Abstract
Objective: Telemedicine (TM) is an evolving method in lower extremity ulcer (LEU) treatment. Previous studies have demonstrated TM as comparable to the same-room care in clinical outcomes measures. Conversely, economic assessments of TM initiative were scarce and inconsistent. This study aims to analyze the costs and benefits of TM in LEU treatment and to propose a TM implementation decision-scoring model.
Approach: This cost minimization analysis was performed at Maccabi Healthcare Services (Israel) during January 1, 2013–June 31, 2017 period. The study was based on cost difference assessment. A decision-scoring model for TM implementation was constructed.
Results: The cost per patient in TM modality, compared to the same-room care, was 7% higher; however, in a proportion similar to same-room care, the cost of TM was lower. The TM implementation decision score was 0.236, while the weight of the direct cost factors is 0.70. Face-to-face only model, compared to the study sample, demonstrated 30% higher costs.
Innovation: The study brings new evidence to an LEU treatment domain with little previous research. Also, a TM decision implementation scoring model has been provided.
Conclusions: The decision support model may be instrumental in the TM implementation process.
Keywords: telemedicine, implementation decision, direct costs, patient's perspective, Israel
Alexander Gamus, MSc.

Introduction
Lower extremity ulcers (LEU) impose a heavy burden on individuals as well as health care organizations.1,2 The estimated annual prevalence of the foot and leg ulcers in published studies varies from 0.18% to 2.1%, reaching 5% in patients older than 65 years.2 In Israel, where the prevalence of diabetes mellitus (DM) is estimated at 8.4%, up to 15% of DM patients may develop diabetic foot ulcers.3 Chronic wounds often coincide with other morbidities, adding physical and psychological strain, further limiting a patient's ability to make their way to a specialist, usually located in urban centers.2,4 Telemedicine (TM) is an evolving method of remote care and has been suggested as a solution in LEU treatment.5,6
Two Israeli laws—Israel National Health Insurance Law (1995) and Israel's Patient's Rights Law (1996)—defined a strategy for equality in quality and efficiency health services available for the central urban and remote populations. Nevertheless, the TM implementation is slow, probably due to its complicated multidisciplinary nature, involving clinical effectiveness, patient's related factors, and organizational, economic, and regulatory issues.7
The economic aspect of the TM has been studied worldwide. However, the results were inconsistent.8,9 Studies of TM, compared to the standard face-to-face care (FTF), have demonstrated equal or nonsignificant differences in clinical outcomes between the two treatment methods.10–12 The TM in LEU care studies as well as the cost analysis trials in Israel have not been found.
This study addresses TM implementation decision questions: what is the cost difference between TM and FTF methods in LEU care and what are the considerations of TM implementation?
Hence, the aim of the study is twofold: (1) to conduct a cost analysis of TM and FTF methods in LEU and (2) to provide a model that would help health care organization decide on TM for LEU implementation.
Clinical problems addressed
LEU are associated with considerable morbidity and even mortality. Their prevalence may further increase as a result of aging and its limited mobility may present a challenge to the health care system. TM is often defined as a process of using the information and communication technologies to provide remote health care to the populations where medical specialist's service availability is limited.
Materials and Methods
A European Wound Management Association has recommended using the Model for Assessment of Telemedicine (MAST) when evaluating TM in wound care. MAST proposes the use of multidisciplinary assessment comprising seven domains.13 This study uses the economics domain for cost per patient calculations, and the sociocultural, ethical and legal aspect domain in the TM implementation decision model assessment.
Settings
This study was performed using the electronic medical records (EMR) database of Maccabi Healthcare Services (MHS), a 2.2-million-member sick fund in Israel. The study period was during January 1, 2013–June 31, 2017 and took place in eight MHS' Northern and Southern District clinics. TM cohort included patients from six outpatient clinics operating through identical telecommunications, information technologies, and videoconferencing infrastructures. The same specialist supervised the treatment in both modalities (TM and FTF) in each district, and at each location (central or remote clinics), the same nurse treated all patients.
