Objectives:
Previous studies have demonstrated that high-dose allopurinol is able to regress left ventricular (LV) mass in cohorts with established cardiovascular disease. The aim of this study was to assess whether treatment with high-dose allopurinol would regress LV mass in a cohort with essential hypertension, LV hypertrophy and well-controlled blood pressure but without established cardiovascular disease.
Methods:
We conducted a mechanistic proof-of-concept randomized, placebo-controlled, double-blind trial of allopurinol (600 mg/day) versus placebo on LV mass regression. Duration of treatment was 12 months. LV mass regression was assessed by Cardiac Magnetic Resonance. Secondary outcomes were changes in endothelial function (flow-mediated dilatation), arterial stiffness (pulse wave velocity) and biomarkers of oxidative stress.
Results:
Seventy-two patients were randomized into the trial. Mean baseline urate was 362.2 ± 96.7 μmol/l. Despite good blood pressure control, LV mass regression was significantly reduced in the allopurinol cohort compared with placebo (LV mass −0.37 ± 6.08 versus −3.75 ± 3.89 g; P = 0.012). Oxidative stress markers (thiobarbituric acid reactive substances) were significantly higher in the allopurinol group versus placebo (0.26 ± 0.85 versus −0.34 ± 0.83 μmol/l; P = 0.007). Other markers of vascular function were not significantly different between the two groups.
Conclusion:
Treatment with high-dose allopurinol in normouricemic controlled hypertensive patients and LV hypertrophy is detrimental. It results in reduced LV mass regression and increased oxidative stress over a 12-month period. This may be because of an adverse impact on redox balance. Cohort selection for future cardiovascular trials with allopurinol is crucial.
Keywords: hypertension, oxidative stress, uric acid
INTRODUCTION
Essential hypertension is an established risk factor for cardiovascular disease. Furthermore, left ventricular hypertrophy (LVH), which is highly prevalent in treated hypertensive patients [1] is an independent risk factor for cardiovascular events, cardiovascular death and overall mortality [2]. Studies have previously demonstrated that high-dose allopurinol, a xanthine oxidase inhibitor, improves vascular function (and therefore, cardiac afterload) independently of urate [3]. Allopurinol has also been shown to regress LVH in patients with ischemic heart disease [4], chronic kidney disease (CKD) [5] and type 2 diabetes [6]. The mechanism for this LVH regression could either be that allopurinol significantly reduces vascular oxidative stress [3] and improves endothelial function resulting in a reduced cardiac afterload, independent of blood pressure or that allopurinol regresses LVH because of a reduction in oxidative stress, which is a known trigger of myocardial hypertrophy [7,8].
Thus, the main aim of this study was to assess whether high-dose allopurinol could regress left ventricular mass (LVM) in a cohort with essential hypertension and LVH but well-treated blood pressure. The secondary aim was to assess the effect of allopurinol on LV volumes, markers of circulating oxidative stress, endothelial function and arterial stiffness in this patient group.
METHODS
Study overview
The study was a single-centre, randomized, double-blind, placebo-controlled mechanistic proof of concept trial with 12 months’ follow-up between 2014 and 2017. The study treatment was allopurinol 600 mg/day, given as 300 mg twice per day or twice daily placebo. The primary objective of the study was to assess LV mass regression after 12 months using cardiac magnetic resonance (CMR). It was approved by the Tayside Research Ethics Committee and was carried out in accordance with the declaration of Helsinki. EudraCT: 2014-002083-33. ClinicalTrials.gov: NCT02237339.
Study participants
Patients with essential hypertension and LVH were identified from the Scottish Primary Care Research Network, Scottish Health Research Register, Cardiovascular Risk clinics or Cardiology databases. All participants provided informed consent prior to inclusion into the study. Consented participants attended for screening echocardiography to assess for LVH at baseline. LVH was defined by the American Society of Echocardiography criteria as a LVM index (LVMI) greater than 115 g/m2 for men and more than 95 g/m2 for women [9]. Participants were excluded if they had a documented intolerance to allopurinol, severe aortic stenosis, active gout or taking allopurinol, secondary cause for their hypertension, severe hepatic disease, CKD 3b or worse, patients taking azathioprine, 6 mercaptopurine, or theophylline, active malignancy or other life-threatening diseases, pregnant or lactating women and any contraindication to MRI. Patient were also required to have a baseline daytime average SBP less than 135 mmHg or 24 h average SBP 130 mmHg or less and on stable antihypertensive medications for at least the preceding 3 months prior to randomization.
