Abstract
It is currently understood that, in order for a tumor to successfully grow, it must evolve means of evading immune surveillance. In the past several decades, researchers have leveraged increases in our knowledge of tumor immunology to develop therapies capable of augmenting endogenous immunity and eliciting strong antitumor responses. In particular, the goal of anticancer vaccination is to train the immune system to properly utilize its own resources in the fight against cancer. Although attractive in principle, there are currently only limited examples of anticancer vaccines that have been successfully translated to the clinic. Recently, there has been a significant push towards the use of nanotechnology for designing vaccine candidates that exhibit enhanced potency and specificity. In this progress report, we discuss recent developments in the field of anticancer nanovaccines. By taking advantage of the flexibility offered by nanomedicine to purposefully program immune responses, this new generation of vaccines has the potential to address many of the hurdles facing traditional platforms. A specific emphasis is placed on the emergence of cell membrane-coated nanoparticles, a novel biomimetic platform that can be used to generate personalized nanovaccines that elicit strong, multi-antigenic antitumor responses.
Keywords: nanomedicine, biomimetic nanoparticle, anticancer vaccination, immunotherapy, antigen presentation
Graphical Abstract
Vaccination represents an attractive approach for the treatment of cancer. By leveraging nanotechnology, it is possible to design nanovaccines with improved potency, specificity, and durability. The recent emergence of cell membrane coating technology has enabled the facile synthesis of biomimetic nanoparticles with enhanced function and antigenicity, potentially paving the way for the future creation of effective and personalized anticancer vaccines.
1. Introduction
Our immune system is a complex network of cells, proteins, and physical barriers that work together to keep the human body free from disease. When mobilized correctly, it has the ability to seek out and eliminate foreign invaders with exquisite specificity. Malfunctioning or underperforming immunity is often the root cause of many disease states. For example, an overactive immune system can result in autoimmunity, which is characterized by proinflammatory states and leads to the destruction of healthy tissue.[1, 2] On the other hand, an underactive immune system can lead to enhanced susceptibility to infection, which is becoming increasingly dangerous given the rise of antibiotic resistance.[3] With regards to tumorigenesis, it has been shown that the immune system is integral in helping to prevent the proliferation of malignant cells.[4] It is now known that, for tumors to successfully grow, cancerous cells must generally go through a prolonged evolutionary process in order to develop mechanisms for immune evasion.[5] Tumors can manipulate the surrounding microenvironment to support growth and suppress host immune responses using cytokine and growth factor secretion,[6] extracellular matrix restructuring,[7] and cellular signaling.[8, 9] It is for this reason that an intense amount of research has been focused on leveraging the immune system to fight off cancer.[10] In general, cancer immunotherapies seek to train, augment, or supplement the body’s own ability to eliminate malignant growths. There are numerous classes of immunotherapy, and they can act on different stages of immunity, ranging from initial antigen presentation up to the final effector stages.[11, 12] Depending on the specific type of cancer being treated, early returns have thus far been promising, and a number of immunotherapies have proven to be highly potent in scenarios where the previous clinical standard of care had little effect.[13–15]
Anticancer vaccination is a class of cancer immunotherapy that focuses largely on training the immune system to recognize and mount a response against tumors in an antigen-specific manner.[12, 16] Over the course of recent human history, vaccines have represented an attractive means of managing the spread of disease, as most are easy to administer and can promote the development of sterilizing immunity.[17] Particularly in the case infectious diseases, vaccination has proven to be highly effective, having likely helped to prevent millions of deaths as a result of large-scale prophylaxis campaigns.[18] Despite the favorable history of antibacterial and antiviral vaccines, anticancer vaccination unfortunately has not achieved the same level of success.[19, 20] Unlike with those against pathogens, there are additional hurdles that must be overcome in order for vaccines against tumors to be effective. One of the main challenges comes from the fact that most tumors are lowly immunogenic and originate from one’s own healthy cells. As such, it is incredibly difficult for the immune system to correctly identify malignant tissue. Additionally, vaccines against established tumors must be administered therapeutically, requiring the need for formulations that are highly potent in addition to being tumor-specific. This has oftentimes necessitated the use of complex strategies for immune system manipulation,[21] many of which are lowly viable in a clinical setting given poor cost-to-benefit ratios. In 2010, the United States Food and Drug Administration approved the first and only therapeutic anticancer vaccine, sipuleucel-T.[22] This autologous cell-based therapy trains patient-derived immune cells against a common prostate cancer antigen before reinfusion of the cells back into the patient. The treatment has been shown to marginally increase patient survival time, but the complex logistics and high cost of manufacturing a personalized cell-based vaccine has limited its commercial viability.
To address the hurdles faced by traditional vaccination schemes against cancer, many researchers have turned towards nanotechnology to help guide the design of nanovaccines capable of producing potent, specific, and durable antitumor responses.[23, 24] Compared with traditional vaccines, those manufactured at the nanoscale have unique physical and material properties that make them better suited for immune manipulation. Through purposeful engineering, nanovaccines can be formulated with antigen and adjuvant payloads in a manner that maximizes immune responses through efficient delivery to specific cellular subsets. Ultimately, the goal is to leverage such platforms for the controlled programming of endogenous immunity to reverse tumor burden. In this review, we start by covering some basic background information regarding anticancer vaccines and the current state of traditional platforms. We then discuss developments in the field of anticancer nanovaccines, focusing on platforms for both nonspecific and antigen-specific immune modulation. Finally, we introduce an emerging class of biomimetic nanoparticles based on cell membrane coating nanotechnology. This top-down strategy directly leverages nature’s own design principles as a means of fabricating multifunctional and multi-antigenic nanosystems, which have the potential to play an important role in the future of anticancer vaccination.
2. Background on Anticancer Vaccination
2.1. Cancer immunology and immunotherapy
Cancer is generally characterized by an accumulation of mutations that allows for uncontrolled cell proliferation. As tumors grow, they are in a constant battle with the immune system and must evolve mechanisms for escape over time.[5] Due to the random nature of the mutations that lead to malignancy, phenotypes can vary greatly among different cancers, as well as among cells within the same tumor. This heterogeneity not only serves as a challenge for traditional cancer therapeutics, but also acts as an immune evasion mechanism, increasing the likelihood of some mutant cell populations remaining undetected.[20, 25] Another immune escape mechanism occurs through antigen shedding.[26] As part of their normal growth, cells generate a large amount of waste products, and these unwanted products are commonly secreted through membrane vesicles. When released in large abundance, this process can also deplete the parent cell of tumor-specific antigens, thus enabling the altered cancer cells to avoid destruction by cytotoxic T cells. Furthermore, shed antigens released into the bloodstream can act as decoys for neutralizing cancer-specific antibodies. Solid tumors can employ additional means of escape, whereby their local microenvironments are remodeled to promote immune tolerance.[27]
A better understanding of how cancer interacts with the immune system has allowed for the development of new and effective therapeutics. The goal of cancer immunotherapies is to leverage a patient’s own immune system to eradicate tumors in a highly specific and relatively safe manner.[28] One example is through an overall activation of the immune system by administering proinflammatory cytokines, which are immunomodulatory molecules released by activated immune cells.[29, 30] Although immune stimulation caused by these molecules are nonspecific, an overall boost in immunity can sometimes strengthen immune cells enough to overcome tumor suppression. More specific, tumor-targeted approaches can be achieved using genetically engineered chimeric antigen receptors (CAR) on T cells.[31, 32] In CAR T cell therapy, T lymphocytes are isolated from a patient or a donor through leukapheresis.[33] The cells are then genetically modified to express a receptor that can recognize tumor-associated antigens, leading to elimination of the corresponding cells. Altered T cells are purified, expanded ex vivo, and finally infused back into patients for treatment. For some cancer types, this CAR approach has displayed striking efficacy in the clinic.
Antibodies have also been widely used to elicit antitumor immunity. For example, tumor-targeted monoclonal antibodies that recognize tumor antigens can opsonize cancer cells and trigger antibody-dependent, cell-mediated cytotoxicity.[34] Furthermore, by conjugating antibodies with chemotherapeutics, these cytotoxic cargos can be more accurately targeted to the tumor site and induce immunogenic cell death.[35] More recently, antibody-based checkpoint inhibitors have been used to directly modulate the function of specific immune cell subsets.[36] Immune checkpoints involve inhibitory receptors such as programmed cell death protein 1 (PD1) and cytotoxic T lymphocyte-associated protein 4 (CTLA4) that regulate T cells. By presenting the corresponding ligands, the cytotoxic activity of T cells can be inhibited by tumor cells and regulatory immune cells. In checkpoint blockade therapy, antibodies target and block these receptor binding sites, thus removing the inhibitory signals on the T lymphocytes and unleashing their full potential for eliminating cancer cells. Despite their ability to elicit strong antitumor responses, efficacy of checkpoint blockades can vary greatly by patient.[37] This discrepancy may be explained by the fact that the therapy generally relies on the presence of preexisting tumor-targeted T cells.[38] For this reason, checkpoint blockades are being actively explored for use in combination with other therapies such as anticancer vaccination, which can help to generate new T cell populations.[39, 40]
2.2. Current state of cancer vaccines
Cancer vaccines introduce tumor-relevant antigenic material in a manner that leads to downstream mobilization of the immune system.[28] As the most immunogenic mutations have likely already been selected out by the time cancer is detected,[5] the presence of tumor antigens alone is usually not sufficient to drive proper immune stimulation. As such, tumor antigens are almost always combined with an adjuvant in order to enhance the immune response.[41] In the basic process, delivered antigens are taken up by professional antigen-presenting cells (APCs), such as dendritic cells, which process and break down the antigens, followed by presentation of the peptide fragments via major histocompatibility complexes (MHCs).[42] With the help of the adjuvant, the APCs mature, enabling engagement and activation of cancer-relevant T cells. Finally, the activated T cells can help to promote tumor elimination, either by further propagating immune activation or by directly seeking out and destroying the cancer cells.
Antigenic delivery to the immune system can be achieved in multiple ways. The most straightforward is the direct administration of tumor antigens. In single-antigen approaches, a tumor antigen overexpressed on cancer cells is administered parenterally.[43] This has been shown to elicit a robust immune response against the target antigen, especially in combination with an adjuvant; however, this approach may ultimately be thwarted by tumor heterogeneity. Whole cell preparations are another source of antigenic material that can theoretically be used to vaccinate against the full breadth of tumor antigens.[44] However, this strategy often suffers from inadequate antitumor immune responses due to the interference from irrelevant proteins. In response to the often weak immunity generated by the above approaches, dendritic cells can be pulsed with an antigen and stimulated ex vivo.[21] Once this process is completed, the cells are then injected back into the patient in a process similar to CAR T cell therapy. The manipulated dendritic cells can subsequently migrate to the body’s immune centers, where they train endogenous T cells. In a final method, antigenic uptake can happen in situ at the tumor site, taking advantage of processes such as immunogenic cell death, which provide autologous tumor antigens under an immunostimulatory context.[45] In situ vaccinations can also be achieved with oncolytic viruses that selectively infect and destroy cancer cells.[46]
In April of 2010, the United States Food and Drug Administration gave its first approval to a therapeutic anticancer vaccine, sipuleucel-T, for the treatment of prostate cancer.[47] In this therapy, patient-derived dendritic cells are pulsed with prostatic acid phosphatase, which is expressed in a significant number of patients with prostate cancer.[48] After exposure to the antigen, along with granulocyte-macrophage colony-stimulating factor, the activated dendritic cells are introduced back into the patient. It was demonstrated in a clinical trial that sipuleucel-T was able to extend median survival by 4.1 months, which paved the way for its eventual approval.[47] The successful translation of this treatment has motivated the further clinical exploration of anticancer vaccine formulations, and a search on ClinicalTrials.gov yields over 200 results for active trials. Examples of current clinical studies include dendritic cell therapies for glioblastoma (NCT01808820), oncolytic viruses for ovarian cancer (NCT00408590), peptide vaccines for recurrent glioblastoma (NCT02754362), and whole cell vaccines for breast cancer (NCT00317603).
Although cancer vaccines have had some success in the clinic, their limited ability to produce strong antitumor responses has hindered their widespread adoption. Despite its regulatory approval, the long-term financial viability of sipuleucel-T has come into question. The labor-intensive processes involved in its manufacture necessitate its high cost, which may be hard to justify given that the treatment only modestly prolongs median survival. Single-antigen peptide vaccines are able to elicit potent immune responses against the tumor cells that display the relevant antigenic epitopes; however, due to the heterogeneity of cancers, antigen-negative cells can eventually escape detection and proliferate without competition.[20] This approach is also not universal, and personalized identification and manufacture of vaccines based on tumor-specific neoantigens may not yet be viable on a large scale.[49, 50] Whole cell vaccination with tumor lysates has the potential to elicit multi-antigenic immunity, but the final immune response is often dampened by the presence of extraneous proteins.[44] This underscores the fact that, even when delivering the correct antigenic material, current vaccination strategies may not have sufficient immunostimulatory capacity to overcome the tolerogenic tumor microenvironment.