Inclusion criteria
Adult patients with a history of LEU for more than 6 weeks. Only data on LEU patients treated at the same clinic throughout the observation period were included.
Exclusion criteria
Patients with less than two treatment interventions, or ulcerations above the knee level were excluded.
Infrastructures
The study employed a commercial videoconferencing system (Lifesize, Inc., Austin, TX) operating through MHS' internal high-availability secure telecommunications network. Both TM and FTF modalities were implemented in an identical treatment setting with the same nurse at each location. During the first consultation stage (anamnesis), the patient was sitting in front of a screen and a camera. Next, the patient's wound was monitored using an additional video camera installed on the adjustable arm, designed specifically for this purpose (Galil-S.L.G. Ltd., Israel). A patient could sit or lay down according to his or her medical condition and the location of the wound. The camera was operated by a nurse, as instructed by a physician. Medical records were available to the specialist and nurse for viewing and updates throughout the session using Maccabi EMR database.
The economic evaluation
Cost minimization analysis (CMA) method was chosen given statistically not significant differences of disease-related outcomes between the TM and FTF modalities in a study sample (mixed model). The studies of LEU treatment clinical outcomes and patient's quality of life were performed previously.5,14–17 Patients' treatments by a nurse were assumed to be similar in costs (dressings, medications, and other procedures) and therefore excluded from the CMA. MHS' direct costs contained a physician's labor and technology expenses for the entire study period. Differences in MHS' and patient-related costs between the treatment modalities were included in a TM implementation decision-scoring model.
Two models were used: (1) study sample with TM and FTF cohorts (mixed model) and (2) FTF-only version (all patients treated by FTF modality). The FTF-only model was based on the physician's ability to assess two to three patients at the time of one FTF session. The TM functionality allows switching among the remote sites by the press of the button. Hence, the time of a wound dressing or other procedure by a nurse in one remote location can be used for assessment of patient status at the other.
The comparison between the two methods of treatment aims at an additional inference on TM implementation implications.
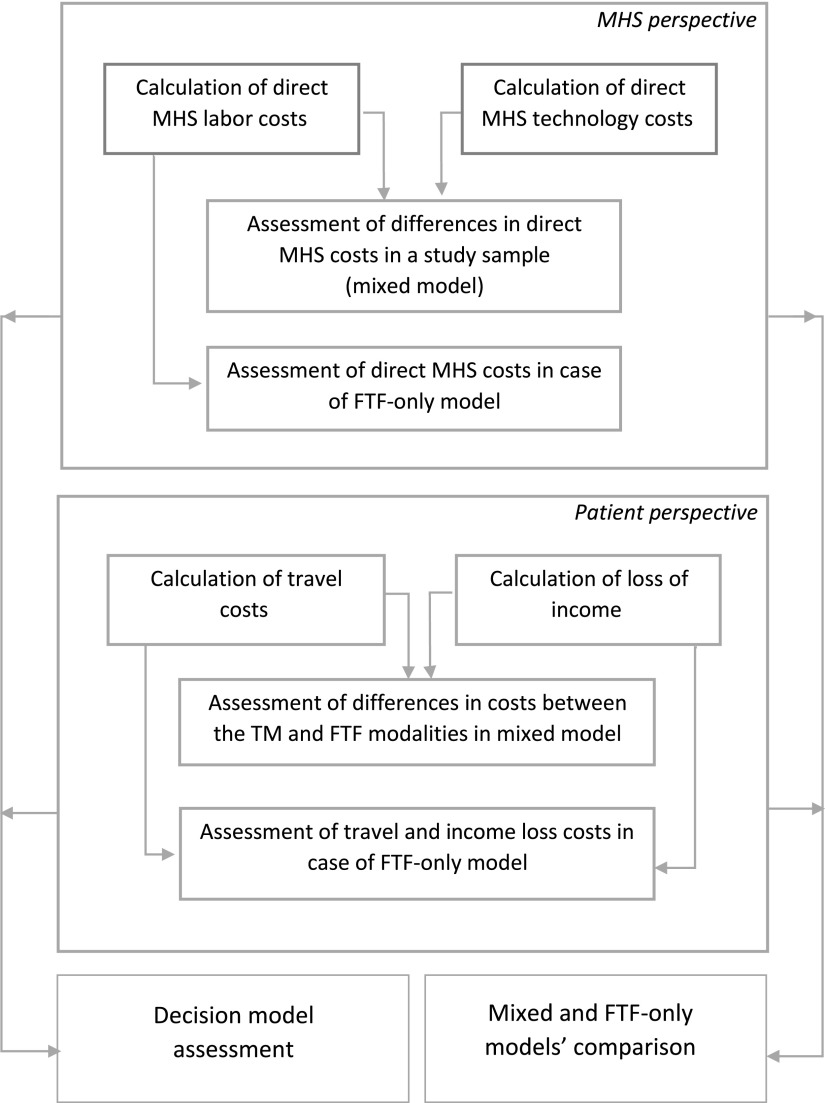
The study method diagram is demonstrated in Figure 1.
