Abstract
Polycystic ovary syndrome (PCOS) is a common reproductive endocrine disorder characterized by theca cell hyperplasia and excessive androgen production. An increasing body of evidence has pointed to a close association between PCOS and low-grade chronic systemic inflammation. However, the mechanistic basis for this linkage is unknown. Therefore, we evaluated the effects of the inflammatory agents lipopolysaccharide (LPS) and IL-1β on rat theca-interstitial cells (TICs). We found that incubation with either LPS or IL-1β elicited a dose-dependent increase in both TIC viability and androgen production. Using RNA sequencing analysis, we found that both of these inflammatory agents also triggered profound and widespread shifts in gene expression. Using a stringent statistical cutoff, LPS and IL-1β elicited differential expression of 5201 and 5953 genes, respectively. Among the genes upregulated by both LPS and IL-1β were key regulatory genes involved in the cholesterol and androgen biosynthesis pathways, including Cyp17a1, Cyp11a1, Hsd3b, and Hmgcr. This provides a molecular explanation for the mechanism of action of inflammatory agents leading to increased androgen production. Gene ontology and pathway analysis revealed that both LPS and IL-1β regulated genes highly enriched for many common functions, including the immune response and apoptosis. However, a large number of genes (n = 2222) were also uniquely regulated by LPS and IL-1β, indicating that these inflammatory mediators have substantial differences in their mechanism of action. Together, these findings highlight the potential molecular mechanisms through which chronic low-grade inflammation contributes to the pathogenesis of androgen excess in PCOS.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder among women of reproductive age; however, its underlying pathophysiology remains poorly understood. In part, this might be because PCOS is a heterogeneous syndrome, which likely results from a combination of genetic, epigenetic, and environmental factors (1). The key features of PCOS include androgen excess, oligo-ovulation or anovulation, and the appearance of polycystic ovaries on ultrasound imaging (2, 3).
In most women with PCOS, androgen excess is the underlying culprit of the symptoms, including irregular ovulation, hirsutism, and acne (3–6). A leading model explaining PCOS is thecal cell dysfunction, the source of androgen biosynthesis in the human ovary (7). In support of this model, PCOS ovaries exhibit enhanced androgen production via the higher theca cell numbers (8) and overexpression of mRNA for the key genes involved in androgen biosynthesis, such as Cyp17a1 and Cyp11a1, compared with controls (9). Although excessive ovarian androgen production via theca cells contributes to many of the phenotypic manifestations of PCOS (3–6, 10), the underlying cause of androgen excess has yet to be determined.
Chronic low-grade inflammation has also been associated with PCOS, increasing the possibility that inflammation might contribute to the androgen excess in PCOS (11, 12). Several lines of evidence have supported this possibility. First, several clinical trials have revealed that agents with anti-inflammatory properties, such as statins (13–18) and resveratrol (19), improve PCOS symptoms and reduce androgen levels (15, 17–19). The converse is unlikely to be the case, because the administration of androgens, including nonaromatizable 5α-DHT, have been found to reduce, rather than increase, the inflammatory responses (20–23). Second, although PCOS has been associated with known proinflammatory metabolic disorders such as obesity, insulin resistance, and diabetes, the association of PCOS with inflammation has persisted in the absence of these confounders (24, 25). For example, several key markers and mediators of systemic inflammation—including C-reactive protein (26, 27), TNF-α (28) and IL-6 (29)—are elevated in women with PCOS compared with body mass index-matched control subjects. Finally, an integral component of many inflammatory reactions—oxidative stress—is elevated in PCOS (30–32). Increased expression of oxidative stress markers in patients with PCOS have been found to be independent of known confounders such as excess weight, suggesting that oxidative stress is not merely a consequence of obesity and, instead, might exert a causal role in PCOS.
In the present study, we investigated the underlying mechanisms by which inflammation might promote PCOS. We hypothesized that low-grade inflammation will trigger increased androgen production by theca cells in the ovary. This increased androgen level could, in turn, drive the development of PCOS. To test this hypothesis, we evaluated the effects of two well-characterized proinflammatory agents: lipopolysaccharide (LPS) and IL-1β. We chose these particular molecules because they are frequently used to induce low-grade systemic inflammatory responses in both in vitro and in vivo experimental model systems (33–37). LPS is a key pathogenic component of the cell wall of gram-negative bacteria. It induces inflammation primarily via activation of macrophages and intracellular pathways in other cells, leading to the production of proinflammatory cytokines. LPS activates cells by binding to Toll-like receptor 4 (TLR4). This leads to a plethora of responses, including upregulation of the inflammasomes (38) and activation of the MAPK and AKT pathways, with consequent effects on cellular proliferation and apoptosis (39, 40). Inflammasomes are multiprotein complexes, belonging to the innate immune system, responsible for the activation of inflammatory responses via proteolytic processing of proinflammatory cytokines such as IL-1β (38, 41–43). IL-1β is first synthesized as an inactive precursor protein, pro-IL-1β, and cleaved by caspase-1 into active IL-1β as a result of inflammasome activation (38). The ovarian IL-1β system plays a major role in ovulatory processes (44, 45); thus, dysregulation of these inflammatory mediators might disrupt this tightly regulated process contributing to ovulatory disruption and anovulation (46).
When administered at high concentrations, both LPS and IL-1β cause sepsis-like symptoms and can lead to death. However, low doses of these substances mimic subclinical endotoxemia by failing to induce negative regulators of inflammatory pathways, allowing chronic low-grade inflammation to persist (37, 47). We hypothesized that this contributes to PCOS through elevation of androgen production by theca cells. The potential sources of low-grade bacterial endotoxins in women with PCOS include altered gut microbiota, resulting in increased intestinal permeability (48, 49) or the persistence of low-grade chronic infection (50–53). In agreement with our hypothesis, we found that low doses of LPS and IL-1β elicited increased expression of androgen biosynthesis genes in theca-interstitial cells (TICs) and increased androgen production in these cells. These proinflammatory agents also had surprisingly widespread effects on gene expression in TICs, which included not only androgen biosynthesis pathways but also many other biochemical pathways, as revealed by RNA sequencing (RNAseq) analysis. LPS and IL-1β regulated hundreds of the same genes but also regulated distinct sets of genes, consistent with their known overlapping, but distinctive, functions (38–42, 47). Together with these findings, our study has provided a foundation to functionally dissect the effects of inflammation on theca cells and PCOS.
Materials and Methods
Isolation of rat TICs
Female Sprague-Dawley rats, aged 24 days, were obtained from Envigo (Placentia, CA). Starting at 27 days of age, the rats were injected subcutaneously with 17β-estradiol (1 mg in 0.3 mL of sesame oil; Millipore Sigma, St. Louis, MO) for 3 days to stimulate ovarian development and growth of antral follicles, as described previously (54). At 24 hours after the last injection, the rats were anesthetized using ketamine (Ketaset; Zoetic, Kalamazoo, MI) and xylazine intraperitoneally (VETone, Boise, ID) and euthanized by intracardiac perfusion using 0.9% saline. The ovaries were collected in ice-cold M199 isolation medium (Millipore Sigma) containing 2-mM l-glutamine, 25 mM HEPES, 1% antibiotic and antimycotic, and 0.1% BSA (pH 7.25). All experimental procedures involving the rats were performed in accordance with the accepted standards of animal care as outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals and a protocol approved by the institutional animal care and use committee at the University of California, San Diego.
The collection and purification of ovarian TICs were performed as described previously (54). In brief, the ovaries were removed from the rats and dissected free of oviducts and fat under a dissecting microscope. The ovaries were gently punctured with a 26-gauge needle in ice-cold M199 isolation medium. The remaining ovaries were washed twice with ice-cold M199 isolation medium (Millipore Sigma) and then were minced and digested in 5 mL of collagenase-DNase solution containing 21.1 mg of collagenase (Worthington, Lakewood, NJ), 1.5 mg of DNase (Worthington), and 50 mg of BSA in M199 isolation medium at 37°C for 60 minutes. After 60 minutes of collagenase-DNase digestion, TICs were purified using discontinuous Percoll (Millipore Sigma) gradient centrifugation. The purified TICs were then washed twice with ice-cold M199 isolation medium, and the pellet was resuspended in a known volume of the ice-cold serum-free McCoy’s 5A culture medium (Gibco, Life Technologies, Carlsbad, CA) supplemented with 1% antibiotic/antimycotic mix, 0.1% BSA, and 2 mM glutamine (Gibco, Life Technologies). The TICs were counted with a hemocytometer, and cell viability, determined by trypan blue exclusion, was routinely in the 90% to 95% range.
