Abstract
Objective: To develop a cost-effective and clinically usable therapy to treat full-thickness skin injuries. We accomplished this by preparing a viscoelastic hydrogel using polyethylene glycol (PEG)-modified platelet-free plasma (PEGylated PFP) combined with human adipose-derived stem cells (ASCs).
Approach: PEGylated PFP hydrogels were prepared by polymerizing the liquid mixture of PEG and PFP±ASCs and gelled either by adding calcium chloride (CaCl2) or thrombin. Rheological and in vitro studies were performed to assess viscoelasticity and the ability of hydrogels to direct ASCs toward a vasculogenic phenotype, respectively. Finally, a pilot study evaluated the efficacy of hydrogels±ASCs using an athymic rat full-thickness skin wound model.
Results: Hydrogels prepared within the range of 11 to 27 mM for CaCl2 or 5 to 12.5 U/mL for thrombin exhibited a storage modulus of ∼62 to 87 Pa and ∼47 to 92 Pa, respectively. The PEGylated PFP hydrogels directed ASCs to form network-like structures resembling vasculature, with a fourfold increase in perivascular specific genes that were confirmed by immunofluorescent staining. Hydrogels combined with ASCs exhibited an increase in blood vessel density when applied to excisional rat wounds compared with those treated with hydrogels (110.3 vs. 95.6 BV/mm2; p < 0.05). Furthermore, ASCs were identified in the perivascular region associated with newly forming blood vessels.
Innovation: This study demonstrates that PFP modified with PEG along with ASCs can be used to prepare cost-effective stable hydrogels, at the bed-side, to treat extensive skin wounds.
Conclusion: These results indicate that PEGylated plasma-based hydrogels combined with ASCs may be a potential regenerative therapy for full-thickness skin wounds.
Keywords: platelet-free plasma, PEGylated hydrogels, adipose-derived stem cells, vascularization, skin regeneration
Robert J. Christy, PhD.

Introduction
Blood and its plasma subcomponents are classified as therapeutic products that can be sourced from one's own body (autologously), which are suitable for immediate clinical application or pooled from multiple donors.1,2 Clinically, autologous platelet-rich plasma (PRP), a combination of residual plasma and platelets, has been used to treat various subacute and chronic tissue injuries.3–9 Before applying to a wound, PRP is typically coagulated or clotted using thrombin, which changes PRP from a liquid to a gel. This process involves molecular interactions between plasma proteins, causing aggregation of platelets within a network of fibrin.10 While PRP has documented successes clinically, a recent review shows its usage has been inconsistent.11 This lack of success may be a result of donor to donor variability in platelet numbers or variations in the preparation of the PRP gels. Specifically, no standard methods exist on the preparation and administration of PRP, including the number of treatments, platelet concentration ranges, level of platelet activation, and degree of white blood cell contamination.12
Fibrin from plasma has been shown to be an excellent biomaterial to prepare biocompatible, less immunogenic, and biodegradable scaffolds. Using the isolated fibrinogen from plasma, clinically useful products such as fibrin sealants have been developed.13,14 Unfortunately, similar to PRP gels, fibrin-based products have been shown to have variability with regard to their mechanical properties and clinical outcomes.11 Still, due to the unique biological properties and scaffold architecture, fibrin has become one of the preferred biomatrices used for soft tissue injuries.15,16 In fact, many clinical studies have used fibrinogen and fibrin-based products for tissue regeneration, such as adipose,17 cardiovascular,18,19 ocular,20 muscle,21,22 and skin.23,24 Unfortunately, the use of fibrin-based gels for tissue regeneration still has several disadvantages, including cost, shrinkage, low mechanical strength, and rapid degradation.18,25 To overcome these problems, fibrin is often modified or mixed with other biomaterial matrices for use as a tissue engineering scaffold. One such approach is copolymerizing fibrin with polyethylene glycol (PEG).26–28 Recently, PEGylated fibrin hydrogels have been shown to be better than collagen and fibrin hydrogels for wound healing.29 Our previous studies demonstrated that PEGylated fibrin hydrogels, when combined with adipose-derived stem cells (ASCs), improved angiogenesis during the wound healing process.30 Clinically, ASCs have been shown to modulate the skin wound healing process, especially with regard to fibroblast activation, proliferation, collagen synthesis, and accelerated re-epithelialization.31
Based on our earlier in vitro and in vivo studies using PEGylated fibrin hydrogels,30 we hypothesized that human blood plasma could be used as a lower-cost alternative source of fibrinogen and the PEG modification would generate a stable hydrogel. Since plasma fibrinogen concentrations in healthy individuals are within a predictable range,25,32 it may be a reliable source material to develop consistent hydrogels. Unlike PRP, which is widely used as an autologous treatment, platelet-free plasma (PFP) is being used as a potential allogeneic treatment, since it avoids complications from the presence of platelets. Platelets are problematic because they release a number of inflammatory chemokines and cytokines, such as interleukin 1 beta (IL-1β), CD40L, and platelet factor 4 (PF4). In addition, the surface receptor on platelets (p-selectin) becomes activated differentially depending on the environmental stimulus. The presence of platelets can also cause activation of antigen-presenting cells, modulate dendritic cell activation, and enhance T cell responses.33,34
Overall, this study has three goals. First, optimization of a PEGylated PFP hydrogel will be conducted by varying the PEG, calcium chloride (CaCl2), and thrombin concentrations. Second, in vitro analysis of PEGylated PFP hydrogels cultured with human ASCs will be utilized to downselect conditions for animal experiments. Finally, using an athymic rat excision wound model, we will determine the angiogenic efficacy of PEGylated PFP hydrogels with and without human ASCs.
Clinical Problem Addressed
Burn patients with full-thickness wounds undergo tangential excision to remove dead or dying tissue, followed by treatment with their own skin (e.g., autograft). In large burns, the availability of donor-site skin for grafting is an issue. FDA-approved dermal substitutes or allografts are used to address this issue. These are temporary wound covers with demonstrated clinical safety and have minimal adverse effects. However, their clinical success has been variable due to their acellular nature and limited integration to the wound.35,36 To reconcile the essential pitfalls of currently available treatment options, we have developed a hydrogel from human PFP as an alternative to treat full-thickness wounds.
One of the immediate host responses to a traumatic injury following a physiological insult is clot formation. An insoluble fibrin clot is formed by cleavage of intrinsic fibrinogen, derived from blood plasma, in the presence of clotting factors, thrombin and calcium. The fibrin clot provides the provisional matrix for the host to secrete chemotactic factors to initiate various cellular responses, including angiogenesis. However, fibrinogen in its normal blood-derived state forms a mechanically weak and unstable clot/gel. To enhance the stability of the gel, we have developed a stable plasma hydrogel using PEG modification (PEGylation). The use of a platelet-free plasma-based hydrogel as a therapeutic adjunct to improve wound healing circumvents the current clinical inconsistencies observed due to the usage of PRP. The presence of platelets and leukocytes may trigger immune responses and therefore, limits its use to autologous, freshly isolated applications.37 This brings up another issue of sourcing which for some patients may not be able to withstand blood withdrawal. PFP hydrogels may widen the scope of allogeneic plasma usage to produce hydrogels and we have implemented this idea in this current study to provide a proof of concept to its clinical applicability.
Materials and Methods
Platelet-free plasma
Human PFP was purchased from South Texas Blood & Tissue Center (STBTC; San Antonio, TX) from healthy donors (n = 4) within the range of 20–50 years of age. Citrated phosphate dextrose was used as an anticoagulant. The samples of PFP were thawed at 37°C for 45 to 60 min, aliquoted in 50-mL conical tubes, and centrifuged at 4,300 g for 30 min at room temperature to ensure complete platelet removal. The PFP (supernatant) was transferred to separate tubes and stored at −80°C until further use. Aliquots of each PFP sample were examined for platelets and red blood cells (RBCs) to confirm purity using an Advia 120 hematology analyzer (Siemens Medical Solutions, Malvern, PA). Each PFP sample was subjected to biochemical analysis using a Siemens Multifibren U Automated coagulation analyzer to determine the concentration of fibrinogen in PFP.
Preparation of PEGylated PFP hydrogels
Our previous studies have explored the use of PEGylated fibrinogen (FPEG) hydrogels consisting of purified PEG and fibrinogen, which are crosslinked using 25 U/mL thrombin in 40 mM of CaCl2 at a final concentration of 12.5 U/mL thrombin.30,38 PEGylated PFP hydrogels were prepared using our previously published protocol for preparing FPEG gels with slight modifications.39,40 Briefly, succinimidyl-glutarate polyethylene glycol (SG-PEG-SG, 3400 Da; NOF America Corporation, White Plains, NY) was dissolved in tris-buffered saline (TBS; Sigma-Aldrich, St. Louis) at a stock concentration of 8 mg/mL, sterilized with a 0.22 μm filter, mixed with PFP (1:10–1:100 ratio of PEG:PFP), and incubated for 20 min in a 5% CO2 humidified incubator at 37°C to allow the PEGylation reaction to occur to form the hydrogels. Gelation of the PEGylated PFP liquid mixture was initiated either using CaCl2 (11–27 mM; Sigma-Aldrich) or thrombin (5–12.5 U; Sigma-Aldrich) from stock concentrations of 1 M CaCl2 or 100 U/mL thrombin, respectively.
Rheological characterization of PEGylated PFP hydrogels
Storage modulus (G′) was determined by testing small-strain oscillatory shear using a rheometer (AR-G2; TA Instruments Ltd., New Castle, DE) with parallel plate geometry. Duplicate PFP hydrogels from all four donors (mixture of 2 mL total volume, 1.8 cm diameter, and 6–7 mm thick) were tested for all thrombin and CaCl2 concentrations by placing the hydrogels on the bottom plate of the rheometer and lowering the top plate until it contacted the entire surface of the gel. Hydrogels were then subjected to frequency and strain sweeps to determine the linear viscoelastic region of the hydrogels. After a short preshear period, the G′ was measured at an angular frequency of 1–100 rad s−1 and a 1% strain.
