Abstract
Objective: Many topicals claim an efficacious role in skin scar management with limited evidence. Our aim is to present a clear format for functional testing of a skin scarring ointment, using noninvasive and invasive measurements, categorizing findings under the physiological, structural, and mechanical parameters of a scar.
Approach: A double-blinded, randomized volunteer research study of 45 subjects receiving an ointment composing of natural ingredients against a widely used antiscarring topical used as a positive control with temporal sequential punch biopsies (up to 16 weeks) was evaluated using noninvasive quantitative devices and validated by gene and protein studies.
Results: Outcome measures included physiological, mechanical, and structural features of scars. Significant non-invasive findings included an increase in skin hydration (p < 0.05) at week (W) 4, 8, and 12, and elasticity (W16; p = 0.009). These findings were validated by immunohistochemistry (IHC) and quantitative real-time PCR (qRT-PCR). Hyaluronic acid IHC (W4 p = 0.014, W12 p = 0.034, and W16 p = 0.042), qRT-PCR (W16 p = 0.049); Collagen I (W16 p = 0.034, and 0.049) IHC and qRT-PCR, respectively. Collagen III qRT-PCR (W12 p = 0.035, and W16 p = 0.32); elastin IHC (W12 p = 0.044); and fibronectin IHC (W4 p = 0.009, W12 p = 0.038, and W16 p = 0.026).
Innovation: Utilizing this model allows for quantitative, objective evaluation of any topical, where previously there has been a paucity of relevant methods to evaluate their effect.
Conclusions: The positive effect of a topical formulation with an unknown mechanism of action on early cutaneous scar maturation over progressive sequential time points is now evidenced using noninvasive and invasive techniques with the findings categorized on the basis of scarring parameters.
Keywords: skin, scars, skin scarring, clinical trial, topicals, MEBO Scar, scar ointments

Ardeshir Bayat, MB, BS, PhD
Introduction
Despite the abundance of topical formulations on the market, the majority are ranked only as category 4, (data from case series), according to the Oxford (United Kingdom) Centre for Evidence-Based Medicine, Levels of Evidence (Supplementary Table S1).1 The perceived lack of relevant methods for objective and quantitative evaluation of topicals for skin scarring, may provide an explanation for the apparent limited level 1 or 2 evidence-based studies to date.
In March 2013, the sale of cosmetic products, which had been tested on animals, was banned in the European Union.2 Even though animal models were widely used, they were not without their own disadvantages, principally, the structural differences to human skin.3,4 Ex vivo organ culture models provide a good alternative to animal models,5,6 but their limited viability does not allow for longer term evaluation.7,8 In addition, in many human skin clinical trials, the evidence is poor due to low patient numbers, poor randomization and blinding, short follow-up periods, disparity in anatomical scar sites, and most notably, the lack of objective and consistent outcome measures.1
Clinical Problem Addressed
A recent study observed some positive clinical effects when MEBO Scar ointment (Julphar Pharma, UAE) was applied to surgical scars, showing improvement in scar appearance and symptomatic relief.9 However, no objective and quantifiable method was applied to evidence these findings, as is the case with many topicals.
Taking all the above factors into account, an in vivo, randomized, blinded research study was devised, comparing this ointment against a positive control. Both noninvasive and invasive quantitative data were obtained in this model. The noninvasive devices measured the evolving scar features, subdivided into the structural, mechanical, and physiological properties of a skin scar. The results were then used to guide relevant subsequent confirmatory gene and protein studies.
Materials and Methods
Volunteer study design
The primary outcome of this study, to assess the role of a topical formulation on the cosmetic appearance of skin scarring, has been registered on the ISRCTN registry (ISRCTN16551998). An independent statistician from the University of Manchester determined sample size and randomization. It was advised that with 40 subjects (based on previous studies by our group where n = 20), the study will have 80% power to detect effect sizes of 0.454 between treatment and control topical arms, based on comparing within-subject differences between the two topicals using a paired t-test at a two-sided 5% significance level. Forty-five healthy volunteer subjects were recruited and followed up (between August 1st, 2016, and May 31st, 2017). Recruitment was through ethically approved advertisement on the University of Manchester intranet and volunteer pages. All subjects were screened by the health care professional conducting the trial and any with a medical history of relevance or keloid scarring was excluded (for patient demographics and full inclusion and exclusion criteria, please see Supplementary Tables S2 and S3). All subjects signed a written consent form (UREC ref 16098) in accordance with Declaration of Helsinki principles, and consent was rechecked verbally at each visit.