Figure 1.
Cost and benefit assessment process. Mixed model = study sample; FTF-only model—cost evaluation in same-room treatment for all patients. FTF, face-to-face care; MHS, Maccabi Healthcare Services.
Data sources
Direct MHS' costs
To avoid disclosure of internal corporate information, all costs are obtained from official sources and expressed in United States Dollar. Labor cost estimated for full-time and part-time medical specialists at a health care organization in Israel is as published by Central Bureau of Statistics. MHS deliver most of its services through third-party providers. In our study, one specialist's labor costs were evaluated by a salary-based reimbursement; the other was a part-time physician. TM-related technologies' costs evaluation based on the official supplier's published price list.18 It is noted that in practice, a health care organization may purchase the required equipment with a considerable discount. In our study, we addressed the costs as they appear at a supplier's website.
Patient-related costs
Patients travel expenses were constructed using official bus tariffs available on the Internet for local and intercity travel.19 The costs of transportation for each patient were adjusted to the age group discounts. An average tariff for local and intercity bus was calculated.
A loss of patients' income due to travel evaluated taking into consideration a travel path to the local or remote TM or FTF centers. A loss of income was considered only for a patient younger than the age of retirement.
Decision model
The proposed model calculates a decision score based on a health care organization's patient-related cost differences and policies. Weight of each cost and benefit item in a decision process may be expressed in proportions of relevance between 0.0 and 1.0, while all factors sum up to 1.0. The costs differences are expressed in proportions as well. Multiplication of proportion of difference by a weight factor results in a score for each item. The policies and regulations were expressed only by the weight of relevance to the provider. The total of items scores means a proportion by which a TM implementation consideration differs from the FTF solution. This score may be negative or positive.
Investigated endpoints
First set of endpoints is related to the study sample using mixed model included direct and patient cost of TM, and FTF from MHS' perspective. The second set of endpoints is within the total direct and patient costs of FTF-only treatment model. The third set of endpoints is the decision assessment scores calculated using the TM implementation model.
Data analyses
All costs were calculated for the entire study period. Patient-related costs were constructed per patient and further analyzed. Average values used for costs were obtained from different sources. The Mann-Whitney U tests were performed in cost data distribution analyses. Microsoft Excel 2016 and IBM SPSS Statistics for Windows, Version 22.0 were used (IBM Corp., Armonk, NY).
Results
Direct MHS' costs
The MHS' direct costs within the study sample (mixed model) are represented in Table 1. The direct costs proportion in TM modality is higher than in FTF by a factor of 0.07 (difference of 28,580 United States Dollar [USD] divided by the costs of TM).
Table 1.
