Supplemental Digital Content is available in the text
Abstract
BACKGROUND
Although prehabilitation programmes for patients undergoing major intra-abdominal cancer surgery have been shown to improve pre-operative physical fitness, the conclusions regarding any postoperative benefits are inconsistent.
OBJECTIVES
The aim of this study was to evaluate the content of and the outcome measures used in studies of prehabilitation programmes for these patients. It was hypothesised that the content of prehabilitation programmes is often therapeutically invalid, and that the postoperative outcomes assessed are inadequate to evaluate the impact of complications.
DESIGN
A systematic review of randomised controlled trials.
DATA SOURCES
Studies published between January 2009 and January 2019 were retrieved from PubMed, Embase and PEDro.
ELIGIBILITY CRITERIA
Studies were included when they investigated the effects of prehabilitation in patients undergoing intra-abdominal surgery for cancer, reported pre-operative and/or postoperative outcome measures and were conducted as a randomised controlled trial. Studies for which the full text was not available were excluded, as were studies of patients undergoing nonabdominal cancer surgery.
RESULTS
Eight studies (565 patients) were included. Therapeutic validity was low in five studies. Most studies included low-risk surgical patients and considerable variation was observed between prehabilitation programmes in terms of supervision, training context, frequency, intensity, duration and training type. Objective monitoring of training progression was typically not performed, and most trials did not include nutritional or psychological support. Postoperative complications were reported in seven studies, but no study reported the impact of postoperative complications, nor on long-term postoperative outcomes.
CONCLUSION
The content of prehabilitation programmes was heterogeneous. Studies with a high therapeutic validity found unequivocal evidence that prehabilitation had beneficial effects on postoperative outcomes. Future research should focus on adequate selection and inclusion of high-risk surgical patients and provide personalised and probably multimodal (partly) supervised prehabilitation, with objective monitoring of progress. Measuring the incidence and impact of postoperative complications may contribute to demonstrating the clinical value of prehabilitation.
Introduction
Despite continuing surgical and anaesthetic advances, invasive cancer treatment remains a challenge that requires substantial physiological and psychological resilience from patients, even in the absence of postoperative complications.1–3 Resilience is defined here as the physical and mental tools and capabilities, which enable patients to cope with the disease and its subsequent treatment. Especially in patients with low physiological and psychological reserves, cancer diagnosis and treatment, including surgery, may lead to the deterioration of physical functioning.4 After treatment, low levels of physical activity by patients result in a further decline in physical functioning, reducing aerobic capacity and muscle function, and these represent obstacles to a swift ‘back-to-baseline’ recovery of physical functioning.5,6
Thus, when psychophysiological reserves are inadequate, as in frail and in less physically fit patients, the risk of postoperative complications increases.7 The aim of prehabilitation is to improve the pre-operative status of patients in the period between diagnosis and treatment by means of physical exercise training, nutritional interventions, psychological support and/or coaching towards lifestyle changes.8 Such prehabilitation is thought to result in the faster recovery of physical functioning, a reduction in postoperative complications, shorter hospital stays and an improved long-term prognosis, as well as in lower direct and indirect healthcare costs.8–10
Although both unimodal and multimodal prehabilitation programmes have been shown to improve physical fitness before surgery, it is surprising that inconsistent conclusions have been drawn about the postoperative benefits.11,12 A possible explanation is that the Clavien-Dindo classification, which seems to be the indicator most frequently used to assess the effects of prehabilitation on postoperative outcomes, may underestimate the benefits of prehabilitation because the personal impact of complications probably varies between patients depending on their psychophysiological reserves.13 Even when complication rates are similar, fitter patients with a higher level of resilience, for example following prehabilitation, may cope better with these stressors and have better postoperative outcomes. This was observed by Hulzebos et al., who reported that postoperative pneumonia had a significantly greater impact on patients in the usual-care-group than patients after prehabilitation: the latter seemed to cope more easily with postoperative hospital-acquired pneumonia.14 In addition, because of the limited availability of evidence-based guidelines for prehabilitation, the content of prehabilitation programmes found in current literature differs in terms of training frequency, intensity, duration, supervision and the number of modalities targeted. It seems fair to assume that these large differences will also be associated with considerable differences in effectiveness and hence the effect size of studies, and this could account for the overall lack of evidence about the effectiveness of prehabilitation in intra-abdominal cancer surgery in terms of postoperative complications, length of stay and quality of life.11,12,15
Many systematic reviews in the current literature have remarked on the heterogeneity of prehabilitation programmes, but there have been no studies that have systematically evaluated the content of pre-operative exercise programmes using clear and predefined criteria. To properly assess the effects of prehabilitation in intra-abdominal cancer surgery, it would seem essential to ensure that the content of prehabilitation programmes is therapeutically valid and that there is an optimal assessment of postoperative outcomes. Because both these factors are of crucial importance in demonstrating the clinical benefits of prehabilitation, the present systematic review aims to assess both these factors.
Materials and methods
Search strategy
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines16 and is registered in the PROSPERO register as CRD42018082720. The electronic databases PubMed, Embase and PEDro were searched to find eligible articles published between January 2009 and January 2019. The MeSH headings used included pre-operative care OR operative surgical procedures or pre-operative period AND colorectal neoplasms OR colonic neoplasms OR abdominal neoplasms OR digestive surgical procedure AND exercise OR physical therapy OR resistance training OR physical education and training OR high-intensity interval training. A detailed description of the search can be found in the Appendix (Supplemental Digital File). Search terms were explored using free text words to avoid the exclusion of recently published articles.
Study selection
Studies were included when they investigated the effects of physical prehabilitation (a pre-operative intervention including physical exercise training with the aim of improving physical fitness) in patients undergoing major intra-abdominal surgery for cancer, reported outcome measures for pre-operative or postoperative levels of physical fitness, postoperative morbidity, postoperative mortality, length of stay and/or quality of life, and were conducted as a randomised controlled trial (RCT). Major surgery was defined here as surgery expected to last more than 2 h, or with an anticipated blood loss greater than 500 ml. Studies for which the full text was not available were excluded, as were studies of patients undergoing nonabdominal cancer surgery.
Data extraction
After the removal of double hits from the search results, two reviewers (GT and RT) independently screened and selected potentially eligible studies. After consensus was reached in this initial selection procedure, both reviewers independently reviewed the full text of the selected studies to determine final suitability for inclusion based on the established inclusion criteria. In order to include additional relevant studies, after full text assessment, reference tracking was performed. A third reviewer (BB) determined study eligibility if the first two reviewers did not reach agreement.