Inflammatory stimuli protocol
TICs were cultured on human fibronectin-coated plates (Corning Life Science, Tewksbury, MA) at 37°C in an atmosphere of 5% carbon dioxide in humidified air in McCoy’s 5A culture medium (Gibco, Life Technologies). To study the effects of inflammatory stimuli on the growth of TICs, the TICs were incubated for ≤96 hours in human fibronectin-coated 96-well plates at a density of l.0 × 104 cells per well. To study the effects of inflammatory stimuli on gene expression, the TICs were incubated for 48 hours in human fibronectin-coated 24-well plate at a density of l.0 to 2.0 × 105 cells per well. The TICs were incubated in the presence of vehicle (control) or in the presence of different doses of recombinant rat IL-1β (0.1 to 10 ng/mL; Gibco, Life Technologies) and LPS (0.01 to 1 µg/mL; Millipore Sigma). The concentrations of LPS and IL-1β chosen for RNAseq analysis (1 µg/mL and 1 ng/mL, respectively) had elicited peak androstenedione and Cyp17A1 expression in previous experiments using four biologic replicates for each treatment condition.
Cell viability assay
Cell viability was estimated using PrestoBlue Cell Viability Reagent 10× solution (Thermo Fisher Scientific, Carlsbad, CA), in accordance with the manufacturer's instructions. In brief, TICs in cell culture medium were seeded at 1 × 104 cells per well in 96-well fibronectin-coated plates treated with vehicle (control) or different doses of LPS and IL-1β for 48 and 72 hours and culture medium only for background control. One hour before the end of the culture period, 10× PrestoBlue was added to 96-well plates, including medium-only wells, and incubated at 37°C for 60 minutes. Cell viability was evaluated by fluorescence, which was determined using a microplate reader (Fluostar Omega; BMG, Durham, NC) with excitation and emission wavelengths of 570 and 610 nm, respectively. Relative cell viability was expressed as a percentage relative to the untreated control cells.
Androstenedione measurement
After 48 hours of treatment, the conditioned culture media were collected for androstenedione quantification and kept frozen at −80°C before testing. The androstenedione concentration was quantified using the Androstenedione ELISA kit [Abcam, Cambridge, MA; RRID: AB_2811019 (55)] using the ELISA kit instructions. The intra-assay and interassay coefficients of variation were 8.5% and 11%, respectively. The samples were assayed in duplicate.
RNA isolation and quantitative PCR
RNA was isolated using the MagMAX-96 Total RNA Isolation kit (Applied Biosystems, Foster City, CA) and the KingFisher robot (Thermo Scientific, Vantaa, Finland). Reverse transcription of total RNA to cDNA was performed using the High Capacity cDNA Reverse Transcription kit for real-time PCR [quantitative PCR (qPCR); Applied Biosystems]. qPCR reactions were set up in 20-μL volumes, consisting of 5 μL of cDNA (5 to 25 ng), 2.5-μL forward and 2.5-μL reverse 200 nM primers, and 10 μL of PowerUp SYBR Green Master Mix (Applied Biosystems). The gene-specific primers used in the present study are listed in Table 1. qPCR was run on an ABI 7300 (Applied Biosystems). The transcription levels of Cyp11a1, Hsd3b, Cyp17a1, and Hmgcr were quantified using the ΔΔCt method using Hprt as the housekeeping reference gene.
Table 1.
Primer Sequences Used
| Gene Symbol | RefSeq | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Product Size, bp |
|---|---|---|---|---|
| Hprt1 | NM_012583 | TTGTTGGATATGCCCTTGACT | CCGCTGTCTTTTAGGCTTTG | 105 |
| Hsd3b1 | NM_001007719 | ATATTGGAGGCCTGCGTCG | CGGCCATCCTTTTGCTGTA | 166 |
| Cyp11a1 | NM_017286.3 | GCTGGAAGGTGTAGCTCAGG | CACTGGTGTGGAACATCTGG | 224 |
| Cyp17a1 | NM_012753.2 | ACTGAGGGTATCGTG GATGC | CCGTCAGGCTGGAGATAGAC | 187 |
| Hmgcr | NM_013134.2 | TGTGGGAACGGTGACACTTA | CTTCAAATTTTGGGCACTCA | 101 |
RNA sequencing
RNA was sequenced using an Illumina HiSeq 4000 at the Institute for Genomic Medicine Genomics Center (University of California, San Diego, La Jolla, CA). Sequencing libraries were generated using the Illumina TruSeq Stranded mRNA kit. The libraries were sequenced using standard methods on an Illumina HiSeq4000 sequencer. The reads were mapped to rat genome (Rnor 6.0) using Tophat, version 2.1.1, and duplicate reads were removed. The reads per gene were counted using the “summarizeOverlaps” function, and differential expression was analyzed using the DESeq2 packages, both in the R program. We have reported on differentially expressed genes (DEGs) shifted in expression by ≥1.5-fold (log2) with an adjusted P value (q-value) of ≤ 0.05. Enriched functional categories were identified using gene ontology (GO) analysis and Ingenuity® Pathway Analysis (IPA).
Statistical analysis and bioinformatics analysis
Statistical analysis was performed using JMP, version 13.0, software (SAS Institute, Cary, NC). The data are presented as mean ± SEM. Statistically significant differences (P < 0.05) between groups were determined using one-way ANOVA, followed by post hoc pairwise comparisons of individual means (Dunnett test). The normality of distribution was assessed using the Shapiro-Wilk W test. In the absence of normality and/or unequal variance, the data were appropriately transformed and/or nonparametric testing (Kruskal-Wallis or Dunn test) was performed.
Results
Inflammatory stimuli augment androgen production and expression of steroid hormone biosynthesis genes in TICs
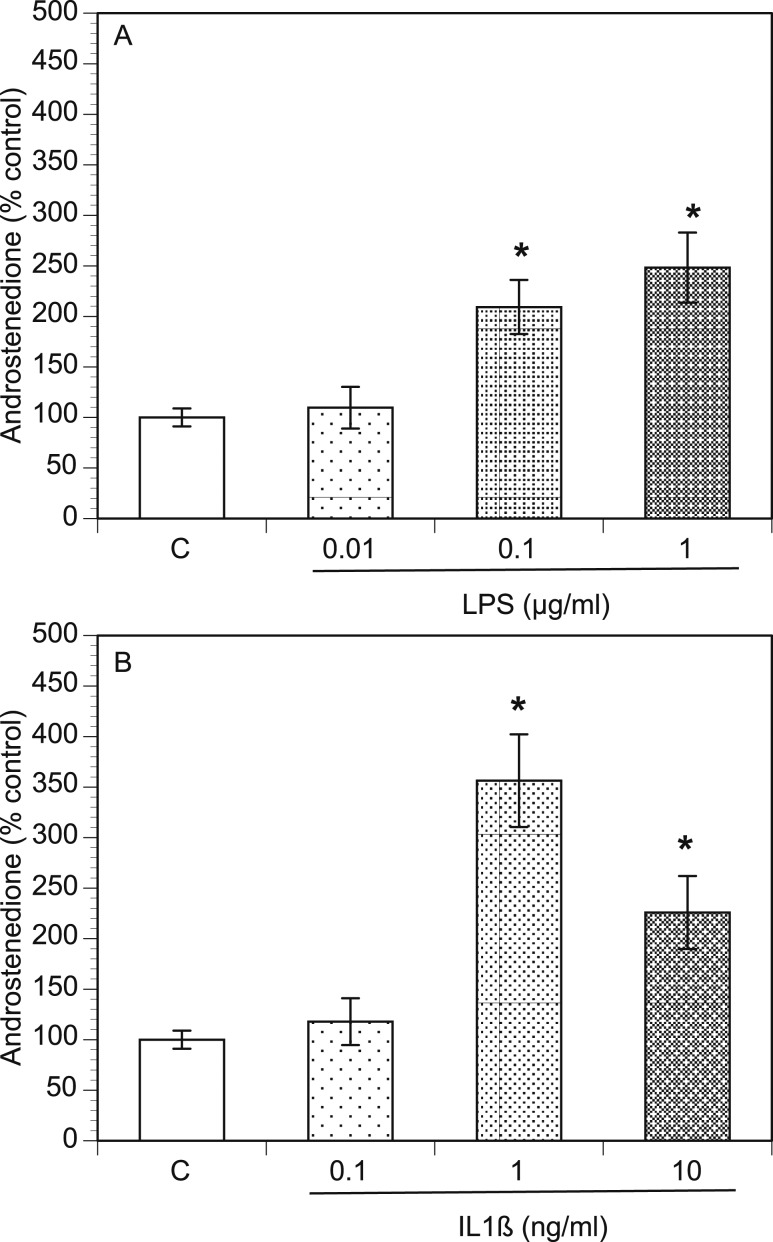
The association of PCOS with both excessive inflammation and hyperandrogenism raises the possibility that inflammation and androgen production have a causal relationship. To test this, we examined whether proinflammatory agents influence theca cell’s primary function (i.e., androgen production). The androstenedione concentration was measured in the culture media after 48 hours of culture with proinflammatory agents. Compared with the control conditions, LPS and IL-1β significantly increased (P < 0.05) the androstenedione level by more than twofold and threefold, respectively (Fig. 1A and 1B). The LPS-mediated increase in the androstenedione level was dose-dependent, but the IL-1β–mediated increase in androstenedione level was biphasic.