Eligible participants were stratified for sex and baseline LVMI (men 116–129 g/m2 or ≥130 g/m2, females 96–114 g/m2 or ≥115 g/m2) and then randomized using a centrally controlled web-based GCP compliant randomization system (TrusT, Health Informatics Centre, University of Dundee) to receive allopurinol or placebo. Patients continued all other medications including antihypertensive medications.
Study visits and drug titration
After recruitment, patients attended six further visits over a 12-month period. An initial dosage of allopurinol, 300 mg/day was dispensed, increased to 600 mg/day after 1 month, and continued for the duration of the trial. Study visits are outlined in Fig. 1. Office BP was measured for all patients at each visit, 24-h ambulatory or home BP monitoring was completed in all patients at the start and end of the study.
FIGURE 1.
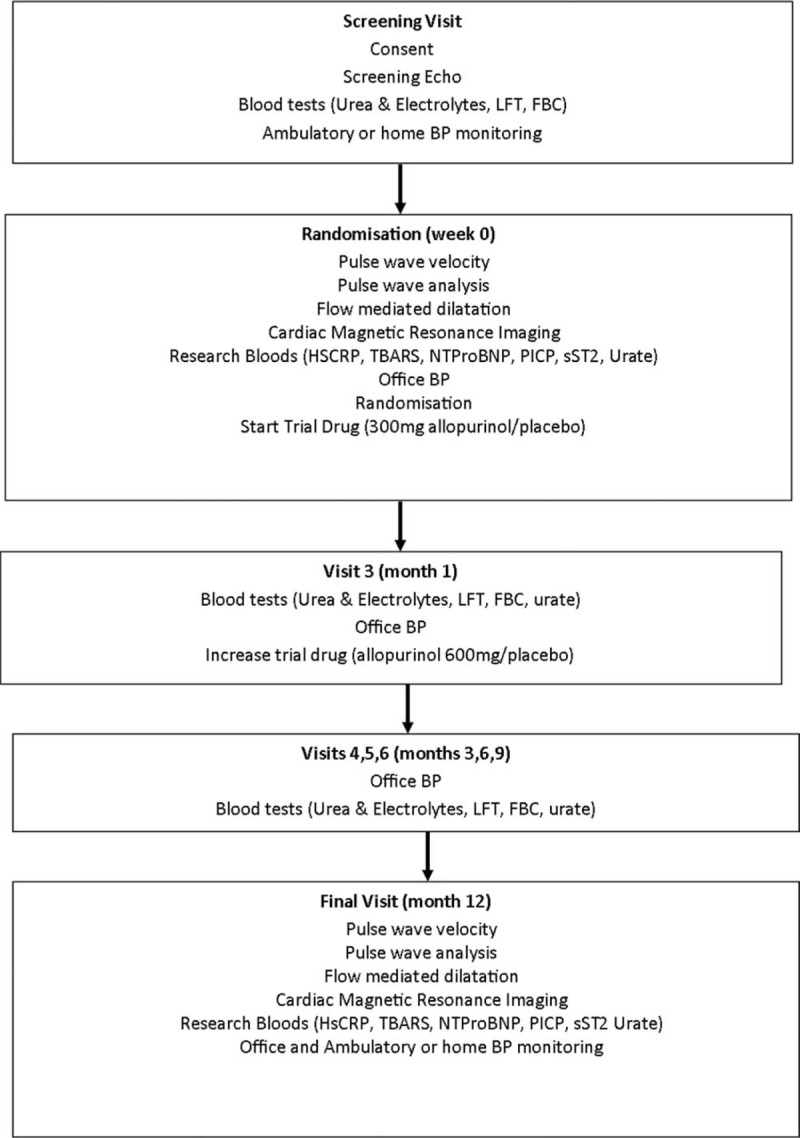
Study visits.
Echocardiography
Echocardiographic measurements were made as per the ASE recommendations [10]. Perpendicular linear measurements of the intraventricular septum, internal and posterior wall of the LV were acquired in the parasternal long axis, at the level of the mitral valve leaflets tips at end-diastole. LV mass was calculated using the cube formula.
Cardiac MRI
Cardiac MRI was performed on a 3T MAGENTOM Trio-PrismaFIT (Siemens, Erlangen, Germany) using dedicated phased array cardiac coils. Serial contiguous short-axis cine images [electrocardiogram-gated (true fast imaging with steady-state precession; TrueFISP)] were acquired from the atrio-ventricular ring to the apex using the vertical and horizontal long axis of the left ventricle as a guide. The short-axis imaging parameters were repetition time (TR) of 2.5 ms, echo time (TE) of 1.1 ms, flip angle (FA) of 60°, and slice thickness 6 and 4 mm gap.
