Abstract
Objective: Increasing numbers of multidrug-resistant bacteria make many antibiotics ineffective; therefore, new approaches to combat microbial infections are needed. In addition, antibiotics are not selective—they kill pathogenic organisms as well as organisms that could positively contribute to wound healing (bio flora).
Approach: Here we report on selective inactivation of Pseudomonas aeruginosa and Staphylococcus epidermidis, potential pathogens involved in wound infections with pulsed electric fields (PEFs) and antibiotics (mix of penicillin, streptomycin, and nystatin).
Results: Using a Taguchi experimental design in vitro, we found that, under similar electric field strengths, the pulse duration is the most important parameter for P. aeruginosa inactivation, followed by the number of pulses and pulse frequency. P. aeruginosa, a potential severe pathogen, is more sensitive than the less pathogenic S. epidermidis to PEF (alone or in combination with antibiotics). Applying 200 pulses with a duration of 60 μs at 2.8 Hz, the minimum electric fields of 308.8 ± 28.3 and 378.4 ± 12.9 V/mm were required to inactive P. aeruginosa and S. epidermidis, respectively. Addition of antibiotics reduced the threshold for minimum electric fields required to inactivate the bacteria.
Innovation: This study provides essential information, such as critical electric field parameters for bacteria inactivation, required for developing in vivo treatment and clinical protocols for using PEF for wound healing.
Conclusion: A combination of PEFs with antibiotics reduces the electric field threshold required for bacteria disinfection. Such an approach simplifies devices required to disinfect large areas of infected wounds.
Keywords: bacterial infection, burn wounds, hurdle technology, pulsed electric fields, electroporation, Pseudomonas aeruginosa PAO1, Staphylococcus epidermidis RP62A
Alexander Golberg, PhD.

Introduction
Wound infection is a stubborn medical and economic problem, which increases hospitalization time and requires more nursing care, additional dressings, and possibly readmission to the hospital and additional surgery.1,2 As a result, wound infection increases treatment costs, and multiple studies have demonstrated the need to increase investments in wound infection prevention to decrease the overall treatment costs in both high- and low-income countries.3–5 The most severe types of infections are invasive bacterial wound infections, which are associated with extreme toxicity, high fever, a hyperdynamic circulatory state, bacteremia, hypotension, and cardiovascular collapse.6 In burn patients, infections remain the major cause of patient death.6,7
Staphylococcus aureus, Pseudomonas aeruginosa, and betahemolytic streptococci are the primary causes of delayed healing and infection in both acute and chronic wounds.8 In addition, the members of a normal skin flora, such as Staphylococcus epidermidis, were shown to slow down the wound-healing process.9–11 Moreover, it successfully forms biofilms on medical devices and implants, leading to additional infection concern.12–15 Based on previous exhaustive work on bacteria inactivation in food systems16–18 and previous work on the low-voltage constant electric fields to facilitate the delivery of antibiotics to otherwise recalcitrant biofilms,19–24 we proposed to use high-voltage but pulsed electric fields (PEFs) for wound and implant disinfection.25–27
PEF is an emerging medical technology28 currently used for tissue ablation by irreversible electroporation,29 cancer treatment by electrochemotherapy,30 and gene electrotransfer.31 The effect of PEF on cells can be explained by the induced change in biological membrane permeability through a phenomenon known as electroporation.32 Current consensus describes electroporation as the formation of aqueous pores in the lipid bilayer that enable molecular transport.32–34 The theory of aqueous pore formation, based on thermodynamics, describes the formation of aqueous pores as started by the penetration of water molecules into the lipid bilayer of the membrane, which leads to the reorientation of adjacent lipids with their polar headgroups toward these water molecules.33 In wound healing, PEF has been used in in vivo experimental models for skin rejuvenation,35 scar treatment,36 and genetic engineering to enhance the expression of healing-enhancing factors.37–39 Our recent in vivo study that examined the normal skin response to PEF in vivo showed complete scarless regeneration in rats.40
Using PEF alone, we showed a reduction of Acinetobacter baumannii in vivo,25,26 and elimination of P. aeruginosa ATCC 19660 (strain 180) biofilm on the surgical mesh.27 However, the application of PEF alone might not be sufficient for clinical applications. Previous studies in food disinfection involving PEF have suggested that a combination of two or more methods simultaneously, known as hurdle technologies,41 could achieve higher disinfection efficiency than each of the methods alone.42,43 Similar results describing the combined effects of PEF and oxacillin were recently shown for the inactivation of blood-isolated S. aureus, Streptococcus pyogenes, Escherichia coli, P. aeruginosa, and Candida albicans in liquid.44
The goal of this work is to determine the electric field thresholds required to inactivate P. aeruginosa, a common wound pathogen, and S. epidermidis, a drug-resistant bacteria that is a part of normal skin flora, but which is considered an “opportunistic pathogen”10 that can slow down the healing process and demonstrates drug resistance similar to that of S. aureus.45 Using a concentric electrode system that allows single-step determination of the critical electric fields,46 we determined the thresholds of electric fields when electric fields were applied alone or in combination with an antibiotics mix in different concentrations in vitro.
Using the Taguchi robust experimental design approach,47 we determined the relative importance of each of the PEF parameters on disinfection efficiency. First, the application of PEF in vivo induces immune system responses,48 which are complex and can interfere with the effect of antibiotics. Second, in vitro experiments allowed us to test a large number of PEF parameters so that we could investigate their impacts and optimize their values. Using our in vitro setup, we followed the 3R principle49 of reduction and significantly reduced the number of animals that would be required to identify the impact of the each of the experimental parameters on inactivation levels of bacteria in future studies. The demonstration of the hurdle approach for inactivation of potential wound pathogens is expected to overcome the problems associated with the current pharmacologic or only physical means of disinfecting wounds.
Clinical Relevance
Currently, local wound infection is addressed by early surgical debridement and skin grafting,8 topical and prophylactic antibiotics,8 an enzymatic detachment of biofilms,50 immunoprophylaxis and immunotherapy,51 photodynamic therapy,52 hyperbaric oxygen therapy,8 or vacuum-assisted wound closure.8 However, in many cases, especially with the emergence of multidrug-resistant strains,53,54 these methods are not efficient, and therefore, additional means of disinfecting wounds are clearly needed. Furthermore, P. aeruginosa and S. epidermidis can cause deep infections in many tissue sites, including joints,10,55 lung, heart,56 liver,57 and implants.10 To address these problems, we recently proposed to use non-thermal, high-voltage PEF technology, previously found to be effective for wounds and surgical mesh disinfection.25–27
Materials and Methods
Bacterial culture
P. aeruginosa PAO1 and S. epidermidis RP62A (RP62A kindly provided by Prof. Micha Fridman, School of Chemistry, Faculty of Exact Sciences, Tel Aviv University) were grown first on electroporation low salt (ELS) media-based solid agar. The ELS media composition was as follows: 0.1 mg/mL NaCl (Merck, Darmstadt, Germany), 0.01 g/mL Bacto-tryptone (Academia, Israel), 0.005 g/mL yeast extract (BD extract of autolyzed yeast, Israel), 0.015 g/mL agar (Bacteriological Agar-Academia, Israel), 0.5 mg/mL glucose-D+ (Sigma-Aldrich, St. Louis, MO), and 0.0239 g/mL HEPES buffer (HEPES 100G-H buffer; Sigma-Aldrich). The reagents were dissolved in the double-distilled water and autoclaved (instrument) for 30 min at 121°C. Each plate was filled with 10 mL ELS media. For starter culture preparation, a single colony was cultured in 2 mL of liquid ELS at conditions of 32°C and 150 rpm for 8 h. One hundred microliters of liquid starter with optical density (OD) 0.22–0.26 (measured using Tecan infinite M200 PRO with 600 nm wave) and pH 7 were spread on solid ELS agar with Dregalski stick and cultivated at 32°C for 8 h before electroporation experiments.