Data collection process and items
The following information was collected and compared for all included studies: general study information (first author, publication year, country), patient characteristics in the intervention and control group [number of patients, age, treatment and American Society of Anesthesiologists (ASA) classification], elements of prehabilitation (such as physical exercise training, nutritional support, psychological support), content of the physical exercise training programme according to the FITT principles (training frequency, training intensity, training time, training type) and outcome measures (such as postoperative complications, postoperative mortality, length of stay).17,18
Assessment of methodological quality and therapeutic validity
Methodological quality was independently assessed by two reviewers (GT and RT) using the Cochrane Collaboration's tool for assessing risk of bias in RCTs, a domain-based evaluation for systematic reviews.19 Selection, performance, detection, attrition and reporting bias were scored as ‘low risk’ (√), ‘high risk’ (×) or ‘unclear’ (?). If the two authors disagreed, a third evaluator (BB) was consulted as a mediator. To systematically assess the content of prehabilitation programmes, its therapeutic validity was assessed independently by the same reviewers using the Consensus on Therapeutic Exercise Training (CONTENT) scale.20 Therapeutic validity was defined as the potential effectiveness of a specific physical exercise training intervention given to a specific group of patients.19 The CONTENT scale assesses the quality of physical exercise training interventions, consisting of nine items covering five critical areas. Patient eligibility, competences and setting, rationale and plausibility of the study, content of the applied intervention and adherence were scored per item as ‘adequately performed’ (√) or ‘not adequately performed’ (×). Up till now, physical exercise training programmes have been evaluated on the methodological quality of the studies in which they were evaluated. With help of the CONTENT scale, this is the first thorough attempt to explicitly evaluate the content of the preoperative physical exercise intervention itself. High therapeutic validity was indicated when ‘adequately performed’ (√) was scored six times or more. Interobserver agreement was calculated by Cohen's Kappa, with poor (<0.20), reasonable (0.21 to 0.40), moderate (0.41 to 0.60), good (0.61 to 0.80) or very good (>0.80) agreement.21
Results
Initially, the literature search identified 4372 manuscripts and, eventually, eight RCTs investigating the effects of prehabilitation in major intra-abdominal cancer surgery were included. Sample sizes of the included studies varied from 21 to 144 patients, representing 565 patients in total, with a mean age ranging between 55 and 71 years in the studies.22–29Figure 1 shows the PRISMA flow diagram for evidence acquisition. The included studies were published between January 2009 and January 2018 and they investigated prehabilitation in colorectal cancer surgery (n = 5), liver cancer surgery (n = 2) and a mixed group of patients undergoing major abdominal surgery (n = 1). General study characteristics can be found in Table 1.
Fig. 1.
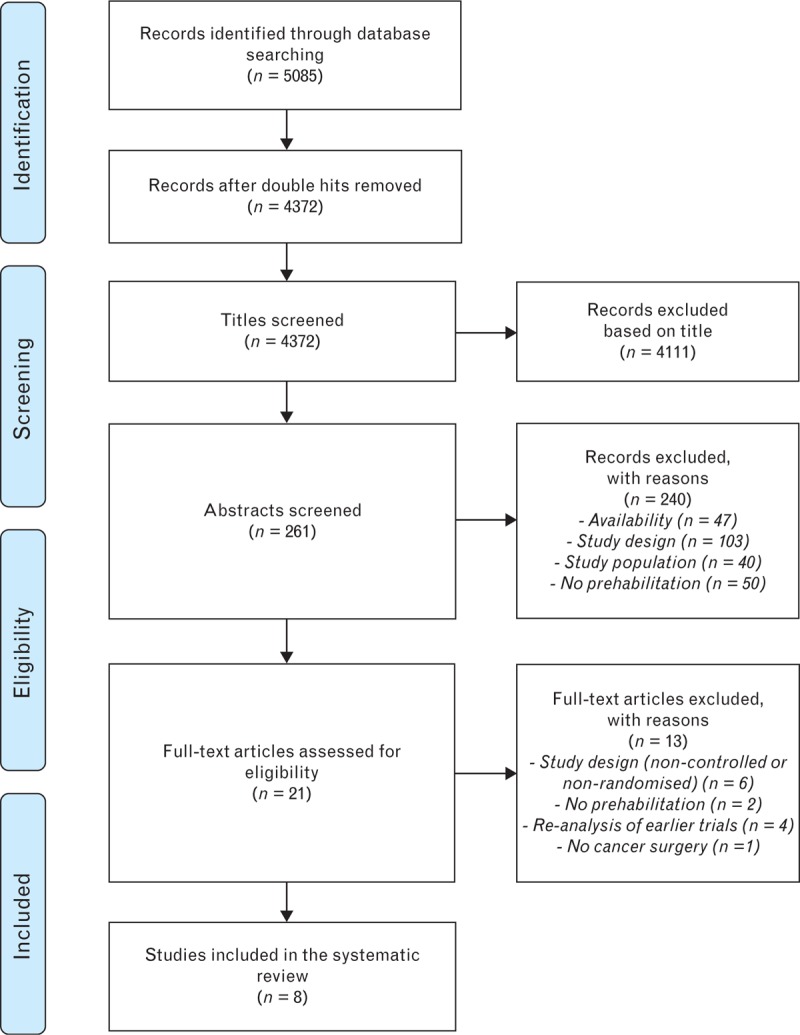
The PRISMA flow diagram for evidence acquisition.
Table 1.
General characteristics of the included studies
| Ref. | Year | Country | Sample size | Disease or treatment | Mean ± SD age (years) | ASA score | Targeted high-risk patients |
| Kim et al.22 | 2009 | USA | I: 14 C: 7 Total: 21 | Colorectal surgery | I: 55 ± 15 C: 65 ± 9 | I-III | No |
| Carli et al.23 | 2010 | Canada | I: 58 C: 54 Total: 112 | Colorectal cancer surgery | I: 61 ± 16 C: 60 ± 15 | I-III | No |
| Dronkers et al.24 | 2010 | The Netherlands | I: 22 C: 20 Total: 42 | Colon cancer surgery | I: 71 ± 6 C: 69 ± 6 | NR | Yesa |
| Kaibori et al.25 | 2012 | Japan | I: 26 C: 25 Total: 51 | Liver cancer surgery | I: 68 ± 9 C: 71 ± 9 | NR | No |
| Gillis et al.26 | 2014 | Canada | I: 38 C: 39 Total: 77 | Colorectal cancer surgery | I: 66 ± 14 C: 66 ± 9 | I-IV | No |
| Dunne et al.27 | 2016 | UK | I: 20 C: 18 Total: 38 | Liver cancer surgery | I: 61 [56 to 66] b C: 62 [53 to 72] b | NR | No |
| Barberan-Garcia et al.28 | 2018 | Spain | I: 73 C: 71 Total: 144 | Major abdominal surgery | I: 71 ± 10 C: 71 ± 11 | II-IV | Yes c |
| Bousquet-Dion et al.29 | 2018 | Canada | I: 41 C:39 Total: 80 | Colorectal cancer surgery | I: 74 [67.5 to 78] b C: 71 [54.5 to 74.5] b | I-IV | No |
ASA, American Society of Anesthesiologists; C, control group; I, intervention group; NR, not reported; SD, standard deviation.
aBased on: age >60 years.
bMedian and interquartile range.
cBased on age, ASA score and Dukes classification.
Methodological quality
Table 2 summarises the methodological quality of the included studies for which the interobserver agreement was ‘very good’ (kappa score of 0.87). None of the studies were blinded. It is noteworthy that the study by Barberan-Garcia et al.28 used a double-informed-consent model in which the control arm was not aware of the existence of an intervention arm and vice versa. Half of the included studies described the blinding of outcome measures in an irreproducible manner or not at all.22,23,25,26
Table 2.