Direct costs evaluation in the study sample (United States Dollar)
| Method | Cost item | Qtya | Cost/unit | Initial cost | Maintenance fee/5 years | Cost/5 years |
|---|---|---|---|---|---|---|
| TM | Subtotal | 406,580 | ||||
| Technologies and support | 112,580 | |||||
| Central system | 1 | 14,321 | 14,321 | 5,728 | 20,049 | |
| Endpoint | 8 | 3,990 | 31,920 | 12,768 | 44,688 | |
| Accessories | 6 | 1,974 | 11,842 | not applicable | 11,842 | |
| Telecom channels | 6 | sunk cost | ||||
| Tech. staff/support | 0.2 | 3,000 | 36,000 | |||
| Labor | 294,000 | |||||
| Medical specialist TM—salary based | 0.46 | 10,000 | 276,000 | |||
| Medical specialist TM—part time | 0.25 | 1,200 | 18,000 | |||
| FTF | Subtotal | 378,000 | ||||
| Medical specialist FTF—salary based | 0.54 | 10,000 | 324,000 | |||
| Medical specialist FTF—part time | 0.75 | 1,200 | 54,000 | |||
| Total for the study | 784,580 |
Qty of labor proportional to treatment method applied; maintenance fee for equipment assumed 10% of the cost of equipment per year; Tech support in 0.1 of technician cost per region.
FTF, face-to-face care; Qty, quantity; TM, telemedicine.
FTF-only version directs cost calculation based upon a conservative approach of only two patient's treatment in TM modality at the time of one in FTF. The additional specialist's cost of 294,000 USD added, resulted in a 966,000 USD total cost for FTF-only version. The total direct cost in the FTF-only model is higher than in the mixed model by a factor of 0.23 (difference related to the mixed model).
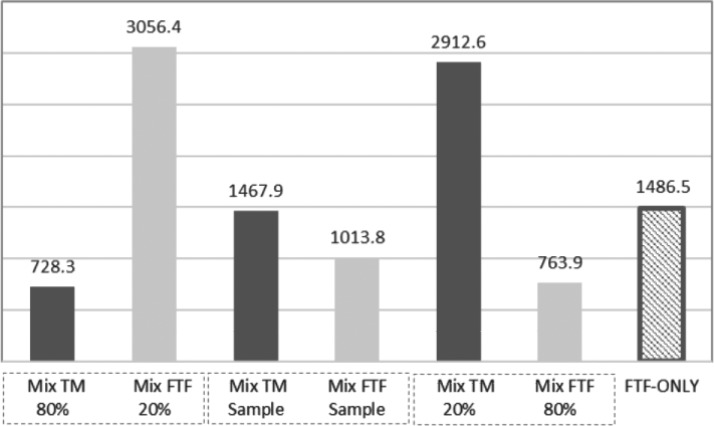
According to the sensitivity analysis, the main per-patient cost driver was a percentage of patients in each modality. This trend is shown in Figure 2: the cost per patient is highest with the lowest number of patients. A total number of patients in the study sample is n = 650, while 277 (42.6%) are from TM cohort and 373 (57.4%) are treated by FTF method. Within the study sample, the cost per patient is higher in TM modality. The theoretical assumption of patient's distribution with 291 (44.8%) in TM and 359 (55.2%) in FTF modality results in a possible break-even point between the TM and FTF cost per patient, 1,010.0 and 1,058.0 USD, respectively, with proportion of difference related to TM equal to 0.047.
Figure 2.
The direct average cost per patient (MHS' perspective). Dotted boxes indicate the study sample sensitivity to the change of TM proportion; FTF-only model costs per patient shown for reference. Mix, mixed model; TM, telemedicine.
Patient-related costs
A comparison between the study sample (mixed model) and FTF-only version shows an income loss of 145,796.0 and 194,656.0 USD, respectively. The higher proportion of the loss of income in FTF-only modality by 0.34 was found. The income loss within the mixed model in FTF modality is higher than in TM by a factor of 2.9. Travel cost comparison between the models shows a higher proportion in an FTF-only version by 0.19, while travel costs in FTF modality within the mixed model is proportionally higher by 0.49 than in TM.