Scanning electron microscopy of PEGylated PFP hydrogels
The ultrastructural characteristics of PEGylated plasma hydrogels prepared with both CaCl2 and thrombin were analyzed using scanning electron microscopic (SEM) technique. For SEM analysis, briefly, the hydrogels were washed three times with phosphate-buffered saline, and carefully dehydrated in a series of graded alcohols (30%, 50%, 70%, 80%, 90%, 95%; 10 min each and finally kept in 100% alcohol overnight). The samples were then dried by critical point drying method using EMS 3100 critical point dryer (Electron Microscopy Sciences, Hatfield, PA). The dried samples were sputter coated with a thin layer (10 nm) of gold and palladium using a Hummer 6.2 sputter coater (Anatech, Union City, CA) and examined on a Zeiss Sigma VP 40 (Zeiss-Leica, Thornwood, NY) in a high-vacuum mode up to 20 kV.
Preparation of PEGylated PFP and ASCs hydrogels
ASC culture
This study was conducted under the protocol reviewed and approved by the U.S. Army Medical Research and Materiel Command Institutional Review Board. Discarded burn tissue samples were collected in accordance with the approved protocol with appropriate written informed consent as to the use of the discarded tissue (H-11-020/M-10128). ASCs were isolated from debrided burn tissue of burn patients at the United States Army Institute of Surgical Research (USAISR) Burn Center, Fort Sam Houston, TX. We have previously shown this tissue contains viable harvestable ASCs that maintain the typical stem cell marker profile and differentiation potential for ASCs.38,41 Briefly, adipose tissue was dissected from discarded skin and finely minced. The minced tissue was suspended in Hank's buffered salt solution (HBSS) and centrifuged for 10 min at 500 g at 16°C. The floating tissue was carefully collected and digested with collagenase type II (3,500 U/mL; Sigma-Aldrich) for 45 to 60 min at 37°C in an orbital shaker incubator at 125 rpm. The undigested tissue was removed by sequential passage through 100 μm and 70 μm filters. The filtrate was centrifuged at 500 g for 10 min at 16°C, treated with lysing buffer (BD Bioscience, San Jose, CA) to remove RBCs, and washed twice with HBSS. The final cell pellets were resuspended in “growth media,” MesenPRO RS™ supplemented with MesenPRO RS growth supplement, antibiotic/antimycotic (100 U/mL of penicillin G, 100 μg/mL of streptomycin sulfate, and 0.25 μg/mL of amphotericin B), and 2 mM of L-glutamine (Life Technologies, Carlsbad, CA). The resulting nucleated cells were cultured in a 5% CO2 humidified incubator at 37°C. After 4 h in culture, the growth media was replaced to remove any floating debris and nonadherent cells. The remaining attached cells were designated as passage 0 (P0) ASCs. All in vitro and in vivo studies were carried out using ASCs of passage 2–4.
ASC-seeded hydrogels
Passage 2 ASCs (50,000 cells/mL) were mixed into the PEGylated PFP solution and gelation of the liquid mixture was initiated either using CaCl2 (15–27 mM) or thrombin (5–12.5 U). The 2 mL PEGylated PFP+ASCs hydrogels were incubated for 20–30 min in a 5% CO2 humidified incubator at 37°C to complete gelation. The hydrogels were cultured in growth media in a 5% CO2 humidified incubator at 37°C for up to 11 days with media changes occurring every 2 days. Phenotypic changes of ASCs within the hydrogels were observed and photomicrographs were taken at 3, 5, 7, and 11 days using an Olympus IX71 inverted microscope and DP71 digital camera (Olympus America, Inc., Center Valley, PA).
Real-time polymerase chain reaction
Total ribonucleic acid (RNA) from ASCs in PEGylated PFP hydrogels was isolated on day 11 using TRIzol LS reagent (Life Technologies) with modifications.30 Briefly, two 2 mL hydrogels from each PEGylated PFP treatment group were pooled together, minced, and incubated on ice for 10–15 min with 8 mL of TRIzol LS reagent. After incubation, 4 mL of chloroform was added, mixed, and the aqueous phase containing RNA was then isolated by centrifugation and purified using mini-spin columns. RNA was extracted from two separate experiments from each PFP sample used (n = 4). The concentration and quality of the purified RNA was determined at OD260/280 using a spectrophotometer (NanoDrop Technologies, Inc., Wilmington, DE). Complementary deoxyribonucleic acid (cDNA) was synthesized using SuperScript III first-strand synthesis supermix with oligo-dT primers (Thermo Fisher Scientific, Waltham, MA). Oligonucleotide primer sequences specific to pericyte markers: chondroitin sulfate proteoglycan/neuroglial factor 2 (NG2), platelet-derived growth factor receptor beta (PDGFRβ), and α-smooth muscle actin (α-SMA); angiogenesis-related growth factors: angiopoietins 1 and 2 (ANGPT1 and ANGPT2), and vascular endothelial growth factor (VEGF) were purchased from SA Biosciences (Frederick, MD). Master mixes containing forward and reverse primers with SYBR® Green ER™, real-time polymerase chain reaction (RT-PCR) supermix (Thermo Fisher Scientific), and the synthesized cDNA were added to appropriate wells. RT-PCR was carried out using a Bio-Rad CFX96 thermal cycler system (Bio-Rad, Hercules, CA). Messenger RNA (mRNA) expression levels were normalized to glyceraldehye-3-phosphate dehydrogenase. Expression level fold changes were determined by 2−ΔΔCT method for each marker listed above.42 Relative fold expression for each gene was calculated by comparing the expression levels of control passage 2 ASCs.
Immunophenotypic characterization of ASCs in PEGylated PFP hydrogels
Passage 2 ASCs (50,000 cells/mL) were characterized after culturing for 11 days within PEGylated PFP hydrogels prepared using 23 mM CaCl2 or 10 U thrombin. The hydrogels were cryopreserved and embedded using a gradient sucrose cryopreservation technique.43 Briefly, the hydrogels were washed with HBSS, fixed with 4% paraformaldehyde (PFA; Electron Microscopy Sciences) for 20 min, serially treated with increasing concentrations of sucrose (from 5% to 20%, 30 min each incubation), and then incubated overnight with 20% sucrose at 4°C. The gels were then embedded in a Histoprep™: 20% sucrose (Fisher, Pittsburgh, PA) mixture, frozen by immersing in liquid nitrogen-cooled isopentane, and stored at −80°C. Cryosections, 10–12 μm thick, were cut using a cryostat (Leica Microsystems, Nussloch, GmbH), washed with sterile HBSS, and fixed with 4% PFA for 20 min. Sections were blocked by incubating for 1 h with 5% donkey serum in HBSS or with 1% bovine serum albumin in HBSS containing 0.01% Triton X-100 and then washed with HBSS. The immunophenotypic changes in ASCs were then determined by staining sections with Alexa Fluor 594 phalloidin (Life Technologies), anti-human α-SMA, NG2, and ANGPT1 (all R&D Systems, Minneapolis, MN) at 4°C overnight. Sections were washed with HBSS and incubated with host species-specific Alexa Fluor 594 secondary antibodies (Life Technologies) for 45 min at room temperature. Finally, all the sections were washed with HBSS and nuclei stained with DAPI (4′,6-diamidino-2-phenylindole, dihydrochloride; Life Technologies) for 20 min at room temperature. Nonspecific fluorescence was evaluated using sections incubated with respective isotype controls and fluorophore-labeled secondary antibodies. Fluorescence images were captured using Olympus IX71 inverted microscope equipped with a reflecting fluorescence system (Olympus America, Inc.) and a DP71 digital camera.
In vivo evaluation of PEGylated PFP hydrogels
Animal research was conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations, and the principles of the Guide for the Care and Use of Laboratory Animals, National Research Council. The facility's Institutional Animal Care and Use Committee approved all research conducted in this study. The facility, where this research was conducted, is fully accredited by AAALAC International. Male rnu nude rats (athymic), deficient in T cell function, weighing 175 to 225 g, were obtained from Harlan Laboratories (Indianapolis, IN). This strain of rats were used to reduce the risk of an immune response toward the human ASCs tested. Rats were housed individually in a temperature-controlled environment with a 12-h light/12-h dark cycle in the AALAAC-approved vivarium at the USAISR with access to water and rat chow ad libitum. A full-thickness skin excision wound of 1.5 cm in diameter was created on the dorsum of anesthetized rats down to the panniculus. Buprenorphine™ SR LAB (1.0–1.2 mg/kg; ZooPharm, Windsor, CO) was subcutaneously administered as analgesia once preoperatively. Anesthesia was maintained at a vaporizer setting of 0.5–3% isoflurane delivered with a nose cone on a Bain circuit hooked to the rodent gas anesthesia machine (VetEquip, Inc., Pleasanton, CA). The back was aseptically prepared by clipping the hair and sterilizing with betadine and 70% alcohol. Animals were randomly divided into four groups with two rats per group and each wound was treated with 2 mL of preformed PEGylated PFP hydrogels. The groups are designated as follows: 23 mM CaCl2–PEGylated PFP gel, 23 mM CaCl2–PEGylated PFP gel containing 50,000 ASCs/mL of hydrogel, 10 U thrombin–PEGylated PFP gel, or 10 U thrombin–PEGylated PFP gel containing 50,000 ASCs/mL of hydrogel. Control animals (n = 4) were treated with saline only. All of the wounds were covered with DuoDERM® hydrocolloid dressing (ConvaTec, Skillman, NJ) to maintain a moist environment. The wound beds, including the margin of healed area surrounding the wound, were harvested on day 8 for histology.