Results of methodological quality according to the Cochrane risk of bias tool and therapeutic validity according to the CONTENT scale
| Methodological qualitya | |||||||
| Ref. | Randomisation (selection bias) | Equal groups (selection bias) | Blinding of participants and personnel (performance bias) | Blinding of outcome assessment (detection bias) | Selective drop-out (attrition bias) | Selective reporting (reporting bias) | Other sources of bias (other bias) |
| Kim et al.22 | ? | ? | X | ? | √ | ? | X |
| Carli et al.23 | √ | ? | X | ? | √ | ? | √ |
| Dronkers et al.24 | √ | √ | X | √ | √ | ? | √ |
| Kaibori et al.25 | ? | ? | X | ? | √ | ? | X |
| Gillis et al.26 | √ | √ | X | ? | √ | ? | √ |
| Dunne et al.27 | √ | √ | X | √ | √ | ? | √ |
| Barberan-Garcia et al.28 | √ | √ | X | √ | √ | √ | √ |
| Bousquet-Dion et al.29 | √ | √ | X | X | √ | √ | √ |
| Therapeutic validityb | ||||||||||
| Ref. | Description patient selection | Adequate patient selection | Eligibility criteria for therapist and setting determined and adequate | Therapeutic exercise based on a priori aims and intentions | Rationale for content and intensity described and plausible | Intensity described | Therapeutic exercise monitored and adjusted when necessary | Exercises personalised and contextualised to individual | Adherence determined and acceptable | Conclusion therapeutic validity c |
| Kim et al. 22 | √ | X | √ | √ | √ | √ | X | √ | X | High |
| Carli et al. 23 | √ | X | X | X | X | √ | X | X | X | Low |
| Dronkers et al. 24 | √ | X | X | √ | √ | √ | √ | √ | √ | High |
| Kaibori et al. 25 | √ | X | X | X | X | √ | X | X | X | Low |
| Gillis et al. 26 | √ | X | √ | √ | X | √ | X | √ | X | Low |
| Dunne et al. 27 | X | X | X | √ | √ | √ | X | √ | √ | Low |
| Barberan-Garcia et al. 28 | √ | √ | √ | √ | √ | √ | √ | √ | √ | High |
| Bousquet-Dion et al. 29 | √ | X | √ | √ | X | √ | X | √ | X | Low |
a√ = low risk of bias; X = high risk of bias; ? = unclear.
b√ = adequately performed; X = inadequately performed.
cHigh therapeutic validity: ≥6 times √; low therapeutic validity: <6 times √.
Therapeutic validity
Only three of the included studies (Table 2) were found to have high therapeutic validity.22,24,28 Interobserver agreement for therapeutic validity was ‘good’ (kappa score of 0.78). Although the selection of patients was described adequately in the majority of the studies, most patients included had low ASA scores and they therefore had a lower risk of postoperative complications. Two studies specifically included high-risk surgical patients, one on the basis of age24 and the other on the basis of age, ASA classification and Duke activity status index score (Table 1).28 None of the studies reported inclusion rates, or possible differences between the baseline characteristics of patients who decided not to participate and those who did. In four studies, patients were supervised during the programme by a researcher, exercise physiologist or physiotherapist to a greater or lesser degree24,27–29: in the other four trials, patients trained without supervision.22,23,25,26 However, the degree of supervision varied: in the study by Gillis et al.,26 no researchers or physicians were present during training sessions. Instead, patients received weekly phone calls to evaluate issues related to prehabilitation programme compliance (training frequency, training intensity, amount of whey protein ingested, use of the relaxation methods). On the contrary, in the study by Dunne et al.,27 all sessions were supervised and took place in the hospital. However, in this study and two other included studies, the background of supervising personnel was not described.24,25,27 In one study in which patients were partly supervised, patients had one supervised session a week at the hospital and were asked to complete the other training sessions unsupervised at home.29
Considerable variation was noted between the prehabilitation programmes in terms of training frequency (ranging from daily to two sessions per week), training intensity (ranging from moderate to high intensity), programme duration (ranging from 2 to 9 weeks) and type of physical exercise (aerobic training, resistance training, high-intensity interval training, stretching exercises, inspiratory muscle training or a combination of these elements) (see Table 3). The personalisation of exercise programmes also varied: the intensity of the aerobic training component was often personalised to some degree using heart rate,22–24,26,29 ventilatory anaerobic threshold,25 oxygen uptake at peak exercise27 or work rate at peak exercise (Table 3).28 The types and location of training were not personalised in most studies,23,25–27,29 but personalisation was seen on the basis of physical condition and/or personal circumstances in studies selecting high-risk patients, for example by adjusting the number of hospital visits needed.24,28 Three studies included hospital-based training.24,27,29 ‘One of these studies combined hospital-based training with home-based training.29’ One study provided community-based training,28 and four studies looked at programmes with home-based training only.22,23,25,26 The monitoring of patient progress throughout the prehabilitation programme and subsequent adjustments to the programme were noted in only two studies: perceived exertion was used in these as a measure for progress.24,26 No study used objective performance measures to assess training progress (to identify responders and nonresponders) and to adjust the training intensity or training programme accordingly. Finally, four trials (50%) investigated a unimodal approach in which physical exercise training was the sole component of prehabilitation,22–24,27 one study investigated a bimodal programme that also included a nutritional component25 and two studies investigated a trimodal programme that also included a psychological component (see Table 4).26,28
Table 3.