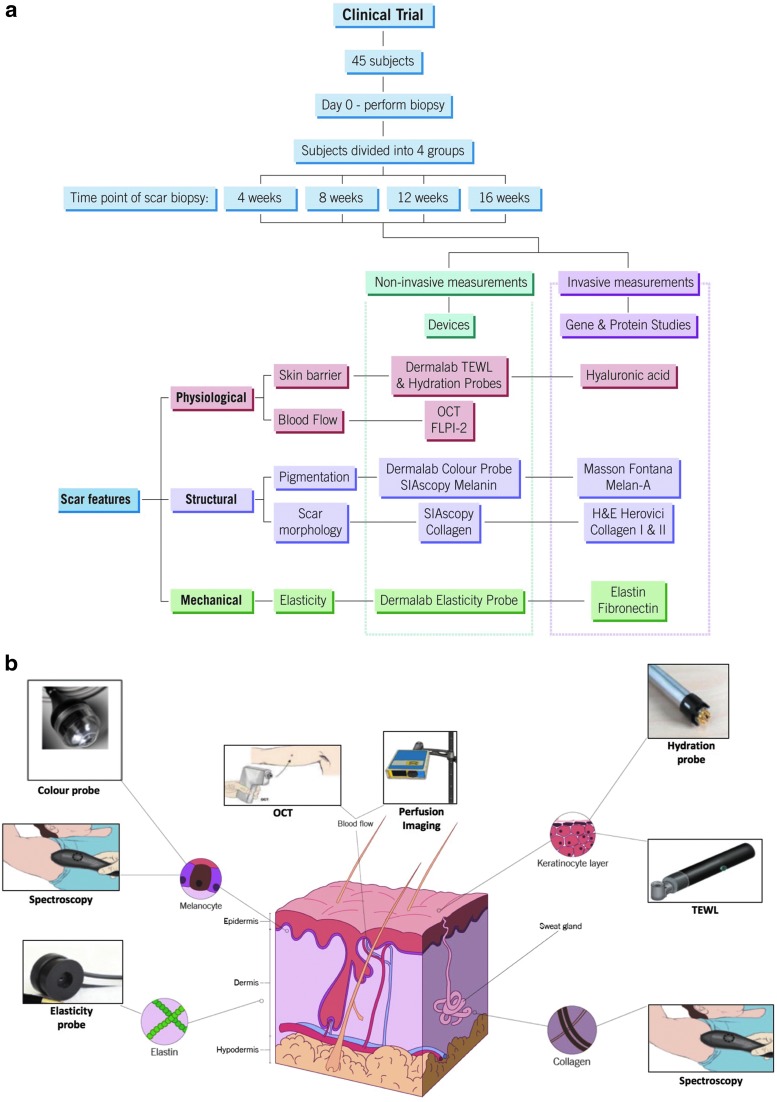
The subjects were divided into four groups—representing a temporal sequential time point: 4 (40 subjects), 8 (30 subjects), 12 (20 subjects), and 16 (10 subjects) weeks. After the initial screening appointment on “Day 0,” a 5 mm punch biopsy (using a punch biopsy kit) was taken under local anesthetic from both upper inner arms to create a uniform scar. Each subject was seen on a fortnightly basis, where all measurements were undertaken, including baseline values on “Day 0”: normal (healthy, non-scarred) skin before injury. No measurements were taken immediately after injury, as the primary focus of this trial was the effect of a topical on scarring, as opposed to wound healing. On the “Day 14” appointment, the first objective measurements of the scars were taken and subjects were given both treatment and control topicals with clear instructions on how exactly to apply them. The subjects were blinded as to which arm received the treatment and control formulations. At the final appointment, they received a 6 mm punch biopsy over the scar site. At that time point, the subject exited the study. All appointments were held at South Manchester University Hospital, part of the Manchester University NHS Foundation Trust (Manchester, United Kingdom). A flowchart summarizing the methodology of this clinical trial and analysis thereafter is shown in Fig. 1a.
Figure 1.
Flow chart and noninvasive devices. (a) Summarizes the methodological approach to this study. The format of the clinical trial is in blue. The findings of both noninvasive and invasive measurements were used to analyze the physiological, structural, and mechanical features of the scar, allowing for thorough assessment of wound healing. (b) The use of noninvasive devices and their anatomical targets within the skin/scar, which allow for assessment of the parameters in 1a. OCT, optical coherence tomography; TEWL, transepidermal water loss.
Tissue samples
All skin samples were appropriately and anonymously labeled with the participants' study ID and stored according to HTA (United Kingdom) guidelines. Biopsies were bisected, half were stored in formalin and the other half in RNA later and stored at −80°C.
Treatment and control topical formulations
MEBO Scar ointment (Julphar Pharma), made from natural ingredients, which was originally formulated and developed in China, a derivative of MEBO™ (Moist Exposed Burn Ointment) (Julphar Pharma), had previously been found to increase vascular endothelial growth factor and fibroblast growth factor expression, affecting angiogenesis and providing anti-inflammatory properties.9–13 This ointment is comprised of sesame, cactus, and beeswax and claims to contain the following “components”: linoleic acid and tyramine, which are thought to improve cosmesis of skin scars.9 All ingredients are existing and approved cosmetic raw materials. For the purpose of our study, this ointment was considered the “treatment” topical for evaluation. The positive “control” topical was kelo-cote® (Sinclair Pharma, United Kingdom), a widely used 100% silicone-based gel topical used principally to prevent abnormal scarring14 and improve symptoms of hypertrophic and keloid scars.15 This trial therefore pursues a noninferiority design, to assess whether there is any clinical benefit to using this experimental treatment (MEBO Scar) in comparison to a silicone-based topical with proven efficacy.
In view of dominant-handedness, the treatment and control formulations were randomized to each arm. The randomization was carried out by an independent medical statistician at the University of Manchester in nQuery Advisor 7.0 using a computer-generated permuted block design with mixed block sizes and random seed, and sent to a different member of the research team (not conducting the trial), who ensured correct dispensing and labeling of topicals and separated them into bags labeled “left arm” and “right arm,” ensuring neither the participant nor the health care professional conducting the trial knew which formulation was in each tube.
