Practice Parameters Committee of American College of Gastroenterology Management of Crohn’s disease in adults. Am J Gastroenterol. 2009;104:465–483; quiz 464, 484. [DOI] [PubMed] [Google Scholar]
- 9. Dotson JL, Nwomeh B, Andridge R, et al. Variation in management of intra-abdominal abscesses in children with Crohn’s disease. Inflamm Bowel Dis. 2013;19:818–825. [DOI] [PubMed] [Google Scholar]
- 10. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn’s disease lesions by magnetic resonance imaging. Am J Gastroenterol. 2015;110:432–440. [DOI] [PubMed] [Google Scholar]
- 11. Hanauer DA, Mei Q, Law J, et al. Supporting information retrieval from electronic health records: a report of university of Michigan’s nine-year experience in developing and using the electronic medical record search engine (EMERSE). J Biomed Inform. 2015;55:290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nguyen DL, Sandborn WJ, Loftus EV Jr, et al. Similar outcomes of surgical and medical treatment of intra-abdominal abscesses in patients with Crohn’s disease. Clin Gastroenterol Hepatol. 2012;10:400–404. [DOI] [PubMed] [Google Scholar]
- 13. Dotson JL, Bashaw H, Nwomeh B, et al. Management of intra-abdominal abscesses in children with Crohn’s disease: a 12-year, retrospective single-center review. Inflamm Bowel Dis. 2015;21:1109–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rieder F, Zimmermann EM, Remzi FH, et al. Crohn’s disease complicated by strictures: a systematic review. Gut. 2013;62:1072–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samimi R, Flasar MH, Kavic S, et al. Outcome of medical treatment of stricturing and penetrating Crohn’s disease: a retrospective study. Inflamm Bowel Dis. 2010;16:1187–1194. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.