Prehabilitation characteristics of the included studies
| Ref. | Context, location | Supervision | Frequency of training | Method used to set training intensity | Overall intensity of training | Objective monitoring of training progression | Time of a training session | Period of training | Type of physical exercise training, including other prehabilitation modalities |
| Kim et al.22 | Home-based | Unsupervised | Daily | Aerobic training: at 40 to 65% of HRR based on CPET | Moderate | No | 20 to 30 min | 4 weeks | Structured aerobic training (20 to 30 min) on a cycle ergometer |
| Carli et al.23 | Home-based | Unsupervised | Daily | Aerobic training: at 50% of HRpeak based on CPET, gradually increased each week by 10%, if tolerable Resistance training: repetitions up to volitional fatigue, with a maximum of 12 repetitions for push-ups, sit-ups and lunges, or up to eight repetitions for biceps, deltoids and quadriceps exercises | Moderate | No | 20 to 45 min | 3 to 9 weeks | Aerobic training (20 to 30 min) on a cycle ergometer (daily) Resistance training (10 to 15 min): push-ups, sit-ups, and standing strides (three times a week) |
| Dronkers et al.24 | Hospital-based | Supervised | Two times a week | Aerobic training: at 55 to 75% of HRmax or a rating of perceived exertion of 11 to 13 on the Borg scale Resistance training: maximum of one set of eight to15 repetitions, consistent with 60 to 80% of 1RM IMT: breathing against a resistance of 10 to 60% of maximal inspiratory pressure | Moderate to high | No | 60 min | 2 to 4 weeks | - Aerobic training (20 to 30 min), combined with 15 min of IMT and resistance training of the lower limb extensors (maximum of one set of eight to 15 repetitions) at the outpatient department of the hospital Additional home-based training: participants were asked to perform moderate-intense exercises (minimum of 30 min walking or cycling), five times a week |
| Kaibori et al.25 | Home-based | Unsupervised | Three times a week | Aerobic training: based on VAT achieved during CPET | Moderate | No | 60 min | 4 weeks | Aerobic training (30 min), walking a Stretching exercises (5 min as warm-up before aerobic training, 20 min of targeted stretching after aerobic training and 5 min as cool-down) Patients also received nutritional support (for patients with hepatitis or liver cirrhosis: daily energy intake was set at 25 to 30 kcal kg body mass−1, with a daily protein intake of 1.0 to 1.2 g/kg, and a daily sodium chloride intake of 5 to 7 g/kg; for patients with diabetes or fatty liver disease, daily energy intake was set at 20 to 25 kcal kg body mass−1; for patients with hypertension, daily sodium chloride intake was set at 6 kcal kg body mass−1) |
| Gillis et al.26 | Home-based | Unsupervised b | Three times a week | Aerobic training: at 40% of HRR, calculated using the Karvonen formula [(220-age) – (resting HR × % intensity) + resting HR], where after intensity was progressed based on perceived exertion, Borg scale >12 | Moderate | No | 50 min | 4 weeks | Trimodal prehabilitation at home, supervised by phone, including: Aerobic (20 min) and resistance (20 min) training, 5 min warm-up and 5 min cool-down Nutritional support (whey protein supplements: 1.2 kcal kg body mass−1) Psychological support (relaxation exercises, imagery and visualization, and breathing exercises), 2 to 3 times a week |
| Dunne et al.27 | Hospital-based | Supervised | Three times a week | HIT: work interval at >90% of VO2peak, rest interval at <60% of VO2peak, based on CPET c | High | No | 40 min | 4 weeks | - HIT (5 min warm-up, 30 min HIT, 5 min cool-down) on a cycle ergometer |
| Barberan-Garcia et al.28 | Community-based | Supervised | One to three times a week d | - HIT: 2-min work interval at ≥70% of WRpeak, based on CPET, in first 2 weeks, thereafter WR was increased by about 5% every week up to a maximum of 85% of WRpeak, 3-min rest interval at ≥40% WRpeak, based on CPET, in first 2 weeks, thereafter WR was increased by about 5% every week up to a maximum of 50% of WRpeak | High | No | 47 min | 6 weeks | Personalised HIT (5 min warm-up, 37 min HIT, 5 min cool-down) on a cycle ergometer Nutritional support (patients suffering from iron-deficiency anaemia received intravenous iron and in patients at a high risk of malnutrition (MUST ≥2), a nutritional intervention was done by registered dieticians) Motivational interviewing aiming to realize a more physically active lifestyle and mindfulness Encouraging to be physically active on a daily base |
| Bousquet-Dion et al.29 | Home and hospital-based e | Partly supervised e | Three to four times a week | Aerobic training: walking, cycling or jogging based on the rate of perceived exertion (Borg scale) and 6MWT performance at 60 to 70% of HRR calculated from the Karvonen formula Resistance training: based on eight repetitions maximum test to provide a submaximal estimation of maximal strength | Moderate | No | 60 min | 4 weeks | - Aerobic training (walking, cycling or jogging for 30 min) and resistance training (30 min) Nutritional support (protein intake aiming for 1.2 kcal kg body mass−1) and supplementation (whey protein) if patients did not reach this target by diet alone Psychological support (home-based relaxation exercises based on visualisation and breathing exercises (two to three times a week), after 60 min supervised relaxation exercises to instruct patients) |
1RM, one-repetition maximum; 6MWT, 6-min walk test; CPET, cardiopulmonary exercise testing; HIT, high-intensity interval training; HR, heart rate; HRmax, maximal heart rate; HRpeak, peak heart rate; HRR, heart rate reserve; IMT, inspiratory muscle training; MUST, malnutrition universal screening tool; VAT, ventilatory anaerobic threshold; VO2peak, peak oxygen uptake; WR, work rate; WRpeak, peak work rate.
aWalking intensity was based on the AT of each patient.
bA limited degree of supervision was performed by phone.
cDuration of work and rest intervals were not reported.
dThe intervention group underwent a personalised prehabilitation programme based on their health conditions and social circumstances.
eOnce a week, a training session was performed in-hospital (supervised by a kinesiologist), and the other sessions were performed at home.
Table 4.
Prehabilitation outcomes of the included studies
| Ref. | Number of modalities | Physical exercise training | Nutritional support | Psychological support | Personalised | Adherence | Reasons for drop-out in prehabilitation group a | Adverse events | Postoperative care | Summary of the effects of the prehabilitation programme |
| Kim et al.22 | Unimodal | √ | X | X | No | 74% | Fatigue and malaise | NR | No rehabilitation | In the prehabilitation group, WRpeak was the only maximal exercise indicator of aerobic capacity that was responsive to the prehabilitation programme (mean ± SD increase of 26 ± 27%; 95% CI 11 to 41). For submaximal indicators of aerobic capacity, HR (-13 ± 15%; 95% CI -10 to -4) and VO2 (-7 ± 6%; 95% CI -21.5 to -4.5) during submaximal exercise were most responsive to prehabilitation in the prehabilitation group. There were no changes in maximal and submaximal indicators of aerobic capacity in the control group. 6MWT distance improved in both groups by ∼30 m. Postoperative outcomes were not evaluated in this study. |
| Carli et al.23 | Unimodal | √ | X | X | No | 59% b | Discontinued participation | NR | NR | Adherence was low. There were no differences between the prehabilitation group (aerobic and resistance training) and the control group (walking and breathing exercises) in mean ± SD 6MWT distance over the prehabilitation programme (-10.6 ± 7.3 versus 8.7 ± 6.8 m, respectively; P = NR) or at postoperative follow-up (-34.4 ± 9.9 versus -12.2 ± 10.9 m, respectively; P-value NR). The proportion showing an improvement in 6MWT distance (≥20 m) was smaller in the prehabilitation group than in the control group after the prehabilitation programme (22 versus 47%, respectively; P = 0.051) and after surgery (11 versus 41%, respectively; P = 0.019). Anxiety did not change in both groups following prehabilitation, whereas depression significantly improved in the prehabilitation group. There was no significant difference in postoperative complications and mean ± SD length of hospital stay (7.4 ± 6.5 versus 6.5 ± 3.6 days; P = NS) between the prehabilitation and control group, respectively. |