Analysis was performed offline by trained observers (C.R.G. and S.J.G.) blinded to the study allocation using Argus software (Version VB15, Siemens Erlangen, Germany). Using the short-axis stack ‘region-of-interest’ contours were placed around the left ventricular endocardial and epicardial borders at end diastole and at end systole to calculate left ventricle ejection fraction (LVEF), left ventricular mass (LVM), end-diastolic (LVEDV), end-systolic (LVESV) and stroke volumes (LVSV). The base and apex were labeled and frames with at least 50% full thickness myocardium were included in the LVM. Papillary muscles were also included in the LVM if the muscle was contiguous with the myocardial wall. Each scan was analyzed twice to ensure consistency, a third measurement was conducted if the LVM varied by more than 5%.
Flow-mediated dilation
Endothelial function was assessed by measuring flow-mediated dilation (FMD) of the brachial artery using a Sequoia 512 (Siemens, Camberley, UK) and an 8-MHz linear array ultrasound probe. Measurements were conducted at baseline and 12 months according to the International Brachial Artery Reactivity Task Force guidelines [11] by a single operator (C.R.G.) blinded to study allocation.
Applanation tonometry
Pulse wave velocity (PWV) and augmentation index (AIx) were measured at baseline and 12 months by a single investigator (C.R.G.) blinded to study allocation. Measurements were recorded with a SphygmoCor (AtCor, Sydney, Australia) machine using a high fidelity micromanometer.
Biomarkers
Blood was collected at the baseline and final visit and analysed for uric acid (colorimetric method; Siemens Healthcare Diagnostics, Erlanger, Germany), N-terminal pro B natriuretic peptide (NTProBNP; multi array assay system; Meso Scale Diagnostics, Maryland, USA), high sensitivity C-reactive protein (HsCRP; ELISA assay, KALON, Aldershot, UK), N-terminal pro b-type natriuretic peptide (PICP; ELISA, Caltag Medsystems, Buckingham, UK), thiobarbituric acid reactive substances (TBARS; trichloroacetic acid method, Cambridge Bioscience, Cambridge, UK) and sST2 (ELISA assay, R&D systems, Minneapolis, Minnesota, USA).
Statistical analysis
Using data from previous LVH regression studies conducted at our unit [4,6] powered for a similar absolute change in LVM, 58 participants (29 per arm) were required to provide 80% power to detect a 5.2 g difference in LVM between study arms. Data for continuous variable are expressed in means and SDs, and percentages and denominators for categorical variables. Comparison between continuous variables was analysed by the Student t test or Mann–Whitney U test, categorical variables were analysed by the chi-squared test. Comparison between arms of the trial was assessed by the regression coefficient for the treatment arm with the final visit LVM as the dependent variable and baseline LVM, baseline BP and sex as covariates in a general linear regression model. All statistical analysis was undertaken using SPSS software version 22 (SPSS, Inc., Chicago, Illinois, USA). A P value less than 0.05 was considered statistically significant.
Endpoints
The primary end-point was to assess whether allopurinol regressed left ventricular mass (LVM) in hypertensive patients with controlled blood pressure. Secondary end-points assessed a change in other LV MRI parameters [EDV, ESV, systolic volume (SV), ejection fraction (EF)], parameters of endothelial function and vascular stiffness (FMD, PWV, PWA), biomarkers (uric acid, HsCRP, TBARS, NTProBNP, PICP, soluble ST2), left atrial MRI parameters (EDV, ESV, SV, EF) and BP.
RESULTS
In total, 72 participants were recruited (consort diagram; Fig. 2), baseline characteristics for participants who completed the study, including class of antihypertensive treatments are shown in Table 1, with no statistically significant differences between the groups at baseline. Blood pressure control was also well matched at the start and for the whole duration of the trial.
FIGURE 2.