Histology and image analysis
Histological analysis was performed on ∼7 μm sections of formalin-fixed paraffin-embedded wound and neighboring normal skin tissue. Deparaffinized sections were hydrated and stained with Masson's trichrome to differentiate between collagen, smooth muscle, and cellular components during the healing process. Sections were stained with Picrosirius Red (PSR; Polysciences, Inc., Warrington, PA) to observe collagen structural organization within the wound bed. Briefly, sections were stained with PSR F3B solution for 1 h, washed in 0.01 N hydrochloric acid, dehydrated, air dried, and mounted (Histomount; National Diagnostics, Atlanta, GA). Polarized images of the stained tissue sections were acquired with an Olympus BX60 microscope (Center Valley, PA) equipped with a polarizing filter. Individual images were stitched together to form a single montage image of the complete tissue section using Research Image Composite Editor (Microsoft Corporation, Redmond, WA).
Blood vessel density quantification
Blood vessel density was quantified using tissue sections from each treatment group by immunolabeling the paraffin-embedded sections with rabbit polyclonal anti-factor VIII (or von Willebrand factor [vWF]) antibody (Cell Marque, Rocklin, CA) using the Ventana ultraView™ automated tissue immunostainer (Ventana Medical Systems, Inc., Tucson, AZ). The central portion of each wound bed (typically 70–120 frames/section) was imaged at high magnification ( × 400). Approximately, 33% of the microscopic images were randomly selected from the center of the wound bed to maintain consistent sizing and density sampling. Blood vessel density was calculated by counting the total number of blood vessels per image using ImageJ 1.45s software with cell counter plugin and dividing by the area of the image. Quantification parameters were (1) blood vessels with intact lumen, (2) blood vessels with positive staining and vessel structure even if lacking a lumen, and (3) blood vessels lacking strong positive staining with a clear lumen containing RBCs were also included. Counts were conducted by two blinded reviewers and their values averaged.
Identification of human ASCs within the wound bed and around host vasculature
Tissue samples were also cryopreserved and sucrose embedded using the gradient sucrose cryopreservation technique.38 Cryosections were incubated with human-specific mitochondrial protein antibody overnight at 4°C (Millipore, Billerica, MA). The sections were washed with HBSS and incubated with Alexa Fluor 594-labeled secondary antibody for 45 min at room temperature. The sections were then washed with HBSS and incubated with rat-specific fluorescein isothiocyanate (FITC)-labeled CD31 antibody (ABD Serotech, Raleigh, NC) for 45 min at room temperature. The nuclei were stained with DAPI and incubated for 20 min at room temperature. Nonspecific fluorescence was determined by using tissue sections incubated with respective isotype controls and with fluorophore-labeled secondary antibodies.
Statistical analysis
Analysis of storage modulus (G′) within the hydrogel samples and blood vessel density in the tissue were performed using one-way analysis of variance (ANOVA) with Bonferroni post hoc test. Statistical significance was noted when p < 0.05.
Results
PFP forms a stable hydrogel after PEGylation
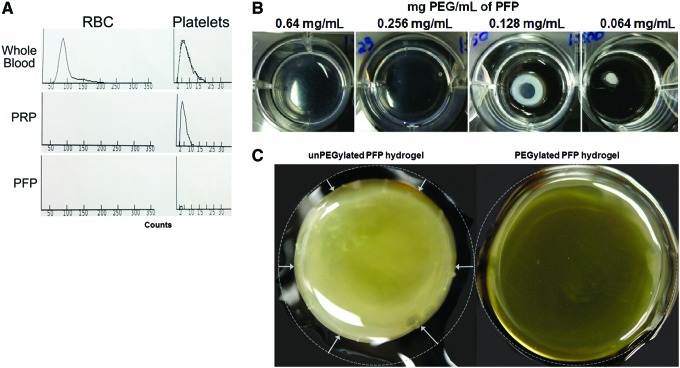
Human PFP contained undetectable numbers of RBCs and platelets in comparison to whole blood and PRP (Fig. 1A). The average concentration of fibrinogen present in the PFP was 2.32 mg/mL (n = 4; assayed in triplicate, data not shown). We screened different final concentrations of PEG (0–2.0 mg/mL) and determined its effect on PFP hydrogel formation. PFP formed stable hydrogels within a PEG concentration range of 0.4–1.2 mg/mL. Hydrogels prepared with higher concentrations of PEG (1.2–2.0 mg/mL) were less viscoelastic, with observable structural deformation. Hydrogels prepared at lower concentrations (<0.2 mg/mL) were also less viscoelastic and had a contracted appearance (Fig. 1B). Therefore, all of our experiments were performed with PEGylated PFP hydrogels prepared at a final PEG concentration of 0.64 mg/mL. Activation of the endogenous fibrinogen in PFP with thrombin (12.5 U) resulted in a hydrogel but when released from the cell culture inserts the hydrogel collapsed with a significant amount of fluid loss (Fig. 1C unPEGylated hydrogel). PEGylation of PFP before activation with thrombin resulted in the formation of stable hydrogels with minimal shrinkage and fluid loss. Representative images show that unPEGylated PFP hydrogels had an opaque precipitated appearance, whereas, PEGylated PFP hydrogels were clear and translucent (Fig. 1C).
Figure 1.
Histogram showing quantification of RBCs and platelets in whole blood, PRP, and PFP preparation using an Advia 120 hematology analyzer (A). Photographs of PFP hydrogels prepared with different concentrations of PEG. Hydrogels formed with 0.64 mg/mL of PFP-formed stable hydrogels, which slowly lost its stability and structure with decreasing concentration of PEG, resulting in hydrogels' shrinkage (B). Images of unPEGylated and PEGylated PFP hydrogels prepared using 12.5 U of thrombin (C). RBCs, red blood cells; PEG, polyethylene glycol; PFP, platelet-free plasma; PRP, platelet-rich plasma. Color images are available online.
Viscoelastic properties of PEGylated PFP hydrogels
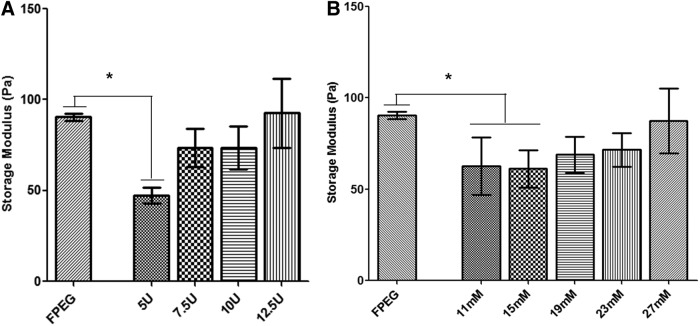
PEGylated PFP hydrogels were generated by reversing the anticoagulant effect using excess calcium chloride thereby activating endogenous thrombin in the PFP or adding thrombin exogenously to convert and crosslink fibrinogen within the PFP. In this study, we were able to generate stable hydrogels using both thrombin and CaCl2. PEGylated PFP crosslinked by either thrombin (5–12.5 U) or the addition of CaCl2 (11–27 mM) resulted in the formation of hydrogels with similar viscoelastic properties. The storage modulus (G′) of the PEGylated PFP hydrogels increased proportionately with different concentrations of both thrombin and calcium (Fig. 2). The storage modulus of the hydrogels prepared with 12.5 U thrombin (92.5 Pa) and 27 mM CaCl2 (87.4 Pa) was comparable to FPEG hydrogels that we have previously tested (90.4 Pa). Although hydrogels prepared using 7.5 U (73.6 Pa) and 10 U (73.5 Pa) of thrombin or 19 mM (68.9 Pa) and 23 mM (71.6 Pa) CaCl2 exhibited slightly lower G′, they were not statistically different from FPEG. Comparative analysis of G′ value within the hydrogel samples using one-way ANOVA detected a statistically significant difference in hydrogels prepared with 5 U thrombin (47.3 Pa, p = 0.0079), 11 mM (62.5 Pa, p = 0.037), and 15 mM CaCl2 (61.1 Pa, p = 0.0337) compared with FPEG (Fig. 2A, B). Similar to previous results, unPEGylated PFP hydrogels prepared with 10 U thrombin or 23 mM CaCl244 did not form stable hydrogels to obtain measurements.
Figure 2.
Rheological measurements of PEGylated PFP hydrogels using different concentrations of thrombin (5–12.5 U) (A) and CaCl2 (11 mM–27 mM) (B) in comparison to FPEG hydrogels. One-way analysis of variance exhibited a significant difference in storage modulus (G′) in hydrogels prepared with 5 U thrombin, 11 mM CaCl2, and 15 mM CaCl2 compared with FPEG hydrogels. *p < 0.05. CaCl2, calcium chloride; FPEG, PEGylated fibrinogen.