| Dronkers et al.24 | Unimodal | √ | X | X | Yes | 97% | Death of spouse Unable to combine training with daily work | 0 | NR | The prehabilitation programme was feasible, with a high compliance and no adverse events. The prehabilitation group increased respiratory muscle endurance preoperatively compared to the control group (from 259 ± 273 to 404 ± 349 J versus 350 ± 299 to 305 ± 323 J, respectively; P < 0.01). Estimated aerobic capacity, functional mobility, level of physical activity and QoL did not reveal significant differences between the two groups after the prehabilitation programme. There was no significant difference in postoperative complications (9 versus 8; P = 0.650) and mean ± SD length of hospital stay (16.2 ± 11.5 versus 21.6 ± 23.7 days; P = 0.310) between the prehabilitation and control group, respectively. |
| Kaibori et al.25 | Bimodal | √ | √ | X | Yes | NR | Tumour recurrence Financial reasons Exacerbation of other disease | NR | NR | There were no statistically significant differences in any postoperative outcomes between both groups; however, mean ± SD hospital length of stay of the prehabilitation (physical exercise training and nutritional support) group was shorter than that of the control (nutritional support) group (13.7 ± 4.0 versus 17.5 ± 11.3 days; P = 0.120). At 6 months postoperatively, the mean ± SD improvement in VO2 at the VAT and VO2peak were significantly greater following prehabilitation in a high-frequency exercise (five to six times a week) subgroup compared with a low-frequency (three times a week) subgroup (115 ± 18 versus 102 ± 14%, respectively; P = 0.038, and 118 ± 11 versus 103 ± 12%, respectively; P = 0.002). |
| Gillis et al.26 | Trimodal | √ | √ | √ | No | 78% c | Emergency surgery Withdrew consent | 0 | ERAS, rehabilitation d | The prehabilitation group improved 6MWT distance (≥20 m) in a higher proportion compared with the rehabilitation group (53 versus 15%; adjusted P = 0.006). Complication rates and duration of hospital stay were similar. The mean ± SD difference between baseline and 8-week postoperative 6MWT distance was significantly better in the prehabilitation group than in the rehabilitation group (+23.7 ± 54.8 versus − 21.8 ± 80.7 m, respectively; mean difference 45.4 m; 95% CI 13.9 to 77.0; adjusted P = 0.020). A higher proportion of the prehabilitation group was also recovered to or above baseline 6MWT distance at 8 weeks postoperatively compared with the rehabilitation group (84 versus 62%, respectively; adjusted P = 0.049). |
| Dunne et al.27 | Unimodal | √ | X | X | Yes | 99% | Insufficient time to complete prehabilitation Distance from tertiary centre | 0 | ERAS | The prehabilitation group improved in aerobic capacity preoperatively (mean increase in VO2 at the VAT +1.0 ml kg−1 min−1; 95% CI -0.2 to 2.1; P = 0.093, and mean increase in VO2peak +2.0 ml kg−1 min−1; 95% CI 0.4 to 3.6; P = 0.019). Compared with the control group, the prehabilitation group demonstrated an improvement in VO2 at the VAT of 1.5 ml kg−1 min−1 (P = 0.023) and in VO2peak of 2.0 ml kg−1 min−1 (P = 0.047). This was associated with improved preoperative QoL. There were no statistically significant differences in any postoperative outcomes between both groups. |
| Barberan-Garcia et al.28 | Trimodal | √ | √ | √ | Yes | NR | Incapacity to perform exercise testing Decided to abandon study | 0 | NR | The prehabilitation group improved in aerobic capacity pre-operatively (mean increase in endurance time +135%; P < 0.001, versus +12% for the control group; P = 0.118), whereas 6MWT distance did not change in both groups. Prehabilitation enhanced postoperative clinical outcomes, as it reduced the number of patients with postoperative complications by 51% (relative risk 0.5; 95% CI 0.3 to 0.8; P = 0.001), reduced the mean ± SD number of complications per patient (0.5 ± 1.0 versus 1.4 ± 1.6; P = 0.001), reduced mean ± SD hospital length of stay (8 ± 8 versus 13 ± 20; P = 0.078) and reduced mean ± SD ICU days of stay (1 ± 2 versus 4 ± 13; P = 0.078). |
| Bousquet-Dion et al.29 | Trimodal | √ | √ | √ | Yes | 98% e | Complications Refused to come | NR | ERAS, rehabilitation d | Both groups were comparable for baseline mean ± SD 6MWT distance (prehabilitation group: 448 ± 118 m versus rehabilitation group: 461 ± 109 m; P = 0.775) and included a similar proportion of patients who improved preoperative 6MWT distance ≥20 m (prehabilitation group: 54% versus rehabilitation group: 38%; P = 0.222). After surgery, changes in 6MWT distance were also similar in both groups. Previously inactive patients were more likely to improve functional capacity due to prehabilitation (OR 7.07; 95% CI 1.10 to 45.51). Length of first stay, emergency department visits and complication rate were similar between both groups. Hospital re-admission and the total duration of hospitalization tended to be higher in the prehabilitation group, but not following intention-to-treat analysis, in which patients who were excluded after surgery due to missing 6MWT at follow-ups were included as well. |
6MWT, 6-min walk test; CI, confidence interval; ERAS, enhanced recovery after surgery; HR, heart rate; NR, not reported; NS, not statistically significant (exact P value not reported); OR, odds ratio; QoL, quality of life; SD, standard deviation; VAT, ventilatory anaerobic threshold; VO2, oxygen uptake; VO2peak, peak oxygen uptake; WRpeak, peak work rate.
aReasons for drop-out other than changes in the surgical plan (timing, other hospital, cancellation).
bAdherence was defined as the percentage of exercise sessions attended.
cAdherence was determined using the CHAMPS (community healthy activities model programme for seniors) activities questionnaire for older adults.
dEight weeks of rehabilitation for the intervention and control group; however, the exact content of this programme was not specified.
eAdherence was only described for the supervised in-hospital sessions and determined using the CHAMPS (community healthy activities model programme for seniors) activities questionnaire for older adults and relating this to American Cancer Society guidelines.
Outcome measures used to evaluate the effects of prehabilitation
Table 5 summarises the outcome measures used to assess the effects of prehabilitation. Postoperative complications were reported in seven of the eight studies included.23–29 Five of these studies also reported postoperative complications using the Clavien-Dindo method (one study using the guidelines of Jammer et al.30 to define complications23,26–29); one study reported on the basis of the presence of complications in hospital records24; one study did not specify assessment methods.25 One study with a cohort of 144 high-risk surgical patients reported a reduction in the number of patients with postoperative complications of 51% in the prehabilitation group.28 None of the other studies reported significant differences in the incidence of postoperative complications.22–27,29 No study reported anything about the impact of postoperative complications on the patients (such as the effect of complications on length of stay, the use of resources or the patient's physical functioning). Mortality was reported in two studies and, in the time windows used, found no differences between the groups.25,28 Most studies also reported length of hospital stay: none of them found a statistically significant difference. ICU admission was reported in two studies, and again, there were no statistically significant differences between groups.27,28 No study reported on long-term postoperative outcomes.
Table 5.