PEF experimental setup for the determination of irreversible electroporation electric field strength threshold with a single step
Concentric ring electroporation as described by Fernand et al. was used.46 The concentric electrode design creates a gradient of disinfection from the center outwards to the periphery. The local electric field strength at each point is described using equation (1) as follows:
 |
where E (V/mm) is field strength, r is distance from the center of the central electrode, ΔV (V) is potential difference between the central and peripheral electrodes, R1 (mm) is the radius of inner electrode, and R2 (mm) is the radius of outer electrode. In this study R1 was 0.75 mm and R2 was 11.95 mm.
Pulses were delivered using a BTX 830 pulse generator (Harvard Apparatus, Inc., Holliston, MA). Currents were measured in vivo using a PicoScope 4224 Oscilloscope with a Pico Current Clamp (60 A, AC/DC) and analyzed with Pico Scope 6 software (Pico technologies, Inc., Cambridgeshire, United Kingdom).
Taguchi robust experimental design to determine the individual impact of a number of pulses, pulse length, and frequency of delivery on minimum electric field strength required to inactivate P. aeruginosa PAO1
The goal in this series of experiments was to determine the effects of PEF parameters of pulse number, duration, and frequency on the minimum strength of electric field (Ec) required to inactivate P. aeruginosa PAO1. The range of PEF parameters and their combinations is large; therefore, to decrease the number of experiments but still allowing to evaluate the impact of each parameter independently, we applied the Taguchi robust design method to the experimental design.58 The key feature of the Taguchi method is the design of the experiment where process factors are tested with orthogonal arrays. We tested the impact of the following range of PEF settings using L9 Taguchi matrix: pulse length of 40, 50, 60 μs; interval between pulses of 350, 400, 450 ms; and pulse number of 100, 150, 200. Supplementary Table S1 summarizes the experiments conducted for the L9 orthogonal Taguchi array needed to determine the individual effects of each of the tested parameters on Ec. At least 12–16 repeats were performed for each experimental condition. Analysis with “minimum the best target” function, the goal of which is to find the smallest Ec at which the bacteria were inactivated,36 was done using Minitab 18 (Minitab, Inc., State College, PA).
Determination of the minimum strength of electric field
The digital image of each experiment was captured with Binocular (Leica M420) and analyzed with Image-J (ver 1.6.0; NIH). rc (mm), the radius from the center where no bacterial growth was observed, was measured at least at four different points. The average of measured radii was taken and used for the calculation of Ec as follows:
 |
where N is number of pulses, tp is duration of a single pulse, and T is interval between pulses. Conversion rate was 90 pixels to 0.2 mm, calibrated with a micrometer with × 5.6 magnification (Leica M420; Leica, Wetzlar, Germany).
Determination of invested energy
Energy, W (J), invested in each treatment was calculated with equation (3).
 |
Hurdle effects of PEF and antibiotics on the inactivation of P. aeruginosa and S. epidermidis
To test the combined hurdle effect and the impact of PEF and antibiotics, we used the following antibiotic mix (Biological Industries 03-032-1C, Cromwell, CT): penicillin (Penicillin G Sodium Salt; 10,000 units/mL); streptomycin (Streptomycin Sulfate; 10 mg/mL); and nystatin (1,250 units/mL). Previous studies have suggested the control of P. aeruginosa with streptomycin59 and nystatin60 and S. aureus with penicillin.61 Two microliters of the diluted antibiotic mixture (1/2 to 1/20 dilution factor) was applied at the spot where the central electrode was positioned. Three replicates were done in the same Petri dish with PEF (1,750 V, 200 pulses, 60 μs, chosen from Taguchi experiments), and three replicates were done with PEF but only with antibiotics. When no PEF treatment was applied in the control samples, the equivalent Ec was calculated with equation (2). The total number of replicates per experimental condition was 6–9 for each dilution.
Statistical analysis
Statistical analysis was done using Minitab18 (Minitab, Inc.), Matlab (ver. 2013; The MathWorks, Inc., Natick, MA), and Excel (ver. 2013; Microsoft Corporation). For randomization, 103 permutation simulations were done. Results show mean and standard deviation. The minimum number of repeats per experimental condition was 15. For the linear regression model developed in this study, we calculated the total relative error (TRE) using equation (4):
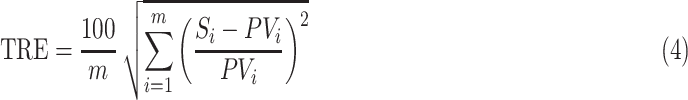 |
where m is number of measurements, Si is measured value, and PVi is predicted value.
Results and Discussion
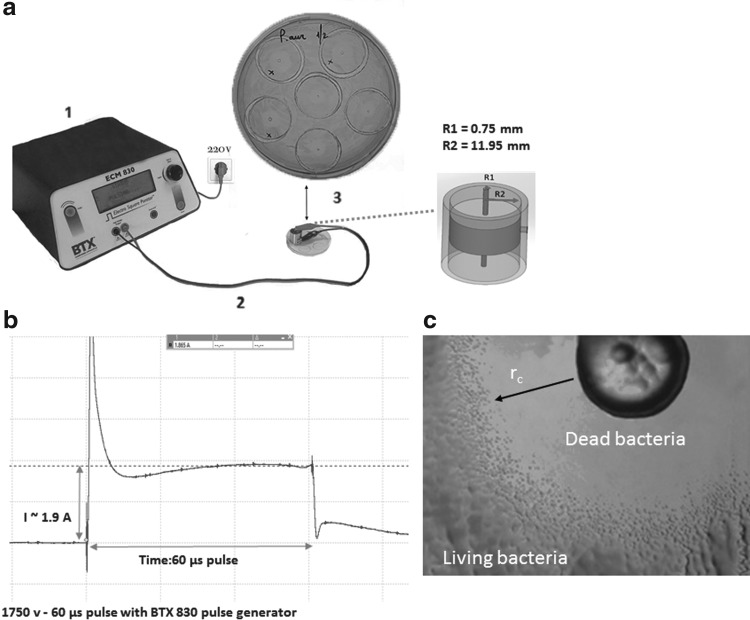
The experimental setup with concentric electrodes for one-step determination of the minimum electric field required for irreversible electroporation of bacteria with PEF is shown in Fig. 1a. The dynamic current of the individual pulse of 60 μs duration is shown in Fig. 1b. Figure 1c shows the PEF effect on the culture of P. aeruginosa. A clean area with dead cells closer to the center is apparent, and unaffected cells remain on the edges, where the strength of the electric field was insufficient to kill the bacteria.
Figure 1.
(a) Experiment setup with concentric electrode electroporation for the determination of critical electric field (Ec) for bacteria inactivation in a single step. (b) The shape of an electric pulse applied to the Petri dish with bacteria. (c) Digital image of the observed disinfected area of Pseudomonas aeruginosa after the application of PEF. PEF, pulsed electric field.