Ultrastructural observations of PFP hydrogels
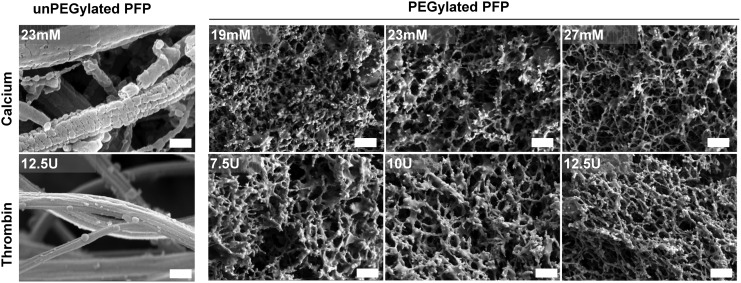
High-resolution SEM images were used to acquire information on ultrastructural details of PEGylated and unPEGylated PFP hydrogels (Fig. 3). The unPEGylated PFP hydrogels, crosslinked with thrombin or CaCl2, exhibited classical open fiber fibrin architecture. The open structure of unPEGylated PFP contributes to the inability of PFP to form a true hydrogel; therefore, it lacked the capacity to hold aqueous volume leading to gel shrinkage (Fig. 1). On the other hand, the PEGylated PFP hydrogels exhibited highly crosslinked, porous fiber architecture and the fibrin network appeared as dense interspersed bundles. Regardless of crosslinking type (thrombin or CaCl2), the fiber network has a fairly uniform appearance with ∼200 nm size pore density. Furthermore, we did not observe a huge variability in bulk property of fibrous structure across different concentrations of thrombin or CaCl2. Therefore, PEGylated PEP hydrogels with similar architecture could be prepared within a wide range of thrombin or CaCl2 concentrations.
Figure 3.
Scanning electron microscope images of unPEGylated and PEGylated hydrogels, crosslinked with either thrombin (5–12.5 U) or CaCl2 (11–27 mM). The PEGylated hydrogel, crosslinked with both CaCl2 and thrombin, exhibits highly porous structures in comparison to an open fiber architecture observed in unPEGylated hydrogels. The images were acquired at the same magnification (scale bar = 500 nm).
Network formation of ASCs within PEGylated PFP hydrogels
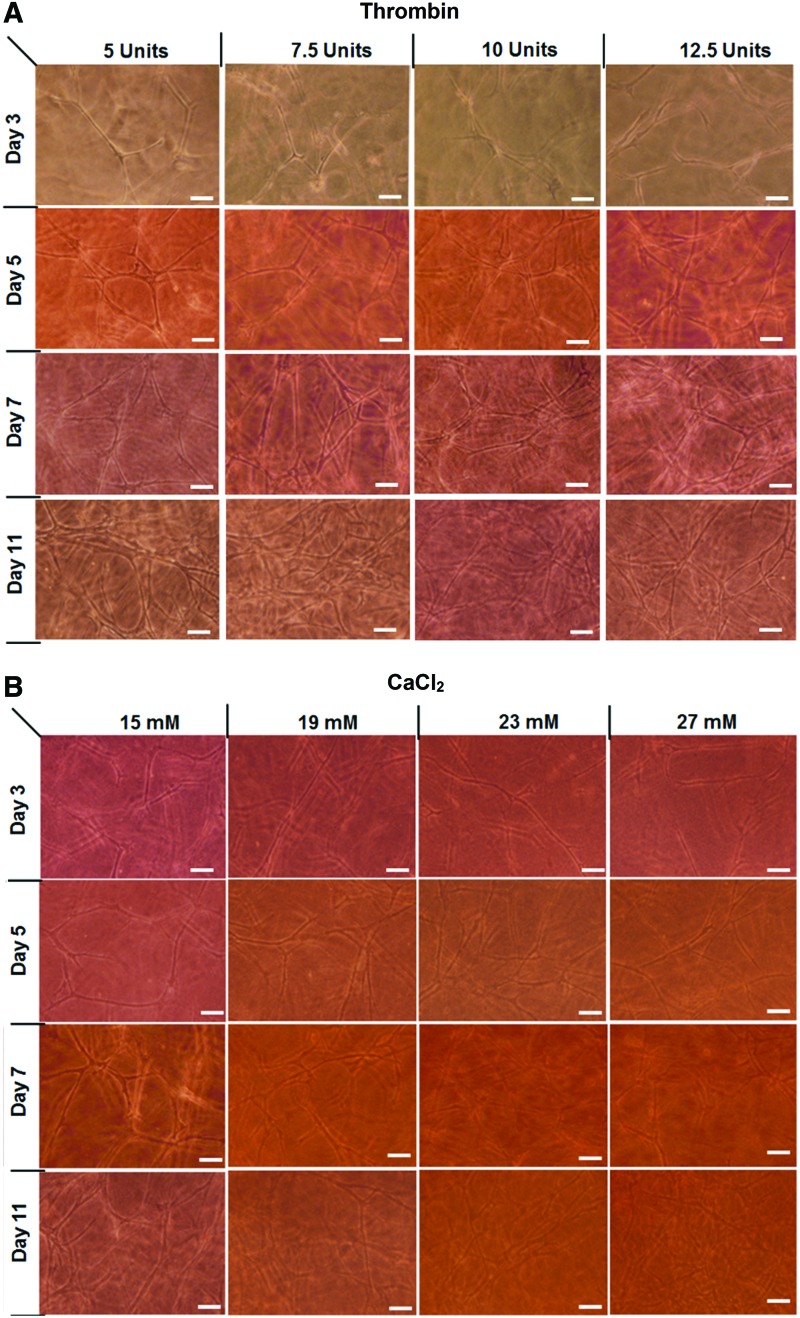
Photomicrographs in Fig. 4A and B represent a time course of ASCs developing network structures in PEGylated PFP hydrogels prepared with different concentrations of thrombin and CaCl2, respectively. Up to day 7, an increase in network density was observed, which increased with thrombin and calcium concentrations. After day 7, the PEGylated PFP hydrogels were saturated with the ASC-laden networks. Hydrogels that were made with 27 mM CaCl2 appeared more granular and opaque on days 11 with significant matrix contraction. Overall, the networks formed within the thrombin-based hydrogels appeared to be more robust at days 7 and 11 in comparison to the CaCl2 hydrogels.
Figure 4.
Light microscopy images of ASCs forming tubular networks within PEGylated PFP hydrogels prepared using 5–12.5 U of thrombin (A) and 15–27 mM of CaCl2 (B). The networks that formed from the ASCs increased in both thrombin and CaCl2 hydrogels up to day 7. In comparison to CaCl2 hydrogels, the networks formed within thrombin-based hydrogels appeared to be more robust at days 7 and 11. Scale bar = 100 μm. ASCs, adipose-derived stem cells. Color images are available online.
ASCs within PEGylated PFP hydrogels express perivascular specific markers
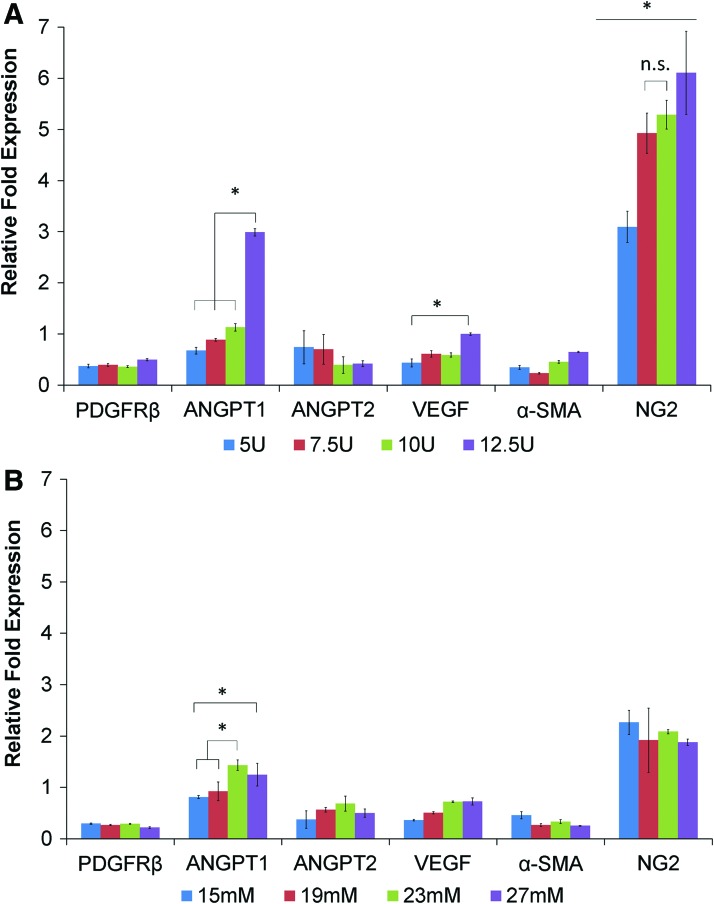
The expression level of NG2, a cell surface marker expressed by differentiating ASCs during the vascularization process, was observed to be upregulated in both thrombin and CaCl2-based PEGylated PFP hydrogels. A dose-dependent increase was detected in the expression levels of NG2 (three to sixfold) with maximum fold change exhibited by hydrogels prepared with 12.5 U of thrombin (6.1-fold) (Fig. 5A). The CaCl2-based hydrogels expressed NG2 (Fig. 5B), with 15 mM CaCl2 exhibiting the highest value (2.26-fold), whereas the other concentrations expressing slightly less. An increase in the levels of ANGPT1 transcript was detected with the highest concentration of thrombin (12.5 U, up to threefold). The CaCl2-based PEGylated PFP hydrogels exhibited a modest fold increase in ANGPT1 (1.4-fold). Expression levels of ANGPT2, a negative regulator of angiogenic signaling, decreased with increasing thrombin concentrations. Surprisingly, no significant increase in expression level of VEGF transcripts was detected in either thrombin or CaCl2-based hydrogels. Other perivascular supporting markers, α-SMA and PDGFRβ, showed a reduced expression (less than onefold) from the baseline levels of ASCs cultured on plastic ware.
Figure 5.