Postoperative outcome measures used in the included studies
| Authors | Postoperative complications | ICU stay | Length of primary hospital stay | In-hospital mortality | Readmission |
| Kim et al.22 | NR | NR | NR | NR | NR |
| Carli et al.23 | I: CD I-II: 16/56 (29%) C: CD I-II: 15/54 (28%) P = NS I: CD III-IV: 6/56 (11%) C: CD III-IV: 3/54 (6%) P = NS | NR | I: mean ± SD days: 11.9 ± 34.6 C: mean ± SD days: 6.6 ± 3.6 P = NS I: mean ± SD days: 7.4 ± 6.5 a C: mean ± SD days: 6.5 ± 3.6a P = NS | NR | NR |
| Dronkers et al.24 | I: complications: 9/21 (43%) C: complications: 8/20 (38%) P = 0.650 I: pulmonary complications: 5/21 (24%) C: pulmonary complications: 5/20 (20%) P = 0.930 I: pneumonia: 1/21 (5%) C: pneumonia: 3/20 (15%) P = 0.270 | NR | I: mean ± SD days: 16.2 ± 11.5 C: mean ± SD days: 21.6 ± 23.7 P = 0.310 | NR | NR |
| Kaibori et al.25 | I: complications: 2/23 (9%) C: complications: 3/23 (13%) P = 0.671 | NR | I: mean ± SD days: 13.7 ± 4.0 C: mean ± SD days: 17.5 ± 11.3 P = 0.120 | I: 0 (0%) C: 0 (0%) | NR |
| Gillis et al.26 | I: 30-day CD I-IV: 12/38 (32%) C: 30-day CD I-IV: 17/39 (44%) P = 0.277 | NR | I: median [IQR]: 4 [3 to 5] C: median [IQR]: 4 [3 to 7] P = 0.812 | NR | I: 30-day readmission: 6/38 (16%) C: 30-day readmission: 5/39 (13%) P = 0.780 |
| Dunne et al.27 | I: CD I-II: 8/19 (42%) C: CD I-II: 7/15 (47%) P = NS I: CD III-IV: 3/19 (16%) C: CD III-IV: 1/15 (7%) P = NS | I: elective admissions: 8/19 (42%) C: elective admissions: 4/15 (27%) P = NS I: median (IQR) days: 1.0 (1 to 2) C: median (IQR) days: 1.5 (1 to 2) P = NS | I: median [IQR]: 5 [4.0 to 6.0] C: median [IQR]: 5 [4.5 to 7.0] P = NS | NR | I: readmission: 4/19 (21%) C: readmission: 0/15 (0%) P-value NS |
| Barberan-Garcia et al.28 | I: complications: 19/62 (31%) C: complications: 39/63 (62%) P = 0.001* | I: mean ± SD days: 1 ± 2 C: mean ± SD days: 4 ± 13 P = 0.078 | I: mean ± SD days: 8 ± 8 C: mean ± SD days: 13 ± 20 P = 0.078 | I: 1 (2%) C: 1 (2%) P = 1.000 | NR |
| Bousquet-Dion et al.29 | I: 30-day complication: 14/37 (38%) C: 30-day complication: 8/26 (31%) P = 0.562 I: most severe CD (I: n = 9; II: n = 3; III: n = 2) C: most severe CD (I: n = 4; II: n = 4; III: n = 0) P = 0.269 | NR | I: median [IQR]: 3 [3 to 4] C: median [IQR]: 3 [2 to 4] P = 0.122 | NR | I: 30-day readmission: 5/37 (14%) C: 30-day readmission: 2/26 (8%) P = 0.415 |
C, control group; CD, Clavien-Dindo; I, intervention group; IQR, interquartile range; NR, not reported; NS, not statistically significant (exact P value not reported); SD, standard deviation.
aData minus one outlier.
*P < 0.01.
Physical fitness was assessed in the majority of the studies, in five of the eight studies using cardiopulmonary exercise testing. Compared with the controls, two studies found a significant benefit in terms of aerobic capacity after prehabilitation (outcome measures used are provided in the supplementary table).22,28 After prehabilitation, one study found significant improvements in multiple variables measuring physical fitness, which were not observed in controls (supplementary table).22 Muscle strength, functional mobility and physical activity were also used as outcome measures to evaluate the effects of prehabilitation, and a significant increase in physical activity was seen after multimodal prehabilitation.28 Data about long-term physical functioning, lifestyle changes or quality of life were not provided in any of the studies.
No adverse events were recorded in any of the studies (Table 4). High adherence to training sessions was reported in the supervised trials (98% on average),24,27,29 whereas unsupervised training was associated with lower patient adherence (70% on average).22,23,26 Adherence was determined using either the number of training sessions attended, or the amount of physical exercise performed by patients. Adherence rates were not reported in two studies.25,28 Adherence during training sessions (as measured by, e.g., prescribed training intensity, unplanned breaks, completion of training sessions) and adherence for other components of a multimodal intervention (such as nutritional or psychological components) were not reported in any of the studies.
Discussion
The aim of this study was to provide a detailed and innovative systematic review of the literature investigating the effectiveness of prehabilitation in patients undergoing major intra-abdominal cancer surgery. By doing so, it should be possible to properly evaluate the effectiveness of prehabilitation trials. More importantly, it should be possible to differentiate between individual trials based on their potential beneficial effects by assessing their content according to the concept of therapeutic validity, as well as by evaluating their use of adequate postoperative outcome measures. The main findings relating to the content of prehabilitation programmes, as assessed using the CONTENT scale for therapeutic validity, were the inclusion of a high proportion of low-risk patients, inadequate monitoring and adjustment of training intensity, and absence of efficient inclusion of prehabilitation in a patient's pre-existent living condition (home, nursing home or hospital). Considerable variation was seen in terms of the content of prehabilitation programmes, with many studies focusing exclusively on physical exercise and failing to include other vital components such as nutritional and psychological support. To determine postoperative outcome, most studies used the incidence of postoperative complications as a measure for the effectiveness of prehabilitation, without taking into account the variability in ability of patients to cope with these postoperative complications.
The heterogeneity seen in the design of prehabilitation programmes, and its likely contribution to different conclusions about the postoperative benefits of prehabilitation, confirms findings from earlier systematic reviews.11,12,15 This variation is not surprising, as the first clinical guideline with recommendations for prehabilitation programmes was published only recently.31 It is recommended that this heterogeneity should be taken into account when investigating physical exercise training interventions.32 For the field of prehabilitation research, which is young and therefore lacks extensively validated measurements, the CONTENT scale may be used. This scale was developed in a four-round Delphi study20 in order to critically evaluate the potential effectiveness of a specific physical exercise training programme given to a potential target group of patients. Although it has been used in various patient populations thus far, it is currently being validated in larger data sets including general and oncological surgery, warranting careful interpretation here. Nevertheless, the present review is the first to provide a systematic evaluation of the therapeutic validity of studies investigating the physical exercise training component of prehabilitation using the CONTENT scale.20 The therapeutic validity of three studies was high and these studies found significant benefits in terms of clinical outcomes, although not all studies were powered to assess the effect on postoperative complications and outcome. In the other studies, therapeutic validity appeared to be insufficient. Surgical patients at a high risk of postoperative complications and functional decline after surgery [i.e. generally frail elderly patients and patients undergoing (neo)adjuvant chemoradiotherapy] may benefit most from prehabilitation.1,31,33,34 The low baseline aerobic capacity and the high incidence of poor nutritional status in these patients means that their capacity to cope with the stressors of disease and treatment is impaired and, consequently, they may need pre-operative optimisation, for example by prehabilitation, to increase their chances of a good outcome after treatment.11,35 However, as most trials do not select high-risk patients pre-operatively, and even seem to exclude them because high-intensity training is considered to be more challenging or even contra-indicated for these patients, therapeutic validity