Devices
The use of measurement devices in a clinical study setting has many advantages, in that some can provide noninvasive and objective quantitative measurements. These devices have been shown to make measurements that span all three phases of cutaneous wound healing, thus providing continual analysis of the physiological, structural, and mechanical parameters, which can be used to define a skin scar. Where the mode of action for a topical is unknown, treatment response to hypothesized improvements in the properties of the scar can be monitored by such noninvasive devices. In this case, three unique skin properties, (1) skin barrier function, and blood flow (physiological), (2) skin thickness and pigmentation (structural), as well as (3) elasticity (mechanical), were analyzed in a quantitative manner (Fig. 1b).
Another advantage of the noninvasive devices specifically selected for this study is that they are multifunctional; the Vivosight optical coherence tomography (OCT) (Michelson Diagnostics, United Kingdom) provides information on the skin architecture and blood vessel formation throughout all phases of wound healing,16 generating high-resolution “near” real-time (<1 s) infra-red images (with a resolution of 10 μm) of the skin. The Moor full-field laser perfusion imager (FLPI-2) (Moor Instruments, United Kingdom) also gives high-resolution images, which provide real-time color-contrast images of changes in vascular perfusion17 (hemoglobin flux) in skin microcirculation at a thickness of 1 mm and capillary diameters up to 10 μm, with flow rates of 0.01–10 mms−1. Spectrophotometric intracutaneous analysis (SIAscopy) (Medx Health, Canada) provides quantitative readings of hemoglobin, melanin, and collagen content, using reflected light when the probe is placed on the skin over an area measuring 12 × 12 mm at a depth of 2 mm and wavelength between 400 and 1,000 nm.18 The Dermalab system (Cortex Technology, Denmark), is a multiprobe device that measures a variety of quantitative measurement functions, including levels of hydration, color, erythema, elasticity, and transepidermal water loss (TEWL).19 In addition, a subjective scale, in the form of a participant diary, was also used to measure reported levels of pain, itching, and redness of the scar on a scale of 1–10 for the duration of the study. Noninvasive data were analyzed using a Wilcoxon signed-rank test.
Histology and immunohistochemistry
Samples were formalin fixed and processed, and then slides were prepared from wax blocks using a microtome (Leica, United Kingdom) set at 5 μm. Slides were dewaxed and rehydrated in xylene and ethanol (Supplementary Tables S2 and S3 for specific stains). Analysis of histological data was carried out using Definiens Tissue Studio (Germany) and using a paired t-test.
Quantitative real-time PCR
Quantitative real-time PCR (qRT-PCR) was used to evaluate whether specific gene targets were also upregulated in the scar samples. RNA was extracted from normal and scarred skin samples stored in RNA later using QIAgen's (Germany) RNEasy mini kit with beta-mercaptoethanol. The RNA quantity and quality were measured using a Nanodrop and converted to cDNA using Roche (Switzerland) EvoScript Universal cDNA master kit. Resultant cDNA was diluted and stored at −20°C until use for qRT-PCR, using a Roche LightCycler 480 and primers and probes from Sigma-Aldrich (United Kingdom). The delta-delta Ct method was used for analysis and a paired t-test.
Results
The design of this study allowed for the evaluation of a topical formulation by assessment of its functional effect on the physiological, structural, and mechanical features of a skin scar. The noninvasive measurements were subsequently validated by relevant histology, immunohistochemistry (IHC), and qRT-PCR studies. There were 40 subjects in the group for the shortest time period (4 weeks); 10 subjects exited at this (and every subsequent) time point, leaving 30 subjects in the week 8 group, 20 subjects in the week 12 group, and finally 10 subjects in the week 16 group.
Physiological
Assessment of skin barrier function
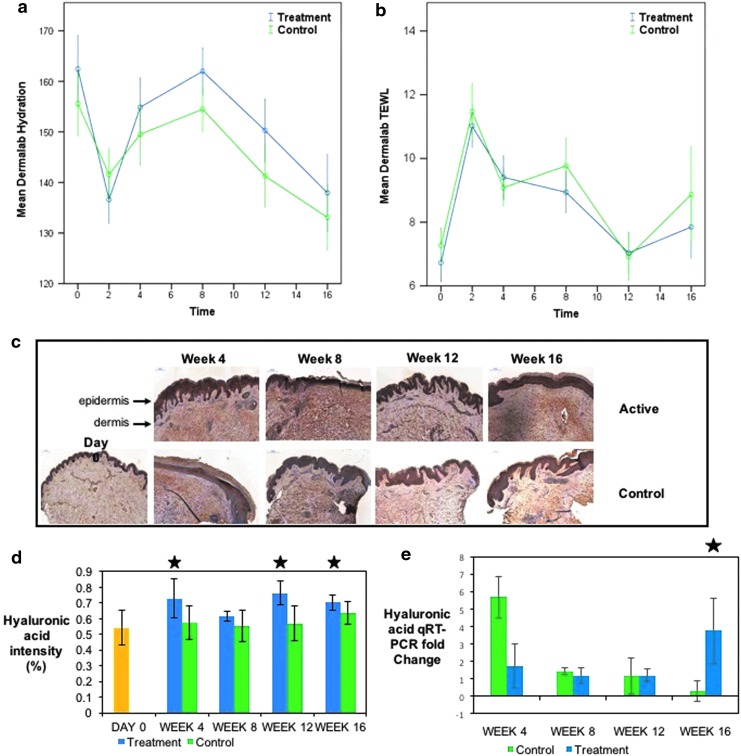
Measurements of hydration and TEWL using the Dermalab System (Cortex Technology) gave an assessment of the skin barrier on normal and scarred skin of each participant. Hydration of the scar in the treatment group was increased significantly from the point of application at week 2 (new scar formation) and through to week 12 (p < 0.05) (Fig. 2a). TEWL decreased as hydration increased in both treatment and control groups, but with no statistical significance between the groups (Fig. 2b.). Hydration of the scar did not show any significant difference between both treatment and control groups compared to normal as opposed to scarred skin; however, with regard to TEWL, at week 16, there was a significant (p = 0.05) improvement in the treatment group versus control compared to normal skin. Hyaluronic acid was used to assess hydration in the formalin-fixed samples and showed significantly higher levels in the scars that received the treatment ointment compared to the control, week 4 p = 0.014, week 12 p = 0.039, and week 16 p = 0.042 (Fig. 2c, d). At week 16, there was also a significant fold increase in hyaluronic acid levels shown by qRT-PCR, p = 0.049 (Fig. 2e).