Quantitative real-time polymerase chain reaction analysis of RNA isolated from ASCs within PEGylated PFP hydrogels made with 5–12.5 U of thrombin (A) and 15–27 mM of CaCl2 on day 11 (B). Relative fold expression levels of perivascular specific markers, NG2 and ANGPT1, proportionately increased with thrombin concentrations, up to approximately six- and threefold, respectively. Although the transcript levels of NG2 and ANGPT1 increased in CaCl2-based hydrogels, they were relatively stable across various concentrations analyzed (15–27 mM), up to ∼2.2-fold and 1.4-fold, respectively. Gene expression levels are represented as mean fold changes (±standard deviation). *p < 0.05 comparing the different groups and n.s. means no significance was detected between those groups. α-SMA, α-smooth muscle actin; ANGPT1, angiopoietin 1; ANGPT2, angiopoietin 2; NG2, neuroglial factor 2; PDGFRβ, platelet-derived growth factor β; RNA, ribonucleic acid; VEGF, vascular endothelial growth factor. Color images are available online.
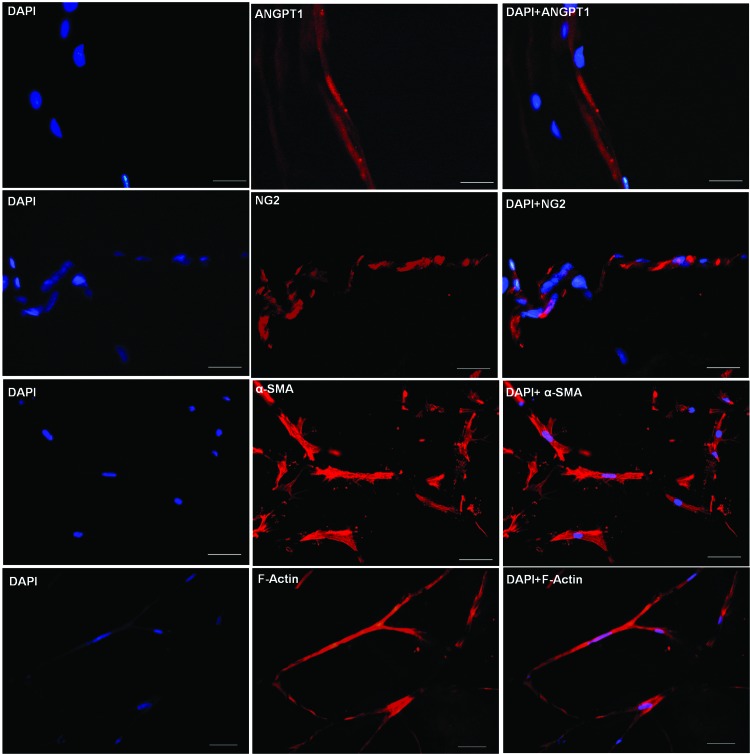
To determine if protein levels correlate with the observed gene expression changes, immunocytochemical analysis was performed. Staining of PEGylated PFP hydrogels made with both 10 U of thrombin and 23 mM CaCl2 was carried out. Both hydrogel types expressed ANGPT1, NG2, α-SMA, and F-actin proteins. A panel of representative fluorescent images of frozen PEGylated PFP hydrogel sections made with thrombin after 11 days of culture is shown in Fig. 6. Multicellular tubular-like networks within the hydrogels are depicted exhibiting positive staining for perivascular specific markers, ANGPT1 and NG2. In addition, other key perivascular protein markers, α-SMA and F-actin, which regulate phenotypic changes in ASCs, were found to be positively expressed within the hydrogels.
Figure 6.
Immunofluorescent images of vascular network-like structure formed within PEGylated plasma gels prepared with 12.5 U of thrombin on day 11. Representative images of frozen sections stained (red) for ANGPT 1, NG2, α-SMA, and actin microfilaments (F-actin). Cell nuclei were stained with DAPI (blue). Scale bar within the panel of DAPI, F-actin, α-SMA, and merge = 50 μm; DAPI, NG2, ANGPT1, and merge = 25 μm. DAPI, 4′,6-diamidino-2-phenylindole, dihydrochloride. Color images are available online.
PEGylated PFP hydrogels improve vascularization in vivo
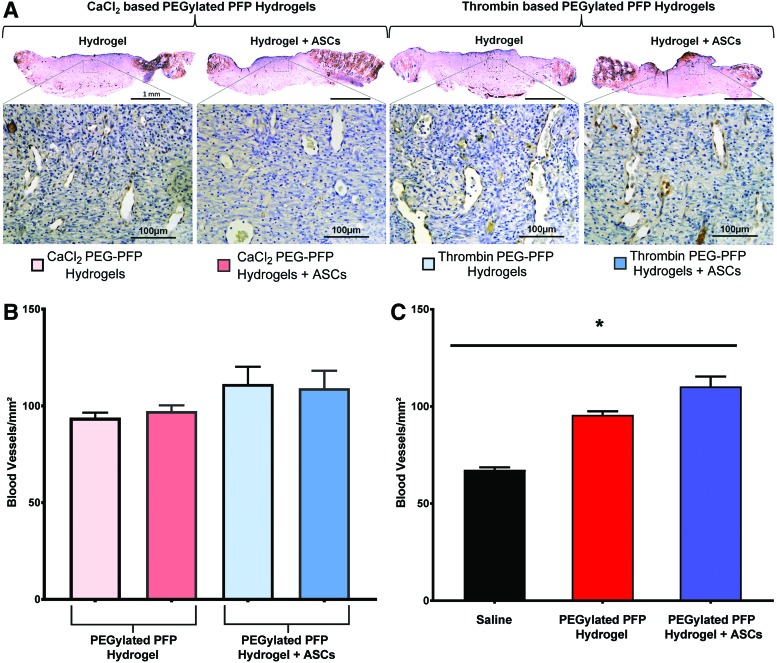
Treatment efficiency of rat excision wounds using PEGylated PFP hydrogels with ASCs was characterized by neovascularization of the wound bed after 8 days of treatment. We determined the presence of functional blood vessels by immunostaining the tissue sections of all the treatment groups using vWF. Figure 7A shows representative light microscopy images of blood vessels stained by vWF antibody. Patent blood vessels are visible in the magnified area of the scanned images. Quantitative assessment of newly formed functional vessels within the wound bed was analyzed using ImageJ and is depicted in Fig. 7B. The density of vessels measured in blood vessels/mm2 showed no statistical difference between the CaCl2 and thrombin PEGylated PFP hydrogel-treated wounds. However, when we performed a grouped analysis comparing hydrogels versus hydrogels with ASCs irrespective of what they were gelled with (CaCl2 or thrombin), a significant difference in vascular density was observed (p < 0.05; Fig. 7C: dark red = [light pink+light red from Fig. 7B] vs. dark blue [light blue+blue from Fig. 7B]).
Figure 7.
Representative light micrograph images of sections taken on day 8 show positively stained vWF+ (brown) blood vessels present within the wound beds of rats treated with various hydrogel treatment groups (scale bar = 1 mm). The magnified area of the scanned image exhibits the presence of patent blood vessels (scale bar = 100 μm) (A). In vivo blood vessel quantification within the wound beds of rats treated with 23 mM CaCl2-based hydrogels and 12.5 U thrombin-based PEGylated PFP hydrogels without or with ASCs, respectively (B). Quantitative assessment of blood vessel densities/mm2 within tissue sections stained positive for vWF+ (C). Both the CaCl2- and thrombin-based PEGylated PFP hydrogels (±ASCs) exhibited the highest blood vessel densities on day 8. Grouped comparing hydrogels versus hydrogels with ASCs irrespective of the type of crosslinking (CaCl2 or thrombin), a significant difference in vascular density was observed (110.3 vs. 95.6 BV/mm2; p < 0.05; dark red [light pink+light red] vs. dark blue [light blue+blue]). A significant difference in vascular density was observed in both PFP hydrogel groups, with or without ASCs (*p = 0.0005 and p < 0.0001 for PFP hydrogel vs. saline and PFP hydrogel+ASCs vs. saline, respectively). vWF, von Willebrand factor. Color images are available online.
ASCs localize to perivascular space during wound revascularization
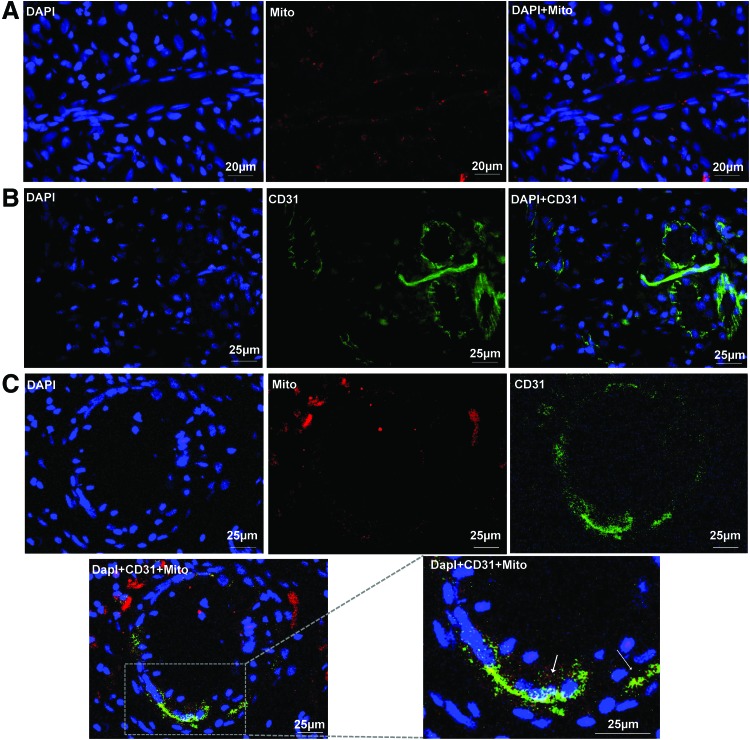
To determine the fate and contribution of human ASCs toward wound healing in the rat excision model, we stained frozen tissue biopsies with human-specific mitochondrial antibody. Representative fluorescent images of skin sections from rats treated with thrombin-based PEGylated PFP hydrogels revealed the presence of positively stained human ASCs for mitochondrial protein (Fig. 8A). Biopsies of wounds treated with CaCl2-based hydrogels showed the presence of human mitochondrial positive cells similar to that observed in the thrombin-based hydrogel-treated wounds (data not shown). A significant number of CD31+ endothelial cells were found within the granulating wound bed, indicating active vascularization following treatment with PEGylated PFP hydrogels and ASCs (Fig. 8B). These results were consistent to our observations of vWF staining experiments. Furthermore, when the complete wound area was probed, we were able to track human ASCs throughout the granulation tissue, providing evidence for their active participation in the cellular events of dermal reorganization. Specifically, some ASCs were found to be associated with newly forming blood vessels. Human ASCs (red fluorescence) were observed around the host vasculature (CD31+; green fluorescence; Fig. 8C) explicitly homing to the perivascular regions of newly formed blood vessels (high-magnification image).