Figure 1.
LPS and IL-1β increase androstenedione concentration in TICs. Rat TICs were cultured in the presence of varying doses of (A) LPS or (B) IL-1β for 48 h. The conditioned culture media were then harvested and androstenedione concentrations (percentage of control) were determined using ELISA. *P < 0.05.
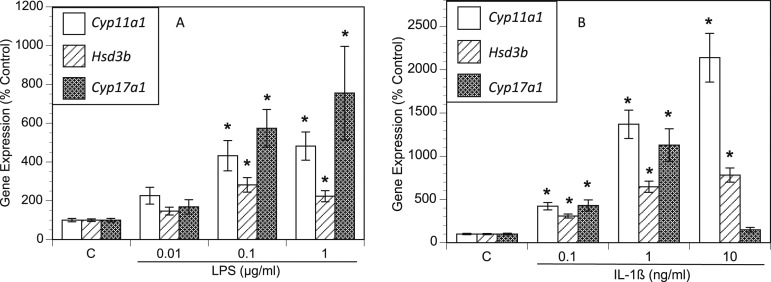
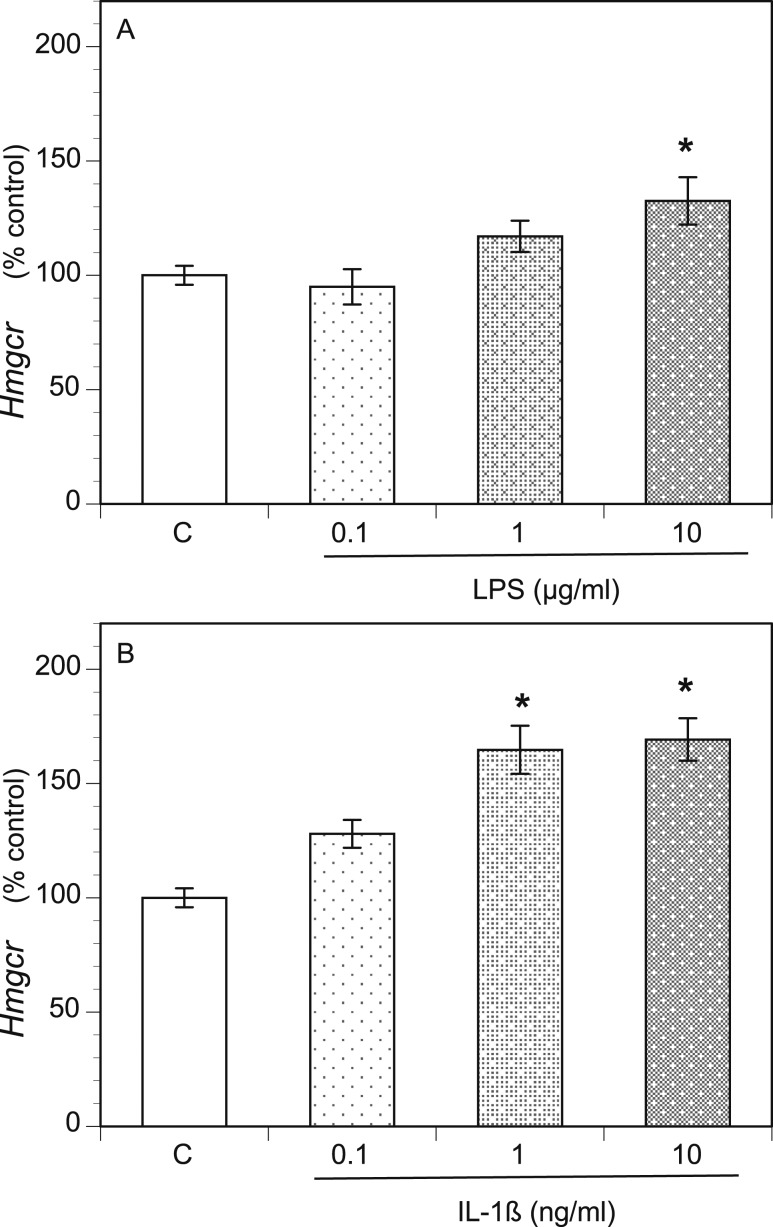
To investigate the underlying mechanism of increased androgen production, we examined the expression of key androgenic genes using qPCR. Compared with the control group, the LPS-treated TICs exhibited increased expression of the genes encoding steroid 17α-monooxygenase (commonly referred to as 17α-hydroxylase) and 17α-hydroxyprogesterone aldolase (17,20 lyase) (Cyp17A1; upregulated up to sevenfold, depending on the LPS dose), 3-β-hydroxysteroid dehydrogenase, Δ5-4 isomerase (Hsd3b; upregulated ≤2.8-fold), and the cholesterol side-chain-cleavage enzyme P450scc (Cyp11A1; upregulated ≤4.3-fold; Fig. 2A). IL-1β treatment increased Cyp17A1 expression ≤11-fold, depending on the dose; Hsd3b ≤7.8-fold; and Cyp11A1 ≤21-fold (Fig. 2B). Because androgens are produced from cholesterol, we also examined the expression of Hmgcr, as it encodes hydroxymethylglutaryl–coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in cholesterol biosynthesis. Hmgcr expression was modestly, but substantially, increased by both LPS treatment (Fig. 3A) and IL-1β treatment (Fig. 3B) in a dose-dependent manner.
Figure 2.
LPS and IL-1β increased expression of key androgen biosynthesis genes. Rat TICs were cultured for 48 h in the presence of varying doses of (A) LPS and (B) IL-1β. RNA was then extracted and reverse transcribed to cDNA, and qPCR was performed for Cyp11a1, Hsd3b, Cyp17a1, and Hprt1. Gene transcript levels are displayed as the percentage of control, Hprt1. *P < 0.05.
Figure 3.
LPS and IL-1β increased expression of the key cholesterol biosynthesis gene, Hmgcr. Rat TICs were cultured for 48 h in the presence of varying doses of (A) LPS and (B) IL-1β. RNA was then extracted and reverse transcribed to cDNA, and qPCR was performed for Hmgcr and Hprt1. Gene transcript levels are displayed as the percentage of control, Hprt1. *P < 0.05.
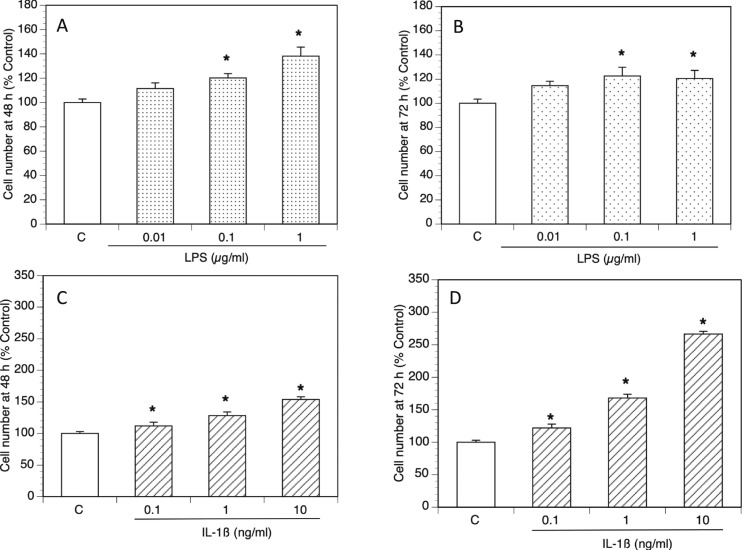
In addition to the increase in key androgenic gene expression, using the PrestoBlue Cell viability reagent, we found that both LPS and IL-1β significantly increased TIC number in a dose-dependent fashion (Fig. 4).
Figure 4.
LPS and IL-1β increased the number of viable TICs. Rat TICs were cultured in varying doses of LPS and IL-1β for 48 and 72 h. Cell viability was assessed by fluorescence. Viable theca cell number (percentage of control) displayed after (A) 48- and (B) 72-h exposure to LPS and (C) 48- and (D) 72-h exposure to IL-1β. *P < 0.05.