Figure 2.
Assessment of the skin barrier. (a) Shows the increase in hydration, which is much greater in the treatment arm compared to the control and was significant in weeks 4–12 (p < 0.05). (b) (TEWL) results (which correlate with hydration). (c) IHC staining for hyaluronic acid. (d) The intensity of staining over sequential time points with p < 0.05 at weeks 4 (p = 0.014), 12 (p = 0.039), and 16 (p = 0.042), and in qRT-PCR (e) at week 16 (p = 0.049) (significant results marked with a star). There were 40 subjects in the group for the shortest time period (4 weeks); 10 subjects exited at this (and every subsequent) time point, leaving 30 subjects in the week 8 group, 20 subjects in the week 12 group, and finally, 10 subjects in the week 16 group. Yellow = normal skin (day 0). IHC, immunohistochemistry; qRT-PCR, quantitative real-time PCR.
Blood flow through the scar
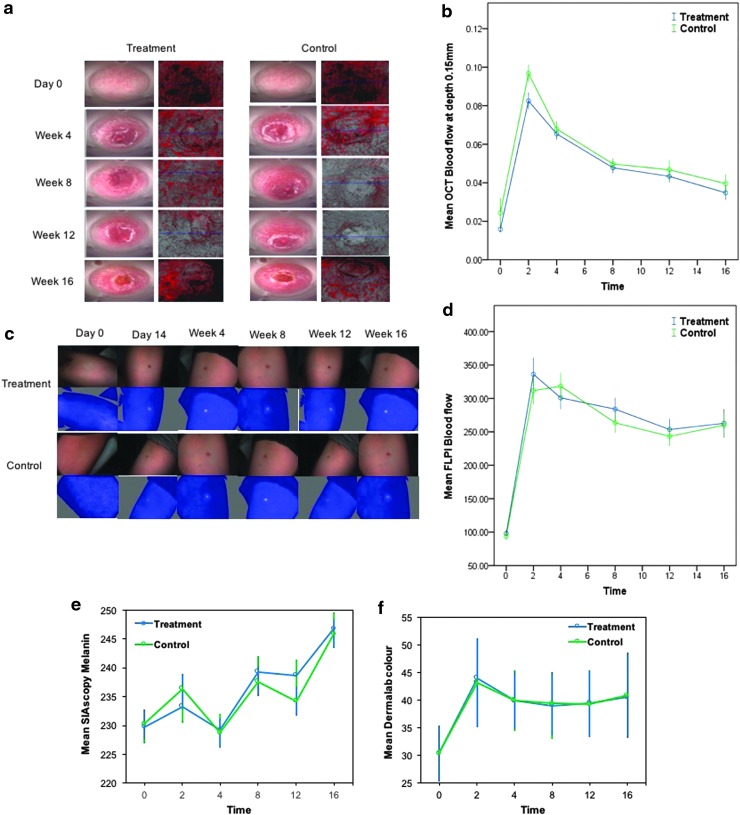
OCT (Michelson Diagnostics) and FLPI-2, (Moor Instruments) were used to measure blood flow. OCT was used to measure blood flow at 0.15, 0.3, and 0.5 mm depth (Fig. 3a). Both treatment and positive control topical formulations demonstrated a sharp increase in blood flow at week 2, reflecting increased levels of angiogenesis, which subsequently decreased, but did not return to baseline levels (Fig. 3b); (no significant difference in blood flow was found between treatment and control in either scarred [from week 2] or normal skin [day 0]). FLPI-2 blood flow results were also raised between 2 and 4 weeks, and then decreased slowly, before increasing again from 12 weeks in both arms (Fig. 3c), reflecting a similar trend to OCT at 0.15 mm (Fig. 3d).
Figure 3.
Assessment of cutaneous blood flow during healing and scar maturation. (a) Pictures from dynamic OCT in both arms. (b) OCT results at 0.15 mm depth. (c) FLPI-2 pictures from both arms. (d) FLPI-2 results. The treatment and control arms displayed a similar pattern with both devices. A sharp increase in blood flow from baseline is evident in both OCT and FLPI, which does decrease slowly, but returns to baseline by week 16. (e) Pigmentation results using SIAscopy continue to rise by week 16 in both arms, with a decrease at weeks 4 and 12. (f) Colorimetry values rise at week 2 and then plateau, but do not return to baseline values by week 16. There were 40 subjects in the group for the shortest time period (4 weeks), 10 subjects exited at this (and every subsequent) time point, leaving 30 subjects in the week 8 group, 20 subjects in the week 12 group, and finally, 10 subjects in the week 16 group. FLPI, full-field laser perfusion imager; SIAscopy, spectrophotometric intracutaneous analysis.