is impaired. Patient selection should start pre-operatively with an adequate assessment of treatment-associated risks. Assessing pre-operative psychophysiological reserves (e.g. by objectively determining aerobic capacity, muscle mass and nutritional status) may identify patient needs in terms of counselling, physical exercise training, nutritional support, psychological support and smoking cessation, with tailored prehabilitation and personalised and patient-centred care as a result.36 Inadequate patient selection in many of the trials included in our review may have led to an underestimation of the benefits of prehabilitation: this supposition may be supported by the finding that two studies that completed pre-operative risk stratification and included high-risk patients found significant improvements in patient physiological parameters24 and in postoperative outcomes.28 The PREHAB trial, which is currently recruiting, may provide an adequate sample size to perform a subgroup analysis of these high-risk surgical patients.37 This may further strengthen scientific evidence for a therapeutic window in these patients, eventually leading to the provision of (cost-)efficient prehabilitation in the right patients. Furthermore, in addition to the adequate personalisation of prehabilitation at commencement, the therapeutic validity (and therefore the success) of prehabilitation may also depend on the appropriate and objective monitoring of progress and the subsequent adjustment of treatment throughout the programme. We found large differences between levels of personalisation in prehabilitation programmes. Although training intensity would seem to have been adequately adapted to baseline physical functioning in most studies, progress, which may differ widely between individual subjects, is often not measured objectively. When measured, training intensity can be adjusted in line with training progress, and the appropriate training stimulus can therefore be maintained throughout the programme. Furthermore, the objective monitoring of progress is essential to identify nonresponders or noncompliant individuals, for whom the researcher, exercise physiologist or physiotherapist should reconsider not only the content of training but also nutrition or elements of psychological support.38 Further personalisation can be achieved when the prehabilitation programme is community or home-based, with patients being taught to train in their own environment with the caregivers and social support already in place being involved. Moreover, high-risk surgical patients are often elderly people who depend on others to get to a hospital and this makes it more difficult for them to participate in a hospital-based prehabilitation programme. Patients who do not live near a hospital are also often unable and/or unwilling to participate in a hospital-based programme.39,40 In addition to improving pre-operative physical fitness, prehabilitation may provide patients with the skills and awareness needed to start mobilising, practise transfers and to be physically active quickly after surgery, enhancing and accelerating the recovery of physical functioning as a result. Prehabilitation at home or in a community-based setting with adequate supervision allows patients to acquire these skills in their own environment, a setting to which they return after hospital discharge, and this makes it more likely that patients will start exercising again soon after surgery.41–43 Most of the studies included did not report on the postoperative clinical care pathway, including adequate discharge criteria, the use of a protocol for enhanced recovery after surgery or the content of rehabilitation, even though postoperative care should also be optimised to establish the full potential of prehabilitation. Finally, the modalities in prehabilitation programmes are highly varied. Many programmes are still unimodular, and they focus exclusively on physical exercise training. Multimodal programmes that consider physical exercise training, nutritional support, psychological support and the interaction between these components may be most effective and should be considered in further research.
The second aim of our systematic review was to assess whether the current literature has used optimal postoperative outcome measures to assess the effects of prehabilitation in major intra-abdominal cancer surgery. Although seven out of eight studies assessed postoperative outcome, different assessment methods were used, for example the prevalence of complications, ICU admission or length of stay. These results indicate that no study used an optimal outcome measure to assess the effects of postoperative complications. Although fitter or prehabilitated patients may also have postoperative complications, the impact may not be as severe, as suggested by the results of Hulzebos et al.14 The impact of such complications is not adequately reflected by simply measuring their incidence with scales such as the Clavien-Dindo classification, comprehensive complication index or postoperative morbidity scale. After prehabilitation and the resulting improvement in aerobic capacity, patients may have better short-term and long-term outcomes, even with similar treatment and equal complication rates. Clinicians and researchers involved in prehabilitation should engage in a debate about the development of outcome measures in which the impact of a complication is also considered, for example by combining a complication with its impact on the use of resources, length of hospital stay or the recovery of a patient's physical functioning. A great step is being made by the COMPAC-stEP group aiming at standardising endpoints in peri-operative trials.44 Measuring the resilience of patients within such a core outcome set could result in a better picture of the potential benefits of prehabilitation in terms of better outcomes and cost-effective care. Furthermore, alternative concepts in terms of outcome could be explored, for example by using the allostatic load index, which takes psychophysiological reserves of patients into account.45 These are novel concepts that have not been described or assessed in the current literature about prehabilitation, for example in the context of cancer surgery. Future studies should investigate multidisciplinary, multimodal programmes and use recent scientific insights to design effective/cost-effective programmes for the right patients, in the right setting and using the right outcome measures.
In conclusion, this systematic review found large variation in the content of prehabilitation in studies investigating its effects in intra-abdominal cancer surgery. Studies with a high therapeutic validity found that prehabilitation had beneficial effects on postoperative outcome. Future research in the field of prehabilitation should focus more on the adequate selection of high-risk surgical patients, and provide personalised, and probably multimodal (partly) supervised prehabilitation with objective monitoring of their progress throughout the programme in order to adjust the intervention as required and thereby minimise the risk of nonresponding patients. In addition, there is a need for consensus-defined standardised endpoints for postoperative outcomes, in which the impact of postoperative complications is taken into consideration. Combining all these elements may allow us finally to clarify the value of prehabilitation in major intra-abdominal cancer surgery.
Supplementary Material
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: we would like to thank Dr. S.P. Thomas for his diligent proofreading of the manuscript.
Financial support and sponsorship: Muhammad R. Tahir was supported by the PANINI programme (Horizon 2020, Marie Curie, Sklodowska, Innovative Training Network, No. 675003).46 The funders had no role in the study design, data collection and analysis, interpretation of data or preparation of the manuscript.
Conflicts of interest: none.
Presentation: none.
Both Gwendolyn Thomas and Muhammad R. Tahir contributed equally to this article.