Figure 8.
Representative immunofluorescent images of day 8 biopsies harvested from wounds treated with thrombin-based hydrogels show the presence of human mitochondrial protein-positive (Mito+, red) ASCs within both the stromal and blood vessel regions of the granulating wound bed (A). The blood vessels within the wound bed stained positive for host endothelial cells (CD31+, green) (B) Bottom panels: Human ASCs (Mito+, red fluorescence) were observed around the host vasculature (CD31+; green fluorescence) and higher magnification image (Mito+ and CD31+ merge) shows human ASCs to home to the perivascular regions of newly formed host blood vessels (C). The arrows in the magnified image indicates CD31 positive host blood vessel. Color images are available online.
PEGylated PFP hydrogels influences wound remodeling
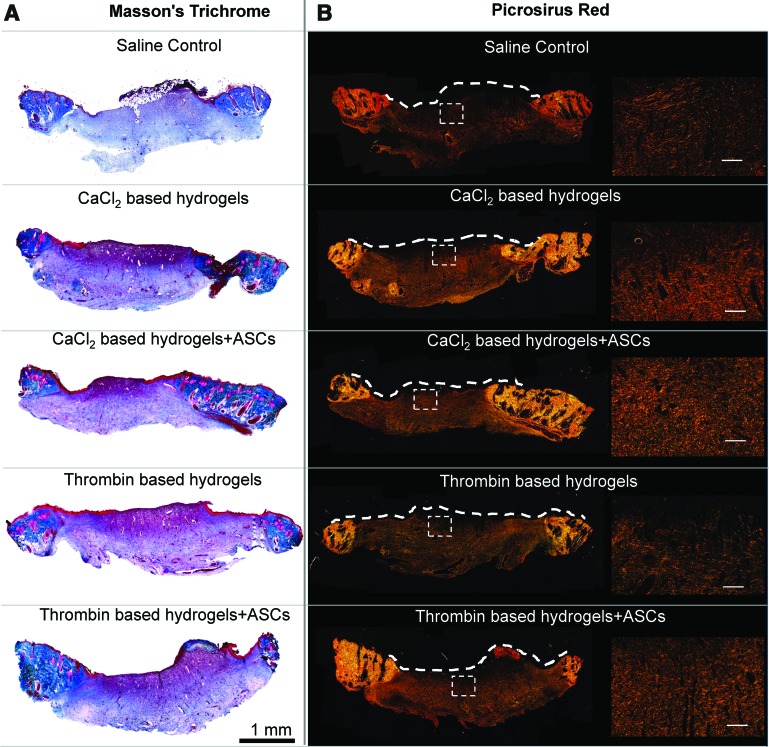
The effect of PEGylated PFP hydrogel (±ASCs) treatments on wound remodeling was assessed by staining tissue biopsies from all treatment groups with Masson's Trichrome stain. Wounds treated with PEGylated PFP hydrogels had a highly vascularized wound bed (Fig. 9A), corroborating our observations from the vascular density measurements. Extracellular remodeling was evaluated by staining tissue sections with PSR stain (Fig. 9B). Examination of birefringent collagen fibers using polarized light revealed appearance of mixed green (collagen type III) and red fiber bundles (collagen type I) in all of the treatment groups.
Figure 9.
Histological images of day 8 wound biopsies stained with MTS and polarized images of Picrosirius Red. (A) The low magnification ( × 40) of wounds treated with 23 mM CaCl2- and 12.5 U thrombin-based PEGylated PFP hydrogels (±ASCs) stained with MTS. (B) The scanned images ( × 40) of the overall organization of collagen fibers in the granulating wound bed. The dashed white line on (B) was traced by superimposing bright field images onto the corresponding polarized image and indicates the areas of wound bed with any remnant hydrogels. The enlarged image to the right is designated by the white box. Scale bar = 40 μm. MTS, Masson's Trichrome stain. Color images are available online.
Discussion
Autologous PRP gel is used clinically as a tissue sealant and in the treatment of soft tissue injuries.45,46 Although unmodified PRP gels are biocompatible, they are highly susceptible to fibrinolysis. The lack of standardization with regard to both the preparation and the administration of PRP may explain its inconsistent clinical and experimental results.12 To reconcile, we have used a simple PEG-based protein modification technique to prepare consistent and stable hydrogels. PEG has been successfully used in modifying fibrinogen, which imparts better resistance to enzymatic degradation.26 In our current investigation, human PFP is modified with a difunctional PEG to create a stable hydrogel. The major advantage of using PFP is because it is cost effective, suitable for bulk production, and easily translatable with minimal regulatory requirement for FDA approval. Furthermore, PEG-modified hydrogels are biocompatible and aid cellular migration and proliferation.47 Similar to PEGylated fibrin hydrogels, PEGylated PFP hydrogels direct ASCs to form structures resembling tubular assemblies of a vasculature.39,40 Furthermore, our study shows that PEGylated plasma-based hydrogels combined with ASCs may be a potential regenerative therapy for full-thickness skin wounds.
Fibrinogen, a major component in the plasma, is present at consistent levels (2–2.5 mg/mL) among healthy individuals.32 The plasma samples we obtained were confirmed to fall within this range (2.3 mg/mL) and yielded a stable hydrogel after PEGylation of the PFP. During this process, difunctional PEG acts as a spacer imparting stability to PFP. The PEGylated PFP hydrogels can be generated by reversing the anticoagulant effect by adding exogenous thrombin or using excess CaCl2, thereby activating endogenous thrombin to convert and crosslink the fibrinogen within the PFP. This gelation of PEGylated PFP resulted in the generation of morphologically stable hydrogels with minimal shrinkage and fluid loss.
After tissue injury, blood vessels are damaged, thereby releasing blood and its components into the wounded area. The body's normal response is to form a fibrin blood clot by the cleavage of fibrinogen after activation of endogenous thrombin from the blood. The strength of the fibrin clot depends on active units of thrombin.48 In particular, thrombin has a great influence on clot strength and also determines the structure of the gel. Although blood plasma has endogenous thrombin, the network structure of plasma fibrinogen is highly influenced by the exogenously added thrombin. The structure of the fibrin network becomes more porous with lower thrombin concentration; however, a huge excess of thrombin after the time of gelation does not qualitatively influence the gel structure.48 A previous study has shown the mean time required to prepare the PRP gels to be less than a minute,44 which we also observed with the thrombin-based PEGylated hydrogels. Still, rheometric measurements suggest that PEGylated PFP can form stable viscoelastic hydrogels with thrombin over a wide concentration range.
Another important factor that influences conversion of fibrinogen to fibrin in PFP is the ionic concentration of calcium (Ca2+).49 The addition of exogenous CaCl2 causes activation of endogenous thrombin to crosslink fibrinogen and may be considered as a viable alternative to form stable PEGylated PFP hydrogels. The fibrin networks formed with added CaCl2 were shown to be heterogeneous with extremely porous links formed initially, followed by the formation of a secondary, superimposed network of a less porous architecture.48 In this study, we observed turbidity of hydrogels formed with higher concentrations of CaCl2 (>27 mM). This may be due to random ligation of fibers with increased Ca2+ concentration.50 However, the hydrogels formed within 11–27 mM CaCl2 were stable and clear suggesting that endogenous thrombin activation is dependent on Ca2+ concentration. Ionic strength of calcium also determines clot strength.51 In the present study, the storage modulus of PEGylated PFP hydrogels prepared with 27 mM CaCl2 (87.4 Pa) was similar to PEGylated fibrin gels (90.4 Pa). The time required for complete hydrogel formation was estimated at 5 min based on visual observation. Depending on the scenario, the CaCl2-based PEGylated PFP hydrogels may not be desirable for immediate clinical application, with regard to the time required for their preparation. These findings make us conclude that the molecular composition of plasma represents an important factor to control the mechanical properties of the PEGylated PFP hydrogels.
Studies based on plasma-rich fibrin matrices suggest that the three-dimensional matrix is required to release angiogenic factors.52 Angiogenesis is a coordinated event involving several factors and receptors.53 Human plasma contains angiogenic factors54 that may allow ASCs to differentiate into vascular-like cells. In our previous studies, ASCs isolated from discarded burn skin samples were embedded within the FPEG hydrogel and tested on an excision wound using athymic rats. The major observation from those studies were that ASCs expressed pericyte/smooth muscle cell markers (α-SMA, PDGFRβ, NG2, and ANGPT1) suggesting that these cells could be involved in a supportive cell role for blood vessels.30,40 ANGPT1 has been shown to stabilize the vasculature and provides a basal signal to maintain quiescence and integrity of the endothelium in mature vessels.55,56 In this study, the thrombin-based PEGylated PFP hydrogels expressed higher levels of ANGPT1 than the CaCl2-based hydrogels. Specifically, the thrombin-based hydrogels exhibited more stable and higher expression levels (up to threefold) indicating that the gel structure influences differentiation of ASCs. The networks formed by ASCs within the PEGylated PFP hydrogels also expressed NG2 and the fold expression increased with time in culture. NG2 has been characterized as a marker of vascular pericytes and may augment the effects of various other growth factors, including platelet-derived growth factor A (PDGF-A) and basic fibroblast growth factor.57,58 Since vascular sprouting is associated with vascular cell proliferation, our results support the previous finding that NG2 is a regulator of angiogenesis. Furthermore, immunofluorescent staining of thrombin-based PEGylated PFP hydrogels confirms stable vascular-like networks formed by ASCs expressing perivascular specific proteins (ANGPT1 and NG2) along with the matrix-associated proteins (α-SMA and F-actin).