Structural
Pigmentation
SIAscopy (Medx Health) and colorimetry from Dermalab (Cortex Technology) were used to assess pigmentation. SIAscopy demonstrated an increase in melanin in both topicals from day 14 to week 16; however, at week 12, there was a significant difference between treatment and control arms (p = 0.025) when comparing scarred skin only (from week 2) (Fig. 3e). The Dermalab colorimetry values showed an increase in pigmentation and no significant difference between treatment and control in either scarred skin or comparing the scars to normal skin (Fig. 3f). To test the validity between the results for pigmentation from the two different devices, the trend in the results was assessed using Pearson's correlation: for each time point (except week 2), and between treatment and control groups, there was a strong correlation (>0.7) (Supplementary Fig. S1), therefore validating the results obtained from each device. Staining for pigmentation using Masson Fontana and Melan-A did not show any significant difference between treatment and control topical across any time point.
Scar morphology
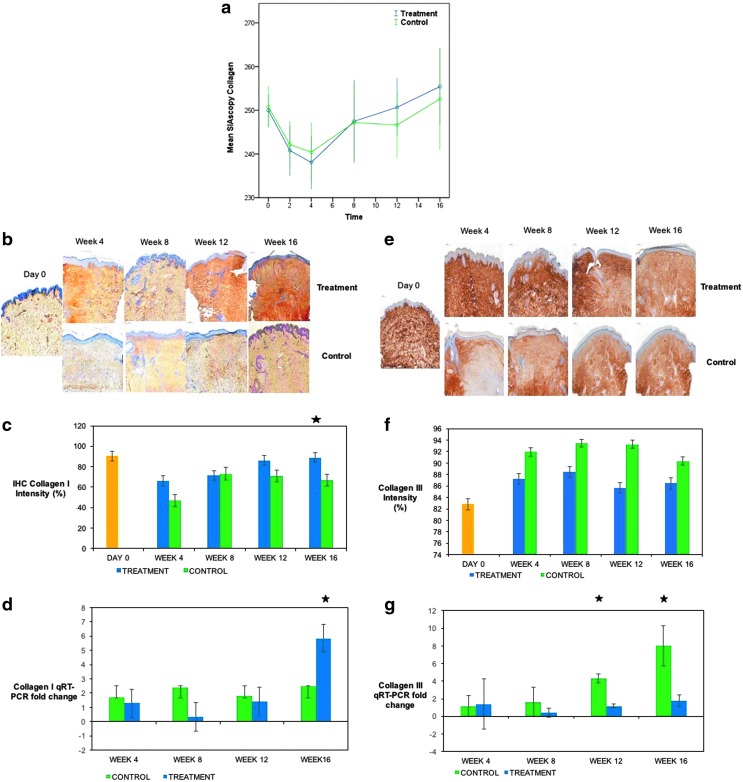
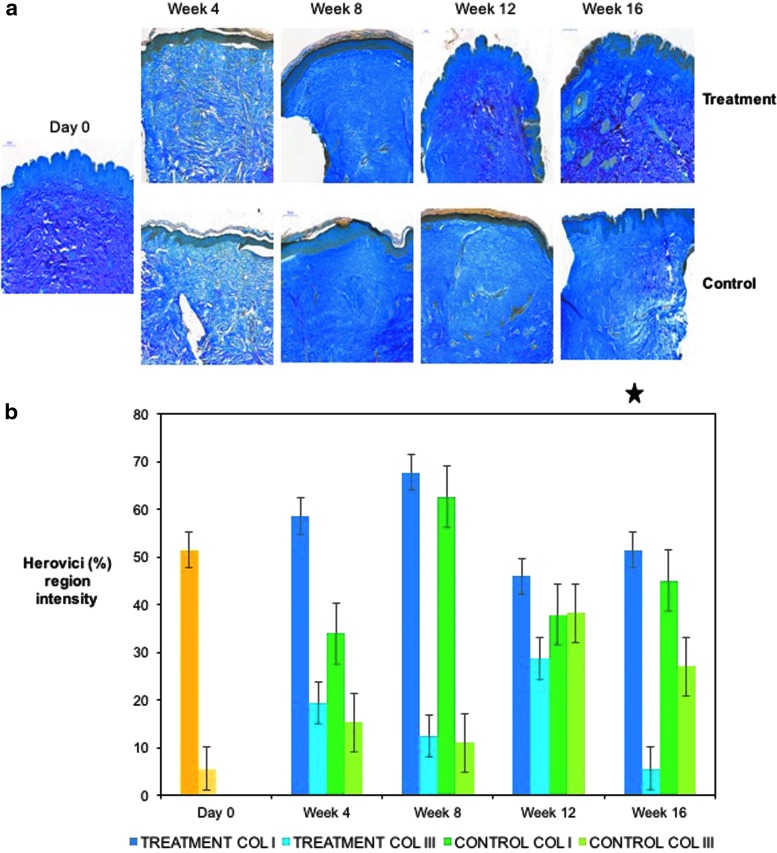
SIAscopy (Medx Health) was used to assess collagen content. An expectant reduction in collagen following scar creation was demonstrated in both arms, which then increased over time at the start of the remodeling phase of wound healing. There was an increase in collagen in both arms; in the treatment arm, the trend showed an 11.2% increase in collagen by week 16 from baseline compared to 6.2% in the control arm, although this was not significant when comparing the difference between the two groups from either week 2 or baseline (day 0) (Fig. 4a). Immunostaining for collagen I was significant at week 16, p = 0.034 (Fig. 4b, c), reflected in the qRT-PCR results, p = 0.049 (Fig. 4d). Collagen III intensity (Fig. 4e, f) was higher in the control arm, which had a significant fold increase in qRT-PCR at weeks 12 and 16 (p = 0.035, and 0.032, respectively) (Fig. 4g). The ratio of Collagen I to III in the treatment arm was also significant at week 16 (p = 0.048), as seen by Herovici staining (Fig. 5a, b).