The Mann-Whitney tests for data distribution across the age categories show a significant difference for all cost items, p < 0.001. The difference is likely to occur due to a higher cost in a group younger than the age of retirement. The retired population has a travel discount of 50%, and in most cases, the loss of income factor is not relevant.
Decision support model
The decision model, shown in Table 2, based on differences in direct costs, patient-related costs, and general policies, demonstrated an advantage of TM in a study sample (mixed model). The significance of the items from the MHS' perspective was divided into three groups: MHS-related costs weight—70%, patient-related costs—20%, and general policies—10%. The resulting decision factor shows the advantage of TM by 23.6% compared to FTF method.
Table 2.
TM implementation model—decision evaluation (significance of Maccabi Healthcare Services cost is 70% of total decision)
| Health Care Provider Related | Patient Related | General/Policies | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Item to compare | Direct costs | Future costs | Travel costs saved | Income saved | Economy | Societal | Regulations | Summarya | |
| Differenceb | −0.070 | — | 0.190 | 2.900 | — | — | — | ||
| Significance Factorc | 0.650 | 0.050 | 0.100 | 0.100 | 0.010 | 0.045 | 0.045 | Checksum: | 1.00 |
| Decision Factors | −0.046 | −0.050 | 0.014 | 0.218 | 0.010 | 0.045 | 0.045 | 0.236 | |
Numbers in italics to be chosen according to decision-making policies.
Positive value indicates TM advantage.
Proportions express difference of values between methods to the value in TM.
Demonstrates the relevance of an item in decision considerations.
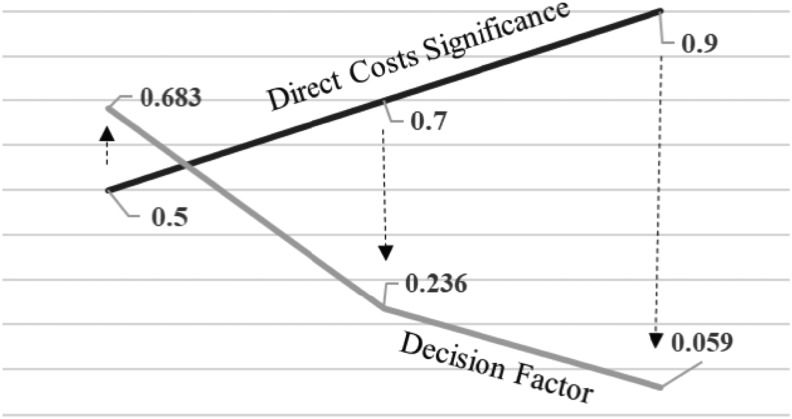
Also, the sensitivity analyses of the proposed model were performed for 50% and 90% differences in MHS-related cost weights. The resulting decision factors are demonstrated in Figure 3.
Figure 3.
Decision score as function of cost significance—study sample. Arrows designate the decision factor dependency on the chosen significance, for example, proportion of 0.9 leads to the score of 0.059, 0.7 to 0.236, and 0.5 to 0.683.
Discussion
This study analysis demonstrates that per-patient LEU treatment costs in TM modality were higher than in FTF mode, 1,467.9 and 1,013.8 USD, respectively. However, in the case of the equal number of patients in both modalities, the costs per patient could be 1,010.0 and 1,058.0 USD in TM and FTF, respectively, and lowering with an increasing number of TM patients, as may be seen in Figure 2. A further increase of TM patients' proportion within the mixed model would benefit a justification to implement the remote treatment. Conversely, the costs of the estimated FTF-only version, where all patients treated in FTF mode, were found to be significantly higher than in a mixed (TM and FTF) model.