Having shown that ASCs utilize PEGylated PFP hydrogels as a platform to form pericytic networks, we then evaluated the efficiency of the PEGylated PFP hydrogels to improve wound vascularization using an athymic rat excision model. During early phases of acute wound healing, the endogenous fibrin matrix acts as a scaffold to support cell migration and further promote granulation tissue deposition. The PEGylated PFP hydrogels function as a stable supportive platform for the host to form the primary capillary plexus, which further assembled into functional blood vessels (Fig. 7A). The PEGylated PFP blended with the host provisional matrix and acted synergistically to provide a more stable cell-inductive platform. This was evident from the increased number of blood vessels formed within the wound bed of rats treated with ASCs (Fig. 7C). Previous studies have shown in vivo stem cell transplantation characterized by poor survival of the transplanted cells.59,60 However, we were able to track ASCs even after 8 days posttransplantation into the wound bed (Fig. 8). The long-term viability of ASCs as a treatment is important since pericytes, regarded as a structural component of blood vessels, regulate vascular contractility, stability, and integrity.61 Immunofluorescent images (Fig. 8C) indicate that ASCs are occupying the perivascular spaces associated with newly forming blood vessels and that the host endothelial cells (CD31+) are homing to the intimal region. These results correlate to the findings made in our previous study, where excisional wounds treated with ASC-seeded FPEG hydrogels also exhibited increased blood vessel density and ASC localization surrounding newly formed blood vessels in the wound bed.30 This finding is of significance, since it is well established that pericyte recruitment around the endothelium is essential for vascular plasticity and ASCs assume the role of pericyte in a matured blood vessel.62 The formation of functional blood vessels not only delivers oxygen and nutrients but also provides instructive regulatory signals to the surrounding cells to deposit extracellular matrix, therefore initiating tissue remodeling (Fig. 9). Collectively, this study provides evidence that hydrogels prepared from PFP are an excellent choice for treating wounds because they are easily prepared, biocompatible, and biodegradable.
Innovation
Human PFP is a cost-effective material and a clinically applicable hydrogel that can be prepared at the bedside to treat full-thickness wounds. ASCs in conjunction with PFP hydrogel provides a nutrient-rich substrate to accelerate the kinetics of neovascularization and overall healing. Early intervention using this hydrogel as an adjunct to a biological substitute will increase the dermal integration and decrease the time needed before grafting, especially in patients with extensive burn wounds. Not limited to skin wounds, PFP hydrogel when combined with appropriate scaffold material may act as a cell-modulatory tool to improve healing of various soft tissue injuries.
Key Findings
We have developed a simple, cost-effective, and clinically usable viscoelastic hydrogel using PEGylated PFP with thrombin or CaCl2 as a crosslinking agent.
Hydrogels from human blood plasma possess comparable strength and network-forming ability to PEGylated fibrin hydrogels.
When combined with ASCs, the PEGylated PFP hydrogel matrices resulted in an improved vasculogenic response.
PEGylated PFP hydrogels and ASCs improved healing of full-thickness wounds by providing a favorable environment for revascularization and remodeling.
Acknowledgments and Funding Sources
The authors would like to thank Ms. Sandra C. Becerra for her technical support. Authors like to also thank Dr. Tao You, PhD, Dental Trauma Research Detachment, USAISR for his support in acquiring SEM images and his technical expertise. This research was supported in part by an appointment to the Postgraduate Research Participation Program at the USAISR administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and USAISR. This study was supported in part by the U.S. Army Medical Research and Materiel Command of the Department of Defense.
Abbreviations and Acronyms
- α-SMA
α-smooth muscle actin
- ANGPT1
angiopoietin 1
- ANGPT2
angiopoietin 2
- ANOVA
analysis of variance
- ASCs
adipose-derived stem cells
- CaCl2
calcium chloride
- DAPI
4′,6-diamidino-2-phenylindole, dihydrochloride
- FPEG
PEGylated fibrinogen
- HBSS
Hank's buffered salt solution
- PDGFRβ
platelet-derived growth factor β
- NG2
neuroglial factor 2
- PEG
polyethylene glycol
- PFP
platelet-free plasma
- PRP
platelet-rich plasma
- PSR
Picrosirius Red
- RBCs
red blood cells
- RNA
ribonucleic acid
- SEM
scanning electron microscopy
- USAISR
United States Army Institute of Surgical Research
- VEGF
vascular endothelial growth factor
- vWF
von Willebrand factor
Author Disclosure and Ghostwriting
The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Department of Defense or Department of Army. The authors are employees of the U.S. Government, and this work was prepared as part of their official duties. No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Shanmugasundaram Natesan, PhD, is a Research Scientist at the Combat Trauma and Burn Injuries Research Task Area, USAISR. Randolph Stone II, PhD, is a Staff Scientist at the Combat Trauma and Burn Injuries Research Task Area, USAISR. Ramon E. Coronado, PhD, is an Executive Director at the Lester Smith Medical Research Institute, San Antonio, TX. Nicole L. Wrice, MS, is a Senior Biological Science Laboratory Technician, Department of Ocular Trauma & Vision Restoration, USAISR, Andrew C. Kowalczewski, BS, is a Research Technician at the Combat Trauma and Burn Injuries Research Task Area, USAISR, David O. Zamora, PhD, is a Research Scientist at the Department of Ocular Trauma & Vision Restoration, USAISR. Robert J. Christy, PhD, is a Research Physiologist and Task Area Manager at the Combat Trauma and Burn Injuries Research Task Area, USAISR.
References
- 1. Dohan Ehrenfest DM, Andia I, Zumstein MA, et al. Classification of platelet concentrates (platelet-rich plasma-PRP, platelet-rich fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J 2014;4:3–9 [PMC free article] [PubMed] [Google Scholar]
- 2. Arnoczky SP, Sheibani-Rad S. The basic science of platelet-rich plasma (PRP): what clinicians need to know. Sports Med Arthrosc 2013;21:180–185 [DOI] [PubMed] [Google Scholar]
- 3. de Leon JM, Driver VR, Fylling CP, et al. The clinical relevance of treating chronic wounds with an enhanced near-physiological concentration of platelet-rich plasma gel. Adv Skin Wound Care 2011;24:357–368 [DOI] [PubMed] [Google Scholar]
- 4. Frykberg RG, Driver VR, Carman D, et al. Chronic wounds treated with a physiologically relevant concentration of platelet-rich plasma gel: a prospective case series. Ostomy Wound Manage 2010;56:36–44 [PubMed] [Google Scholar]
- 5. Henderson JL, Cupp CL, Ross EV, et al. The effects of autologous platelet gel on wound healing. Ear Nose Throat J 2003;82:598–602 [PubMed] [Google Scholar]
- 6. Kazakos K, Lyras DN, Verettas D, Tilkeridis K, Tryfonidis M. The use of autologous PRP gel as an aid in the management of acute trauma wounds. Injury 2009;40:801–805 [DOI] [PubMed] [Google Scholar]
- 7. Mehta S, Watson JT. Platelet rich concentrate: basic science and current clinical applications. J Orthop Trauma 2008;22:432–438 [DOI] [PubMed] [Google Scholar]
- 8. Yan Y, Larson DL. Acceleration of full-thickness wound healing in porcine model by autologous platelet gel. Wounds 2007;19:79–86 [PubMed] [Google Scholar]
- 9. Zhou B, Ren J, Ding C, et al. Rapidly in situ forming platelet-rich plasma gel enhances angiogenic responses and augments early wound healing after open abdomen. Gastroenterol Res Pract 2013;2013:926764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shida N, Kurasawa R, Maki Y, et al. Study of plasma coagulation induced by contact with calcium chloride solution. Soft Matter 2016;12:9471–9476 [DOI] [PubMed] [Google Scholar]
- 11. Everts PA, Knape JT, Weibrich G, et al. Platelet-rich plasma and platelet gel: a review. J Extra Corpor Technol 2006;38:174–187 [PMC free article] [PubMed] [Google Scholar]
- 12. Sanchez M, Anitua E, Andia I. Poor standardization in platelet-rich therapies hampers advancement. Arthroscopy 2010;26:725–726; author reply 726 [DOI] [PubMed] [Google Scholar]
- 13. McGill V, Kowal-Vern A, Lee M, et al. Use of fibrin sealant in thermal injury. J Burn Care Rehabil 1997;18:429–434 [DOI] [PubMed] [Google Scholar]
- 14. Foster K. The use of fibrin sealant in burn operations. Surgery 2007;142:S50–S54 [DOI] [PubMed] [Google Scholar]
- 15. Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult-to-heal wounds: a pilot study. Transfusion 2004;44:1013–1018 [DOI] [PubMed] [Google Scholar]