Figure 4.
Assessment of collagen during healing and scar maturation. (a) SIAscopy demonstrates after an initial decrease in collagen following the biopsy, collagen continues to rise in both treatment and control arms. (b) Images for IHC staining for collagen I. (c) IHC intensity of collagen I in treatment and control arms, significant at week 16 (p = 0.034) between both groups. (d) qRT-PCR for collagen I in treatment versus control arms, significant at week 16 (p = 0.049). (e) Intensity of collagen III IHC stain. (f) Staining for Collagen III, matching trend in collagen III q-RT PCR (g), significant at weeks 12 (p = 0.035) and 16 (p = 0.032), treatment versus control. There were 40 subjects in the group for the shortest time period (4 weeks), 10 subjects exited at this (and every subsequent) time point, leaving 30 subjects in the week 8 group, 20 subjects in the week 12 group, and finally, 10 subjects in the week 16 group. Yellow = normal (healthy non-scarred) skin (day 0). Star = significant p value at time point indicated.
Figure 5.
Herovici staining during healing and scar maturation. (a) Images of Herovici staining in both arms. (b) Ratio of collagen I to III (significant in the treatment arm at week 16 p = 0.048). Yellow = normal (healthy non-scarred) skin (day 0). Star = significant p value at time point indicated.
Mechanical
Elasticity
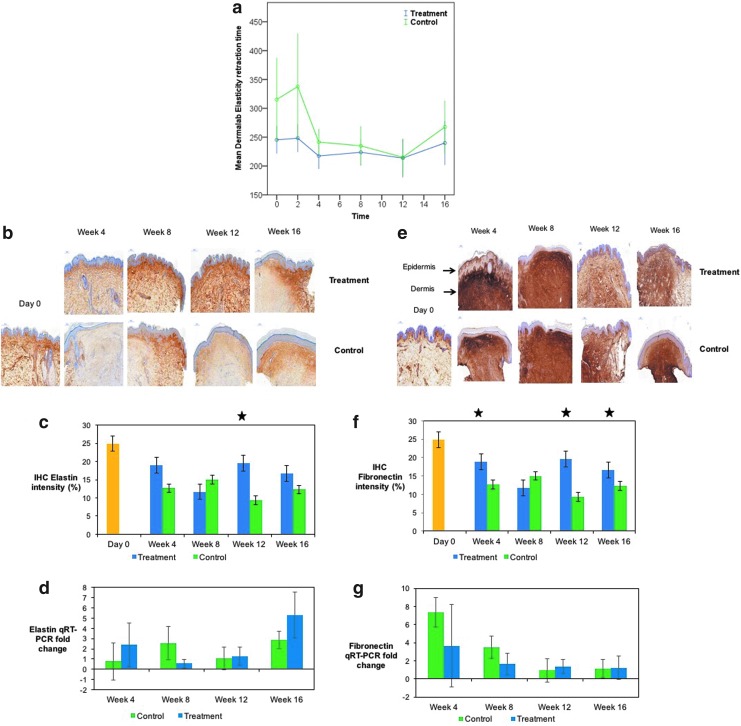
Noninvasive measurements of elasticity were taken with the Dermalab (Cortex Technology) system. A significant increase (p < 0.05) in elasticity in the control group compared to treatment group at week 2 from day 0 is evident in Fig. 6a, although the variation in day 0 values for both arms may suggest a technical or operator-dependent error at this data point, and thus a potential limitation of these results. Elasticity is decreased in both arms, but at week 16, there was a significant increase in the treatment group (p = 0.009) compared to control (Fig. 6a) from week 2. IHC stains for elastin (Fig. 6b, c) showed an increase at week 12, p = 0.044 (no significance in qPCR (Fig. 6d), and fibronectin (Fig. 6e, f) (IHC week 4, p = 0.009, week 12, p = 0.038, week 16, p = 0.026) for the treatment group.
Figure 6.
Assessment of elasticity during healing and scar maturation. (a) Dermalab elasticity results significantly increased in treatment arm (p = 0.009) at week 16. (b) IHC elastin staining in both arms. (c) Elastin intensity significantly increased at week 12 (p = 0.044) in treatment arm versus control. (d) qRT-PCR fold change in elasticity. (e) Fibronectin intensity significantly increased in the treatment arm compared to the control arm at weeks 4 (p = 0.009), 12 (p = 0.038), and 16 (p = 0.026). (f) Comparison of fibronectin staining in both arms. (g) qRT-PCR fold change of fibronectin in both arms. There were 40 subjects in the group for the shortest time period (4 weeks), 10 subjects exited at this (and every subsequent) time point, leaving 30 subjects in the week 8 group, 20 subjects in the week 12 group, and finally, 10 subjects in the week 16 group. Yellow = normal (healthy non-scarred) skin (day 0). Star = significant p value at time point indicated.
Subjective
All subjects completed a “patient diary” using a numerical scale (1–10) to describe their symptoms of pain, redness, and itching. The trend showed a mean value of “10” (the maximum score allocation for each symptom) between weeks 2 and 4, and a mean value of “0” (i.e., pain, redness, or itching) from weeks 12 to 16. This trend was identified in both arms, with no significance between treatment and control, implying that within the subjective data, the length of time, rather than the topical applied, was the principal factor improving the symptomatic features of skin scarring.