LPS and IL-1β trigger widespread changes in gene expression in TICs
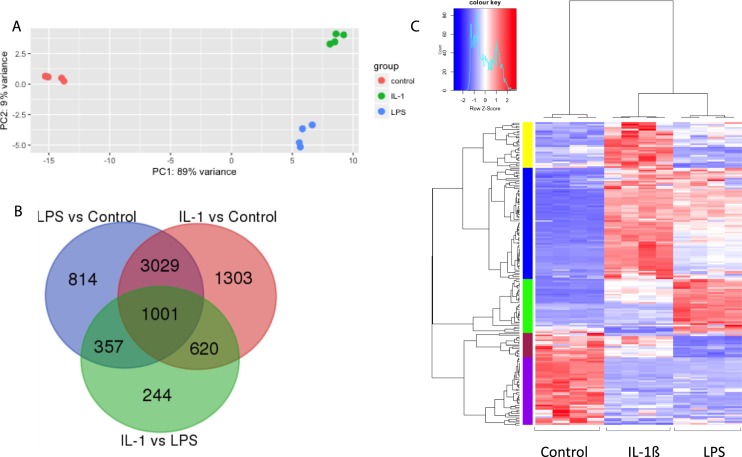
To elucidate the global effects of proinflammatory agents on TICs, we performed RNAseq analysis. Principal component analysis of the transcriptome revealed a tight clustering among the four biologic replicates (Fig. 5A). Both IL-1β and LPS treatment caused a large shift along the first principal component compared with the control, with a variance of 89%, indicative of a dramatic shift in gene expression. DESeq2 analysis identified 5201 DEGs in TICs treated with LPS compared with control (when using a stringent statistical cutoff of q < 0.01; Fig. 5B). A similarly large number of genes (n = 5953) exhibited significantly altered expression in response to IL-1β treatment (Fig. 5B). Most of these DEGs (n = 4030) were shifted in their expression by both inflammatory treatments (Fig. 5B). Among these common DEGs were the key cholesterol and androgen biosynthesis genes—Hmgcr, Cyp11a1, Hsd3b, and Cyp17a1—which we had shown by qPCR are induced by both LPS and IL-1β treatment (Figs. 3 and 4).
Figure 5.
LPS and IL-1β induced genome-wide shifts in gene expression in RNAseq analysis. (A) Two-dimensional view of principal component analysis performed with global gene expression information on control (red dots), IL-1β (green dots), and LPS (blue dots). (B) Heat map using supervised hierarchical cluster analysis of control, IL-1β, and LPS treatments (n = 4 per treatment group). Expression values were transformed to z-scores. The color saturation is proportional to the magnitude of the measured fold change, with bright red and bright blue representing higher and lower levels of gene expression, respectively. Genes are arranged horizontally and experimental treatments vertically. (C) Venn diagram of DEGs among IL-1β, LPS, and control groups using the cutoff of q < 0.01.
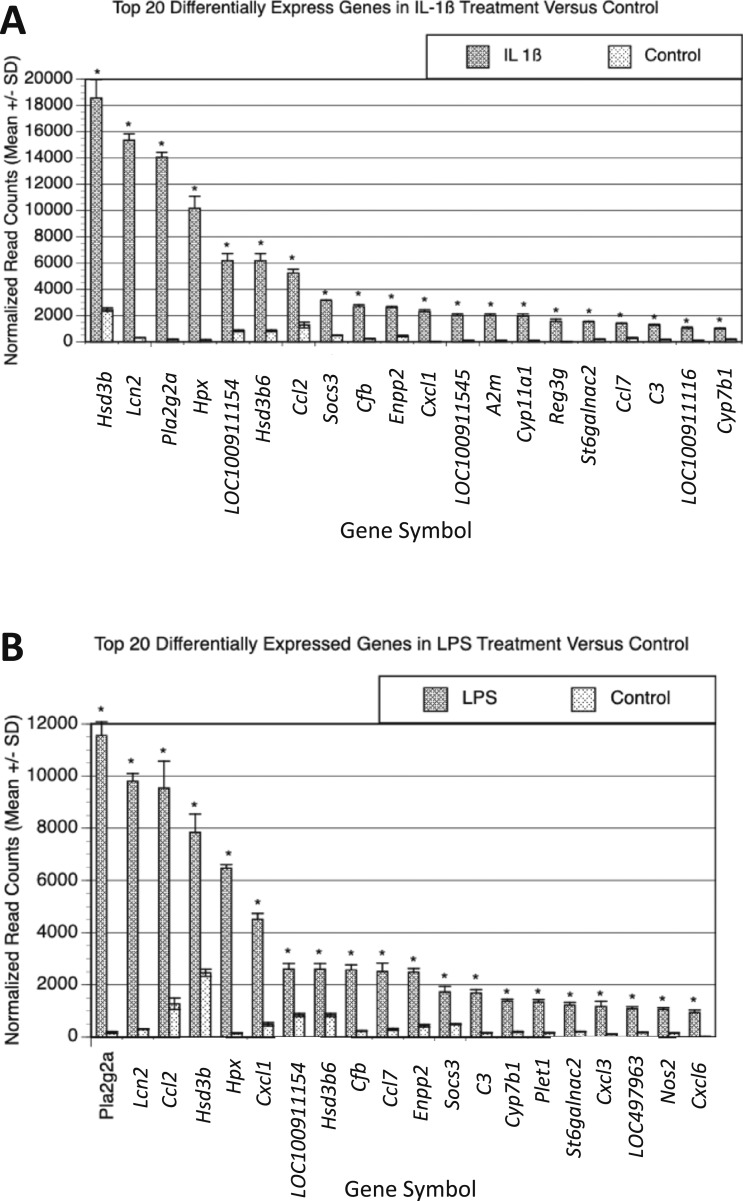
Unsupervised hierarchical clustering of the top DEGs in response to these two inflammatory stimuli segregated the three groups into distinct clusters (Fig. 5C). The top 20 genes most differentially expressed in response to LPS or IL-1β are shown in Fig. 6A and 6B. Several of these genes (14) overlap, highlighting the shared effects of these two inflammatory agents. Among these shared DEGs, many are involved in cholesterol and steroid biosynthesis (Hsd3b, LOC1000911154, Hsd3b6, and Cyp7b1) and the innate immune response (Lcn2, Pla2g2a, CCl2, Socs3, Cfb, Cxcl1, Ccl7, and C3). Although many genes responded similarly to both inflammatory agents, we also identified 2222 genes that were differentially regulated in response to LPS vs IL-1β (Fig. 5B). This suggests that although these two inflammatory agents have common effects, they also have substantial differences in their mechanism of action.
Figure 6.
Top 20 DEGs in LPS and IL-1β showing overlap between treatment conditions compared with control. After 48 h of treatment with LPS (1 µg/mL) and/or IL-1β (1 ng/mL) using four biologic replicates, RNA was isolated and sequenced. (A) Normalized read counts of top 20 DEGs in IL-1β compared with control (mean ± SD). (B) Normalized read counts of top 20 DEGs in LPS compared with control (mean ± SD). *P < 0.05.
The RNAseq data are available in the Gene Expression Omnibus (accession number GSE138275; available at: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE138275).
Proinflammatory stimuli trigger widespread changes in expression of immune and mevalonate pathway genes in TICs
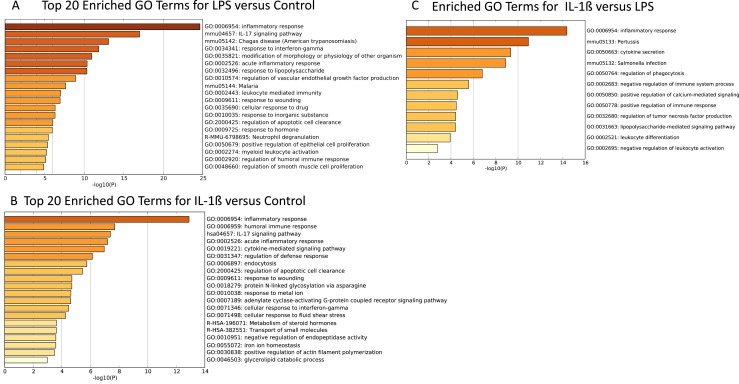
To begin to define the physiological significance of the genome-wide shifts in gene expression triggered by proinflammatory stimuli in TICs, we performed GO analysis using the Metascape program. DEGs defined using a stringent cutoff (q < 0.05 and log twofold change > 2.5) were targeted for this analysis. As anticipated, owing to the treatment, immune-related responses were the most highly enriched GO categories for both the LPS and IL-1β treatment groups compared with the control groups (Fig. 7A and 7B). For LPS-treated cells, the response to LPS was significantly enriched statistically, as were many other immune system-related categories, including inflammatory response, acute inflammatory response, leukocyte-mediated immunity, and neutrophil degranulation (Fig. 7A). Cytokine signaling pathways were also triggered by LPS, based on enrichment for the IL-17 signaling pathway and the response to interferon-γ GO categories. Similar to LPS, IL-1β regulated the inflammatory response genes and many other immune-related GO categories (Fig. 7B). However, the particular immune response GO categories enriched for the LPS and IL-1β treatment groups generally differed (Fig. 7C). IL-1β–specific GO categories included cytokine secretion, regulation of TNF production, regulation of phagocytosis, negative regulation of immune system process, and negative regulation of leukocyte activation. The microorganisms associated with the two proinflammatory stimuli also differed. IL-1β triggered altered expression of the genes associated with the response to pertussis and Salmonella infection (Fig. 7C), although LPS altered the expression of genes known to respond to the parasites that cause Chagas disease and malaria (Fig. 7A). Other GO categories enriched in the IL-1β treatment group were apoptotic cell clearance, adenylate cyclase–activating G protein–coupled receptor signaling pathway, and metabolism of steroid hormones (Fig. 7B).