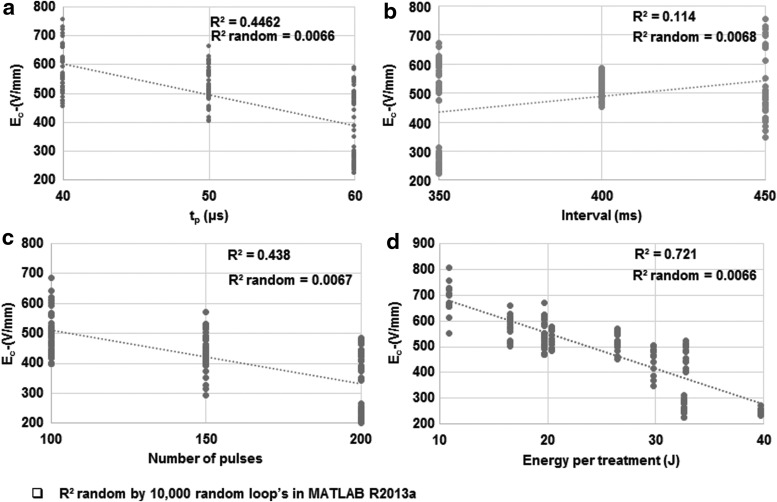
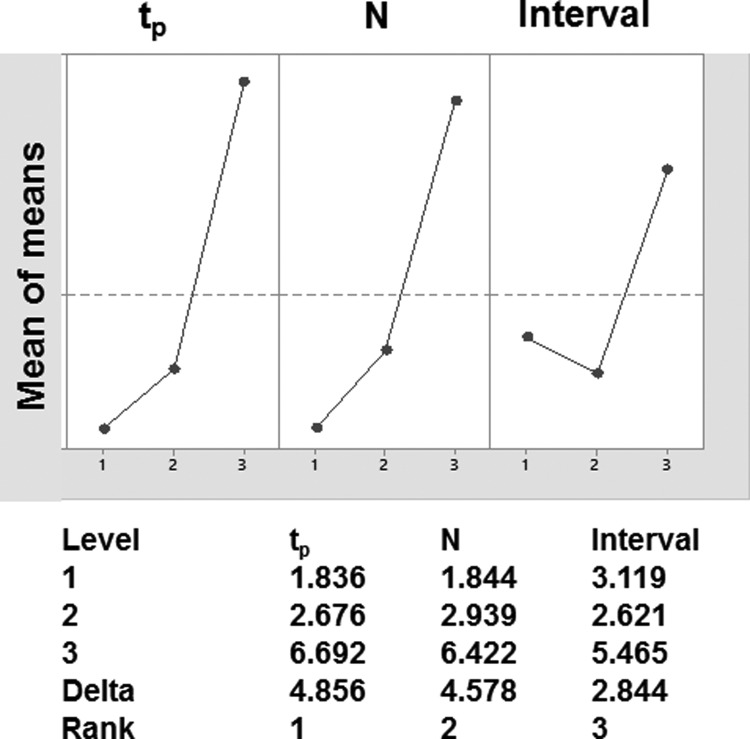
In the tested ranges, increasing pulse duration (tp), increasing number of pulses, and energy invested in the whole treatment decreased Ec (Figs. 2a, c, d and 3 and Table 1). Increasing the pulse interval had almost no effect on Ec (Figs. 2b and 3). In studies using Taguchi orthogonal arrays and the individual parameters of a pulse, pulse duration had the strongest effect on Ec, followed by pulse number (Fig. 3). The interval between pulses had the lowest impact on Ec (Fig. 3). The lowest Ec was observed when 200 pulses of 60 μs duration were delivered with 350 ms interval.
Figure 2.
Individual impacts of PEF parameters on the threshold of electric field required to inactivate P. aeruginosa as done using Taguchi orthogonal array described in Table 2. The impacts of (a) pulse duration, (b) interval between pulses, (c) total number of pulses, and (d) total invested energy in the treatment are shown.
Figure 3.
Analysis of process factor impacts and their ranking of importance on the threshold of electric field required to inactivate P. aeruginosa as done using Taguchi methodology. The top plot shows the impact of change of each of the process factors on Ec. The bottom shows the ranking of each of the parameters.
Table 1.
L9 Taguchi matrix of pulsed electric field inactivation of Pseudomonas aeruginosa
| Experiment Number | Voltage at R1 | Pulse Length (μs) | Number of Pulses | Pulse Interval (ms) | Ec(V/mm) |
|---|---|---|---|---|---|
| 1 | 1,700 | 40 | 100 | 450 | 705.7 ± 65.4 |
| 2 | 1,700 | 50 | 150 | 400 | 552.7 ± 35.6 |
| 3 | 1,700 | 60 | 200 | 350 | 295.3 ± 35.3 |
| 4 | 1,725 | 40 | 150 | 350 | 593.1 ± 52.5 |
| 5 | 1,725 | 50 | 200 | 450 | 478.2 ± 38.5 |
| 6 | 1,725 | 60 | 100 | 400 | 527.4 ± 40.4 |
| 7 | 1,750 | 40 | 200 | 400 | 520.9 ± 34.1 |
| 8 | 1,750 | 50 | 100 | 350 | 581.8 ± 43.8 |
| 9 | 1,750 | 60 | 150 | 450 | 452.9 ± 49.1 |
Using a multivariable regression approach, we constructed a linear regression model to describe the dependence of Ec (V/mm) of P. aeruginosa on the tested parameters of the electric pulse (n = 150) as in equation (5),
 |
where 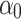 is intercept, and
is intercept, and 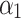 ,
, 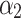 ,
, 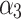 are linear coefficients of pulse duration (tp, μs), N is number of pulses, I is interval between pulses (ms), and ɛ is model error.
are linear coefficients of pulse duration (tp, μs), N is number of pulses, I is interval between pulses (ms), and ɛ is model error.
The determined coefficients were: 962.15 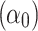 , −9.55
, −9.55 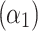 , −1.89
, −1.89 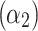 , 0.77
, 0.77 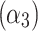 , indicating that tp is the strongest predictor among measured parameters in the tested ranges of Ec. The model p-value was <2.2 10−16, the adjusted R2 was 0.868, and the corresponding TRE was 0.78%.
, indicating that tp is the strongest predictor among measured parameters in the tested ranges of Ec. The model p-value was <2.2 10−16, the adjusted R2 was 0.868, and the corresponding TRE was 0.78%.
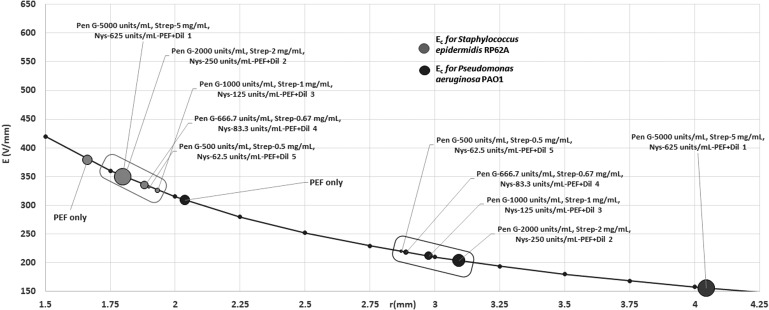
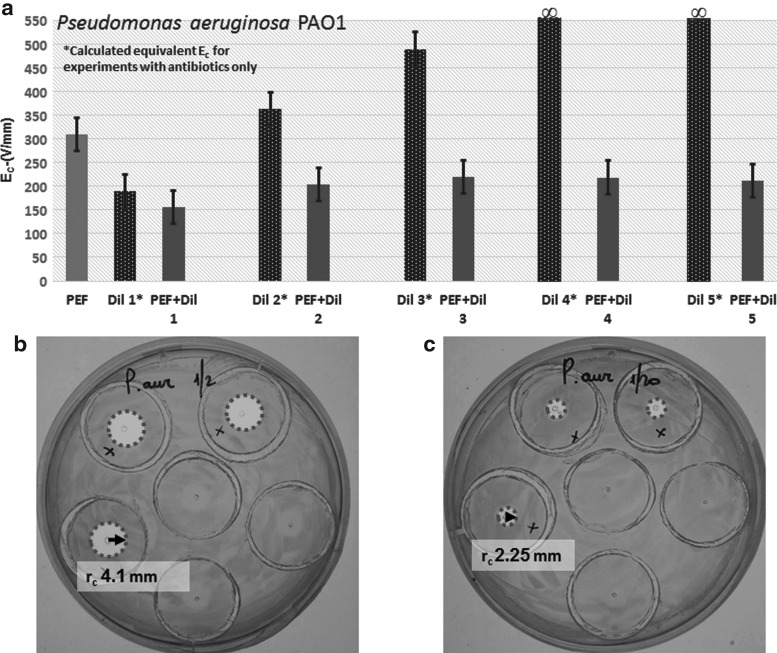
Next, using the PEF protocol with the smallest Ec (tp = 60 μs, I = 350 ms, N = 200), we investigated the combined effect of PEF and antibiotics on P. aeruginosa and S. epidermidis. For P. aeruginosa, the addition of antibiotics reduced Ec in comparison to PEF alone (Figs. 4 and 5 and Table 2). The hurdle effect of PEF and antibiotics was also stronger than the effect of the same dose of antibiotics alone [equivalent Ec was calculated from the inhibition radius when no PEF was applied using the same Eq. (1)] (Fig. 5 and Table 2). For example, Ec was reduced from 308.8 V/mm at PEF-alone treatments to 155.6 V/mm at PEF+Pen G 5,000 units/mL, Strep 5 mg/mL, and Nys 625 units/mL (p = 0.000); at antibiotic mix alone, the equivalent Ec was 189.6 V/mm. Increasing the concentration of antibiotics significantly reduced Ec in the tested range of concentrations (Fig. 5 and Table 2).