Discussion
Despite the number of topical formulations, which exists commercially and purported to improve skin scarring, the evidence to support their claims remains poor and unconvincing, and cannot be substantiated.20 Thus, there is a need for an improved quantitative approach and relevant tools for functional evaluation of the effects of topical formulations, which exist for skin scar management.
Previous clinical studies9–13 had observed positive improvement in scars after application of the treatment topical; however, these results were based on case series and reports of patients with a variety of scars of different ages and from different anatomical sites. In addition, these reports had limited quantifiable data of scar outcome and absence of comprehensive noninvasive measurements, and lacked the use of subjective visual analogue scar scales.9–13 Using this study design, we demonstrate how positive clinical outcomes observed in practice can easily be validated in an objective and quantifiable manner using a randomized blinded design with a positive control.
Quantitative changes were observed in both noninvasive and invasive approaches to our study. The treatment topical showed evidence of retaining moisture, with significantly less compromise to the skin barrier in the scars that received it compared to the control from weeks 4 to 12. Evidence of increased hyaluronic acid levels in dermis was also demonstrated by IHC, corroborated by upregulated gene expression as shown by qRT-PCR in the treated skin samples. In addition, this study demonstrated the response within the structural components of the scars, as the treated arm demonstrated a higher collagen content by SIAscopy measurements, of which a larger proportion was mature (type I) collagen evidenced by Herovici staining. However, there was a lack of reduction of inflammation or pigmentation with regard to physiological and structural parameters, respectively, in the noninvasive or invasive data. Finally, within mechanical parameters, elasticity was increased in both the treated and control arms, although to a significant level in the treated arm by week 16. IHC supported this finding, with significantly increased elastin also by week 16, and fibronectin, at weeks 4, 8, and 16.
In this case, it would be prudent to imply that the best indicators of scarring assessment were structural changes, and physiological improvements in hydration. This methodology, however, allows for other parameters to be evidenced. For example, with a topical claiming to possess anti-inflammatory properties, the primary focus would be on the physiological features of a scar, specifically changes in erythema and blood flow. Previous claims and case series reports are therefore invaluable to this methodology as they can guide the investigator on the best indicators for scarring, and have the added advantage of building a picture of clinical effects through noninvasive data before the commitment of invasive investigations. In addition, having a statistically adequate number of human volunteers was an advantage. Volunteers could then receive sequential skin biopsies and undergo continual monitoring of the observed effect of the treatment topical against the placebo with 4-week increments over a total period of 16 weeks.
There are some limitations to this approach, namely, the unavoidable use of a positive control, that is, a topical with a different base in the absence of knowing the key active component/s of the treatment topical. It would also have been useful to extend the study period beyond 16 weeks to assess whether longer term, the observed findings would have persisted and/or if topical would have affected redness and pigmentation. However, compliance remains the major issue with longer-term studies. Technical errors both machine and operator dependent may also occur, as is the case when looking at the noninvasive data for elasticity. On day 0, both arms are comparing normal “healthy” skin, so variability at this point is a potential limitation of using the Dermalab system for measurements in elasticity.
This clinical research study demonstrates how using noninvasive quantifiable measures and invasive techniques, including biopsy-derived gene and protein studies, can objectively evaluate the clinical effects of any topical formulation, in human skin scarring.
Innovation
There is a paucity of relevant methods for topical evaluation: sequential time points, as opposed to static evaluation of wound healing, is one advantage, in addition to using devices. There is no single device that can measure all parameters.21 Many provide data on multiple features, strengthening validity of data. IHC and qRT-PCR corroborate noninvasive findings with positive upregulation of protein and gene expression, respectively, for each significant finding observed clinically, presenting the findings under physiological, structural, and mechanical parameters. MEBO Scar ointment demonstrates how this methodology can provide significant findings even when active compounds within a topical are unknown.
Key Findings
The benefit of this double-blinded, randomized trial using sequential biopsies in human skin allows assessment of wound healing over time, and evaluates the impact of topical application of wound healing and scarring.
Noninvasive measurements are used to guide gene and protein studies and outcome measures documented under the physiological, structural, and mechanical properties of scars.
Meboscar™ demonstrated an increase in elasticity and hydration, and mature collagen formation compared to a positive control, supported by IHC and qRT-PCR data.
This model provides objective and quantitative evaluation of a topical for skin scarring, despite an unknown mechanism of action.
Supplementary Material
Abbreviations and Acronyms
- FLPI-2
full-field laser perfusion imaging
- IHC
immunohistochemistry
- OCT
optical coherence tomography
- qRT-PCR
quantitative real-time PCR
- SIAscopy
spectrophotometric intracutaneous analysis
- TEWL
transepidermal water loss
Acknowledgments and Funding Sources
This study was partially funded by Julphar Gulf Pharmaceutical Industries, UAE. Thanks to Katie Stocking for additional statistics and Glen Cutwerk for the illustrations. This study was selected to be presented at the Young Investigator Award, Wound Healing Society (WHS) Conference in Charlotte, North Carolina, in April 2018.