Figure 7.
LPS and IL-1β treatments shared similar and distinct gene ontologies. After RNAseq analysis, enriched functional categories were identified using GO analysis of enriched biologic functional categories comparing (A) LPS and (B) IL-1β with control DEGs using a cutoff of q < 0.05 and log twofold change >2.5, and all enriched GO categories comparing (C) IL-1β and LPS using a cutoff of q < 0.05 and log twofold change >1.5.
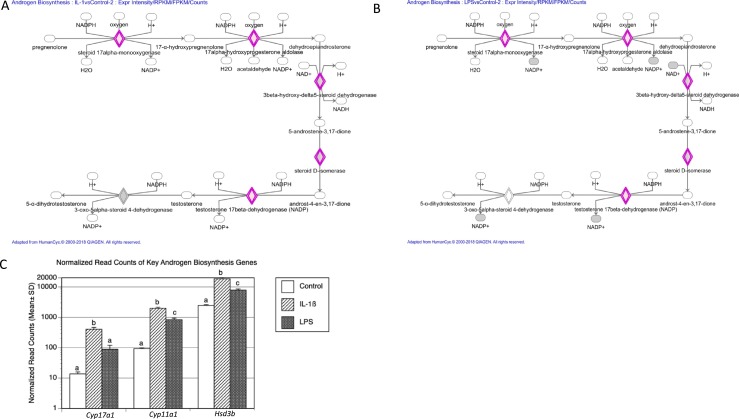
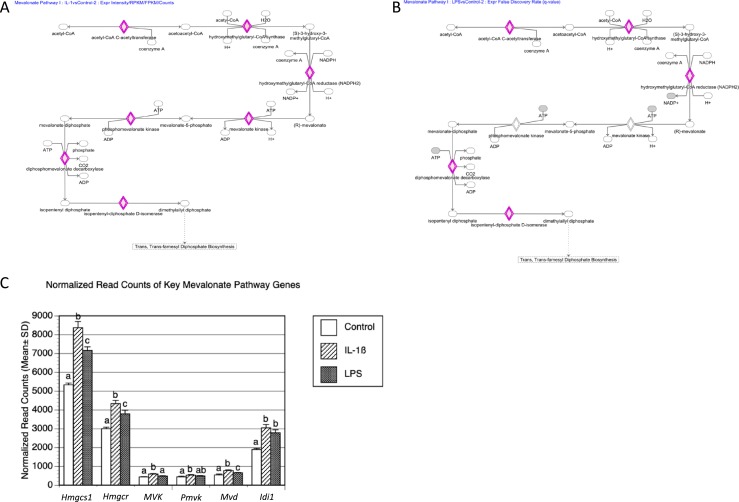
To further investigate the molecular responses of TICs to these two proinflammatory stimuli, we used the IPA program. Unlike Metascape GO analysis, which ranks the GO terms associated with the provided gene lists, IPA identifies the top canonical pathways to predict gene networks. The top three significantly statistically enriched canonical pathways associated with both the LPS and the IL-1β treatment groups were axonal guidance signaling, hepatic fibrosis, and integrin signaling (available at: https://data.mendeley.com/datasets/hyzpkbv376/draft#file-0d7286f6-adde-400a-a8f1-aab2d5bd29a1). In addition, IPA identified the androgen biosynthesis pathway as a substantially enriched network for both the LPS and IL-1β treatment groups (Fig. 8A and 8B). The genes regulated by both of these proinflammatory agents in this androgen biosynthesis network were Cyp17a1, Cyp11a, and Hsd3b. This confirmed and extended the results of our qPCR analysis, showing that these androgen biosynthesis genes are induced by LPS and IL-1β treatment (Fig. 8C). Another substantially enriched category was the mevalonate pathway (Fig. 9A and 9B), the biosynthetic pathway responsible for generating isopentenyl pyrophosphate and dimethylallyl pyrophosphate, the building blocks of a large number of biomolecules, including steroid hormones such as androgens. Mevalonate pathway genes differentially expressed in response to either LPS and IL-1β treatment were those encoding HMG-CoA reductase, the rate-controlling enzyme of the mevalonate pathway (Hmgcr), HMG-CoA synthase (Hmgcs1), mevalonate kinase (Mvk), phosphomevalonate kinase (Pmvk), diphosphomevalonate decarboxylase (Mvd), and isopentyl-diphosphate d-isomerase (Idi1).
Figure 8.
Androgen biosynthesis pathway was enriched in both LPS and IL-1β treatments. IPA was performed to identify enriched pathways. Using a cutoff of log2 >2.5-fold change and adjusted P value < 0.05, the canonical androgen biosynthesis pathway was found to be enriched in (A) IL-1β and (B) LPS treatments vs control (DEGs highlighted in pink). (C) Normalized read counts of key androgen biosynthesis genes. Different letters were used to indicate statistically significant differences using the Tukey honestly significant difference test (P < 0.05).
Figure 9.
Mevalonate pathway was enriched after both LPS and IL-1β treatment. IPA was performed to identify enriched pathways. Using a cutoff of log2 > 2.5-fold change and adjusted P value of <0.05, the canonical mevalonate pathway was enriched in (A) IL-1β and (B) LPS treatments vs control (DEGs highlighted in pink). (C) Normalized read counts of key mevalonate and cholesterol synthesis genes. Different letters were used to indicate statistically significant differences using the Tukey honestly significant difference test (P < 0.05).
Discussion
In the present study, we demonstrated that the inflammatory stimuli, LPS and IL-1β, increased androgen production, triggered widespread alterations in gene expression, and increased the number of viable rat TICs. Consistent with the elevated levels of androstenedione (Fig. 1), the key genes involved in androgen synthesis—Cyp17a1, Hsd3b, and Cyp11a1—were upregulated by both proinflammatory treatments, according to both qPCR analysis (Fig. 2) and RNAseq analysis (Fig. 8C). qPCR analysis demonstrated a dose-dependent increase in the expression of these genes with increasing doses of LPS (Fig. 2A). IL-1β treatment elicited a dose-dependent response in Cyp11a1 and Hsd3b expression and a biphasic response in Cyp17a1 (Fig. 2B).
RNAseq analysis revealed that both proinflammatory agents regulated many common genes, including those involved in cholesterol biosynthesis, androgen biosynthesis, and innate immunity. This was expected, because both inflammatory mediators are associated with inflammasome activation, with LPS a potent inducer of this pathway and IL-1β activation the end product (41, 42). An example of a gene upregulated by both LPS and IL-1β is Hmgcr (Figs. 3 and 9), which encodes HMG-CoA reductase, the rate-limiting enzyme of the mevalonate pathway. Previous studies have suggested inflammatory stimuli might upregulate HMG-CoA reductase–mediated cholesterol synthesis and might increase resistance to statins (56, 57). These findings highlight a potential mechanism through which anti-inflammatory treatments such as statins and resveratrol, both Hmgcr inhibitors, can reduce testosterone levels in women with PCOS (17, 18, 58).
RNAseq analysis also identified other biologically relevant genes upregulated by LPS and IL-1β, including those encoding Tlr4 and Nfkb (nuclear factor-κB; available at: https://data.mendeley.com/datasets/hyzpkbv376/draft#file-0d7286f6-adde-400a-a8f1-aab2d5bd29a1), both involved in activation of the innate immune system and regulation of the inflammasome. Altogether, these results support the hypothesis that LPS and IL-1β increase TIC androgen production via activation of the inflammasome and upregulation of key steroidogenic genes.
Both LPS and IL-1β increased the number of viable TICs in a dose-dependent fashion, with the most pronounced effect occurring at 1 µg/mL of LPS and 10 ng/mL of IL-1β (Fig. 4). Although the underlying mechanisms for this are not known, a likely possibility is that it results from increased proliferation and/or decreased apoptosis. Our RNAseq analysis identified genes upregulated by LPS and IL-1β that are candidates to be responsible, including Pi3k/Akt and Erk1/2, both of which encode proproliferation factors, and Bcl-2 and Bcl-x, which encode apoptosis inhibitors. In further support of this, statistically enriched GO categories associated with both LPS and IL-1β treatment included regulation of vascular endothelial growth factor production, regulation of apoptotic cell clearance, and regulation of smooth muscle cell proliferation (Fig. 7A and 7B). Our finding that LPS and IL-1β increased the number of viable TICs is interesting because of previous studies demonstrating that oxidative stress stimulates TIC proliferation in vitro (31). Oxidative stress and chronic inflammation are closely related in a vicious circle (59), as has been observed in chronic disease states such as atherosclerosis and obesity, in which inflammation can induce generation of reactive oxygen species and oxidative stress can promote inflammation (59). PCOS has been associated with oxidative stress (32) and with chronic low-grade inflammation.