Figure 4.
The threshold of electric field strength for the inactivation of P. aeruginosa and Staphylococcus epidermidis by PEF and antibiotics mix. The values of Ec for a specific dose of the antibiotic mix are shown in dots on the electric field curve that starts from the center of the concentric system. r is distance from the central point. PEF parameters: pulse duration = 60 μs; interval between pulses = 350 ms; number of pulses = 200. For each point, N = 48 for P. aeruginosa and 36 for S. epidermidis.
Figure 5.
(a) The threshold of electric field, Ec, required to inactivate P. aeruginosa by PEF and antibiotics. For antibiotic mix treatment only, we calculated the equivalent Ec from the observed radius of inactivation. Dil 1–5 are as described in Table 3. n = 63. (b) Digital image of a plate with PEF: 1,750 V, 200 pulses, 60 μs pulse length, 350 ms pulse interval combined with Pen G 5,000 units/mL, Strep 5 mg/mL, Nys 625 units/mL (Dil 1). (c) Digital image of a plate with PEF: 1,750 V, 200 pulses, 60 μs pulse length, 350 ms pulse interval combined with Pen G 500 units/mL, Strep 0.5 mg/mL, Nys 62.5 units/mL (Dil 5). Dil, dilution; Nys, Nystatin; Pen, Penicillin; Strep, Streptomycin.
Table 2.
Hurdle effects of pulsed electric field and antibiotics on P. aeruginosa inactivation
| Ec/p-Value | PEF Alone | Dil 1 | Dil 2 | Dil 3 | Dil 4 | Dil 5 | PEF+Dil 1 | PEF+Dil 2 | PEF+Dil 3 | PEF+Dil 4 | PEF+Dil 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEF alone | 308.86 V/mm/p = 0.000 | ||||||||||
| Dil 1 | p = 0.000 | 189.65 V/mm* | |||||||||
| Dil 2 | p = 0.004 | p = 0.000 | 362.99 V/mm* | ||||||||
| Dil 3 | p = 0.025 | p = 0.003 | p = 0.015 | 488.93 V/mm* | |||||||
| Dil 4 | ∞**/p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | ∞** | ||||||
| Dil 5 | ∞**/p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | — | ∞** | |||||
| PEF+Dil 1 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | 155.67 V/mm | ||||
| PEF+Dil 2 | p = 0.000 | p = 0.022 | p = 0.000 | p = 0.002 | p = 0.000 | p = 0.000 | p = 0.000 | 203.53 V/mm | |||
| PEF+Dil 3 | p = 0.000 | p = 0.022 | p = 0.000 | p = 0.002 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.013 | 219.07 V/mm | ||
| PEF+Dil 4 | p = 0.001 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.002 | p = 0.112 | p = 0.282 | 217.78 V/mm | |
| PEF+Dil 5 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.022 | p = 0.062 | p = 0.118 | 211.34 V/mm |
PEF parameters: pulse duration = 60 μs; interval between pulses = 350 ms; number of pulses = 200; n = 63. Dil 1: Pen G 5,000 units/mL, Strep 5 mg/mL, Nys 625 units/mL; Dil 2: Pen G 2,000 units/mL, Strep 2 mg/mL, Nys 250 units/mL; Dil 3: Pen G 1,000 units/mL, Strep 1 mg/mL, Nys 125 units/mL; Dil 4: Pen G 666.7 units/mL, Strep 0.67 mg/mL, Nys 83.3 units/mL; Dil 5: Pen G 500 units/mL, Strep 0.5 mg/mL, Nys 62.5 units/mL.
Equivalent to Ec.
∞ no delay effect.
—, no value; Dil, dilution; Nys, Nystatin; PEF, pulsed electric field; Pen, Penicillin; Strep, Streptomycin.
Gray shading shows no significant difference.
In comparison, in our preliminary work, we showed that for tp = 50 μs, I = 500 ms, and N = 150, the Ec for P. aeruginosa biofilms on surgical mesh was 235 ± 6.1 V/mm; and for N = 300 it was 121 ± 14 V/mm.27 Interestingly, low-voltage (5 V), high-frequency (200 Hz) electric fields were shown to prevent biofilm formation of P. aeruginosa.62 Future combinations of high- and short-voltage fields could provide an effective protection from P. aeruginosa biofilms by simultaneous killing and developmental prevention.63 It is important to mention that an 8-h culture of P. aeruginosa was treated in this work. This may be a limitation, since the treatment of old, stable cultures may be required to truly simulate clinical infections. Additional work on the impact of culture age on PEF resistance is warranted.
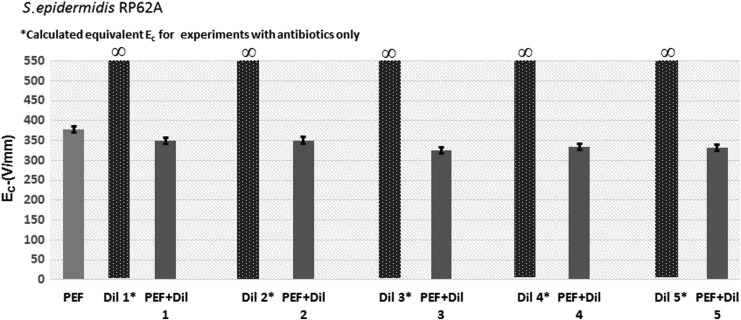
For S. epidermidis, the addition of antibiotics reduced Ec in comparison with PEF alone (Figs. 3 and 6 and Table 3). The hurdle effect of PEF and antibiotics was also stronger than the effect of the same dose of antibiotics alone [equivalent Ec was calculated from the inhibition radius when no PEF was applied using the same Eq. (2)]. For example, Ec was reduced from 378.41 V/mm at PEF-alone treatments to 348.51 V/mm at PEF+Pen G 5,000 units/mL, Strep 5 mg/mL, and Nys 625 units/mL (p = 0.020). Using antibiotic mix alone, the equivalent Ec was ∞ V/mm (Table 3). However, unlike P. aeruginosa cultures, increasing the concentration of antibiotics significantly did not significantly reduce Ec in most of the tested concentration ranges for S. epidermidis (Table 3). This can be potentially explained by the fact that we may have reached drug saturation and membrane damage, and hence effectiveness on this bacteria. Stronger field strengths should be tested in future studies to determine if additional synergistic effects are possible. To the best of our knowledge, there are no previous reports describing the inactivation of S. epidermidis with PEF and electroporation technology. Eradicating S. epidermidis with PEF alone or in combination with antibiotics, as shown in this study, could provide a new direction for treating wounds64,65 and disinfection of medical equipment,14 where biofilms are problematic and lead to infections.
Figure 6.
The threshold of electric field, Ec, required to inactivate S. epidermidis by PEF and antibiotics. For antibiotic mix treatment only, we calculated the equivalent Ec from the observed radius of inactivation. n = 36. Dil 1–5 are as described in Table 4.