2.3. Advantages of nanovaccines
Nanotechnology offers many opportunities for improving the treatment efficacy of cancer vaccine formulations compared to traditional strategies (Figure 1). A major advantage is the ability to formulate the antigen and adjuvant components together in a manner that maximizes immune stimulation.[51] Flexibility in nanoparticle synthesis methods and material choice allows for the incorporation of different classes of molecules, such as proteins, polysaccharides, nucleic acids, lipids, proteins, and polymers. For example, electrostatic interactions can be used to bind nanoparticles and payloads with opposite charges together,[52] or lipid-based cargoes can be incorporated into the bilayer of liposomes through an insertion technique.[53, 54] Cargoes can also be encapsulated through chemical conjugation,[55] or they can be decorated onto the nanoparticle surface.[56] Oftentimes the nanocarriers themselves can also be fabricated using biologically active vaccine components. For example, in has been demonstrated that both calcium phosphate,[57] a mineral-based adjuvant, and certain antigen proteins[58] can be made into nanoparticulate form.
Figure 1.
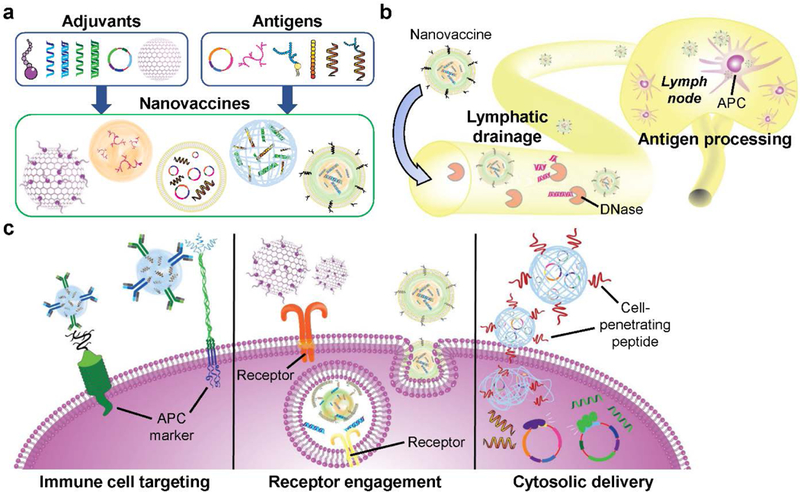
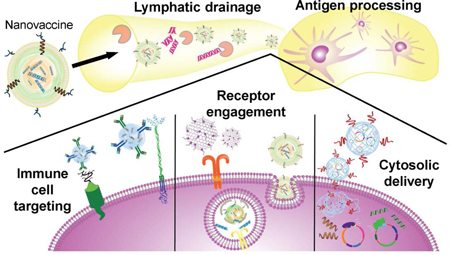
Advantages of nanoparticles for vaccine design. a) Various combinations of adjuvants and antigens can be formulated using nanoparticle platforms such as liposomes, emulsions, nanogels, and many others. b) Nanovaccines can access the lymphatic drainage system for lymph node delivery while protecting cargoes from environmental degradation. Once at the lymph nodes, the nanocarriers can deliver their cargoes to antigen-presenting cells (APCs) for immune processing. c) Nanovaccine properties can be tuned to efficiently deliver their cargoes for maximum immune activation. For example, nanoparticles can be modified to target specific subsets of immune cells. They can also be delivered to specific intracellular compartments, where receptors for immune pathways can be triggered.
Loading of antigen and adjuvant into nanoparticles can serve a variety of purposes. Encapsulation of vaccine components has been shown to increase immunogenicity by protecting the integrity of the molecules from enzymes in the body, such as nucleases, proteases, and phosphatases.[59] Nanoparticulate delivery not only protects the adjuvant from degradation, but can also protect the body from the systemic toxicity of the adjuvants, which can cause side effects such as fever, lethargy, diarrhea, and nausea.[60] Nanoencapsulation can also be used to enhance immune responses by providing extended release properties. Certain gel-like or polymeric nanoparticle platforms can as depots, slowly releasing adjuvants and antigens over a long period of time.[61] Finally, there are a wide range of techniques available for loading both antigens and adjuvants into the same nanocarrier, which has been shown to dramatically increase antigen-specific immune responses by unifying the pharmacokinetics of the co-encapsulated payloads.[51]
In terms of payload delivery, nanoparticles can be designed to better target immune cells and immune-rich organs. At their size range, nanoparticulate vaccine formulations more easily drain into the lymphatic system after administration, enabling efficient delivery to the lymph nodes,[62, 63] which contain high densities of immune cells. The localization of the nanoparticles can be further improved by modifying their outer layer to display ligands specific to immune cell surface receptors.[64, 65] Nanoformulations can also be designed to promote intracellular localization in a manner that maximizes the biological activity of the payloads. For example, nucleotide-binding oligomerization domain-like agonists and siRNA can be delivered directly to the cytosol using nanoparticles designed to penetrate through cell membranes,[66] and toll-like receptors (TLRs) can be engaged by various agonists when delivered into cells via an endosomal pathway.[67] Overall, careful choices in the use of materials, loading methods, and synthesis techniques for nanoparticle-based formulations can all lead to improved vaccine efficacy.
3. Nanoparticle-Based Cancer Vaccines
3.1. Nonspecific modulation
Some immunomodulatory nanoparticle platforms work to nonspecifically boost immune system function. While not strictly considered vaccines, these systems do rely on a patient’s own tumor as the source of antigenic material and work by augmenting immune processes such as antigen processing and antigen presentation. This is generally achieved by manipulating the immune system in a way that reduces immunosuppression or activates specific immune cell subsets to potentiate a response against cancer cells. In some cases, these formulations can also be combined with tumor cell killing mechanisms to increase exposure to tumor-associated antigens.
3.1.1. Enhancing physical proximity of immune cells
An intuitive method for boosting antitumor immune activity is to bring the principal immune cells responsible for tumor elimination closer to their target. To achieve this, nanoparticles can be decorated with two different antibodies, one to target and/or activate immune cells, and another to target the tumor cells. By using these bifunctional nanoparticles, nearby immune cells can be targeted to tumors, increasing the chance of exposure to released tumor antigens or apoptotic cancer cells while enhancing immune stimulation. In a first example, biodegradable poly(lactic acid) nanoparticles were decorated with antibodies against the dendritic cell co-stimulatory marker CD40, as well as an antibody against human epidermal growth factor receptor 2 (HER2)/neu, a common tumor antigen overexpressed in human breast cancer.[68] The anti-CD40 antibody was found to both bind and activate dendritic cells, inducing a strong proinflammatory immune response that could be directed towards neu+ tumors. Intratumoral injection of the nanoparticles yielded 100% rejection, while systemic injections resulted in 70% of mice rejecting neu+ tumors. Importantly, rechallenge of mice that rejected the primary tumor did not lead to any subsequent tumor growth. In another example, polystyrene nanoparticles were conjugated with antibodies against HER2/neu and calreticulin, a protein that facilitates phagocytosis in APCs.[69] Macrophages treated with these multivalent bispecific nanobioconjugate engagers were able to better take up HER2+ cancer cells and presented tumor-associated antigens via MHC surface complexes. Intratumoral and intravenous injections of the nanoparticles led to higher infiltration of CD8+ T cells and inhibited the growth of HER2-expressing tumors. Upon rechallenge, treated mice rejected HER2+ cancer cells but not HER2- cells, demonstrating the specificity of the treatment and the durability of the response. Instead of binding APCs to tumor cells, it has also been demonstrated that antigen-specific T cells can be linked to cancer cells in a similar manner.[70] Conjugation of nanoparticles with SIY-MHC complexes effectively enabled binding to 2C T cells, while the inclusion of anti-CD19 allowed for crosslinking with CD19+ Raji cancer cells. Shortly after intratumoral injection of the nanoparticles, mice were infused with adoptively transferred 2C T cells, which led to significant retardation of tumor growth.
3.1.2. Reduction of immunosuppression
The immunosuppressive tumor microenvironment is a hurdle for most anticancer immunotherapy treatments, as effector cells can be rendered ineffective by inhibitory proteins or anti-inflammatory cytokines. For example, a melanoma-specific peptide vaccine was found to be effective for early stage melanoma, but it failed to demonstrate efficacy at later disease stages due to increased levels of immunosuppressive cytokines like tumor growth factor β (TGFβ) in the tumor microenvironment.[71] To address this, a liposome-protamine-hyaluronic acid nanoparticle was designed to deliver siRNA against TGFβ into tumor cells.[72] Injection of the nanoparticles halved the levels of TGFβ in the tumor microenvironment while doubling the efficacy of the vaccine. This improvement was discovered to be caused by an increase in CD8+ T cells in the late stage tumor tissue along with a marked decrease in regulatory T cell levels. Other immunosuppressive efforts focus on the expression of signaling proteins on tumor tissue that interact with immune cells. Well-known pathways such PD1 can be intercepted using checkpoint blockades, but systemic administration can have toxic side effects, potentially leading to the development of autoimmune diseases and pathological inflammation.[73] In one recent work, platelet-derived microparticles were used as a carrier for antibodies against programmed death-ligand 1 (PDL1).[74] After tumor resection, residual cancer cells can oftentimes start to regrow the tumor or be released into circulation. These remaining cells can express PDL1 in response to inflammation, making it highly difficult for the immune system to destroy them and prevent tumor recurrence. Due to the abundance of exposed collagen in wound sites, platelet microparticles were chosen as the delivery vehicle for anti-PDL1 given their inherent targeting ability. Intravenous injection of the microparticles immediately after incomplete tumor resections was shown to greatly reduce tumor regrowth and metastasis formation in both B16-F10 melanoma and triple-negative 4T1 breast cancer mouse models. Similarly, immunotherapy mediated by low dose doxorubicin has been shown to have partial efficacy against B-Raf proto-oncogene mutant melanoma, but it failed at long-term efficacy likely due to the emergence of the Wnt family member 5a (Wnt5a) protein on cancer cells. Wnt5a can induce dendritic cell tolerance and cause fibrosis of tumor tissue, as well as prevent T cell infiltration. A lipid-protamine-DNA nanoparticle loaded with plasmid DNA encoding for a Wnt5a trap was able to transiently reduce Wnt5a levels in the tumor microenvironment and significantly boost treatment efficacy using doxorubicin.[75]
3.1.3. Immune system activation
The immune system can be boosted through the introduction of immunostimulatory payloads, including pathogen-associated molecular patterns (PAMPs), co-stimulatory markers, cytokines, and other signaling proteins. Adjuvant administration has been found to be a powerful nonspecific modulator to aid in cancer immunotherapy. PAMPs such as single-stranded DNA, double-stranded RNA, and lipopolysaccharides are recognized by the TLRs found on immune cells and help to promote downstream inflammatory responses. Many of these PAMPs, such as CpG oligonucleotides (ODNs) recognized by endosomal TLR9, have been extensively used as adjuvants in conjunction with a co-injection of proteins or peptides to promote specific immune responses.[76–80] Other TLR-targeted PAMPs such as monophosphoryl lipid A (MPLA)[81, 82] and imidazoquinoline[83] have been used in nanoparticle formulations as adjuvants, and some PAMPs have even been coloaded together to simultaneously engage multiple different TLRs.[84]
Cyclic dinucleotides (CDNs), small nucleic acids characteristic of invading microbes, are a family of type I interferon (IFN)-producing PAMPs. These CDNs are in phase I clinical trials, but they require very high dosages to ensure that adequate amounts can get into the cytosol to interact with their stimulator of interferon genes (STING) receptor. Encapsulation of CDNs into nanoparticles can improve cytosolic delivery and enhance immune responses at lower concentrations. In one work, cyclic diguanylate was encapsulated into polyethylene glycol-functionalized lipid nanoparticles and used to adjuvant soluble ovalbumin (OVA) protein.[85] After vaccination, a significant increase in both CD8+ and CD4+ T cells was observed, and T cells restimulated with OVA produced 5-fold increases in IFNγ and tumor necrosis factor α (TNFα). Further, a CDN-adjuvanted B16-F10 vaccine formulation induced a 7-fold higher frequency of gp100-specific CD8+ T cells and significantly delayed B16-F10 tumor growth. CDNs have also been incorporated into nanoparticles consisting of a cationic poly(β-amino ester) (PBAE), a polymer widely used for cytosolic delivery of DNA.[86] Delivery of cyclic diguanylate to THP-1 cells using a PBAE carrier yielded an equivalent amount of IFN regulatory factor 3 activation as free CDN, but at a 100-fold lower dose of adjuvant. When the nanoparticles were given as an intratumoral injection along with anti-PD1 antibodies, complete remission of B16-F10 tumors was seen at an order of magnitude lower CDN dosage than the soluble form.