The TM implementation decision model demonstrated a reliance of the final decision score on the significance (relative importance) given to the direct costs in the decision-making process, shown in Figure 3. While a high significance of 90% results in a relatively low decision factor of 5.9%, meaning low priority to the TM implementation, the probably more realistic figure of 70% results in a score of 23.6% to the TM advantage. The decision model may result in a negative or positive score, given 100% significance to direct costs difference. Direct costs significance of 100% in our study would result in −9.6% score, derived from a negative difference between the TM and FTF direct costs. However, we believe that in most circumstances, patient-related costs, as well as national/governmental policies, may also be relevant to the remote care implementation decision, leading to a lower than 100% direct costs significance in the decision-making process. Also, the proposed model is resilient to changes in relative proportions of significance of direct costs, and patient-related and general policies in the decision-making process. We used a 70%, 20%, and 10%, respectively, as probably appropriate in Israeli health care environment. However, other proportions may be chosen according to other health care organizations and government policies.
A published systematic review on TM cost-effectiveness has shown that there is no conclusive evidence of TM interventions being more cost-effective compared to conventional care.20 The review concluded that most of the studies were undertaken for a period less than 2 years and a relatively small number of observations, which may not reflect the true value of TM cost efficiency. Additional systematic review of TM cost studies showed mixed results for rural service delivery, and concluded that key factors of synchronous video TM cost-effectiveness are “settings and particular models of health service delivery.”8 The cost-effectiveness of TM in diabetic ulcers patients' study demonstrated no significant differences between the TM and FTF care.9 Also, the study has suggested that the result “transferability may be limited due to the difference in settings and treatment protocols.”9
Conversely, to the published studies, we assessed the costs related to given morbidity using assisted care protocol. The proposed decision model includes considerations relevant to the Israeli health care policies. Subsequently, the generalization of the results may be relevant where legal and organizational considerations are similar.
Some study limitations may be pointed out. All costs are for the 5-year period of study based on the official price lists, tariffs, and statistics relevant to 2017. The actual costs may differ during the study period. Also, due to the ethical requirement, the patient's identity could not be known. Subsequently, patient-related costs, like travel or income loss, could only be estimated by a travel distance and time spent. However, the impact of these variations on costs differences, and therefore on the study results, most likely are minimal. The actual costs of TM technologies may be lower, taking into consideration purchasing variations and the economic value of consolidation of technologies with other medical treatment applications. Centrally located equipment and applications may serve all synchronous video applications within the organization. Subsequently, the initial investment in the central system would be considered a sunk cost for additional expansion of TM applications. Also, a remote unit may be utilized in favor of the additional type of medical care, if feasible. These and mobile technology addition in LEU care may be a subject of future studies.
Innovation
The study brings new evidence to an LEU treatment domain with little previous research. The multidisciplinary nature of TM implementation may challenge health care organizations. Hence, our proposed costs and benefits evaluation model may prove to be instrumental in management of decisions support.
Key Findings
The TM implementation costs per patient may be lower than the FTF method in case of equal proportion of the TM and FTF patients.
The mixed TM and FTF method of treatment was found to be less costly than the FTF-only alternative.
The health care decision on TM implementation feasibility may be evaluated by the proposed model of providers, patients, and other considerations scoring.
Acknowledgments and Funding Sources
Maccabi Healthcare Services ethics committee approved the study according to the 1964 Helsinki declaration, permission number 42/2015. No funding was provided for this study.
Abbreviations and Acronyms
- CMA
cost minimization analysis
- DM
diabetes mellitus
- EMR
electronic medical records
- FTF
face-to-face care
- LEU
lower extremities ulcers
- MAST
Model for Assessment of Telemedicine
- MHS
Maccabi Healthcare Services
- TM
telemedicine
- USD
United States Dollar
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Alexander Gamus, MSc, is presently a doctoral candidate in Epidemiology Department, Sackler Faculty of Medicine at Tel-Aviv University, Tel-Aviv, Israel. Gabriel Chodick, PhD, is a professor in Epidemiology Department, Sackler Faculty of Medicine at Tel-Aviv University, Tel-Aviv, Israel.