Table 3.
Hurdle effects of pulsed electric field and antibiotics on S. epidermidis inactivation
| Ec/p-Value | PEF Alone | Dil 1 | Dil 2 | Dil 3 | Dil 4 | Dil 5 | PEF+Dil 1 | PEF+Dil 2 | PEF+Dil 3 | PEF+Dil 4 | PEF+Dil 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEF alone | 378.41 V/mm/p = 0.000 | ||||||||||
| Dil 1 | p = 0.000 | ∞* | |||||||||
| Dil 2 | p = 0.000 | — | ∞* | ||||||||
| Dil 3 | p = 0.000 | — | — | ∞* | |||||||
| Dil 4 | p = 0.000 | — | — | — | ∞* | ||||||
| Dil 5 | p = 0.000 | — | — | — | — | ∞* | |||||
| PEF+Dil 1 | p = 0.020 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | 348.51 V/mm | ||||
| PEF+Dil 2 | p = 0.010 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.438 | 350.12 V/mm | |||
| PEF+Dil 3 | p = 0.001 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.025 | p = 0.033 | 325.67 V/mm | ||
| PEF+Dil 4 | p = 0.003 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.013 | p = 0.039 | p = 0.226 | 334.04 V/mm | |
| PEF+Dil 5 | p = 0.006 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.000 | p = 0.039 | p = 0.024 | p = 0.355 | p = 0.281 | 331.10 V/mm |
PEF parameters: pulse duration = 60 μs; interval between pulses = 350 ms; number of pulses = 200; n = 36. Dil 1: Pen G 5,000 units/mL, Strep 5 mg/mL, Nys 625 units/mL; Dil 2: Pen G 2,000 units/mL, Strep 2 mg/mL, Nys 250 units/mL; Dil 3: Pen G 1,000 units/mL, Strep 1 mg/mL, Nys 125 units/mL; Dil 4: Pen G 666.7 units/mL, Strep 0.67 mg/mL, Nys 83.3 units/mL; Dil 5: Pen G 500 units/mL, Strep 0.5 mg/mL, Nys 62.5 units/mL.
Equivalent to Ec.
∞ no delay effect.
—, no value.
Gray shading shows no significant difference.
Using a combination of PEF with antibiotics, we showed that a much lower dose of antibiotics is needed to inactivate both organisms when PEF is used. This suggests that a combined therapy where antibiotics are assisted by PEF, as adjuvant, could dramatically reduce the volumes of used antibiotics, contributing to the minimization of antibiotic resistance.66,67 Previous studies in food preservation also showed the hurdle effects of PEF with various antibiotic compounds.68,69 We found that P. aeruginosa is more sensitive to PEF alone or in combination with antibiotics than S. epidermidis. These findings are important as P. aeruginosa is a much more infectious agent (25% patients with surgical wound infections had P. aeruginosa vs. 7% who had S. epidermidis70), suggesting that milder protocols would be used more often in clinical applications.
The observed higher sensitivity of P. aeruginosa to PEF than S. epidermidis could partially be explained by the difference in cell size and shape that affects induced transmembrane potential.71 Previous work has shown that rod cells experience 15% higher induced transmembrane potential than elliptical cells.72 P. aeruginosa cells are rods of 0.3–0.5 × 3 μm,73 and the S. epidermidis shape is closer to spheroidal with 1–2 μm radius.74 Previous theoretical analysis of the induced transmembrane voltage (equations 6–8 in Ref.71) suggests that P. aeruginosa cells will experience unequal induced transmembrane potential depending on the angle between surface vector of the membrane and external electric field lines. A higher induced transmembrane voltage develops on the cell membrane when the long side of the rod is orthogonal to the lines of external electric fields.71 At the same time, spherical cells of S. epidermidis will experience equal induced transmembrane potential in all parts of the membrane. These differences imply that larger areas of the P. aeruginosa cell surface are exposed to larger induced critical transmembrane potential than surface areas of S. epidermidis.71 We previously showed, in the example of Listeria monosetogenes, that large electroporated fractions of the cell membranes are correlated with cell death.75
As in this study, we used a concentric electrode setup, where the disinfected area around the central electrode (Fig. 1a) shows the potential disinfected areas for actual disinfection applications around a single needle (Table 4). Increasing the drug concentration increased the treated areas for P. aeruginosa, but had no significant effect on S. epidermidis disinfected areas. This could probably be explained by differences in mechanisms of bacterial resistance to drugs76 and differences in membrane structure,77 which impacts the PEF. Similar differences were shown for resistance to cold plasma.78 Such an approach could address the issue of large infected surfaces if a multi-needle device is developed. Previous studies have shown that electrode shapes with a single needle could create a point of singularity that create high electric fields without electrolysis around the electrodes.79–81 Multi-needle electrode configurations, previously developed for precise tissue volume ablation and electrochemotherapy, could be used for large surface disinfection with the parameters found in this study.82–85
Table 4.
Disinfected areas with a single-needle electrode area
| P. aeruginosa (mm2 ± SD) | S. epidermidis (mm2 ± SD) | |
|---|---|---|
| PEF | 13.30 ± 2.24 | 8.71 ± 0.61 |
| PEF+Dil 1 | 53.87 ± 15.47 | 10.28 ± 0.81 |
| PEF+Dil 2 | 30.24 ± 3.16 | 10.20 ± 0.96 |
| PEF+Dil 3 | 26.32 ± 4.24 | 11.81 ± 1.31 |
| PEF+Dil 4 | 26.55 ± 3.72 | 11.18 ± 0.82 |
| PEF+Dil 5 | 27.98 ± 2.34 | 11.47 ± 1.52 |
PEF parameters: pulse duration = 60 μs; interval between pulses = 350 ms; number of pulses = 200; n = 48 for P. aeruginosa and n = 36 for S. epidermidis. Dil 1: Pen G 5,000 units/mL, Strep 5 mg/mL, Nys 625 units/mL. Dil 2: Pen G 2,000 units/mL, Strep 2 mg/mL, Nys 250 units/mL. Dil 3: Pen G 1,000 units/mL, Strep 1 mg/mL, Nys 125 units/mL. Dil 4: Pen G 666.7 units/mL, Strep 0.67 mg/mL, Nys 83.3 units/mL. Dil 5: Pen G 500 units/mL, Strep 0.5 mg/mL, Nys 62.5 units/mL.
This in vitro study allowed us to determine the effective protocol for bacterial inactivation by PEF alone or in combination with antibiotics. Our previous in vivo work on burn disinfection25,26 showed the feasibility for the use of PEF alone in small animal models. Further translation of the PEF to wound healing clinics will require detailed safety studies as PEF will affect both bacteria and host cells. Studies on irreversible electroporation safety in humans have demonstrated that the procedure is safe,86,87 especially if the delivery of pulses is electrocardiographically synchronized.88 Pain studies on patients with deep-tissue tumors showed no difference in comparison with other ablation methods.89,90 In addition, skin DNA vaccination with PEF in a pain study reported that the procedure is well tolerated.91 However, it is important to note that the parameters used in our studies have not been tested for pain in patients. Although we have recently demonstrated a full regeneration of normal rat skin ablated by PEF,40 human skin is different, and further studies on normal or wounded skin responses to PEF in humans are needed. Moreover, the effect of the rapid release of bacterial content in tissues after PEF on procedure safety is still to be investigated.
Furthermore, we and others have shown that PEF cell inactivation is not a deterministic, but rather a statistical event.92,93 Numerous previous works on bacteria inactivation in the food industry led to the development of a function, which describes bacteria inactivation levels as a function of process parameters.93,94 This implies that complete, 100%, kill of bacteria by PEF alone is not expected, and additional effects either from activated immune system responses or antibiotics are needed.