The repetitive protein structure of viral capsids self-assembled into nanoparticles can also serve as a PAMP. For example, cowpea mosaic virus is a non-infectious agent that self-assembles into hollow, icosahedral 30 nanometer virus-like particles, which can have strong antitumor immunotherapeutic activity (Figure 2).[87, 88] Inhalation of the virus-like particles by B16-F10 tumor-bearing mice increased tumor-infiltrating neutrophils, activated neutrophils in the lung microenvironment, and elevated levels of neutrophil-secreted cytokines. Significantly delayed tumor growth was seen after injections of the nanoparticles via various routes in multiple different tumor models. In particular, the virus-like particles were able to eliminate primary B16-F10 tumors in half of mice upon intratumoral injection, as well as provide long-term antitumor immunity as shown by rejection of a contralateral B16-F10 rechallenge. Other virus-like particles such as the papaya mosaic virus,[89] influenza virus,[90] and tomato yellow leaf curl virus[91] have also shown strong adjuvanting properties that can be taken advantage of for immune modulation.
Figure 2.
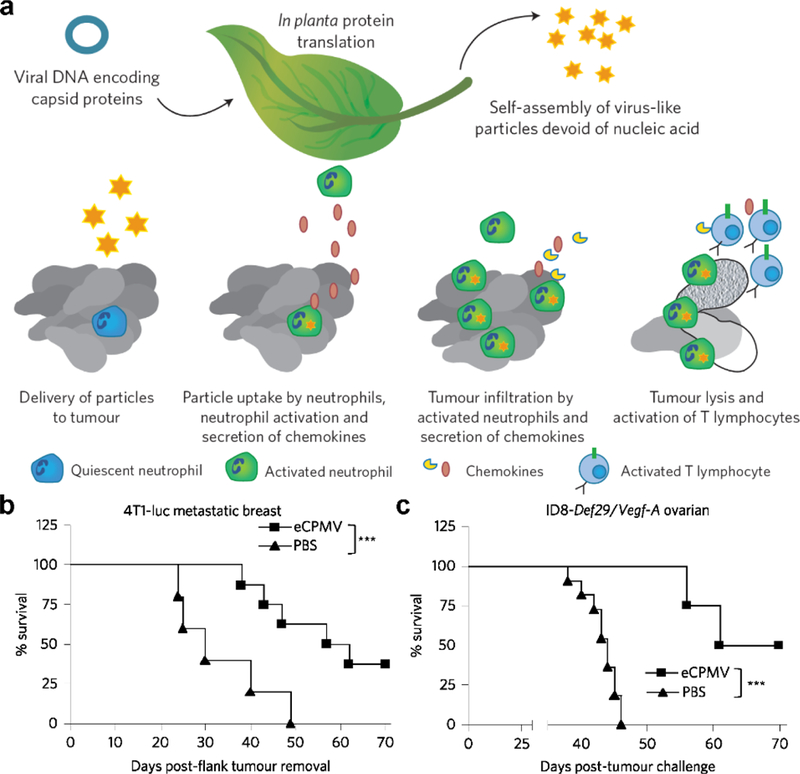
Virus-like nanoparticles for in situ anticancer vaccination. a) Schematic depicting the synthesis of virus-like nanoparticles (eCPMV) and their expected mechanism of action for tumor treatment. b,c) When used to treat tumor-bearing mice, virus-like nanoparticles significantly enhanced survival in both a 4T1-luc metastatic breast cancer model (b) and an ID8-Def29/Vegf-A ovarian cancer model (c). Reproduced with permission.[87, 88] Copyright 2016, Nature Publishing Group.
Cytokines serve a very important role in the adaptive immune system and can also be used for potent immune activation. For instance, mast cells can influence dendritic cell migration to the lymph nodes and upregulate inflammatory responses through the release of granules full of immune mediators like TNF. To mimic this natural boosting of the immune system, synthetic mast cell granules were synthesized by trapping TNF into a nanoparticle matrix of chitosan-heparin.[92] Like real mast cell granules, the particles drained to lymph nodes and promoted germinal center formation. Due to the modular nature of the nanoparticles, TNF could be replaced with interleukin-12 (IL12) to promote polarization of immune cells toward pro-inflammatory phenotypes, such as IFNγ-secreting T cells. Delivery of IL2, a crucial cytokine for T cell survival and proliferation, has also been explored as a method to enhance T cell-mediated immunotherapy. Hydroxyethyl starch nanocapsules were coupled with IL2 using copper-free click chemistry, and incubation with T cells resulted in a high level of uptake and a 4-fold increase in division index compared to unmodified nanocapsules. It has been shown previously that nanoparticles delivering a combination of different classes of immune-activating adjuvants can promote increased therapeutic efficacy.[93] Combinations of cytokines with other molecules, such as PAMPs[94] and co-stimulatory ligands,[95] have also been shown to synergistically activate immune cells.
3.1.4. Immune activation and immunosuppressive intervention combination
Beyond combining different methods of activating immune cells, simultaneous use of immunosuppressive intervention and immune activation can also yield impressive results. For example, combining IL10 siRNA and CpG ODN into a pathogen-mimicking nanoparticle resulted in a balanced Th1/Th2 cytokine response that improved antitumor efficacy.[96] Immune activating R848 has also been delivered to T cells by encapsulation in nanoparticles that were targeted to T cells expressing PD1.[97] To enhance co-stimulation while reducing immunosuppression, dual-targeted nanoparticles have been developed with both agonistic and antagonistic antibodies conjugated onto the same surface. In one case, anti-4–1BB was attached onto particles to activate the 4–1BB co-stimulation pathway on CD8+ T cells, while the conjugation of anti-PDL1 served to block PDL1 expressed on the surface of cancer cells.[98] Alternatively, nanoparticles decorated with anti-OX40 and anti-PD1 were able to target T cells expressing both receptors, simultaneously activating them and preventing their anergy.[99] In both cases above, T cells were less inhibited by the immunosuppressive tumor microenvironment, leading to enhanced antitumor efficacy in a variety of mouse cancer models.
3.1.5. Combination with traditional anticancer therapies
In the examples discussed thus far, it can be understood that the immunostimulatory nanoparticle platforms relied on the natural immune processing of tumor cells as the source of antigenic material. To facilitate the generation of tumor antigens and downstream immune activation, another strategy is to actively promote the release of material from tumors while concurrently introducing nonspecific immune modulators. For example, administration of the immunotherapeutic potato virus X alone caused a modest decrease in the growth rate of B16-F10 cancer cells, similar to monotherapy with doxorubicin. However, co-administration of both components led to a significant improvement in antitumor efficacy.[100] In another work, cytotoxic cationic silica nanoparticles were used to induce necrotic cell death while delivering a STING agonist to the immune cells in the tumor microenvironment.[101] Finally, “sticky” nanoparticles were designed to capture antigens in situ before being phagocytosed by immune cells.[102] After administration of anti-PD1 antibodies, primary tumors were irradiated and then injected with the antigen-capturing nanoparticles. Taking advantage of the abscopal effect, protein-loaded nanoparticles could then travel to the lymph nodes to facilitate an adaptive immune response, which led to the eventual destruction of a secondary tumor in 20% of mice.
3.2. Specific modulation
The ultimate goal of vaccination is to stimulate the immune system while simultaneously guiding a specific response against the desired target. For cancer immunotherapy, this target is often a lowly immunogenic antigen that is differentially expressed by tumor cells. As a result, an ideal cancer vaccine requires delivery of the relevant antigens along with a potent immunological adjuvant, which can be used to force the immune system to mount an antitumor response. In recent research, nanotechnology has been employed to further improve the efficacy of cancer vaccines using several strategies, including inherent nanoparticle adjuvancy, co-delivery of antigen and adjuvant, targeted delivery to immune cells, enhanced immune cell uptake and cross presentation, and cytosolic delivery.
3.2.1. Inherent nanoparticle adjuvancy
There is a wide variety of materials and structures that can be made into nanoparticles, and one strategy for the formulation of nanovaccines is to carefully choose a material that is naturally immunostimulatory. This can help to streamline nanoparticle fabrication by reducing the complexity of the final formulation. As an example, nanoparticles made of viral capsids naturally activate the immune system, largely due to the conservation of repetitive protein structures or the retention of nucleic acid-based PAMPs. These virus-like particles can engage TLRs in immune cells while delivering an antigenic payload. Even very lowly immunogenic tumor-associated antigens like idiotypic immunoglobulin from B cell lymphomas can elicit a strong humoral response when delivered by nanoparticles made of potato virus X viral coat proteins.[103] Other gel-like nanoparticles can be made by crosslinking materials that mimic the structure of PAMPs, such as hydrophobic polymers,[104] peptides,[105, 106] or DNA[107], while also encapsulating antigens. D-tetra-peptide hydrogels in particular show promise as a vaccine adjuvant. Nanoformulations made by mixing irradiated tumor cells with a self-assembling hydrogel made of the D configuration of naphthylacetic acid-modified GFFY peptide were able to significantly protect mice from both E.G7 and 4T1 tumor challenges.[108]
Immune responses to antigens can also be naturally boosted by carefully tuning their release over time. Nanogels are especially adept at this, as protein-to-polymer ratios can be precisely varied to change matrix spacing and cargo release rates.[109, 110] Some formulations have shown impressive sustained protein release, such as a PBAE layer-by-layer microparticle that extended release half-life from 4.9 hours to 143.9 hours,[111] or a hyaluronic acid-based nanogel that released proteins for over one week in rats.[112] Antigen delivery can be further improved by modifying nanogels to be retained at the immunization site, promoting sustained release of the payload in the presence of immune cells.[113] Polymeric nanoparticles can also provide sustained protein release profiles, as in the case of a poly(lactic-co-glycolic acid) (PLGA)-based formulation that was shown to release OVA protein for over a week.[114] When modified to carry gp100 or B16-F10 lysate, the same particles could produce approximately 3-fold greater T cell activation compared to equivalent doses of protein in soluble form, and this resulted in superior B16-F10 tumor suppression.
3.2.2. Co-delivery of antigen and adjuvant
In general, delivery of antigens alone is not enough to trigger a strong immune response, requiring the use of an adjuvant to boost immune activation. For example, OVA antigen conjugated to poly(propylene sulfide) nanoparticles showed no anti-OVA immune response in mice, but high levels of dendritic cell maturation and OVA-specific T cell generation were observed when the same particles were delivered along with an administration of CpG, resulting in protection against influenza-OVA challenge.[115] Furthermore, vaccines generally work best when the antigen and adjuvant are delivered concurrently to the same APC, which can be readily accomplished using nanoparticle-based systems. This idea was shown systematically with a model cancer vaccine consisting of a polymeric nanoparticle loaded with an OVA peptide and the TLR7/8 agonist R848.[116] Administration of a nanoparticle encapsulating both payloads resulted in higher anti-OVA IgG production compared to either component in free form, one component in free form and the other encapsulated, or both components encapsulated separately. In addition, co-delivery of both components together enhanced downstream T cell-mediated lysis of OVA-expressing cells and elicited increased local cytokine production. Many platforms have been designed for the co-delivery of antigen and adjuvant together, including interbilayer-crosslinked multilamellar vesicles loaded with OVA antigen inside and MPLA interspersed throughout their lipid bilayers.[117] Immunization with this formulation lead to an impressive 28% of CD8+ T cells exhibiting OVA specificity, which was 14 times greater than observed when using soluble OVA and MPLA. These specific T cells also retained their functionality, as shown by high IFNγ production upon restimulation with OVA ex vivo.
When vaccinating against a heterogenous target like cancer cells, multi-epitope vaccine formulations can be employed to prevent immune escape and tumor recurrence.[20] Modular vaccine designs, exemplified by recent work describing designer nanodiscs,[118] can help overcome this barrier. Synthetic high-density lipoprotein nanodiscs were mixed with cholesterol-modified CpG ODN for immunogenicity and further functionalized with cysteine-modified, tumor-specific neoantigens for specificity. Mice immunized with nanodiscs harboring a combination of three antigens experienced an expansion in their pool of antigen-specific CD8+ and CD4+ T cells when compared to those receiving soluble formulations. The multi-antigen formulation also showed significantly better control of B16-F10 tumor growth compared to single-antigen or dual-antigen formulations. Impressively, when mice were vaccinated in combination with anti-PD1 and anti-CTLA4, 90% were cured of their tumor burden.