IPA analysis identified many enriched canonical pathways shared between LPS and IL-1β. The top three pathways enriched in each inflammatory treatment were axonal guidance signaling, hepatic fibrosis/hepatic stellate cell activation, and integrin signaling (available at: https://data.mendeley.com/datasets/hyzpkbv376/draft#file-0d7286f6-adde-400a-a8f1-aab2d5bd29a1). The potential clinical relevance of these pathways in the theca cell deserves further investigation. Each of these pathways has been implicated in the pathophysiology of PCOS. The potential relevance of the enriched axonal guidance signaling pathway might be related to an increasing body of evidence supporting the hypothesis that noradrenergic fibers might contribute to the development of PCOS (60, 61). The GO function hepatic fibrosis/hepatic stellate cell activation might have been enriched because of the increased prevalence of nonalcoholic fatty liver disease in women with PCOS (62). Finally, enrichment of the integrin signaling GO category might be related to previously described aberrations in integrin signaling in the endometrium of women with PCOS (63–65).
Despite the coregulation of many genes, LPS and IL-1β each had unique effects on gene expression. In total, 2222 genes were significantly regulated by LPS or IL-1β but not by both (Fig. 5B). Using unsupervised hierarchical clustering of top DEGs, we found that these proinflammatory treatments segregated into distinct clusters (Fig. 5C), suggesting they might also have diverging mechanisms of action. The top enriched GO categories for IL-1β vs LPS treatment pertained primarily to the response to inflammation, including inflammatory response, cytokine secretion, negative regulation of immune system process, positive regulation of immune response, LPS-mediated signaling pathway, leukocyte differentiation, and negative regulation of leukocyte activation (Fig. 7C). This was not surprising because these proinflammatory stimuli act at different levels of the inflammasome activation pathway (43). LPS primes the inflammasome via binding to TLR4, and bioactive IL-1β is secreted as a result inflammasome activation (43). These unique effects of LPS and IL-1β on gene expression could explain why these two proinflammatory agents have modest differences in their effects on androstenedione synthesis and theca cell viability.
Our results are in contrast to previous in vitro studies that have shown either no effect of LPS on androstenedione concentration (66) or a decrease in androstenedione secretion (34). The methods used in these studies differed from ours in that their TICs were cultured in the presence of tonic levels of LH and we had cultured TICs without LH. LH is a well-characterized agonist of TICs via its binding to the LH receptor on TICs (67). Thus, we suggest that the apparent discrepancy between the findings from our study and those from the earlier studies can be attributed to a subadditive effect of two agonists of varying potency—a concept first described by Loewe (68)—in which a partial agonist in the presence of a full agonist exhibits a net decrease in receptor activation compared with the full agonist alone. Also, LH is released in a pulsatile fashion in vivo and, thus, might act on the theca cell differently than that seen with tonic LH exposure in vitro. We are currently developing an in vitro theca cell culture model with LH pulsatility capability to further test this hypothesis.
Correlative studies have demonstrated that low-grade chronic systemic inflammation is associated with the PCOS phenotype, as evidenced by increased levels of C-reactive protein (24), white blood cells (69), and inflammatory cytokines (70, 71). We chose a low concentration of proinflammatory agents for our studies to mimic such low-dose “subclinical” endotoxemia. Although the inflammatory response to an endotoxin challenge is similar between human and murine models, the dose required to induce this response varies dramatically between these species (36, 72, 73). Rodents are much more tolerant of LPS and require up to ∼250-fold greater LPS doses to produce an LPS-induced inflammatory response comparable to the response that occurs in humans (72). A 58-ng/mL LPS serum concentration in mice elicits a similar response as a 0.1- to 0.2-ng/mL LPS serum concentration in humans (36, 74). This serum concentration in humans is within the range of subclinical LPS levels found in several chronic disease states such as cardiovascular disease, diabetes, obesity, and PCOS (48, 74, 75). The potential sources of low-grade bacterial endotoxins in women with PCOS include altered gut microbiota, resulting in increased intestinal permeability (48) or the persistence of low-grade chronic infections, such as Chlamydia pneumonia, Chlamydia trachomatis, Helicobacter pylori, and chronic periodontal disease (50–53). In support of the possibility that the microbiome could have a causal role in PCOS, clinical studies have shown substantial alterations of the gut microbiome of women with PCOS compared with controls (49, 76–78). Furthermore, these changes were associated with abnormal clinical parameters, including hyperandrogenism and oligomenorrhea and amenorrhea (49, 79). Regarding the mechanism, dysbiosis of the gut microbiome can lead to increased intestinal permeability, thereby allowing gut-derived LPS to enter the circulation and induce systemic low-grade inflammation (i.e., metabolic endotoxemia) (48, 74, 76, 80, 81). In contrast to high-dose LPS, which mimics septic shock-like conditions, low-dose LPS can activate the TLR4 pathway without fully activating the NF-κB pathway and compensatory anti-inflammatory mediators (75). This might enable low-grade inflammation to propagate and initiate and/or exacerbate chronic inflammatory disease. Although our current in vitro model supports the hypothesis that chronic inflammatory states might promote ovarian hyperandrogenism and induce PCOS, further in vivo and translational clinical studies are necessary to fully define this relationship.
In conclusion, our findings support the hypothesis that inflammatory stimuli alter the function of TICs in a manner consistent with the phenotype seen in PCOS. Our genome-wide analysis revealed a diverse and complex array of genes regulated by simulated chronic proinflammatory conditions, thereby providing a number of potential targets for treating PCOS.
Acknowledgments
We thank the IGM Genomics Center (University of California, San Diego, La Jolla, CA) for performing Illumina platform library preparation and sequencing.
Financial Support: The present study was supported by the National Institutes of Health (Grant T32 HD007203 to C.W.F.) and the National Centers for Translational Research in Reproduction and Infertility (Grant P50 HD012303 to R.J.C.).
Glossary
Abbreviations:
- DEG
differentially expressed gene
- GO
gene ontology
- HMG-CoA
hydroxymethylglutaryl–coenzyme A
- IPA
Ingenuity® Pathway Analysis
- LPS
lipopolysaccharide
- PCOS
polycystic ovary syndrome
- qPCR
quantitative PCR
- RNAseq
RNA sequencing
- TIC
theca-interstitial cell
- TLR4
Toll-like receptor 4
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or data repositories.