The important still open question for future studies is the role of the survived bacteria in the wound-healing process and if these bacteria could develop resistance to PEF. A previous study that used PEF to eliminate Pseudomonas putida in the wastewater has shown that the inactivation rate (percentage of survived bacteria) remained constant over 30 generations when each generation was grown from the survival fraction of the PEF-treated culture.95 The fraction of bacteria could survive because of the natural variance in the membrane structure. We showed previously that a variation in membrane surface charge leads to a variance in the bacteria survival ratio.75 One of the approaches to keep bacteria concentration lower than the level that might lead to an abnormal healing could be the intermittent delivery of PEF treatment, shown by us to be effective in water and food systems.63,96 Although, in these previous studies, we did not find increased bacteria resistance to PEF with treatment cycles,63,96 additional tests with a much larger number of generations are needed to investigate the long-term impact of PEF on bacteria resistance.
Innovation
Although the burden of wound infection is a major clinical and economical problem, no single approach to date has been found to be effective in preventing deep infection and biofilm formation in infected patients.97 We found that the combination of PEF with antibiotics decreases the minimum threshold required to inactivate bacteria. In addition, we determined the parameters needed to disinfect specific areas with a single electrode by PEF alone or in combination with antibiotics. Larger disinfection areas were achieved using a combined approach than by antibiotics alone, suggesting that this approach could reduce the overuse of antibiotics that might lead to the emergence of antibiotic-resistant strains.
Key Findings.
PEFs inactivate P. aeruginosa PAO1 and S. epidermidis RP62A.
Combination of PEFs with antibiotics reduces the threshold of the electric field required for inactivation.
Combination of PEFs with antibiotics increases the disinfection radius for a single-point electrode.
Supplementary Material
Acknowledgments and Funding Sources
The authors acknowledge Bi-National USA-Israel Science Foundation (BSF) for the support of this study (BSF grant no. 2015286). The study was also partially funded by the NJ Commission on spinal cord research (grant no. CSCR17ERG006).
Abbreviations and Acronyms
- ΔV
potential difference between the central and peripheral electrodes (V)
- E
electric field strength (V/mm)
- Ec
critical electric field required for bacteria inactivation (V/mm)
- ELS
electroporation low salt
- N
number of pulses
- Nys
nystatin
- PEF
pulsed electric field
- Pen
penicillin
- R1
radius of the inner electrode (mm)
- R2
radius of the outer electrode (mm)
- rc
critical radius with no bacteria growth (mm), radius of inactivation
- Strep
streptomycin
- p
single pulse duration (μs)
- TRE
total relative error
Author Disclosure and Ghostwriting
No competing financial interests exist. The authors listed expressly wrote the content of this article. No ghostwriters were used to write this article.
About the Authors
Andrey Ethan Rubin, BSc, is a master's student at the PSEES. He is a biologist working in the interface of sciences, technology, and engineering to advance human health. Osman Berk Usta, PhD, is an assistant professor of surgery at HMS. His work lies at the intersection between micro-tissue engineering, biopreservation, and computational modeling. Rene Schloss, PhD, is an assistant research professor at Rutgers University. Her research is in the field of inflammation and wound healing. Martin Yarmush, MD, PhD, is a professor of biomedical engineering at Rutgers University, and director of the Center for Engineering in Medicine at HMS. His research is in the fields of metabolic engineering, wound healing, and technology development for critical medical applications. Alexander Golberg, PhD, is a senior lecturer at the PSEES, whose major research interests are in the development of new technologies for human health with a specific emphasis on burns and wound healing. He is the recipient of the Robert B. Lindberg Award from American Burn Association in 2015 for the development of electroporation and pulsed electric field technologies for burn wound healing.
References
- 1. Wenzel RP. Minimizing surgical-site infections. N Engl J Med 2010;3621:75–77 [DOI] [PubMed] [Google Scholar]
- 2. Urban JA. Cost analysis of surgical site infections. Surg Infect (Larchmt) 2006;7 (Suppl 1):S19–S22 [DOI] [PubMed] [Google Scholar]
- 3. Badia JM, Casey AL, Petrosillo N, et al. Impact of surgical site infection on healthcare costs and patient outcomes: a systematic review in six European countries. J Hosp Infect 2017;96:1–15 [DOI] [PubMed] [Google Scholar]
- 4. Broex ECJ, van Asselt ADI, Bruggeman CA, et al. Surgical site infections: how high are the costs? J Hosp Infect 2009;72:193–201 [DOI] [PubMed] [Google Scholar]
- 5. Allegranzi B, Nejad SB, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet 2011;377:228–241 [DOI] [PubMed] [Google Scholar]
- 6. Sheridan RL. Burns: A Practical Approach to Immediate Treatment and Long-Term Care. London: Manson Publishing, 2012. [Google Scholar]
- 7. Church D, Elsayed S, Reid O, et al. Burn wound infections. Clin Microbiol Rev 2006;19:403–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bowler PG, Duerden BI, Armstrong DG. Wound microbiology and associated approaches to wound management. Clin Microbiol Rev 2001;14:244–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vuong C, Otto M. Staphylococcus epidermidis infections. Microbes Infect 2002;4:481–489 [DOI] [PubMed] [Google Scholar]
- 10. Otto M. Staphylococcus epidermidis—the “accidental” pathogen. Nat Rev Microbiol 2009;7:555–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blum RA, Rodvold KA. Recognition and importance of Staphylococcus epidermidis infections. Clin Pharm 1987;6:464–475 [PubMed] [Google Scholar]
- 12. Vadyvaloo V, Otto M. Molecular genetics of Staphylococcus epidermidis biofilms on indwelling medical devices. Int J Artif Organs 2005;28:1069–1078 [DOI] [PubMed] [Google Scholar]
- 13. Chessa D, Ganau G, Spiga L, et al. Staphylococcus aureus and Staphylococcus epidermidis virulence strains as causative agents of persistent infections in breast implants. PLoS One 2016;11:e0146668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. McCann MT, Gilmore BF, Gorman SP. Staphylococcus epidermidis device-related infections: pathogenesis and clinical management. J Pharm Pharmacol 2008;60:1551–1571 [DOI] [PubMed] [Google Scholar]
- 15. Vuong C, Kocianova S, Yao Y, et al. Increased colonization of indwelling medical devices by quorum-sensing mutants of Staphylococcus epidermidis in vivo. J Infect Dis 2004;190:1498–1505 [DOI] [PubMed] [Google Scholar]
- 16. Golberg A, Fischer J, Rubinsky B. The use of irreversible electroporation in Food Preservation In: Rubinsky B, ed. Irreversible Electroporation. Basel, Switzerland: Springer, 2010:273–312 [Google Scholar]
- 17. FDA. Kinetics of microbial inactivation for alternative food processing technologies—pulsed electric fields. https://www.fda.gov/downloads/food/foodborneillnesscontaminants/ucm545175.pdf(last accessed November282018).