- 16. Anitua E, Prado R, Orive G. Endogenous morphogens and fibrin bioscaffolds for stem cell therapeutics. Trends Biotechnol 2013;31:364–374 [DOI] [PubMed] [Google Scholar]
- 17. Cho SW, Kim I, Kim SH, et al. Enhancement of adipose tissue formation by implantation of adipogenic-differentiated preadipocytes. Biochem Biophys Res Commun 2006;345:588–594 [DOI] [PubMed] [Google Scholar]
- 18. Jockenhoevel S, Zund G, Hoerstrup SP, et al. Fibrin gel—advantages of a new scaffold in cardiovascular tissue engineering. Eur J Cardiothorac Surg 2001;19:424–430 [DOI] [PubMed] [Google Scholar]
- 19. Mol A, van Lieshout MI, Dam-de Veen CG, et al. Fibrin as a cell carrier in cardiovascular tissue engineering applications. Biomaterials 2005;26:3113–3121 [DOI] [PubMed] [Google Scholar]
- 20. Han B, Schwab IR, Madsen TK, Isseroff RR. A fibrin-based bioengineered ocular surface with human corneal epithelial stem cells. Cornea 2002;21:505–510 [DOI] [PubMed] [Google Scholar]
- 21. Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol (1985) 2005;98:706–713 [DOI] [PubMed] [Google Scholar]
- 22. Rowe SL, Lee S, Stegemann JP. Influence of thrombin concentration on the mechanical and morphological properties of cell-seeded fibrin hydrogels. Acta Biomater 2007;3:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hojo M, Inokuchi S, Kidokoro M, et al. Induction of vascular endothelial growth factor by fibrin as a dermal substrate for cultured skin substitute. Plast Reconstr Surg 2003;111:1638–1645 [DOI] [PubMed] [Google Scholar]
- 24. Kober J, Gugerell A, Schmid M, Kamolz LP, Keck M. Generation of a fibrin based three-layered skin substitute. Biomed Res Int 2015;2015:170427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawasumi M, Kitoh H, Siwicka KA, Ishiguro N. The effect of the platelet concentration in platelet-rich plasma gel on the regeneration of bone. J Bone Joint Surg Br 2008;90:966–972 [DOI] [PubMed] [Google Scholar]
- 26. Dikovsky D, Bianco-Peled H, Seliktar D. The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials 2006;27:1496–1506 [DOI] [PubMed] [Google Scholar]
- 27. Almany L, Seliktar D. Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials 2005;26:2467–2477 [DOI] [PubMed] [Google Scholar]
- 28. Gonen-Wadmany M, Goldshmid R, Seliktar D. Biological and mechanical implications of PEGylating proteins into hydrogel biomaterials. Biomaterials 2011;32:6025–6033 [DOI] [PubMed] [Google Scholar]
- 29. Chung E, Rytlewski JA, Merchant AG, et al. Fibrin-based 3D matrices induce angiogenic behavior of adipose-derived stem cells. Acta Biomater 2015;17:78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zamora DO, Natesan S, Becerra S, et al. Enhanced wound vascularization using a dsASCs seeded FPEG scaffold. Angiogenesis 2013;16:745–757 [DOI] [PubMed] [Google Scholar]
- 31. Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24 [DOI] [PubMed] [Google Scholar]
- 32. Cieslik-Bielecka A, Choukroun J, Odin G, Dohan Ehrenfest DM. L-PRP/L-PRF in esthetic plastic surgery, regenerative medicine of the skin and chronic wounds. Curr Pharm Biotechnol 2012;13:1266–1277 [DOI] [PubMed] [Google Scholar]
- 33. Levi M, van der Poll T, Buller HR. Bidirectional relation between inflammation and coagulation. Circulation 2004;109:2698–2704 [DOI] [PubMed] [Google Scholar]
- 34. Nurden AT. Platelets, inflammation and tissue regeneration. Thromb Haemost 2011;105 Suppl 1:S13–S33 [DOI] [PubMed] [Google Scholar]
- 35. Neill JS, Lineaweaver WC. Tissue response to bovine fetal collagen extracellular matrix in full-thickness skin wounds. Am J Clin Pathol 2013;140:248–252 [DOI] [PubMed] [Google Scholar]
- 36. Wainwright DJ, Bury SB. Acellular dermal matrix in the management of the burn patient. Aesthet Surg J 2011;31:13s–23s [DOI] [PubMed] [Google Scholar]
- 37. Frautschi RS, Hashem AM, Halasa B, Cakmakoglu C, Zins JE. Current evidence for clinical efficacy of platelet rich plasma in aesthetic surgery: a systematic review. Aesthet Surg J 2017;37:353–362 [DOI] [PubMed] [Google Scholar]
- 38. Natesan S, Zamora DO, Wrice NL, Baer DG, Christy RJ. Bilayer hydrogel with autologous stem cells derived from debrided human burn skin for improved skin regeneration. J Burn Care Res 2013;34:18–30 [DOI] [PubMed] [Google Scholar]
- 39. Zhang G, Hu Q, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A 2008;14:1025–1036 [DOI] [PubMed] [Google Scholar]
- 40. Natesan S, Zhang G, Baer DG, et al. A bilayer construct controls adipose-derived stem cell differentiation into endothelial cells and pericytes without growth factor stimulation. Tissue Eng Part A 2011;17:941–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Natesan S, Wrice NL, Baer DG, Christy RJ. Debrided skin as a source of autologous stem cells for wound repair. Stem Cells 2011;29:1219–1230 [DOI] [PubMed] [Google Scholar]
- 42. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408 [DOI] [PubMed] [Google Scholar]
- 43. Barthel LK, Raymond PA. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem 1990;38:1383–1388 [DOI] [PubMed] [Google Scholar]
- 44. Wu X, Ren J, Luan J, Yao G, Li J. Biochemical, mechanical, and morphological properties of a completely autologous platelet-rich wound sealant. Blood Coagul Fibrinolysis 2012;23:290–295 [DOI] [PubMed] [Google Scholar]
- 45. Achora S, Muliira JK, Thanka AN. Strategies to promote healing of split thickness skin grafts: an integrative review. J Wound Ostomy Continence Nurs 2014;41:335–339; quiz E331–E332 [DOI] [PubMed] [Google Scholar]
- 46. Marck RE, Gardien KL, Stekelenburg CM, et al. The application of platelet-rich plasma in the treatment of deep dermal burns: a randomized, double-blind, intra-patient controlled study. Wound Repair Regen 2016;24:712–720 [DOI] [PubMed] [Google Scholar]
- 47. Chiu YC, Cheng MH, Engel H, et al. The role of pore size on vascularization and tissue remodeling in PEG hydrogels. Biomaterials 2011;32:6045–6051 [DOI] [PubMed] [Google Scholar]
- 48. Blomback B, Carlsson K, Fatah K, Hessel B, Procyk R. Fibrin in human plasma: gel architectures governed by rate and nature of fibrinogen activation. Thromb Res 1994;75:521–538 [DOI] [PubMed] [Google Scholar]
- 49. Reganon E, Vila V, Aznar J. Effect of calcium ions on fibrin gel formation in normal plasma. Thromb Res 1984;35:365–369 [DOI] [PubMed] [Google Scholar]
- 50. Ryan EA, Mockros LF, Weisel JW, Lorand L. Structural origins of fibrin clot rheology. Biophys J 1999;77:2813–2826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nair CH, Shah GA, Dhall DP. Effect of temperature, pH and ionic strength and composition on fibrin network structure and its development. Thromb Res 1986;42:809–816 [DOI] [PubMed] [Google Scholar]
- 52. Anitua E, Orive G. Endogenous regenerative technology using plasma- and platelet-derived growth factors. J Control Release 2012;157:317–320 [DOI] [PubMed] [Google Scholar]
- 53. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011;473:298–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mammoto T, Jiang A, Jiang E, Mammoto A. Platelet rich plasma extract promotes angiogenesis through the angiopoietin1-Tie2 pathway. Microvasc Res 2013;89:15–24 [DOI] [PubMed] [Google Scholar]
- 55. Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. Direct cell adhesion to the angiopoietins mediated by integrins. J Biol Chem 2001;276:26516–26525 [DOI] [PubMed] [Google Scholar]
- 56. Dallabrida SM, Ismail N, Oberle JR, Himes BE, Rupnick MA. Angiopoietin-1 promotes cardiac and skeletal myocyte survival through integrins. Circulation Res 2005;96:e8–e24 [DOI] [PubMed] [Google Scholar]
- 57. Goretzki L, Burg MA, Grako KA, Stallcup WB. High-affinity binding of basic fibroblast growth factor and platelet-derived growth factor-AA to the core protein of the NG2 proteoglycan. J Biol Chem 1999;274:16831–16837 [DOI] [PubMed] [Google Scholar]
- 58. Ozerdem U, Grako KA, Dahlin-Huppe K, Monosov E, Stallcup WB. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn 2001;222:218–227 [DOI] [PubMed] [Google Scholar]
- 59. Bhang SH, Park J, Yang HS, Shin J, Kim BS. Platelet-rich plasma enhances the dermal regeneration efficacy of human adipose-derived stromal cells administered to skin wounds. Cell Transplant 2013;22:437–445 [DOI] [PubMed] [Google Scholar]
- 60. Vaquero J, Otero L, Bonilla C, et al. Cell therapy with bone marrow stromal cells after intracerebral hemorrhage: impact of platelet-rich plasma scaffolds. Cytotherapy 2013;15:33–43 [DOI] [PubMed] [Google Scholar]
- 61. von Tell D, Armulik A, Betsholtz C. Pericytes and vascular stability. Exp Cell Res 2006;312:623–629 [DOI] [PubMed] [Google Scholar]
- 62. Geevarghese A, Herman IM. Pericyte-endothelial crosstalk: implications and opportunities for advanced cellular therapies. Transl Res 2014;163:296–306 [DOI] [PMC free article] [PubMed] [Google Scholar]