Author Disclosure and Ghostwriting
R.A.K. is an employee of Julphar Gulf Pharmaceutical Industries, UAE. For R.B., M.B., P.F., and A.B., no competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
Supplementary Material
About the Authors
Rubinder Basson, MB, BS, is a clinical doctor and surgical trainee in Manchester, currently doing a PhD at the University of Manchester, United Kingdom. Mohamed Baguneid, MD, is the Chair of Surgery, Al Ain Hospital, SEHA, Abu Dhabi Emirate, UAE. Philip Foden, MSc, is a medical statistician based at the University of Manchester, United Kingdom. Rawya Al Kredly, BSc, works in R&D at Julphar Pharma, UAE. Ardeshir Bayat, MB, BS, PhD, is a clinician scientist and Reader at the University of Manchester, United Kingdom; he is Principal Investigator and corresponding author for this article.
References
- 1. Bayat A, McGrouther DA, Ferguson MWJ. Skin scarring. Br Med J 2003;326:88–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sidgwick G, McGeorge D, Bayat A. A comprehensive evidence-based review on the role of topicals and dressings in the management of skin scarring. Arch Dermatol Res 2015;207:461–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfuhler S, Fautz R, Ouedraogo G, et al. The cosmetics Europe strategy for animal-free genotoxicity testing: project status update. Toxicol In Vitro 2014;28:18–23 [DOI] [PubMed] [Google Scholar]
- 4. Harunari N, Zhu KQ, Armendariz RT, et al. Histology of the thick scar on the female, red Duroc pig: final similarities to human hypertrophic scar. Burns 2006;32:669–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon GA, Maibach HI. The pig as an experimental animal model of percutaneous permeation in man: qualitative and quantitative observations—an overview. Skin Pharmacol Appl Skin Physiol 2000;13:229–234 [DOI] [PubMed] [Google Scholar]
- 6. Jepps OG, Dancik Y, Anissimov YG, Roberts MS. Modeling the human skin barrier—towards a better understanding of dermal absorption. Adv Drug Deliv Rev 2013;65:152–168 [DOI] [PubMed] [Google Scholar]
- 7. Mathes SH, Ruffner H, Graf-Hausner U. The use of skin models in drug development. Adv Drug Deliv Rev 2014;69:81–102 [DOI] [PubMed] [Google Scholar]
- 8. Andrade TA, Aguiar AF, Guedes FA, et al. Ex vivo model of human skin (hOSEC) as an alternative to animal use for cosmetic tests. Proc Eng 2015;110:67–73 [Google Scholar]
- 9. Majeed MUA. The use of MEBO scar ointment in the treatment and prevention of post-operative wounds and scars. Int J Pharm Res 2016;4:1171–1178 [Google Scholar]
- 10. Atiyeh BS, El-Musa KA, Dham R. Scar quality and physiologic barrier function restoration after moist and moist-exposed dressings of partial thickness wounds. Dermatol Surg 2003;29:14–20 [DOI] [PubMed] [Google Scholar]
- 11. Atiyeh BS, Loannovich J, Al-Amm CA, El-Musa KA, Dham R. Improving scar quality: a prospective clinical study. Aesthetic Plast Surg 2002;26:470–476 [DOI] [PubMed] [Google Scholar]
- 12. El-Hadidy MR, El-Hadidy AR, Bhaa A, Asker SA, Mazroa SA. Role of epidermal stem cells in repair of partial-thickness burn injury after using Moist Exposed Burn Ointment (MEBO(®)) histological and immunohistochemical study. Tissue Cell 2014;46:144–151 [DOI] [PubMed] [Google Scholar]
- 13. Tang QL, Han SS, Feng J, et al. Moist exposed burn ointment promotes cutaneous excisional wound healing in rats involving VEGF and bFGF. Mol Med Rep 2014;9:1277–1282 [DOI] [PubMed] [Google Scholar]
- 14. Mustoe TA. Evolution of silicone therapy and mechanism of action in scar management. Aesth Plast Surg 2008;32:82–92 [DOI] [PubMed] [Google Scholar]
- 15. Fabbrocini G, Marasca C, Ammad S, et al. Assessment of the combined efficacy of needling and the use of silicone gel in the treatment of c-section and other surgical hypterophic scars and keloids. Adv Skin Wound Care 2016;29:408–411 [DOI] [PubMed] [Google Scholar]
- 16. Welzel J, Reinhardt C, Lankenau E, Winter C, Wolff H. Changes in function and morphology of normal human skin: evaluation using optical coherence tomography. Br J Dermatol 2004;150:220–225 [DOI] [PubMed] [Google Scholar]
- 17. Greaves N, Morris J, Benater B, Alonso-Rasgado T, Baguneid M, Bayat A. Acute cutaneous wounds treated with human decellularised dermis show enhanced angiogenesis during healing. PLoS One 2015;10:e00113209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moncrieff M, Cotton S, Claridge E, Hall P. Spectrophotometric intracutaneous analysis: a new technique for imaging pigmented skin lesions. Br J Dermatol 2002;146:448–457 [DOI] [PubMed] [Google Scholar]
- 19. Hua W, Fan LM, Dai R, et al. Comparison of two series of non-invasive instruments used for the skin physiological properties measurements: the DermaLab® from Cortex Technology vs. the series of detectors from Courage & Khazaka. Skin Res Technol 2017;23:70–78 [DOI] [PubMed] [Google Scholar]
- 20. Zurada JM, Kriegel D, Davis IC. Topical treatments for hypertrophic scars. J Am Acad Derm 2006;55:1024–1031 [DOI] [PubMed] [Google Scholar]
- 21. Ud-din S, Bayat A. Non-invasive objective devices for monitoring the inflammatory, proliferative and remodelling phases of cutaneous wound healing and skin scarring. Expo Derm 2016;25:579–585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.