Published online 10 Jun 2019
References
- 1.West M, Parry M, Lythgoe D, et al. Cardiopulmonary exercise testing for the prediction of morbidity risk after rectal cancer surgery. Br J Surg 2014; 101:1166–1172. [DOI] [PubMed] [Google Scholar]
- 2.McDermott F, Heeney A, Kelly M, et al. Systematic review of preoperative, intraoperative and postoperative risk factors for colorectal anastomotic leaks. Br J Surg 2015; 102:462–479. [DOI] [PubMed] [Google Scholar]
- 3.Van Leersum N, Snijders H, Henneman D, et al. The Dutch surgical colorectal audit. Eur J Surg Oncol 2013; 39:1063–1070. [DOI] [PubMed] [Google Scholar]
- 4.International Surgical Outcomes Study group. Global patient outcomes after elective surgery: prospective cohort study in 27 low-, middle-and high-income countries. Br J Anaesth 2016; 117:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson R, Davies S, Yates D, et al. Impaired functional capacity is associated with all-cause mortality after major elective intra-abdominal surgery. Br J Anaesth 2010; 105:297–303. [DOI] [PubMed] [Google Scholar]
- 6.Kerr J, Anderson C, Lippman SM. Physical activity, sedentary behaviour, diet, and cancer: an update and emerging new evidence. Lancet Oncol 2017; 18:e457–e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McEwen BS. Stress: homeostasis, rheostasis, allostasis and allostatic load. In: Fink G. Stress science: neuroendocrinology. Oxford: Academic Press, 2010:10–14. [Google Scholar]
- 8.Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil 2013; 92:715–727. [DOI] [PubMed] [Google Scholar]
- 9.Christensen T, Bendix T, Kehlet H. Fatigue and cardiorespiratory function following abdominal surgery. Br J Surg 1982; 69:417–419. [DOI] [PubMed] [Google Scholar]
- 10.Dimick JB, Chen SL, Taheri PA, et al. Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 2004; 199:531–537. [DOI] [PubMed] [Google Scholar]
- 11.Moran J, Guinan E, McCormick P, et al. The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 2016; 160:1189–1201. [DOI] [PubMed] [Google Scholar]
- 12.Bruns E, Heuvel B, Buskens C, et al. The effects of physical prehabilitation in elderly patients undergoing colorectal surgery: a systematic review. Colorectal Dis 2016; 18:O263–277. [DOI] [PubMed] [Google Scholar]
- 13.Dindo D, Demartines N, Clavien P-A. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hulzebos EH, Helders PJ, FaviÚ NJ, et al. Preoperative intensive inspiratory muscle training to prevent postoperative pulmonary complications in high-risk patients undergoing CABG surgery: a randomized clinical trial. JAMA 2006; 296:1851–1857. [DOI] [PubMed] [Google Scholar]
- 15.Santa Mina D, Clarke H, Ritvo P, et al. Effect of total-body prehabilitation on postoperative outcomes: a systematic review and meta-analysis. Physiotherapy 2014; 100:196–207. [DOI] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 7:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winters-Stone KM, Neil SE, Campbell KL. Attention to principles of exercise training: a review of exercise studies for survivors of cancers other than breast. Br J Sports Med 2014; 48:987–995. [DOI] [PubMed] [Google Scholar]
- 18.Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM's new preparticipation health screening recommendations from ACSM's guidelines for exercise testing and prescription. Curr Sports Med Rep 2013; 12:215–217. [DOI] [PubMed] [Google Scholar]
- 19.Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011; 343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoogeboom TJ, Oosting E, Vriezekolk JE, et al. Therapeutic validity and effectiveness of preoperative exercise on functional recovery after joint replacement: a systematic review and meta-analysis. PloS One 2012; 5:e38031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petrie A, Sabin C. Medical statistics at a glance. 2013; Oxford, UK: Wiley-Blackwell, 1-180. [Google Scholar]
- 22.Kim DJ, Mayo NE, Carli F, et al. Responsive measures to prehabilitation in patients undergoing bowel resection surgery. Tohoku J Exp Med 2009; 217:109–115. [DOI] [PubMed] [Google Scholar]
- 23.Carli F, Charlebois P, Stein B, et al. Randomized clinical trial of prehabilitation in colorectal surgery. Br J Surg 2010; 97:1187–1197. [DOI] [PubMed] [Google Scholar]
- 24.Dronkers J, Lamberts H, Reutelingsperger I, et al. Preoperative therapeutic programme for elderly patients scheduled for elective abdominal oncological surgery: a randomized controlled pilot study. Clin Rehab 2010; 24:614–622. [DOI] [PubMed] [Google Scholar]
- 25.Kaibori M, Ishizaki M, Matsui K, et al. Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg 2013; 206:202–209. [DOI] [PubMed] [Google Scholar]
- 26.Gillis C, Li C, Lee L, et al. Prehabilitation versus rehabilitation: a randomized control trial in patients undergoing colorectal resection for cancer. Anesthesiology 2014; 121:937–947. [DOI] [PubMed] [Google Scholar]
- 27.Dunne D, Jack S, Jones R, et al. Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 2016; 103:504–512. [DOI] [PubMed] [Google Scholar]
- 28.Barberan-Garcia A, Ubré M, Roca J, et al. Personalised prehabilitation in high-risk patients undergoing elective major abdominal surgery: a randomized blinded controlled trial. Ann Surg 2018; 267:50–56. [DOI] [PubMed] [Google Scholar]
- 29.Bousquet-Dion G, Awasthi R, Loiselle S-È, et al. Evaluation of supervised multimodal prehabilitation programme in cancer patients undergoing colorectal resection: a randomized control trial. Acta Oncol 2018; 57:849–859. [DOI] [PubMed] [Google Scholar]
- 30.Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015; 32:88–105. [DOI] [PubMed] [Google Scholar]
- 31.Tew G, Ayyash R, Durrand J, Danjoux G. Clinical guideline and recommendations on preoperative exercise training in patients awaiting major noncardiac surgery. Anaesthesia 2018; 73:750–768. [DOI] [PubMed] [Google Scholar]
- 32.Pinto BM, Floyd A. Methodologic issues in exercise intervention research in oncology. Semin Oncol Nurs 2007; 4:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levett D, Jack S, Swart M, et al. Perioperative cardiopulmonary exercise testing (CPET): consensus clinical guidelines on indications, organization, conduct, and physiological interpretation. Br J Anaesth 2018; 120:484–500. [DOI] [PubMed] [Google Scholar]
- 34.Richardson K, Levett D, Jack S, Grocott M. Fit for surgery? Perspectives on preoperative exercise testing and training. Br J Anaesth 2017; 119:i34–i43. [DOI] [PubMed] [Google Scholar]
- 35.West M, Asher R, Browning M, et al. Validation of preoperative cardiopulmonary exercise testing-derived variables to predict in-hospital morbidity after major colorectal surgery. Br J Surg 2016; 103:744–752. [DOI] [PubMed] [Google Scholar]
- 36.Glance LG, Osler TM, Neuman MD. Redesigning surgical decision making for high-risk patients. N Engl J Med 2014; 370:1379–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Rooijen S, Carli F, Dalton S, et al. Multimodal prehabilitation in colorectal cancer patients to improve functional capacity and reduce postoperative complications: the first international randomized controlled trial for multimodal prehabilitation. BMC Cancer 2019; 19:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montero D, Lundby C. Refuting the myth of nonresponse to exercise training:‘nonresponders’ do respond to higher dose of training. J Physiol 2017; 595:3377–3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berkel AE, Bongers BC, van Kam M-JS, et al. The effects of prehabilitation versus usual care to reduce postoperative complications in high-risk patients with colorectal cancer or dysplasia scheduled for elective colorectal resection: study protocol of a randomized controlled trial. BMC Gastroenterol 2018; 18:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodfield J, Zacharias M, Wilson G, et al. Protocol, and practical challenges, for a randomised controlled trial comparing the impact of high intensity interval training against standard care before major abdominal surgery: study protocol for a randomised controlled trial. Trials 2018; 19:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Verbrugge LM, Jette AM. The disablement process. Soc Sci Med 1994; 38:1–14. [DOI] [PubMed] [Google Scholar]
- 42.Gill TM, Robison JT, Williams CS, Tinetti ME. Mismatches between the home environment and physical capabilities among community-living older persons. J Am Geriatr Soc 1999; 47:88–92. [DOI] [PubMed] [Google Scholar]
- 43.Siemonsma PC, Blom JW, Hofstetter H, et al. The effectiveness of functional task exercise and physical therapy as prevention of functional decline in community dwelling older people with complex health problems. BMC Geriatr 2018; 18:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Myles P, Grocott M, Boney O, et al. Standardizing end points in perioperative trials: towards a core and extended outcome set. Br J Anaesth 2016; 116:586–589. [DOI] [PubMed] [Google Scholar]
- 45.Juster R-P, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010; 35:2–16. [DOI] [PubMed] [Google Scholar]
- 46.Whittaker AC, Delledonne M, Finni T, et al. Physical Activity and Nutrition INfluences In ageing (PANINI): consortium mission statement. Aging Clin Exp Res 2018; 30:685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