3.2.3. Immune cell targeting
Due to the easy surface functionalization properties of nanoparticles, the efficacy and efficiency of nanovaccines can be improved by including an immune cell targeting modality. Vaccine processing mainly takes place in APCs, and thus the most common immune cells targeted are dendritic cells and macrophages. A variety of surface markers can be targeted, such as the C-type lectin mannose receptor (CD206) by the inclusion of mannose on the nanovaccine surface.[71, 119, 120] In one example, the targeting ability of mannose was examined, and it was observed that functionalization could increase particle uptake into bone marrow-derived dendritic cells.[121] Strong localized signal of a fluorescently labeled targeted nanovaccine was seen in the draining lymph nodes at 24 hours, while particles without mannose started to lose signal as early as 12 hours after injection. Other surface markers such as CD11c,[122] scavenger receptor class B type 1,[123] DEC205,[124, 125] and macrophage galactose-specific C-type lectin[55] have also been commonly targeted.
3.2.4. Efficient cytosolic entry
Traditional cancer vaccines suffer from difficulty in entering the cytosol of immune cells. Cytosolic entry can help to facilitate the presentation of antigens by MHC-I and subsequent mobilization of CD8+ effector T cells. In addition, there are several maturation pathways and pathogen recognition receptors located in the cytosol that can be leveraged to boost the potency of vaccine formulations. As most nanoparticles are taken up endosomally, there exist many strategies for facilitating endosomal escape. Due to the characteristic acidic environment of the endosomal compartment, redox-responsive nanovaccines can be used to achieve this goal. For example, some polymeric nanoparticles can act as proton sponges and induce lysosome swelling and rupture when encountering low pH environments.[126] Lysosomal rupture-triggered reactive oxygen species have also been shown to enhance proteasome activation, which can help to trigger MHC-I antigen presentation.[55] In one case, the common transfection agent, polyethylenimine, was coated onto the surface of antigen-loaded polymeric nanoparticles, and this helped to facilitate cross-presentation of the loaded antigen after uptake.[127] Similar reducible polymeric systems like poly(γ-glutamic acid) nanoparticles[128] and cationic dextran nanogels[129] have also shown a similar ability for facilitating MHC-I restriction. Besides endosomal escape, there are other ways to enter the cytosol from the endosomal compartment. OVA-loaded α-alumina nanoparticles can engage non-canonical autophagy, where antigens are diverted into autophagosomes and the delayed antigen degradation allows for increased cross-presentation.[130] By taking advantage of this process, significant levels of OVA-specific T cells could be induced, enabling mice to completely reject established B16-OVA tumors in vivo. In another strategy, nanoparticles can be designed to directly cross cell membranes by incorporating cell penetrating peptides onto their surfaces.[131–133] Macropinocytosis of lipid-coated nanovaccines has also been reported.[134]
Cytosolic localization gives delivered antigens access to MHC-I presentation, but it can also be leveraged to enhance immune stimulation. Recent work has shown that retinoic acid-inducible protein 1 ligands and STING ligands may be stronger activators of the immune system than traditional TLR-based adjuvants like CpG and MPLA.[85, 135] PC7A synthetic nanoparticles have been used to deliver antigen while simultaneously activating the STING pathway (Figure 3).[136, 137] When loaded with OVA, the nanoformulation induced a 3-fold increase in antigen cross-presentation due to endosomal disruption by the redox-responsive PC7A. Once in the cytosol, the PC7A also engages the STING receptor, resulting in higher immune activation compared to poly(I:C) or other polymeric nanoparticle groups. The combination of potent STING activation and efficient antigen cross-presentation led to significant antitumor efficacy against loaded antigens in B16-OVA, B16-F10, MC38, and TC-1 mouse tumor models.
Figure 3.
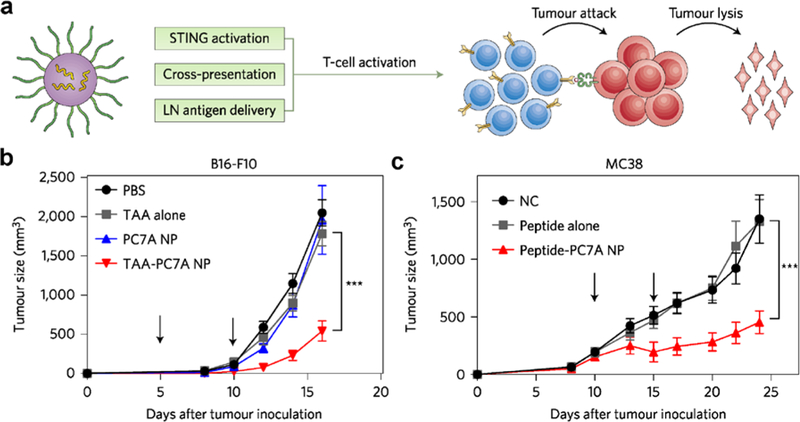
Synthetic nanoparticles activating the STING pathway for antitumor vaccination. a) Schematic depicting an antigen-loaded synthetic nanocarrier (PC7A) and its proposed mechanism of action. b,c) When used to treat tumor-bearing mice, antigen-loaded PC7A nanoparticles significantly enhanced survival in both a B16-F10 melanoma model (b) and an MC38 colon cancer model (c). Reproduced with permission.[136, 137] Copyright 2017, Nature Publishing Group.
Instead of delivering antigens directly to the cytosol, some recent work has also focused on delivery of antigen-encoding RNA for in situ transcription and antigen production.[120, 134, 138] Acid-dissolvable calcium phosphate nanoparticles carrying mRNA encoding the tumor-associated tyrosinase-related protein 2 (TRP2) could elicit stronger antigen-specific T cell responses and humoral responses against B16-F10 melanoma compared to peptide delivery.[120] In addition, PDL1 siRNA could be delivered to directly downregulate PDL1 in dendritic cells to reduce immunosuppression. Cytosolic delivery of both mRNAs was shown to have a potent antitumor effect, significantly better than cytosolic delivery of either component alone. This strategy of RNA antigen sourcing has also been implemented using a highly modular RNA-lipoplex platform.[134] RNA-containing lipoplexes were optimized to target the spleen by modifying the charge ratios of the components, and the resulting formulation was shown to be taken up into the cytoplasm of dendritic cells and macrophages via macropinocytosis. The nanoparticles also induced TLR7-triggered IFNα production and IFN-α/β receptor-dependent activation of APCs. Introduction of antigen-encoding RNA induced generation of functional antigen-specific T cells and memory cells, which resulted in potent antitumor efficacy in several tumor models. Moving towards clinical translation, three human patients with advanced malignant melanoma received five doses of the nanovaccine encoding for four tumor antigens. All three patients showed systemic IFNα production, along with de novo priming and amplification of T cells against the vaccine antigens.
3.2.5. Artificial antigen presentation
Most cancer vaccines work by manipulating APCs, which can then further stimulate antigen-specific T cells and B cells. Recently, there has been significant interest in developing artificial APCs (aAPCs) that are capable of directly stimulating effector cells.[139] This strategy was originally developed in order to effectively expand T cells ex vivo for adoptive cell therapies such as CAR T cell therapy.[140] These aAPCs, which include both live cell-based or synthetic micro/nanoparticle-based platforms, mimic professional APCs and can strongly activate T cells while avoiding the intensive labor, high cost, and difficulty in quality control when using autologous APCs. Similar to their natural counterparts, aAPCs require at least two signals to induce T cell activation. The first signal, a peptide-MHC complex, binds to its cognate T cell receptor (TCR) and establishes antigen specificity. To become fully activated, T cells require a second signal in the form of co-stimulatory molecules such as CD80 and CD86, which engage their corresponding receptor on the T cell surface.[139] With these two signals, aAPCs have the potential to act as a vaccine-like platform that can expand antigen-specific T cell populations, but without the use of immunological adjuvants. In addition to the minimum two signal requirement, at times a third signal, in the form of soluble cytokines, can further enhance the survivability of the activated T cells.[141]
To generate nanoscale aAPCs capable of engaging and activating T cells, MHC-Ig along with a co-stimulatory signal, in the form of CD80 or anti-CD28, have been decorated onto the surface of nanoparticles (Figure 4).[142] When administered subcutaneously, nanoscale aAPCs exhibited greater lymphatic drainage compared with microscale aAPCs, which largely remained at the injection site. When administered into tumor-bearing mice that received adoptively transferred antigen-specific T cells, the nanoparticles were able to help significantly control tumor growth. It has also been demonstrated that aAPCs can be fashioned using magnetic nanomaterials.[143] After incubation with their cognate T cells, these magnetic aAPCs helped to induce significant proliferation and could also guide the T cells to tumors with the use of a magnetic field. In the future, such a platform may be directly used in vivo to promote antitumor activity. Interestingly, it has been found that the shape of nanoscale aAPCs can have a significant impact on their biological activity.[144] Ellipsoid nanoparticles were fabricated by stretching spherical PLGA nanoparticles, followed by conjugation with anti-CD28 and MHC-Ig loaded with a gp100 tumor antigen epitope. After intravenous injection, it was observed that the ellipsoid particles could induce more antigen-specific T cells in circulation compared with their spherical counterparts. Although there are currently limited examples of nanoparticulate aAPCs being used in vivo, this nanovaccine-like platform holds significant potential given its ability to help bypass the complicated processes of antigen processing and presentation.
Figure 4.
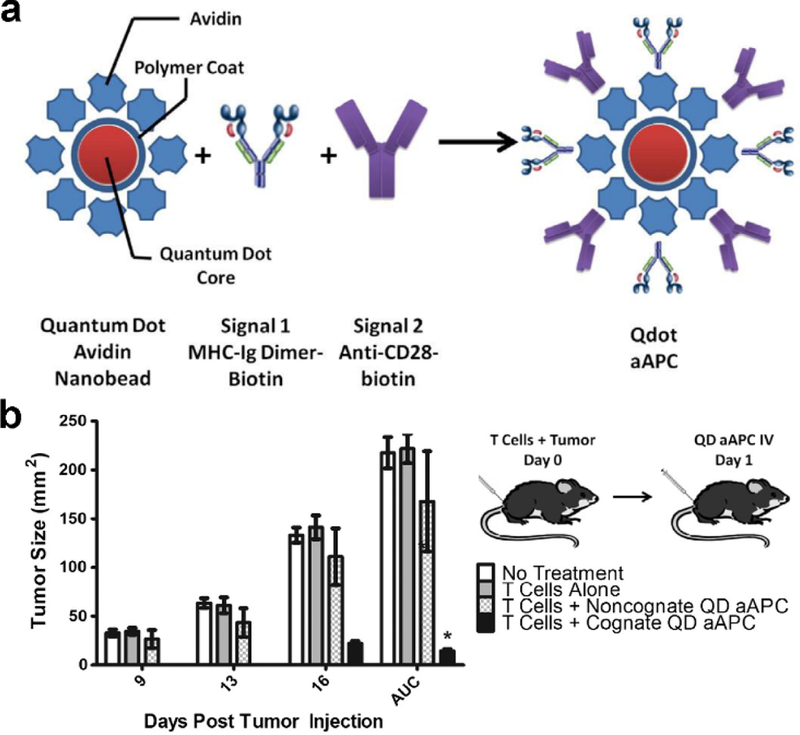
Quantum dot (QD) nanoparticles for artificial antigen presentation. a) Schematic depicting the artificial antigen presenting cell (aAPC) structure, where both signals are attached to the nanoparticle surface using biotin-avidin interactions. b) When injected intravenously into B16 tumor-bearing mice that were also adoptively transferred with antigen-specific T cells, the aAPCs were able to significantly control tumor growth. Reproduced with permission.[142] Copyright 2014, Elsevier Ltd.