- 18. Reineke K, Schottroff F, Meneses N, et al. Sterilization of liquid foods by pulsed electric fields-an innovative ultra-high temperature process. Front Microbiol 2015;6:400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Del Pozo JL, Rouse MS, Patel R. Bioelectric effect and bacterial biofilms. A systematic review. Int J Artif Organs 2008;31:786–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shirtliff ME, Bargmeyer A, Camper AK. Assessment of the ability of the bioelectric effect to eliminate mixed-species biofilms. Appl Environ Microbiol 2005;71:6379–6382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Costerton JW, Ellis B, Lam K, et al. Mechanism of electrical enhancement of efficacy of antibiotics in killing biofilm bacteria. Antimicrob Agents Chemother 1994;38:2803–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jass J, Costerton JW, Lappin-Scott HM. The effect of electrical currents and tobramycin on Pseudomonas aeruginosa biofilms. J Ind Microbiol 1995;15:234–242 [DOI] [PubMed] [Google Scholar]
- 23. Barki KG, Das A, Dixith S, et al. Electric field based dressing disrupts mixed-species bacterial biofilm infection and restores functional wound healing. Ann Surg 2017. DOI: 10.1097/SLA.0000000000002504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jaideep B, Piya DG, Sashwati R, et al. Silver-zinc redox-coupled electroceutical wound dressing disrupts bacterial biofilm. PLoS One 2015;10:e0119531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Golberg A, Broelsch GF, Vecchio D, et al. Pulsed electric fields for burn wound disinfection in a murine model. J Burn Care Res 2014;36:7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Golberg A, Broelsch GF, Vecchio D, et al. Eradication of multidrug-resistant A. baumannii in burn wounds by antiseptic pulsed electric field. Technology 2014;2:153–160 [PMC free article] [PubMed] [Google Scholar]
- 27. Khan SI, Blumrosen G, Vecchio D, et al. Eradication of multidrug-resistant pseudomonas biofilm with pulsed electric fields. Biotechnol Bioeng 2016;113:643–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yarmush ML, Golberg A, Serša G, et al. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng 2014;16:295–320 [DOI] [PubMed] [Google Scholar]
- 29. Davalos RV, Mir LM, Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng 2005;33:223–231 [DOI] [PubMed] [Google Scholar]
- 30. Okino M, Mohri H. Effects of a high-voltage electrical impulse and an anticancer drug on in vivo growing tumors. Jpn J Cancer Res 1987;72:1319–21 [PubMed] [Google Scholar]
- 31. Nomura M, Nakata Y, Inoue T, et al. In vivo induction of cytotoxic T lymphocytes specific for a single epitope introduced into an unrelated molecule. J Immunol Methods 1996;193:41–49 [DOI] [PubMed] [Google Scholar]
- 32. Kotnik T, Kramar P, Pucihar G, et al. Cell membrane electroporation—part 1: the phenomenon. IEEE Electr Insul Mag 2012;28:14–23 [Google Scholar]
- 33. Weaver JC, Chizmadzhev YA. Theory of electroporation: a review. Bioelectrochem Bioenerg 1996;41:135–160 [Google Scholar]
- 34. Spugnini EP, Arancia G, Porrello A, et al. Ultrastructural modifications of cell membranes induced by “electroporation” on melanoma xenografts. Microsc Res Tech 2007;70:1041–1050 [DOI] [PubMed] [Google Scholar]
- 35. Golberg A, Khan S, Belov V, et al. Skin rejuvenation with non-invasive pulsed electric fields. Sci Rep 2015;5:10187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Golberg A, Villiger M, Khan S, et al. Preventing scars after injury with partial irreversible electroporation. J Invest Dermatol 2016;136:2297–2304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yao F, Eriksson E. Gene therapy in wound repair and regeneration. Wound Repair Regen 2000;8:443–451 [DOI] [PubMed] [Google Scholar]
- 38. Kos S, Vanvarenberg K, Dolinsek T, et al. Gene electrotransfer into skin using noninvasive multi-electrode array for vaccination and wound healing. Bioelectrochemistry 2017;114:33–41 [DOI] [PubMed] [Google Scholar]
- 39. Rezende FC, Gomes HC, Lisboa B, et al. Electroporation of vascular endothelial growth factor gene in a unipedicle transverse rectus abdominis myocutaneous flap reduces necrosis. Ann Plast Surg 2010;64:242–246 [DOI] [PubMed] [Google Scholar]
- 40. Golberg A, Villiger M, Felix Broelsch G, et al. Skin regeneration with all accessory organs following ablation with irreversible electroporation. J Tissue Eng Regen Med 2017. DOI: 10.1002/term.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Arroyo C, Lyng JG. Pulsed electric fields in hurdle approaches for microbial inactivation. In: Miklavcic D, ed. Handbook of Electroporation. Berlin, Germany: Springer International, 2017:1–30 [Google Scholar]
- 42. Álvarez I, Heinz V. Hurdle technology and the preservation of food by pulsed electric fields. In: Lelieveld HLM, Notermans S, de Haan SWH, eds. Food Preservation by Pulsed Electric Fields: From Research to Application. Sawston, United Kingdom: Woodhead Publishing, 2007:165–177 [Google Scholar]
- 43. Noci F, Riener J, Walkling-Ribeiro M, et al. Ultraviolet irradiation and pulsed electric fields (PEF) in a hurdle strategy for the preservation of fresh apple juice. J Food Eng 2008;85:141–146 [Google Scholar]
- 44. Korem M, Goldberg NS, Cahan A, et al. Clinically applicable irreversible electroporation for eradication of micro-organisms. Lett Appl Microbiol 2018;67:15–21 [DOI] [PubMed] [Google Scholar]
- 45. Campoccia D, Montanaro L, Baldassarri L, et al. Antibiotic resistance in Staphylococcus aureus and Staphylococcus epidermidis clinical isolates from implant orthopedic infections. Int J Artif Organs 2005;28:1186–1191 [DOI] [PubMed] [Google Scholar]
- 46. Fernand F, Rubinsky L, Golberg A, et al. Variable electric fields for high throughput electroporation protocol design in curvilinear coordinates. Biotechnol Bioeng 2012;109:2168–2171 [DOI] [PubMed] [Google Scholar]
- 47. Rao RS, Kumar CG, Prakasham RS, et al. The Taguchi methodology as a statistical tool for biotechnological applications: a critical appraisal. Biotechnol J 2008;3:510–523 [DOI] [PubMed] [Google Scholar]
- 48. Al-Sakere B, André F, Bernat C, et al. Tumor ablation with irreversible electroporation. PLoS One 2007;2:e1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kroeger M. How omics technologies can contribute to the “3R” principles by introducing new strategies in animal testing. Trends Biotechnol 2006;24:343–346 [DOI] [PubMed] [Google Scholar]
- 50. Kaplan JB, Ragunath C, Velliyagounder K, et al. Enzymatic detachment of Staphylococcus epidermidis biofilms. Antimicrob Agents Chemother 2004;48:2633–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Van Mellaert L, Shahrooei M, Hofmans D, et al. Immunoprophylaxis and immunotherapy of Staphylococcus epidermidis infections: challenges and prospects. Expert Rev Vaccines 2012;11:319–334 [DOI] [PubMed] [Google Scholar]
- 52. Hashimoto MCE, Prates RA, Kato IT, et al. Antimicrobial photodynamic therapy on drug-resistant Pseudomonas aeruginosa-induced infection. An in vivo study. Photochem Photobiol 2012;88:590–595 [DOI] [PubMed] [Google Scholar]
- 53. Nolff MC, Reese S, Fehr M, et al. Assessment of wound bio-burden and prevalence of multi-drug resistant bacteria during open wound management. J Small Anim Pract 2016;57:255–259 [DOI] [PubMed] [Google Scholar]
- 54. Pîrvănescu H, Bălăşoiu M, Ciurea ME, et al. Wound infections with multi-drug resistant bacteria. Chirurgia (Bucur) 2014;109:73–79 [PubMed] [Google Scholar]
- 55. Rogers KL, Fey PD, Rupp ME. Coagulase-negative staphylococcal infections. Infect Dis Clin North Am 2009;23:73–98 [DOI] [PubMed] [Google Scholar]