References
- 1. Shubhandi V. Chronic leg ulcers: epidemiology, aetiopathogenesis and management. Ulcers 2013;2013:413–604 [Google Scholar]
- 2. Setacci C, de Donato G, Settaci F, Chisi E. Diabetic patients: epidemiology and global impact. J Cardivasc Surg 2009;50:263–273 [PubMed] [Google Scholar]
- 3. Israel Center for Disease Control (ICDC). National Diabetes Registry (total population) 2015;14 http://www.health.gov.il/PublicationsFiles/INHIS_3main_findings.pdf(last accessed October2, 2018) [Google Scholar]
- 4. Gray LC, Armfield NR, Smith AC. Telemedicine for wound care: current practice and future potential. Wound Pract Res 2010;18:158–163 [Google Scholar]
- 5. Nordheim LV, Haavind MT, Iversen MM. Effect of telemedicine follow-up care of leg and foot ulcers: a systematic review. BMC Health Serv Res 2014;14:565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chittoria R. Telemedicine for wound management. Indian J Plast Surg 2012;45:412–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moffat C, Vowden K, Price P, Vowden P. European Wound Management Association (EWMA). Position Document: Hard-to -Heal wounds: A holistic approach. London: MEP Ltd., 2008 [Google Scholar]
- 8. Wade VA, Karnon J, Elshaug AG, Hiller JE. A systematic review of economic analyses of telehealth services using real time video communication. BMC Health Serv Res 2010;10:2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fasterholdt I, Gerstrom M, Schnack B, et al. Cost-effectiveness of telemonitoring of diabetic foot ulcer patients. Health Informatics J 2018;24:245–258 [DOI] [PubMed] [Google Scholar]
- 10. Chanussot-Deprez C, Contreras-Ruiz J. Telemedicine in wound care: a review. Adv Skin Wound Care 2013;26:78–82 [DOI] [PubMed] [Google Scholar]
- 11. Iversen MM, Espehaug B, Hausken MF, et al. Telemedicine versus standard follow-up care for diabetes-related foot ulcers: Protocol for a cluster randomized controlled noninferiority trial (DiaFOTo). JMIR Res Protoc 2016;5:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wootton R. Twenty years of telemedicine in chronic disease management—an evidence synthesis. J Telemed Telecare 2012;18:211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kidholm K, Ekeland AG, Jensen LK, et al. A model for assessment of telemedicine applications: mast. Int J Technol Assess 2012;28:44–51 [DOI] [PubMed] [Google Scholar]
- 14. Gamus A, Kaufman H, Chodick G. Synchronous video telemedicine in lower extremities chronic ulcers treatment - retrospective cohort study. In: Proceedings of 5th International Conference on Medical Informatics & Telemedicine Prague, Czech Republic: Conference Series, 2017 [Google Scholar]
- 15. Wilbright WA, Birke JA, Patout CA, Varnado M, Horswell R. The Use of telemedicine in the management of diabetic-related foot ulceration: a pilot study. Adv Skin Wound Care 2004;17:232–238 [DOI] [PubMed] [Google Scholar]
- 16. Clemmensen J, Larsen SB, Kirkevold M, Ejskjaer N. Treatment of diabetic foot ulcers in the home: video consultations as an alternative to outpatient hospital care. Int J Telemed App 2008:132890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Smith-Strom H, Igland J, Ostbye T, et al. The effect of telemedicine follow-up care on diabetes-related foot ulcers: a cluster-randomized controlled non-inferiority trial. Diabetes Care 2018;41:96–103 [DOI] [PubMed] [Google Scholar]
- 18. Video Conferencing Supply. Video Conferencing Systems. https://www.videoconferencingsupply.com/video-conferencing-systems/?filter_bes8o1wpm5=Lifesize (last accessed January13, 2019)
- 19. Egged. Fares and Codes (Effective February 1, 2016). www.egged.co.il/Article-1593-Fares-and-Codes.aspx (last accessed January13, 2019)
- 20. Mistry H. Systematic review of studies of the cost-effectiveness of telemedicine and telecare. Changes in the economic evidence over twenty years. J Telemed Telecare 2012;18:1–6 [DOI] [PubMed] [Google Scholar]