4. Cell Membrane-Coated Nanovaccines
4.1. Background
As discussed thus far, nanoparticle technology has the potential to significantly alter the landscape of anticancer vaccination, enabling the design of new nanovaccines with improved efficacy compared with traditional formulations. More recently, there has been a noticeable paradigm shift within the field of nanomedicine in which a greater emphasis has been placed on biomimetic design principles.[145–148] Along these lines, a new cell membrane coating approach has emerged in which nanoparticles are cloaked with a layer of cell-derived membrane.[149–151] In contrast to traditional bottom-up synthetic strategies, top-down membrane coating directly leverages naturally occurring biological material for the fabrication of multifunctional nanoparticles. Using red blood cells (RBCs) as the source of membrane material, it was demonstrated that RBC membrane-coated nanoparticles gained the ability to avoid immune clearance and circulate for extended periods of time (Figure 5).[152] The cell-mimicking properties of these biomimetic nanoparticles results from the transference of the originating cell’s membrane proteins onto the surface of the nanoparticle substrate.[153] This approach for functionalization has proven to be highly generalizable, allowing for the delivery of a wide range of cargoes using different types of materials for the inner core.[154, 155] The outer membrane layer can also be modified with further functionality by facile means, affording additional flexibility to membrane-coated platforms.[54, 156]
Figure 5.
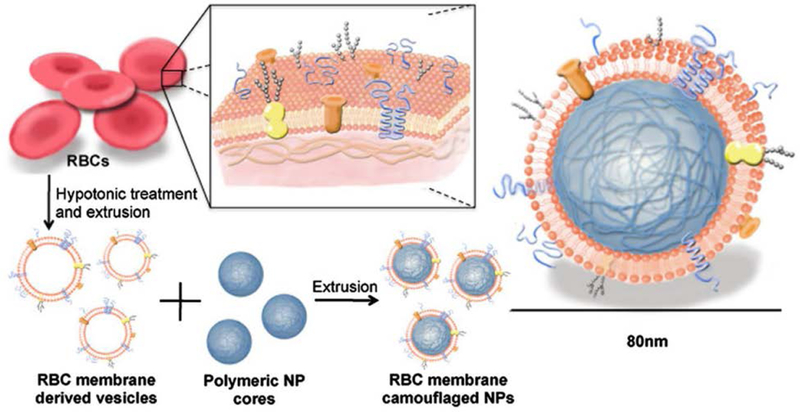
Functionalization of nanoparticles with cell membrane coating. Schematic depicting the fabrication of red blood cell (RBC) membrane-coated nanoparticles. RBC vesicles are obtained by hypotonic treatment, followed by coating onto polymeric nanoparticle cores using extrusion. The resulting membrane-coated nanoparticle exhibits a characteristic core-shell structure. Reproduced with permission.[152] Copyright 2011, National Academy of Sciences.
Since the first work on RBC membrane-coated nanoparticles was reported, research on cell membrane coatings has expanded in multiple directions. In addition to modulating the material composition of the inner core, the membrane can be sourced from a plethora of cell types, each resulting in unique formulations with novel properties. For example, platelet membrane-coated nanoparticles exhibit the ability to target bacteria and damaged vasculature,[157, 158] while cancer cell membrane-coated nanoparticles can homotypically target cancer cells.[159] White blood cell membrane, with its various toxin and cytokine receptors, has utility for treating sepsis.[160] Other membrane-coated formulations have also been reported using stem cell membrane,[161] endothelial cell membrane,[162] and even hybrid membranes generated from multiple cell types.[163] As a result of all the complex functionalities that can be incorporated, this approach has enabled the resulting biomimetic nanoparticles to excel in nontraditional areas of nanomedicine. A major example is detoxification, where membrane-coated particles can act as nanosponges to neutralize toxins by taking advantage of their interactions with cell membranes.[164–166] By neutralizing these toxins and preventing them from attacking healthy cells, these nanoscale decoys have utility for the treatment of bacterial infections, animal envenoming, and even exposure to chemical warfare agents. The ability of cell membrane-coated nanoparticles to bind and present multiple antigens, combined with the flexibility of choosing various core materials, has also made them suitable for vaccine design.[23, 24]
4.2. Cell membrane-coated nanoparticles for antibacterial vaccination
Overall, vaccines represent one of the most efficient methods of reducing the global health burden posed by bacterial infections.[167] Toxoid vaccination represents an effective means of disarming bacteria of their virulent proteins, making it harder for the pathogens to colonize their host. This strategy is currently used in the clinic to vaccinate against tetanus and diphtheria.[168] In order to make bacterial toxins safe for administration, they are generally inactivated with harsh chemical or heat treatments that can damage antigenicity and reduce vaccination efficacy. In contrast, RBC nanosponges have demonstrated the ability to naturally detain and neutralize bacterial toxins when the two are mixed together, forming what are referred to as nanotoxoids.[167, 169] Using methicillin-resistant Staphylococcus aureus (MRSA) and its major virulence factor α-hemolysin as a model system, the corresponding nanotoxoid was able to generate significant antitoxin titers, improving overall antibacterial immunity compared to a heat-denatured toxoid formulation.[170] While the control toxoid required 60 minutes of high heat exposure to achieve an acceptable safety profile, the nanotoxoid demonstrated excellent safety on a number of cell types at the outset. In animal models of both of systemic and skin toxin burden, nanotoxoid vaccination on a prime with two boosts schedule resulted in almost complete protection. A later study also demonstrated the efficacy of this approach against live MRSA infection.[171]
As the mechanism of toxin binding to membrane-coated nanoparticles relies on function rather than the specific structure of the toxin, the nanotoxoid platform can be easily generalized. To generate a multi-antigenic nanotoxoid, RBC nanosponges were mixed with a crude hemolytic protein fraction isolated from MRSA culture (Figure 6).[172] It was confirmed that the nanotoxoids contained several toxins on their surface, including α-hemolysin, γ-hemolysin, and Panton-Valentine leukocidin. Further, the nanotoxoids were found to be completely safe, whereas intense heat treatment of the hemolytic protein fraction could not completely abrogate its toxicity. When used as a vaccine, the multivalent nanotoxoids were capable of generating antibody titers against all of the aforementioned toxins, which helped to reduce bacterial burden upon live MRSA challenge. In addition to the nanotoxoid approach, another method of generating multi-antigenic vaccines is to directly employ bacteria-derived membrane. Outer membrane vesicles (OMVs) are secreted from bacteria and are important in pathogenesis as well as cell-to-cell signaling.[173] Some vaccines employing OMVs as the antigenic material have already been used in the clinic, as is the case with a formulation against meningococcal infection.[174] OMVs are attractive for use as antibacterial vaccines because they often share a similar biochemical membrane profile to their parent cell.[175] The utility of OMVs can be further improved by coating the material around a nanoparticulate core. In one instance, Escherichia coli OMVs were coated onto small gold nanoparticles, which provided increased stability and size control compared with free OMVs.[176] Due to the ability to finely control their size, the membrane-coated particles efficiently localized to the lymph nodes, leading to strong and durable immune activation.
Figure 6.
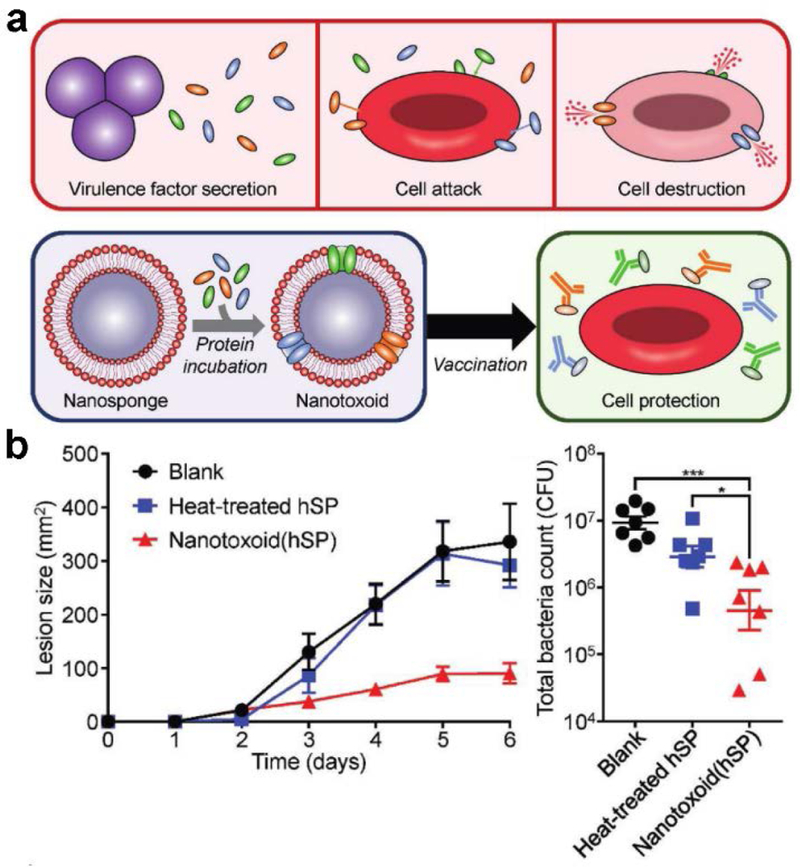
Membrane-coated nanoparticles for antibacterial vaccination. a) Schematic depicting the nanotoxoid concept, which can be used to develop vaccines against bacteria-secreted toxins. b) Vaccination using multi-antigenic nanotoxoids fabricated with a hemolytic secreted protein (hSP) fraction from methicillin-resistant Staphylococcus aureus (MRSA) significantly inhibited lesion formation caused by subcutaneous MRSA challenge, leading to decreased bacteria counts. Reproduced with permission.[172] Copyright 2017, Wiley-VCH.
4.3. Cell membrane-coated nanoparticles for anticancer vaccination
As a whole, antibacterial vaccines have been extremely successful in reducing mortality rates related to infection. Unfortunately, the same level of clinical success has not been achieved for formulations against cancer. Recently, the extension of cell membrane-coated nanoparticles to anticancer vaccination has become an active area of research. In one example, an RBC membrane-based nanocarrier was designed to deliver an hgp100 tumor antigen peptide and the adjuvant MPLA.[177] The platform was further modified with mannose on the surface to better target dendritic cells, and this led to enhanced localization to the draining lymph nodes. Both prophylactic and therapeutic efficacy were demonstrated in a B16-F10 subcutaneous tumor model, resulting in a slowing of tumor growth and a reduction in metastasis.
Since cancer cell membranes contain a plethora of autologous tumor antigens, utilizing the purified membrane of cancer cells as the antigenic material can be an effective approach in the design of nanoparticulate anticancer vaccines. This was initially demonstrated using B16-F10 melanoma membrane-coated nanoparticles incorporated with MPLA.[159] The formulation significantly increased the maturation of bone marrow-derived dendritic cells and enhanced the stimulation of antigen-specific T cells. More recently, an in-depth set of studies was conducted using a platform in which cancer cell membrane was coated around CpG ODN-loaded polymer cores (Figure 7).[178] CpG ODN 1826, a potent TLR9 agonist in mice, was encapsulated into PLGA cores through a double emulsion process, and B16-F10 membrane was coated onto the adjuvant-loaded cores by bath sonication. When the formulation was administered subcutaneously into mice, increased maturation of dendritic cells in the draining lymph nodes was observed, as indicated by the upregulation of protein markers such as CD40, CD80, CD86, and MHC-II, when compared to various controls. Notably, CpG encapsulated in nanoparticulate form was able to activate the immune system significantly better than free CpG, likely due to the preferential cellular uptake of the nanoparticles.[179–182] Additionally, it should be noted that TLR9 is located within the endosomal compartment, which highlights the power of leveraging the inherent properties of nanoparticles to purposefully manipulate immune responses. Mice vaccinated with the nanovaccine were able to generate antigen-specific CD8+ T cells against gp100 and TRP2, both of which are melanoma-associated antigens.[183] When immunized mice were challenged with B16-F10 cancer cells, 86% of the mice exhibited no tumor growth, even after 150 days. In a therapeutic setting, it was demonstrated that the nanoformulation, along with a cocktail of anti-PD1 and anti-CTLA4 checkpoint inhibitors, was able to extend the survival of the tumor-bearing mice compared to either treatment alone.
Figure 7.