References and Notes
- 1. Diamanti-Kandarakis E, Piperi C, Spina J, Argyrakopoulou G, Papanastasiou L, Bergiele A, Panidis D. Polycystic ovary syndrome: the influence of environmental and genetic factors. Hormones (Athens). 2006;5(1):17–34. [DOI] [PubMed] [Google Scholar]
- 2. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- 3. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Androgen Excess Society. Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91(11):4237–4245. [DOI] [PubMed] [Google Scholar]
- 4. Azziz R. Androgen excess is the key element in polycystic ovary syndrome. Fertil Steril. 2003;80(2):252–254. [DOI] [PubMed] [Google Scholar]
- 5. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF; Task Force on the Phenotype of the Polycystic Ovary Syndrome of the Androgen Excess and PCOS Society. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009;91(2):456–488. [DOI] [PubMed] [Google Scholar]
- 6. Rosenfield RL, Ehrmann DA. The pathogenesis of polycystic ovary syndrome (PCOS): the hypothesis of PCOS as functional ovarian hyperandrogenism revisited. Endocr Rev. 2016;37(5):467–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Erickson GF, Magoffin DA, Dyer CA, Hofeditz C. The ovarian androgen producing cells: a review of structure/function relationships. Endocr Rev. 1985;6(3):371–399. [DOI] [PubMed] [Google Scholar]
- 8. Mahajan DK. Steroidogenesis in human polycystic ovary. Endocrinol Metab Clin North Am. 1988;17(4):751–769. [PubMed] [Google Scholar]
- 9. Jakimiuk AJ, Weitsman SR, Navab A, Magoffin DA. Luteinizing hormone receptor, steroidogenesis acute regulatory protein, and steroidogenic enzyme messenger ribonucleic acids are overexpressed in thecal and granulosa cells from polycystic ovaries. J Clin Endocrinol Metab. 2001;86(3):1318–1323. [DOI] [PubMed] [Google Scholar]
- 10. McGee WK, Bishop CV, Bahar A, Pohl CR, Chang RJ, Marshall JC, Pau FK, Stouffer RL, Cameron JL. Elevated androgens during puberty in female rhesus monkeys lead to increased neuronal drive to the reproductive axis: a possible component of polycystic ovary syndrome. Hum Reprod. 2012;27(2):531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. González F, Rote NS, Minium J, Kirwan JP. Increased activation of nuclear factor kappaB triggers inflammation and insulin resistance in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(4):1508–1512. [DOI] [PubMed] [Google Scholar]
- 12. González F. Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids. 2012;77(4):300–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Banaszewska B, Pawelczyk L, Spaczynski RZ, Dziura J, Duleba AJ. Effects of simvastatin and oral contraceptive agent on polycystic ovary syndrome: prospective, randomized, crossover trial. J Clin Endocrinol Metab. 2007;92(2):456–461. [DOI] [PubMed] [Google Scholar]
- 14. Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Comparison of simvastatin and metformin in treatment of polycystic ovary syndrome: prospective randomized trial. J Clin Endocrinol Metab. 2009;94(12):4938–4945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Banaszewska B, Pawelczyk L, Spaczynski RZ, Duleba AJ. Effects of simvastatin and metformin on polycystic ovary syndrome after six months of treatment. J Clin Endocrinol Metab. 2011;96(11):3493–3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Duleba AJ, Banaszewska B, Spaczynski RZ, Pawelczyk L. Simvastatin improves biochemical parameters in women with polycystic ovary syndrome: results of a prospective, randomized trial. Fertil Steril. 2006;85(4):996–1001. [DOI] [PubMed] [Google Scholar]
- 17. Sathyapalan T, Kilpatrick ES, Coady AM, Atkin SL. The effect of atorvastatin in patients with polycystic ovary syndrome: a randomized double-blind placebo-controlled study. J Clin Endocrinol Metab. 2009;94(1):103–108. [DOI] [PubMed] [Google Scholar]
- 18. Raval AD, Hunter T, Stuckey B, Hart RJ. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565. [DOI] [PubMed] [Google Scholar]
- 19. Banaszewska B, Wrotyńska-Barczyńska J, Spaczynski RZ, Pawelczyk L, Duleba AJ. Effects of resveratrol on polycystic ovary syndrome: a double-blind, randomized, placebo-controlled trial. J Clin Endocrinol Metab. 2016;101(11):4322–4328. [DOI] [PubMed] [Google Scholar]
- 20. Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J Clin Endocrinol Metab. 2004;89(7):3313–3318. [DOI] [PubMed] [Google Scholar]
- 21. Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602. [DOI] [PubMed] [Google Scholar]
- 22. Pergola C, Rogge A, Dodt G, Northoff H, Weinigel C, Barz D, Rådmark O, Sautebin L, Werz O. Testosterone suppresses phospholipase D, causing sex differences in leukotriene biosynthesis in human monocytes. FASEB J. 2011;25(10):3377–3387. [DOI] [PubMed] [Google Scholar]
- 23. Vignozzi L, Cellai I, Santi R, Lombardelli L, Morelli A, Comeglio P, Filippi S, Logiodice F, Carini M, Nesi G, Gacci M, Piccinni MP, Adorini L, Maggi M. Antiinflammatory effect of androgen receptor activation in human benign prostatic hyperplasia cells. J Endocrinol. 2012;214(1):31–43. [DOI] [PubMed] [Google Scholar]
- 24. Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N. Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab. 2001;86(6):2453–2455. [DOI] [PubMed] [Google Scholar]
- 25. Orio F Jr, Palomba S, Cascella T, Di Biase S, Manguso F, Tauchmanovà L, Nardo LG, Labella D, Savastano S, Russo T, Zullo F, Colao A, Lombardi G. The increase of leukocytes as a new putative marker of low-grade chronic inflammation and early cardiovascular risk in polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90(1):2–5. [DOI] [PubMed] [Google Scholar]
- 26. Toulis KA, Goulis DG, Mintziori G, Kintiraki E, Eukarpidis E, Mouratoglou SA, Pavlaki A, Stergianos S, Poulasouchidou M, Tzellos TG, Makedos A, Chourdakis M, Tarlatzis BC. Meta-analysis of cardiovascular disease risk markers in women with polycystic ovary syndrome. Hum Reprod Update. 2011;17(6):741–760. [DOI] [PubMed] [Google Scholar]
- 27. Escobar-Morreale HF, Luque-Ramirez M, Gonzalez F. Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis . Fertil Steril. 2011;95(3):1048–1058.e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzalez F, Thusu K, Abdel-Rahman E, Prabhala A, Tomani M, Dandona P. Elevated serum levels of tumor necrosis factor alpha in normal-weight women with polycystic ovary syndrome. Metabolism. 1999;48(4):437–441. [DOI] [PubMed] [Google Scholar]
- 29. Peng Z, Sun Y, Lv X, Zhang H, Liu C, Dai S. Interleukin-6 levels in women with polycystic ovary syndrome: a systematic review and meta-analysis. PLoS One. 2016;11(2):e0148531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19(3):268–288. [DOI] [PubMed] [Google Scholar]
- 31. Duleba AJ, Foyouzi N, Karaca M, Pehlivan T, Kwintkiewicz J, Behrman HR. Proliferation of ovarian theca-interstitial cells is modulated by antioxidants and oxidative stress. Hum Reprod. 2004;19(7):1519–1524. [DOI] [PubMed] [Google Scholar]
- 32. González F, Rote NS, Minium J, Kirwan JP. Reactive oxygen species-induced oxidative stress in the development of insulin resistance and hyperandrogenism in polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91(1):336–340. [DOI] [PubMed] [Google Scholar]
- 33. Yoo K, Lee SH. Effect of lipopolysaccharide (LPS) exposure on the reproductive organs of immature female rats. Dev Reprod. 2016;20(2):113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Taylor CC, Terranova PF. Lipopolysaccharide inhibits rat ovarian thecal-interstitial cell steroid secretion in vitro. Endocrinology. 1995;136(12):5527–5532. [DOI] [PubMed] [Google Scholar]
- 35. Liang W, Lindeman JH, Menke AL, Koonen DP, Morrison M, Havekes LM, van den Hoek AM, Kleemann R. Metabolically induced liver inflammation leads to NASH and differs from LPS- or IL-1β-induced chronic inflammation. Lab Invest. 2014;94(5):491–502. [DOI] [PubMed] [Google Scholar]
- 36. Lew WY, Bayna E, Molle ED, Dalton ND, Lai NC, Bhargava V, Mendiola V, Clopton P, Tang T. Recurrent exposure to subclinical lipopolysaccharide increases mortality and induces cardiac fibrosis in mice. PLoS One. 2013;8(4):e61057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Geng S, Chen K, Yuan R, Peng L, Maitra U, Diao N, Chen C, Zhang Y, Hu Y, Qi CF, Pierce S, Ling W, Xiong H, Li L. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat Commun. 2016;7(1):13436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Afonina IS, Müller C, Martin SJ, Beyaert R. Proteolytic processing of interleukin-1 family cytokines: variations on a common theme. Immunity. 2015;42(6):991–1004. [DOI] [PubMed] [Google Scholar]
- 39. Price JC, Bromfield JJ, Sheldon IM. Pathogen-associated molecular patterns initiate inflammation and perturb the endocrine function of bovine granulosa cells from ovarian dominant follicles via TLR2 and TLR4 pathways. Endocrinology. 2013;154(9):3377–3386. [DOI] [PubMed] [Google Scholar]
- 40. Xie T, Xu Q, Wan H, Xing S, Shang C, Gao Y, He Z. Lipopolysaccharide promotes lung fibroblast proliferation through autophagy inhibition via activation of the PI3K-Akt-mTOR pathway. Lab Invest. 2019;99(5):625–633. [DOI] [PubMed] [Google Scholar]
- 41. Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140(6):821–832. [DOI] [PubMed] [Google Scholar]
- 42. Jo EK, Kim JK, Shin DM, Sasakawa C. Molecular mechanisms regulating NLRP3 inflammasome activation. Cell Mol Immunol. 2016;13(2):148–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guo H, Callaway JB, Ting JP. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat Med. 2015;21(7):677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gérard N, Caillaud M, Martoriati A, Goudet G, Lalmanach AC. The interleukin-1 system and female reproduction. J Endocrinol. 2004;180(2):203–212. [DOI] [PubMed] [Google Scholar]
- 45. Smolikova K, Mlynarcikova A, Scsukova S. Role of interleukins in the regulation of ovarian functions. Endocr Regul. 2012;46(4):237–253. [DOI] [PubMed] [Google Scholar]
- 46. Duffy DM, Ko C, Jo M, Brannstrom M, Curry TE. Ovulation: parallels with inflammatory processes. Endocr Rev. 2019;40(2):369–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871–882. [DOI] [PubMed] [Google Scholar]
- 48. Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57(6):1470–1481. [DOI] [PubMed] [Google Scholar]
- 49. Torres PJ, Siakowska M, Banaszewska B, Pawelczyk L, Duleba AJ, Kelley ST, Thackray VG. Gut microbial diversity in women with polycystic ovary syndrome correlates with hyperandrogenism. J Clin Endocrinol Metab. 2018;103(4):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Dursun E, Akalın FA, Güncü GN, Çınar N, Aksoy DY, Tözüm TF, Kılınc K, Yıldız BO. Periodontal disease in polycystic ovary syndrome. Fertil Steril. 2011;95(1):320–323. [DOI] [PubMed] [Google Scholar]
- 51. Morin-Papunen LC, Duleba AJ, Bloigu A, Järvelin MR, Saikku P, Pouta A. Chlamydia antibodies and self-reported symptoms of oligo-amenorrhea and hirsutism: a new etiologic factor in polycystic ovary syndrome? Fertil Steril. 2010;94(5):1799–1804. [DOI] [PubMed] [Google Scholar]
- 52. Özçaka Ö, Ceyhan BO, Akcali A, Biçakci N, Lappin DF, Buduneli N. Is there an interaction between polycystic ovary syndrome and gingival inflammation? J Periodontol. 2012;83(12):1529–1537. [DOI] [PubMed] [Google Scholar]
- 53. Yavasoglu I, Kucuk M, Cildag B, Arslan E, Gok M, Kafkas S. A novel association between polycystic ovary syndrome and Helicobacter pylori. Am J Med Sci. 2009;338(3):174–177. [DOI] [PubMed] [Google Scholar]
- 54. Ortega I, Villanueva JA, Wong DH, Cress AB, Sokalska A, Stanley SD, Duleba AJ. Resveratrol potentiates effects of simvastatin on inhibition of rat ovarian theca-interstitial cells steroidogenesis. J Ovarian Res. 2014;7(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. RRID: AB_2811019, https://scicrunch.org/resolver/AB_2811019.