- 56. Lin T-I, Huang Y-F, Liu P-Y, et al. Pseudomonas aeruginosa infective endocarditis in patients who do not use intravenous drugs: analysis of risk factors and treatment outcomes. J Microbiol Immunol Infect 2014:1–7. DOI: 10.1016/j.jmii.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 57. Bang JH, Jung Y, Cheon S, et al. Pseudomonas aeruginosa bacteremia in patients with liver cirrhosis: a comparison with bacteremia caused by Enterobacteriaceae. BMC Infect Dis 2013;13:332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kacker RN, Lagergren ES, Filliben JJ. Taguchi's orthogonal arrays are classical designs of experiments. J Res Natl Inst Stand Technol 1991;96:577–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Torres-Barceló C, Arias-Sánchez FI, Vasse M, et al. A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages. PLoS One 2014;9:e106628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. May I, Abu-Khdeir M, Blackwood RA. An usual approach to treatment of a case of multidrug resistance Pseudomonas aeruginosa peritonitis: parenteral and intraperitoneal aminoglycosides and parenteral colistin. Infect Dis Rep 2012;4:e36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hagstrand Aldman M, Skovby A, I Påhlman L. Penicillin-susceptible Staphylococcus aureus: susceptibility testing, resistance rates and outcome of infection. Infect Dis 2017;49:454–460 [DOI] [PubMed] [Google Scholar]
- 62. Perez-Roa RE, Tompkins DT, Paulose M, et al. Effects of localised, low-voltage pulsed electric fields on the development and inhibition of Pseudomonas aeruginosa biofilms. Biofouling 2006;22:383–390 [DOI] [PubMed] [Google Scholar]
- 63. Golberg A, Kandel J, Belkin M, et al. Intermittently delivered pulsed electric fields for sterile storage of turbid media. IEEE Trans Plasma Sci 2010;38:3211–3218 [Google Scholar]
- 64. Schierle CF, De La Garza M, Mustoe TA, et al. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound Repair Regen 2009;17:354–359 [DOI] [PubMed] [Google Scholar]
- 65. Wilson APR, Grüneberg RN, Treasure T, et al. Staphylococcus epidermidis as a cause of postoperative wound infection after cardiac surgery: assessment of pathogenicity by a wound‐scoring method. Br J Surg 1988;75:168–170 [DOI] [PubMed] [Google Scholar]
- 66. Lee CR, Cho IH, Jeong BC, et al. Strategies to minimize antibiotic resistance. Int J Environ Res Public Health 2013;10:4274–4305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wright GD. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol 2016;24:862–871 [DOI] [PubMed] [Google Scholar]
- 68. Martín-Belloso O, Sobrino-López A. Combination of pulsed electric fields with other preservation techniques. Food Bioprocess Technol 2011;4:954–968 [Google Scholar]
- 69. Sobrino-López A, Martín-Belloso O. Use of nisin and other bacteriocins for preservation of dairy products. Int Dairy J 2008;18:329–343 [Google Scholar]
- 70. Giacometti A, Cirioni O, Schimizzi AM, et al. Epidemiology and microbiology of surgical wound infections. J Clin Microbiol 2000;38:918–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kotnik T, Pucihar G, Miklavčič D. Induced transmembrane voltage and its correlation with electroporation-mediated molecular transport. J Membr Biol 2010;236:3–13 [DOI] [PubMed] [Google Scholar]
- 72. El-Hag AH, Jayaram SH, Rodríguez-González O, et al. The influence of size and shape of microorganism on pulsed electric field inactivation. IEEE Trans Nanobioscience 2011;10:133–138 [DOI] [PubMed] [Google Scholar]
- 73. Mitik-Dineva N, Wang J, Truong VK, et al. Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus attachment patterns on glass surfaces with nanoscale roughness. Curr Microbiol 2009;58:268–273 [DOI] [PubMed] [Google Scholar]
- 74. Johansson KE. VetBact—culturing bacteriological knowledge for veterinarians. Vet Rec 2014;174:162–164 [DOI] [PubMed] [Google Scholar]
- 75. Golberg A, Rae CS, Rubinsky B. Listeria monocytogenes cell wall constituents exert a charge effect on electroporation threshold. Biochim Biophys Acta 2012;1818:689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Wimmerstedt A, Kahlmeter G. Associated antimicrobial resistance in Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Streptococcus pneumoniae and Streptococcus pyogenes. Clin Microbiol Infect 2008;14:315–321 [DOI] [PubMed] [Google Scholar]
- 77. Ouberai M, El Garch F, Bussiere A, et al. The Pseudomonas aeruginosa membranes: a target for a new amphiphilic aminoglycoside derivative? Biochim Biophys Acta 2011;1808:1716–1727 [DOI] [PubMed] [Google Scholar]
- 78. Mai-Prochnow A, Clauson M, Hong J, et al. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci Rep 2016;6:38610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Troszak GD, Rubinsky B. A theoretical analysis of the feasibility of a singularity-induced micro-electroporation system. PLoS One 2011;6:e18523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Troszak GD, Rubinsky B. Self-powered electroporation using a singularity-induced nano-electroporation configuration. Biochem Biophys Res Commun 2011;414:419–424 [DOI] [PubMed] [Google Scholar]
- 81. Lyu C, Wang J, Powell-Palm M, et al. Simultaneous electroporation and dielectrophoresis in non-electrolytic micro/nano-electroporation. Sci Rep 2018;8:2481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Golberg A, Rubinsky B. Towards electroporation based treatment planning considering electric field induced muscle contractions. Technol Cancer Res Treat 2012;11:189–201 [DOI] [PubMed] [Google Scholar]
- 83. Bommakanti S, Agoramurthy P, Campana L, et al. A simulation analysis of large multi-electrode needle arrays for efficient electrochemotherapy of cancer tissues. In: 2011 Annual Report Conference on Electrical Insulation and Dielectric Phenomena. IEEE, 2011:187–190. DOI: 10.1109/CEIDP.2011.6232628 [DOI] [Google Scholar]
- 84. Campana LG, Dughiero F, Forzan M, et al. A prototype of a flexible grid electrode to treat widespread superficial tumors by means of Electrochemotherapy. Radiol Oncol 2016;50:49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Castiello M, Dughiero F, Scandola F, et al. A new grid electrode for electrochemotherapy treatment of large skin tumors. IEEE Trans Dielectr Electr Insul 2014;21:1424–1432 [Google Scholar]
- 86. Buijs M, van Lienden KP, Wagstaff PG, et al. Irreversible electroporation for the ablation of renal cell carcinoma: a prospective, human, in vivo study protocol (IDEAL phase 2b). JMIR Res Protoc 2017;6:e21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Van Den Bos W, De Bruin DM, Muller BG, et al. The safety and efficacy of irreversible electroporation for the ablation of prostate cancer: a multicentre prospective human in vivo pilot study protocol. BMJ Open 2014;4:e006382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Thomson KR, Cheung W, Ellis SJ, et al. Investigation of the safety of irreversible electroporation in humans. J Vasc Interv Radiol 2011;22:611–621 [DOI] [PubMed] [Google Scholar]
- 89. Li J, Sheng S, Zhang K, et al. Pain analysis in patients with pancreatic carcinoma: irreversible electroporation versus cryoablation. Biomed Res Int 2016;2016:2543026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Sorokin I, Lay AH, Reddy NK, et al. Pain after percutaneous irreversible electroporation of renal tumors is not dependent on tumor location. J Endourol 2017;31:751–755 [DOI] [PubMed] [Google Scholar]
- 91. Wallace M, Evans B, Woods S, et al. Tolerability of two sequential electroporation treatments using MedPulser DNA delivery system (DDS) in healthy adults. Mol Ther 2009;17:922–928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Golberg A, Rubinsky B. A statistical model for multidimensional irreversible electroporation cell death in tissue. Biomed Eng Online 2010;9:13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Peleg M. A model of microbial survival after exposure to pulsed electric fields. J Sci Food Agric 1995;67:93–99 [Google Scholar]
- 94. Peleg M, Normand MD, Damrau E. Mathematical interpretation of dose-response curves. Bull Math Biol 1997;59:747–761 [Google Scholar]
- 95. Gusbeth C, Frey W, Volkmann H, et al. Pulsed electric field treatment for bacteria reduction and its impact on hospital wastewater. Chemosphere 2009;75:228–233 [DOI] [PubMed] [Google Scholar]
- 96. Golberg A. Long-term Listeria monocytogenes proliferation control in milk by intermittently delivered pulsed electric fields, implications for food security in the low-income countries. Technology 2015;3:1–6 26167518 [Google Scholar]
- 97. Barret JP, Herndon DN. Effects of burn wound excision on bacterial colonization and invasion. Plast Reconstr Surg 2003;111:744–750 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.