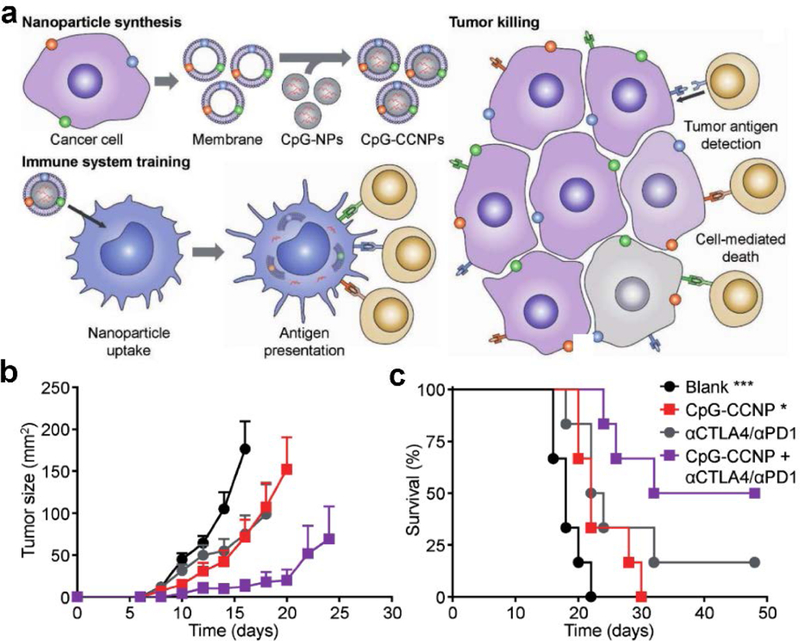
Membrane-coated nanoparticles for anticancer vaccination. a) Schematic depicting the fabrication of adjuvant-loaded cancer cell membrane-coated nanoparticles (CpG-CCNPs) and their proposed mechanism of action. b,c) When combined with a cocktail of checkpoint blockades (anti-CTLA4 and anti-PD1), treatment of established B16-F10 melanoma with the CpG-CCNP nanovaccine resulted in significantly slowed tumor growth (b) and improved survival (c). Reproduced with permission.[178] Copyright 2017, Wiley-VCH.
Building upon the concept of using cancer cell membrane-coated nanoparticles for antitumor vaccination, various strategies have been employed to augment immune responses. For example, mannose was introduced to bestow immune cell-targeting properties, helping to enhance uptake by dendritic cells and subsequently promoting their maturation.[184] As a result of this additional functionality, the targeted nanovaccine was able to offer better protection for vaccinated mice. It was claimed that this triple combination of an adjuvant, cancer cell membrane antigens, and a targeting ligand could work together to generate a robust anticancer immune response similar to levels generated against bacterial infections. In another example, immune stimulation was enhanced via the concurrent delivery of multiple adjuvants in an artificial cancer cell membrane-coated nanoparticle.[185] CpG-encapsulated calcium phosphate cores were fabricated by a water-in-oil microemulsion process and then coated with a membrane-mimicking liposome layer. Then, OVA-expressing B16-F10 cancer cell membrane proteins were purified by dialyzing the membrane against a detergent solution. The membrane proteins, along with the danger-associated molecular pattern Hsp70, were incorporated onto the nanoparticle surface to create the final formulation. This dual-adjuvant formulation was able to significantly upregulate maturation markers such as CD80, CD86, and MHC-II, and treated mice had fewer lung metastasis compared to formulations with just the CpG adjuvant. In all, the works described in this section demonstrate that cell membrane-coated nanoparticles have significant potential to be used as nanovaccines. Armed with the versatility to easily modulate both the adjuvant and the cancer membrane material, which may eventually be derived from a patient’s own tumor, this platform may ultimately pave the way for potent, personalized anticancer vaccine therapies.
5. Conclusions and Perspectives
In this review, we have discussed the progress of using nanotechnology towards the design of cancer vaccines. In theory, vaccination represents an attractive option for cancer therapy, but in practice there are many challenges that need to be overcome in order for such platforms to achieve widespread clinical adoption. Generally, it is highly difficult for the immune system to generate a potent response against established tumors, which can employ various means to lower their immunogenicity over time. With the help of nanoscale delivery vehicles, researchers are exploring the design of novel vaccine formulations that can elicit immune responses capable of overcoming tumor immunosuppression. Nanocarriers offer many advantages, including the effective localization of payloads to the desired immune cell populations, loading of multiple cargoes into a single nanoparticle, and prolonged release characteristics.
More recently, a novel type of biomimetic platform, the cell membrane-coated nanoparticle, has emerged as a strong candidate to drive the further improvement of nanovaccine platforms. Membrane coating presents a facile means of introducing multiple functionalities onto the same nanoparticle without the need for complicated synthetic techniques. Regarding anticancer vaccination, the use of cancer cell membrane as the coating material offers an approach for creating vaccine formulations rich in tumor antigens. Combined with a nanoparticulate core carrying potent immune stimulators and the ability to easily target the resulting nanoparticles to antigen presenting cells, cancer cell membrane-coated nanoparticles can achieve strong inhibition of tumor growth. These nanoformulations may be further improved through the continued optimization of adjuvant and membrane antigen combinations. Methods can also be developed for obtaining membrane material from the resected tumors of patients, enabling the facile fabrication of personalized vaccines.
Looking towards clinical translation, a main challenge will be scaling up nanoparticle production in an efficient and cost-effective manner. Nanoformulations will avoid many expenses required for live-cell vaccines, but there will likely need to be a substantial investment of time and resources to adapt current lab-scale manufacturing procedures to high-throughput workflows capable of production at the scale necessary for human patients. These workflows will also need to align with good manufacturing practices to meet quality requirements for regulatory approval. Finally, significant work will also need to be done on evaluating the synergy between vaccines and other types of cancer therapies. By simultaneously tackling the challenge of cancer treatment on multiple fronts, it may one day be possible to eliminate tumors altogether, regardless of their underlying characteristics.
Acknowledgements
This work was supported by the Defense Threat Reduction Agency Joint Science and Technology Office for Chemical and Biological Defense under Grant Number HDTRA1–18-1–0014. J.Z. was supported by a National Institutes of Health 5T32CA153915 training grant from the National Cancer Institute.
Biographies:
 Ashley V. Kroll is a graduate student researcher in the Department of NanoEngineering at the University of California San Diego. She received her B.S. in NanoEngineering at the University of California San Diego in 2014. Her research interests lie in the development of biomimetic nanoparticles for improved treatment of human diseases. She is currently working on cell membrane-coated nanoparticles for cancer immunotherapy.
Ashley V. Kroll is a graduate student researcher in the Department of NanoEngineering at the University of California San Diego. She received her B.S. in NanoEngineering at the University of California San Diego in 2014. Her research interests lie in the development of biomimetic nanoparticles for improved treatment of human diseases. She is currently working on cell membrane-coated nanoparticles for cancer immunotherapy.
 Ronnie H. Fang is an Assistant Project Scientist in the Department of NanoEngineering at the University of California San Diego. He received his Ph.D. in NanoEngineering at the University of California San Diego. His research is focused on leveraging biomimetic nanoparticles for drug delivery and immunoengineering applications.
Ronnie H. Fang is an Assistant Project Scientist in the Department of NanoEngineering at the University of California San Diego. He received his Ph.D. in NanoEngineering at the University of California San Diego. His research is focused on leveraging biomimetic nanoparticles for drug delivery and immunoengineering applications.
 Liangfang Zhang is a Professor in the Department of NanoEngineering and Moores Cancer Center at the University of California San Diego. He received his Ph.D. in Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign. His research aims to create cutting-edge biomimetic nanotechnologies and exploit them for various biomedical applications with a particular focus on drug delivery, biodetoxification and vaccination.
Liangfang Zhang is a Professor in the Department of NanoEngineering and Moores Cancer Center at the University of California San Diego. He received his Ph.D. in Chemical and Biomolecular Engineering at the University of Illinois at Urbana-Champaign. His research aims to create cutting-edge biomimetic nanotechnologies and exploit them for various biomedical applications with a particular focus on drug delivery, biodetoxification and vaccination.
References
- [1].Rosenblum MD, Remedios KA, Abbas AK, J. Clin. Invest. 2015, 125, 2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang L, Wang FS, Gershwin ME, J. Intern. Med. 2015, 278, 369. [DOI] [PubMed] [Google Scholar]
- [3].Arias CA, Murray BE, N. Engl. J. Med. 2009, 360, 439. [DOI] [PubMed] [Google Scholar]
- [4].Swann JB, Smyth MJ, J. Clin. Invest. 2007, 117, 1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kim R, Emi M, Tanabe K, Immunology 2007, 121, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Mocellin S, Wang E, Marincola FM, J. Immunother. 2001, 24, 392. [PubMed] [Google Scholar]
- [7].Leight JL, Drain AP, Weaver VM, Annu. Rev. Cancer Biol. 2017, 1, 313. [Google Scholar]
- [8].Chaudhary B, Elkord E, Vaccines (Basel) 2016, 4, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Schaaf MB, Garg AD, Agostinis P, Cell Death Dis. 2018, 9, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Farkona S, Diamandis EP, Blasutig IM, BMC Med. 2016, 14, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Pardoll DM, Nat. Rev. Cancer 2012, 12, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rosenberg SA, Yang JC, Restifo NP, Nat. Med. 2004, 10, 909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M, N. Engl. J. Med. 2012, 366, 2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ, N. Engl. J. Med. 2010, 363, 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, Pitot HC, Hamid O, Bhatia S, Martins R, Eaton K, Chen S, Salay TM, Alaparthy S, Grosso JF, Korman AJ, Parker SM, Agrawal S, Goldberg SM, Pardoll DM, Gupta A, Wigginton JM, N. Engl. J. Med. 2012, 366, 2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY, Adv. Cancer Res. 2013, 119, 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Plotkin S, Proc. Natl. Acad. Sci. U. S. A. 2014, 111, 12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Whitney CG, Zhou F, Singleton J, Schuchat A, C. Centers for Disease, Prevention, MMWR Morb. Mortal. Wkly. Rep. 2014, 63, 352. [PMC free article] [PubMed] [Google Scholar]
- [19].Finn OJ, Nat. Rev. Immunol. 2003, 3, 630. [DOI] [PubMed] [Google Scholar]
- [20].van der Burg SH, Arens R, Ossendorp F, van Hall T, Melief CJ, Nat. Rev. Cancer 2016, 16, 219. [DOI] [PubMed] [Google Scholar]
- [21].Banchereau J, Palucka AK, Nat. Rev. Immunol. 2005, 5, 296. [DOI] [PubMed] [Google Scholar]
- [22].Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS, N. Engl. J. Med. 2010, 363, 411. [DOI] [PubMed] [Google Scholar]
- [23].Fang RH, Kroll AV, Zhang L, Small 2015, 11, 5483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Fang RH, Zhang L, Annu. Rev. Chem. Biomol. Eng. 2016, 7, 305. [DOI] [PubMed] [Google Scholar]
- [25].Dagogo-Jack I, Shaw AT, Nat. Rev. Clin. Oncol. 2017, 15, 81. [DOI] [PubMed] [Google Scholar]