- 56. Chen Y, Ku H, Zhao L, Wheeler DC, Li LC, Li Q, Varghese Z, Moorhead JF, Powis SH, Huang A, Ruan XZ. Inflammatory stress induces statin resistance by disrupting 3-hydroxy-3-methylglutaryl-CoA reductase feedback regulation. Arterioscler Thromb Vasc Biol. 2014;34(2):365–376. [DOI] [PubMed] [Google Scholar]
- 57. Chen Y, Zhao L, Li Q, Wheeler DC, Varghese Z, Moorhead JF, Powis SH, Ruan XZ. Inflammatory stress reduces the effectiveness of statins in the kidney by disrupting HMGCoA reductase feedback regulation. Nephrol Dial Transplant. 2014;29(10):1864–1878. [DOI] [PubMed] [Google Scholar]
- 58. Wong DH, Villanueva JA, Cress AB, Sokalska A, Ortega I, Duleba AJ. Resveratrol inhibits the mevalonate pathway and potentiates the antiproliferative effects of simvastatin in rat theca-interstitial cells. Fertil Steril. 2011;96(5):1252–1258. [DOI] [PubMed] [Google Scholar]
- 59. Hulsmans M, Holvoet P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J Cell Mol Med. 2010;14(1-2):70–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lara HE, Dissen GA, Leyton V, Paredes A, Fuenzalida H, Fiedler JL, Ojeda SR. An increased intraovarian synthesis of nerve growth factor and its low affinity receptor is a principal component of steroid-induced polycystic ovary in the rat. Endocrinology. 2000;141(3):1059–1072. [DOI] [PubMed] [Google Scholar]
- 61. Espinoza JA, Alvarado W, Venegas B, Domínguez R, Morales-Ledesma L. Pharmacological sympathetic denervation prevents the development of polycystic ovarian syndrome in rats injected with estradiol valerate. Reprod Biol Endocrinol. 2018;16(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rocha ALL, Faria LC, Guimarães TCM, Moreira GV, Cândido AL, Couto CA, Reis FM. Non-alcoholic fatty liver disease in women with polycystic ovary syndrome: systematic review and meta-analysis. J Endocrinol Invest. 2017;40(12):1279–1288. [DOI] [PubMed] [Google Scholar]
- 63. Kim JY, Song H, Kim H, Kang HJ, Jun JH, Hong SR, Koong MK, Kim IS. Transcriptional profiling with a pathway-oriented analysis identifies dysregulated molecular phenotypes in the endometrium of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94(4):1416–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Giudice LC. Endometrium in PCOS: implantation and predisposition to endocrine CA. Best Pract Res Clin Endocrinol Metab. 2006;20(2):235–244. [DOI] [PubMed] [Google Scholar]
- 65. Donaghay M, Lessey BA. Uterine receptivity: alterations associated with benign gynecological disease. Semin Reprod Med. 2007;25(6):461–475. [DOI] [PubMed] [Google Scholar]
- 66. Herath S, Williams EJ, Lilly ST, Gilbert RO, Dobson H, Bryant CE, Sheldon IM. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134(5):683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Allen JJ, Herrick SL, Fortune JE. Regulation of steroidogenesis in fetal bovine ovaries: differential effects of LH and FSH. J Mol Endocrinol. 2016;57(4):275–286. [DOI] [PubMed] [Google Scholar]
- 68. Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3(6):285–290. [PubMed] [Google Scholar]
- 69. Herlihy AC, Kelly RE, Hogan JL, O’Connor N, Farah N, Turner MJ. Polycystic ovary syndrome and the peripheral blood white cell count. J Obstet Gynaecol. 2011;31(3):242–244. [DOI] [PubMed] [Google Scholar]
- 70. Glintborg D, Andersen M, Richelsen B, Bruun JM. Plasma monocyte chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1alpha are increased in patients with polycystic ovary syndrome (PCOS) and associated with adiposity, but unaffected by pioglitazone treatment. Clin Endocrinol (Oxf). 2009;71(5):652–658. [DOI] [PubMed] [Google Scholar]
- 71. Kaya C, Pabuccu R, Berker B, Satiroglu H. Plasma interleukin-18 levels are increased in the polycystic ovary syndrome: relationship of carotid intima-media wall thickness and cardiovascular risk factors. Fertil Steril. 2010;93(4):1200–1207. [DOI] [PubMed] [Google Scholar]
- 72. Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D; Inflammation and the Host Response to Injury Investigators. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12(1):60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Amandi-Burgermeister E, Tibes U, Kaiser BM, Friebe WG, Scheuer WV. Suppression of cytokine synthesis, integrin expression and chronic inflammation by inhibitors of cytosolic phospholipase A2. Eur J Pharmacol. 1997;326(2-3):237–250. [DOI] [PubMed] [Google Scholar]
- 74. Manco M, Putignani L, Bottazzo GF. Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr Rev. 2010;31(6):817–844. [DOI] [PubMed] [Google Scholar]
- 75. Maitra U, Deng H, Glaros T, Baker B, Capelluto DG, Li Z, Li L. Molecular mechanisms responsible for the selective and low-grade induction of proinflammatory mediators in murine macrophages by lipopolysaccharide. J Immunol. 2012;189(2):1014–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zeng B, Lai Z, Sun L, Zhang Z, Yang J, Li Z, Lin J, Zhang Z. Structural and functional profiles of the gut microbial community in polycystic ovary syndrome with insulin resistance (IR-PCOS): a pilot study. Res Microbiol. 2019;170(1):43–52. [DOI] [PubMed] [Google Scholar]
- 77. Liu R, Zhang C, Shi Y, Zhang F, Li L, Wang X, Ling Y, Fu H, Dong W, Shen J, Reeves A, Greenberg AS, Zhao L, Peng Y, Ding X. Dysbiosis of gut microbiota associated with clinical parameters in polycystic ovary syndrome. Front Microbiol. 2017;8:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Insenser M, Murri M, Del Campo R, Martínez-García MA, Fernández-Durán E, Escobar-Morreale HF. Gut microbiota and the polycystic ovary syndrome: influence of sex, sex hormones, and obesity. J Clin Endocrinol Metab. 2018;103(7):2552–2562. [DOI] [PubMed] [Google Scholar]
- 79. Lindheim L, Bashir M, Münzker J, Trummer C, Zachhuber V, Leber B, Horvath A, Pieber TR, Gorkiewicz G, Stadlbauer V, Obermayer-Pietsch B. Alterations in gut microbiome composition and barrier function are associated with reproductive and metabolic defects in women with polycystic ovary syndrome (PCOS): a pilot study. PLoS One. 2017;12(1):e0168390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Frazier TH, DiBaise JK, McClain CJ. Gut microbiota, intestinal permeability, obesity-induced inflammation, and liver injury. JPEN J Parenter Enteral Nutr. 2011; 35(5 suppl)14S–20S. [DOI] [PubMed] [Google Scholar]
- 81. Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, Waget A, Delmée E, Cousin B, Sulpice T, Chamontin B, Ferrières J, Tanti JF, Gibson GR, Casteilla L, Delzenne NM, Alessi MC, Burcelin R. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56(7):1761–1772. [DOI] [PubMed] [Google Scholar]