- [26].Law SK, Clin. Exp. Immunol. 1991, 85, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rabinovich GA, Gabrilovich D, Sotomayor EM, Annu. Rev. Immunol. 2007, 25, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mellman I, Coukos G, Dranoff G, Nature 2011, 480, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rosenberg SA, J. Immunol. 2014, 192, 5451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chada S, Ramesh R, Mhashilkar AM, Curr. Opin. Mol. Ther. 2003, 5, 463. [PubMed] [Google Scholar]
- [31].Fesnak AD, June CH, Levine BL, Nat. Rev. Cancer 2016, 16, 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brudno JN, Kochenderfer JN, Nat. Rev. Clin. Oncol. 2017, 15, 31. [DOI] [PubMed] [Google Scholar]
- [33].Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME, Nat. Rev. Cancer 2008, 8, 299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Weiner LM, Surana R, Wang S, Nat. Rev. Immunol. 2010, 10, 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Gerber HP, Sapra P, Loganzo F, May C, Biochem. Pharmacol. 2016, 102, 1. [DOI] [PubMed] [Google Scholar]
- [36].Ribas A, Wolchok JD, Science 2018, 359, 1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Yarchoan M, Hopkins A, Jaffee EM, N. Engl. J. Med. 2017, 377, 2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Suen H, Brown R, Yang S, Ho PJ, Gibson J, Joshua D, Leukemia 2015, 29, 1621. [DOI] [PubMed] [Google Scholar]
- [39].Morse MA, Lyerly HK, Vaccine 2015, 33, 7377. [DOI] [PubMed] [Google Scholar]
- [40].Overwijk WW, Curr. Opin. Immunol. 2017, 47, 103. [DOI] [PubMed] [Google Scholar]
- [41].Dubensky TW Jr., Reed SG, Semin. Immunol. 2010, 22, 155. [DOI] [PubMed] [Google Scholar]
- [42].Petersen TR, Dickgreber N, Hermans IF, Crit. Rev. Immunol. 2010, 30, 345. [DOI] [PubMed] [Google Scholar]
- [43].Slingluff CL, Cancer J Sci. Am. 2011, 17, 343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Keenan BP, Jaffee EM, Semin. Oncol. 2012, 39, 276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hammerich L, Binder A, Brody JD, Mol. Oncol. 2015, 9, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Parato KA, Senger D, Forsyth PAJ, Bell JC, Nat. Rev. Cancer 2005, 5, 965. [DOI] [PubMed] [Google Scholar]
- [47].Cheever MA, Higano CS, Clin. Cancer Res. 2011, 17, 3520. [DOI] [PubMed] [Google Scholar]
- [48].Graddis TJ, McMahan CJ, Tamman J, Page KJ, Trager JB, Int. J. Clin. Exp. Pathol. 2011, 4, 295. [PMC free article] [PubMed] [Google Scholar]
- [49].Schumacher TN, Schreiber RD, Science 2015, 348, 69. [DOI] [PubMed] [Google Scholar]
- [50].Gros A, Parkhurst MR, Tran E, Pasetto A, Robbins PF, Ilyas S, Prickett TD, Gartner JJ, Crystal JS, Roberts IM, Trebska-McGowan K, Wunderlich JR, Yang JC, Rosenberg SA, Nat. Med. 2016, 22, 433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Fischer NO, Rasley A, Corzett M, Hwang MH, Hoeprich PD, Blanchette CD, J. Am. Chem. Soc. 2013, 135, 2044. [DOI] [PubMed] [Google Scholar]
- [52].An M, Li M, Xi J, Liu H, ACS Appl. Mater. Interfaces 2017, 9, 23466. [DOI] [PubMed] [Google Scholar]
- [53].Hanson MC, Bershteyn A, Crespo MP, Irvine DJ, Biomacromolecules 2014, 15, 2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Fang RH, Hu CM, Chen KN, Luk BT, Carpenter CW, Gao W, Li S, Zhang DE, Lu W, Zhang L, Nanoscale 2013, 5, 8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wang C, Li P, Liu L, Pan H, Li H, Cai L, Ma Y, Biomaterials 2016, 79, 88. [DOI] [PubMed] [Google Scholar]
- [56].Radovic-Moreno AF, Chernyak N, Mader CC, Nallagatla S, Kang RS, Hao L, Walker DA, Halo TL, Merkel TJ, Rische CH, Anantatmula S, Burkhart M, Mirkin CA, Gryaznov SM, Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].He Q, Mitchell AR, Johnson SL, Wagner-Bartak C, Morcol T, Bell SJ, Clin. Diagn. Lab. Immunol. 2000, 7, 899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Deng L, Mohan T, Chang TZ, Gonzalez GX, Wang Y, Kwon Y-M, Kang S-M, Compans RW, Champion JA, Wang B-Z, Nat. Commun. 2018, 9, 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bishop CJ, Kozielski KL, Green JJ, J. Control. Release 2015, 219, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Petrovsky N, Drug Saf. 2015, 38, 1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Tam HH, Melo MB, Kang M, Pelet JM, Ruda VM, Foley MH, Hu JK, Kumari S, Crampton J, Baldeon AD, Sanders RW, Moore JP, Crotty S, Langer R, Anderson DG, Chakraborty AK, Irvine DJ, Proc. Natl. Acad. Sci. U. S. A. 2016, 113, E6639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Bachmann MF, Jennings GT, Nat. Rev. Immunol. 2010, 10, 787. [DOI] [PubMed] [Google Scholar]
- [63].Reddy ST, van der Vlies AJ, Simeoni E, Angeli V, Randolph GJ, O’Neil CP, Lee LK, Swartz MA, Hubbell JA, Nat. Biotechnol. 2007, 25, 1159. [DOI] [PubMed] [Google Scholar]
- [64].Shannahan JH, Bai W, Brown JM, Receptors Clin. Investig. 2015, 2, e811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chen P, Liu X, Sun Y, Zhou P, Wang Y, Zhang Y, Hum. Vaccin. Immunother. 2016, 12, 612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Bale SS, Kwon SJ, Shah DA, Banerjee A, Dordick JS, Kane RS, ACS Nano 2010, 4, 1493. [DOI] [PubMed] [Google Scholar]
- [67].Behzadi S, Serpooshan V, Tao W, Hamaly MA, Alkawareek MY, Dreaden EC, Brown D, Alkilany AM, Farokhzad OC, Mahmoudi M, Chem. Soc. Rev. 2017, 46, 4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dominguez AL, Lustgarten J, Vaccine 2010, 28, 1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Yuan H, Jiang W, von Roemeling CA, Qie Y, Liu X, Chen Y, Wang Y, Wharen RE, Yun K, Bu G, Knutson KL, Kim BYS, Nat. Nanotechnol. 2017, 12, 763. [DOI] [PubMed] [Google Scholar]
- [70].Schütz C, Varela JC, Perica K, Haupt C, Oelke M, Schneck JP, Oncotarget 2016, 7, 68503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Xu Z, Ramishetti S, Tseng Y-C, Guo S, Wang Y, Huang L, Control J. Release 2013, 172, 259. [DOI] [PubMed] [Google Scholar]
- [72].Xu Z, Wang Y, Zhang L, Huang L, ACS Nano 2014, 8, 3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Mullard A, Nat. Rev. Drug Discov. 2013, 12, 489. [DOI] [PubMed] [Google Scholar]
- [74].Wang C, Sun W, Ye Y, Hu Q, Bomba HN, Gu Z, Nat. Biomed. Eng. 2017, 1, 0011. [Google Scholar]
- [75].Liu Q, Zhu H, Tiruthani K, Shen L, Chen F, Gao K, Zhang X, Hou L, Wang D, Liu R, Huang L, ACS Nano 2018, 12, 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].de Titta A, Ballester M, Julier Z, Nembrini C, Jeanbart L, van der Vlies AJ, Swartz MA, Hubbell JA, Proc. Natl. Acad. Sci. U. S. A. 2013, 110, 19902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Chinnathambi S, Chen S, Ganesan S, Hanagata N, Sci. Rep. 2012, 2, 534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Thomas SN, Vokali E, Lund AW, Hubbell JA, Swartz MA, Biomaterials 2014, 35, 814. [DOI] [PubMed] [Google Scholar]
- [79].Fan Y, Kuai R, Xu Y, Ochyl LJ, Irvine DJ, Moon JJ, Nano Lett. 2017, 17, 7387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Molino NM, Neek M, Tucker JA, Nelson EL, Wang S-W, ACS Biomater. Sci. Eng. 2017, 3, 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Diwan M, Elamanchili P, Lane H, Gainer A, Samuel J, J. Drug Target. 2003, 11, 495. [DOI] [PubMed] [Google Scholar]
- [82].Elamanchili P, Lutsiak CME, Hamdy S, Diwan M, Samuel J, J. Immunother. 2007, 30, 378. [DOI] [PubMed] [Google Scholar]
- [83].Dowling DJ, Scott EA, Scheid A, Bergelson I, Joshi S, Pietrasanta C, Brightman S, Sanchez-Schmitz G, Van Haren SD, Ninković J, Kats D, Guiducci C, de Titta A, Bonner DK, Hirosue S, Swartz MA, Hubbell JA, Levy O, J. Allergy Clin. Immunol. 2017, 140, 1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Fox CB, Sivananthan SJ, Duthie MS, Vergara J, Guderian JA, Moon E, Coblentz D, Reed SG, Carter D, J. Nanobiotechnology 2014, 12, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hanson MC, Crespo MP, Abraham W, Moynihan KD, Szeto GL, Chen SH, Melo MB, Mueller S, Irvine DJ, J. Clin. Invest. 2015, 125, 2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wilson DR, Sen R, Sunshine JC, Pardoll DM, Green JJ, Kim YJ, Nanomedicine 2018, 14, 237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Lizotte PH, Wen AM, Sheen MR, Fields J, Rojanasopondist P, Steinmetz NF, Fiering S, Nat. Nanotechnol. 2016, 11, 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Peruzzi PP, Chiocca EA, Nat. Nanotechnol. 2016, 11, 214. [DOI] [PubMed] [Google Scholar]
- [89].Lebel M-È, Chartrand K, Tarrab E, Savard P, Leclerc D, Lamarre A, Nano Lett. 2016, 16, 1826. [DOI] [PubMed] [Google Scholar]
- [90].Schumacher R, Adamina M, Zurbriggen R, Bolli M, Padovan E, Zajac P, Heberer M, Spagnoli GC, Vaccine 2004, 22, 714. [DOI] [PubMed] [Google Scholar]
- [91].Gilbert SC, Mol. Biotechnol. 2001, 19, 169. [DOI] [PubMed] [Google Scholar]
- [92].St John AL, Chan CY, Staats HF, Leong KW, Abraham SN, Nat. Mater. 2012, 11, 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Sagiv-Barfi I, Czerwinski DK, Levy S, Alam IS, Mayer AT, Gambhir SS, Levy R, Sci. Transl. Med. 2018, 10, eaan4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Margaroni M, Agallou M, Kontonikola K, Karidi K, Kammona O, Kiparissides C, Gaitanaki C, Karagouni E, Eur. J. Pharm. Biopharm. 2016, 105, 18. [DOI] [PubMed] [Google Scholar]
- [95].Zhang Y, Li N, Suh H, Irvine DJ, Nat. Commun. 2018, 9, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Pradhan P, Qin H, Leleux JA, Gwak D, Sakamaki I, Kwak LW, Roy K, Biomaterials 2014, 35, 5491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Schmid D, Park CG, Hartl CA, Subedi N, Cartwright AN, Puerto RB, Zheng Y, Maiarana J, Freeman GJ, Wucherpfennig KW, Irvine DJ, Goldberg MS, Nat. Commun. 2017, 8, 1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Kosmides AK, Sidhom J-W, Fraser A, Bessell CA, Schneck JP, ACS Nano 2017, 11, 5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Mi Y, Smith CC, Yang F, Qi Y, Roche KC, Serody JS, Vincent BG, Wang AZ, Adv. Mater. 2018, 30, 1706098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lee KL, Murray AA, Le DHT, Sheen MR, Shukla S, Commandeur U, Fiering S, Steinmetz NF, Nano Lett. 2017, 17, 4019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].An M, Yu C, Xi J, Reyes J, Mao G, Wei W-Z, Liu H, Nanoscale 2018, 10, 9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Min Y, Roche KC, Tian S, Eblan MJ, McKinnon KP, Caster JM, Chai S, Herring LE, Zhang L, Zhang T, DeSimone JM, Tepper JE, Vincent BG, Serody JS, Wang AZ, Nat. Nanotechnol. 2017, 12, 877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Jobsri J, Allen A, Rajagopal D, Shipton M, Kanyuka K, Lomonossoff GP, Ottensmeier C, Diebold SS, Stevenson FK, Savelyeva N, PLoS One 2015, 10, e0118096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Purwada A, Tian YF, Huang W, Rohrbach KM, Deol S, August A, Singh A, Adv. Healthc. Mater. 2016, 5, 1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Rudra JS, Mishra S, Chong AS, Mitchell RA, Nardin EH, Nussenzweig V, Collier JH, Biomaterials 2012, 33, 6476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Wang H, Luo Z, Wang Y, He T, Yang C, others, Adv. Funct. Mater. 2016, 26, 1822. [Google Scholar]
- [107].Umeki Y, Mohri K, Kawasaki Y, others, Adv. Funct. Mater. 2015, 25, 5758. [Google Scholar]
- [108].Luo Z, Wu Q, Yang C, Wang H, He T, Wang Y, Wang Z, Chen H, Li X, Gong C, Others, Adv. Mater. 2017, 29, 1601776. [DOI] [PubMed] [Google Scholar]
- [109].Singh A, Agarwal R, Diaz-Ruiz CA, Willett NJ, Wang P, Lee LA, Wang Q, Guldberg RE, García AJ, Adv. Healthc. Mater. 2014, 3, 1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Soni KS, Desale SS, Bronich TK, J. Control. Release 2016, 240, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Sakr OS, Jordan O, Borchard G, Drug Deliv. 2016, 23, 2747. [DOI] [PubMed] [Google Scholar]
- [112].Nakai T, Hirakura T, Sakurai Y, Shimoboji T, Ishigai M, Akiyoshi K, Macromol. Biosci. 2012, 12, 475. [DOI] [PubMed] [Google Scholar]
- [113].Nochi T, Yuki Y, Takahashi H, Sawada S-I, Mejima M, Kohda T, Harada N, Kong IG, Sato A, Kataoka N, Tokuhara D, Kurokawa S, Takahashi Y, Tsukada H, Kozaki S, Akiyoshi K, Kiyono H, Nat. Mater. 2010, 9, 572. [DOI] [PubMed] [Google Scholar]
- [114].Solbrig CM, Saucier-Sawyer JK, Cody V, Saltzman WM, Hanlon DJ, Mol. Pharm. 2007, 4, 47. [DOI] [PubMed] [Google Scholar]
- [115].Nembrini C, Stano A, Dane KY, Ballester M, van der Vlies AJ, Marsland BJ, Swartz MA, Hubbell JA, Proc. Natl. Acad. Sci. U. S. A. 2011, 108, E989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Ilyinskii PO, Roy CJ, O’Neil CP, Browning EA, Pittet LA, Altreuter DH, Alexis F, Tonti E, Shi J, Basto PA, Iannacone M, Radovic-Moreno AF, Langer RS, Farokhzad OC, von Andrian UH, Johnston LPM, Kishimoto TK, Vaccine 2014, 32, 2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Moon JJ, Suh H, Bershteyn A, Stephan MT, Liu H, Huang B, Sohail M, Luo S, Um SH, Khant H, Goodwin JT, Ramos J, Chiu W, Irvine DJ, Nat. Mater. 2011, 10, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kuai R, Ochyl LJ, Bahjat KS, Schwendeman A, Moon JJ, Nat. Mater. 2017, 16, 489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Silva JM, Zupancic E, Vandermeulen G, Oliveira VG, Salgado A, Videira M, Gaspar M, Graca L, Préat V, Florindo HF, J. Control. Release 2015, 198, 91. [DOI] [PubMed] [Google Scholar]
- [120].Wang Y, Zhang L, Xu Z, Miao L, Huang L, Mol. Ther. 2018, 26, 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Shi G-N, Zhang C-N, Xu R, Niu J-F, Song H-J, Zhang X-Y, Wang W-W, Wang Y-M, Li C, Wei X-Q, Kong D-L, Biomaterials 2017, 113, 191. [DOI] [PubMed] [Google Scholar]
- [122].Liang R, Xie J, Li J, Wang K, Liu L, Gao Y, Hussain M, Shen G, Zhu J, Tao J, Biomaterials 2017, 149, 41. [DOI] [PubMed] [Google Scholar]
- [123].Qian Y, Jin H, Qiao S, Dai Y, Huang C, Lu L, Luo Q, Zhang Z, Biomaterials 2016, 98, 171. [DOI] [PubMed] [Google Scholar]
- [124].Bandyopadhyay A, Fine RL, Demento S, Bockenstedt LK, Fahmy TM, Biomaterials 2011, 32, 3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Saluja SS, Hanlon DJ, Sharp FA, Hong E, Khalil D, Robinson E, Tigelaar R, Fahmy TM, Edelson RL, Int. J. Nanomedicine 2014, 9, 5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP, Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Song C, Noh Y-W, Lim YT, Int. J. Nanomedicine 2016, 11, 3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Uto T, Toyama M, Nishi Y, Akagi T, Shima F, Akashi M, Baba M, Results Immunol. 2013, 3, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Li D, Sun F, Bourajjaj M, Chen Y, Pieters EH, Chen J, van den Dikkenberg JB, Lou B, Camps MGM, Ossendorp F, Hennink WE, Vermonden T, van Nostrum CF, Nanoscale 2016, 8, 19592. [DOI] [PubMed] [Google Scholar]
- [130].Li H, Li Y, Jiao J, Hu H-M, Nat. Nanotechnol. 2011, 6, 645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Wang K, Yang Y, Xue W, Liu Z, Mol. Pharm. 2018, 15, 975. [DOI] [PubMed] [Google Scholar]
- [132].Steinbach JM, Seo Y-E, Saltzman WM, Acta Biomater. 2016, 30, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Wu Z, Chen K, Yildiz I, Dirksen A, Fischer R, Dawson PE, Steinmetz NF, Nanoscale 2012, 4, 3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Hüsemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Türeci Ö, Sahin U, Nature 2016, 534, 396. [DOI] [PubMed] [Google Scholar]
- [135].Goodwin TJ, Huang L, Vaccine 2017, 35, 2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Luo M, Wang H, Wang Z, Cai H, Lu Z, Li Y, Du M, Huang G, Wang C, Chen X, Porembka MR, Lea J, Frankel AE, Fu Y-X, Chen ZJ, Gao J, Nat. Nanotechnol. 2017, 12, 648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Fiering S, Nat. Nanotechnol. 2017, 12, 615. [DOI] [PubMed] [Google Scholar]
- [138].Oberli MA, Reichmuth AM, Dorkin JR, Mitchell MJ, Fenton OS, Jaklenec A, Anderson DG, Langer R, Blankschtein D, Nano Lett. 2017, 17, 1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Eggermont LJ, Paulis LE, Tel J, Figdor CG, Trends Biotechnol. 2014, 32, 456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Turtle CJ, Riddell SR, Cancer J. 2010, 16, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Steenblock ER, Fahmy TM, Mol. Ther. 2008, 16, 765. [DOI] [PubMed] [Google Scholar]
- [142].Perica K, De León Medero A, Durai M, Chiu YL, Bieler JG, Sibener L, Niemöller M, Assenmacher M, Richter A, Edidin M, Oelke M, Schneck J, Nanomedicine 2014, 10, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang Q, Wei W, Wang P, Zuo L, Li F, Xu J, Xi X, Gao X, Ma G, Xie H-Y, ACS Nano 2017, 11, 10724. [DOI] [PubMed] [Google Scholar]
- [144].Meyer RA, Sunshine JC, Perica K, Kosmides AK, Aje K, Schneck JP, Green JJ, Small 2015, 11, 1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Hu CMJ, Fang RH, Zhang LF, Adv. Healthc. Mater. 2012, 1, 537. [DOI] [PubMed] [Google Scholar]
- [146].Fang RH, Hu CMJ, Zhang LF, Expert Opin. Biol. Th. 2012, 12, 385. [DOI] [PubMed] [Google Scholar]
- [147].Dehaini D, Fang RH, Zhang L, Bioeng. Transl. Med. 2016, 1, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Yoo JW, Irvine DJ, Discher DE, Mitragotri S, Nat. Rev. Drug Discov. 2011, 10, 521. [DOI] [PubMed] [Google Scholar]
- [149].Fang RH, Kroll AV, Gao WW, Zhang LF, Adv. Mater. 2018, 30, 1706759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kroll AV, Fang RH, Zhang LF, Bioconjug. Chem. 2017, 28, 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Fang RH, Jiang Y, Fang JC, Zhang LF, Biomaterials 2017, 128, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Hu C-MJ, Zhang L, Aryal S, Cheung C, Fang RH, Zhang L, Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 10980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Hu CM, Fang RH, Luk BT, Chen KN, Carpenter C, Gao W, Zhang K, Zhang L, Nanoscale 2013, 5, 2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Aryal S, Hu CM, Fang RH, Dehaini D, Carpenter C, Zhang DE, Zhang L, Nanomedicine (Lond.) 2013, 8, 1271. [DOI] [PubMed] [Google Scholar]
- [155].Gao W, Hu CM, Fang RH, Luk BT, Su J, Zhang L, Adv. Mater. 2013, 25, 3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Chai Z, Hu X, Wei X, Zhan C, Lu L, Jiang K, Su B, Ruan H, Ran D, Fang RH, Zhang L, Lu W, J. Control. Release 2017, 264, 102. [DOI] [PubMed] [Google Scholar]
- [157].Wei XL, Ying M, Dehaini D, Su YY, Kroll AV, Zhou JR, Gao WW, Fang RH, Chien S, Zhang LF, ACS Nano 2018, 12, 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Hu C-MJ, Fang RH, Wang K-C, Luk BT, Thamphiwatana S, Dehaini D, Nguyen P, Angsantikul P, Wen CH, Kroll AV, Carpenter C, Ramesh M, Qu V, Patel SH, Zhu J, Shi W, Hofman FM, Chen TC, Gao W, Zhang K, Chien S, Zhang L, Nature 2015, 526, 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Fang RH, Hu C-MJ, Luk BT, Gao W, Copp JA, Tai Y, O’Connor DE, Zhang L, Nano Lett. 2014, 14, 2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Thamphiwatana S, Angsantikul P, Escajadillo T, Zhang Q, Olson J, Luk BT, Zhang S, Fang RH, Gao W, Nizet V, Zhang L, Proc. Natl. Acad. Sci. U. S. A. 2017, 114, 11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Lai PY, Huang RY, Lin SY, Lin YH, Chang CW, RSC Adv. 2015, 5, 98222. [Google Scholar]
- [162].Silva AKA, Di Corato R, Pellegrino T, Chat S, Pugliese G, Luciani N, Gazeau F, Wilhelm C, Nanoscale 2013, 5, 11374. [DOI] [PubMed] [Google Scholar]
- [163].Dehaini D, Wei X, Fang RH, Masson S, Angsantikul P, Luk BT, Zhang Y, Ying M, Jiang Y, Kroll AV, Gao W, Zhang L, Adv. Mater. 2017, 29, 1606209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Chen Y, Chen M, Zhang Y, Lee JH, Escajadillo T, Gong H, Fang RH, Gao W, Nizet V, Zhang L, Adv. Healthc. Mater. 2018, 1701366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Hu CMJ, Fang RH, Copp J, Luk BT, Zhang LF, Nat. Nanotechnol. 2013, 8, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Fang RH, Luk BT, Hu CM, Zhang L, Adv. Drug Deliv. Rev. 2015, 90, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Angsantikul P, Thamphiwatana S, Gao W, Zhang L, Vaccines 2015, 3, 814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Mendoza N, Ravanfar P, Satyaprakah A, Pillai S, Creed R, Dermatol. Ther. 2009, 22, 129. [DOI] [PubMed] [Google Scholar]
- [169].Angsantikul P, Fang RH, Zhang LF, Bioconjug. Chem. 2018, 29, 604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Hu C-MJ, Fang RH, Luk BT, Zhang L, Nat. Nanotechnol. 2013, 8, 933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Wang F, Fang RH, Luk BT, Hu C-MJ, Thamphiwatana S, Dehaini D, Angsantikul P, Kroll AV, Pang Z, Gao W, Lu W, Zhang L, Adv. Funct. Mater. 2016, 26, 1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Wei X, Gao J, Wang F, Ying M, Angsantikul P, Kroll AV, Zhou J, Gao W, Lu W, Fang RH, Zhang L, Adv. Mater. 2017, 29, 1701644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Wang S, Gao J, Wang Z, Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, e1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Petousis-Harris H, Paynter J, Morgan J, Saxton P, McArdle B, Goodyear-Smith F, Black S, Lancet 2017, 390, 1603. [DOI] [PubMed] [Google Scholar]
- [175].Rosenthal JA, Chen L, Baker JL, Putnam D, DeLisa MP, Curr. Opin. Biotechnol. 2014, 28, 51. [DOI] [PubMed] [Google Scholar]
- [176].Gao W, Fang RH, Thamphiwatana S, Luk BT, Li J, Angsantikul P, Zhang Q, Hu C-MJ, Zhang L, Nano Lett. 2015, 15, 1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Guo Y, Wang D, Song Q, Wu T, Zhuang X, Bao Y, Kong M, Qi Y, Tan S, Zhang Z, ACS Nano 2015, 9, 6918. [DOI] [PubMed] [Google Scholar]
- [178].Kroll AV, Fang RH, Jiang Y, Zhou J, Wei X, Yu CL, Gao J, Luk BT, Dehaini D, Gao W, Zhang L, Adv. Mater. 2017, 29, 1703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Oyewumi MO, Kumar A, Cui Z, Expert Rev. Vaccines 2010, 9, 1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Joshi VB, Geary SM, Salem AK, AAPS J. 2013, 15, 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF, Eur. J. Immunol. 2008, 38, 1404. [DOI] [PubMed] [Google Scholar]
- [182].Foged C, Brodin B, Frokjaer S, Sundblad A, Int. J. Pharm. 2005, 298, 315. [DOI] [PubMed] [Google Scholar]
- [183].Castelli C, Rivoltini L, Andreola G, Carrabba M, Renkvist N, Parmiani G, J. Cell. Physiol. 2000, 182, 323. [DOI] [PubMed] [Google Scholar]
- [184].Yang R, Xu J, Xu L, Sun X, Chen Q, Zhao Y, Peng R, Liu Z, ACS Nano 2018, 12, 5121. [DOI] [PubMed] [Google Scholar]
- [185].Kang T, Huang Y, Zhu Q, Cheng H, Pei Y, Feng J, Xu M, Jiang G, Song Q, Jiang T, Chen H, Gao X, Chen J, Biomaterials 2018, 164, 80. [DOI] [PubMed] [Google Scholar]