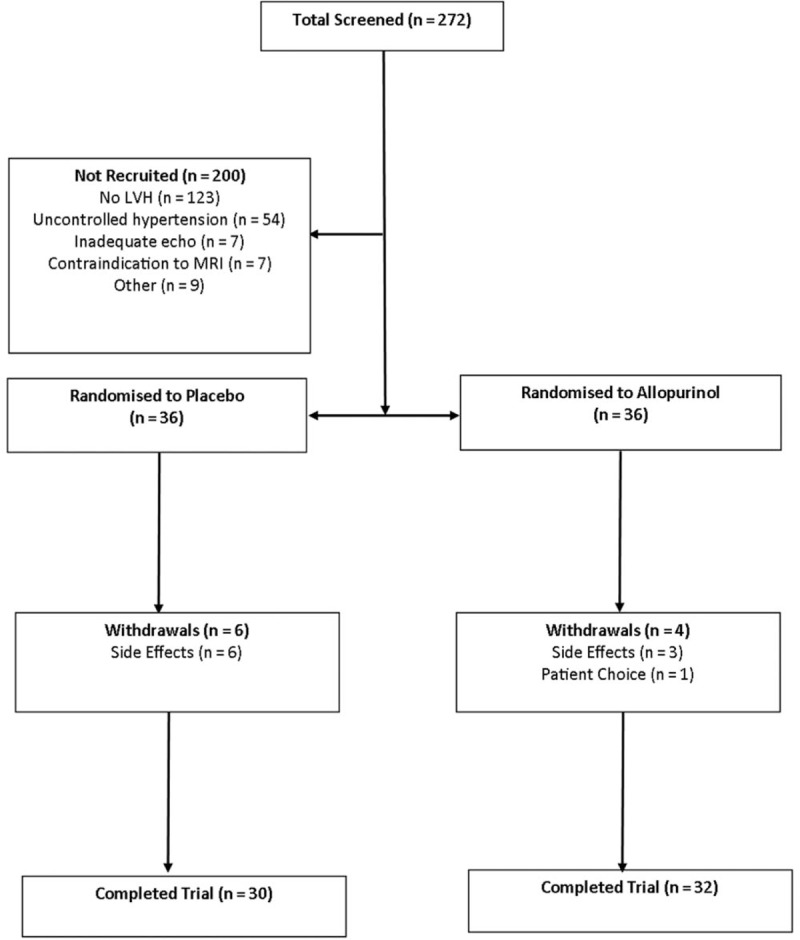
Consort diagram.
TABLE 1.
Baseline characteristics of trial participants
| Variable | All patients | Placebo | Allopurinol | P value |
| Total patients | n = 62 | n = 30 | n = 32 | |
| Mean age (years) | 65.9 ± 9.4 | 65.4 ± 9.0 | 66.4 ± 9.9 | 0.561 |
| Male | 38 (61) | 18 (60) | 20 (63) | 0.840 |
| BMI (kg/m2) | 30.9 ± 5.1 | 31.1 ± 5.3 | 30.7 ± 5.0 | 0.754 |
| Daytime SBP (mmHg) ABPM/home monitoring | 124.8 ± 8.3 | 125.3 ± 7.5 | 124.3 ± 9.0 | 0.620 |
| Daytime DBP (mmHg) ABPM/home monitoring | 73.5 ± 8.7 | 74.1 ± 7.2 | 73.0 ± 10.0 | 0.624 |
| Duration of HTN (years) | 12.7 ± 8.8 | 13.4 ± 10.0 | 12.0 ± 7.6 | 0.553 |
| IHD | 2 (3) | 0 (0) | 2 (9) | 0.164 |
| Dyslipidaemia | 27 (44) | 14 (47) | 13 (41) | 0.632 |
| CVA/TIA | 7 (11) | 4 (13) | 3 (9) | 0.623 |
| DM | 4 (6) | 3 (10) | 1 (3) | 0.271 |
| PVD | 1 (1) | 1 (3) | 0 (0) | 0.298 |
| Smoker | 4 (6) | 3 | 1 | 0.521 |
| Ex-smoker | 27 (44) | 12 | 15 | |
| Never smoked | 31 (50) | 15 | 16 | |
| ACE-I | 29 (47) | 16 (53) | 13 (41) | 0.316 |
| ARB | 24 (39) | 10 (33) | 14 (44) | 0.400 |
| B blocker | 18 (29) | 6 (20) | 12 (38) | 0.129 |
| CCB | 44 (71) | 22 (73) | 22 (69) | 0.691 |
| α blocker | 14 (23) | 6 (20) | 8 (25) | 0.638 |
| Thiazide diuretic | 23 (37) | 13 (43) | 10 (31) | 0.325 |
| Loop diuretic | 5 (8) | 3 (10) | 2 (6) | 0.588 |
| MRA | 4 (7) | 2 (7) | 2 (6) | 0.947 |
| Centrally acting anti-hypertensive | 1 (2) | 0 (0) | 1 (3) | 0.329 |
| Renin blocker | 1 (2) | 1 (3) | 0 (0) | 0.298 |
| Number of antihypertensive medications | 2.6 ± 1.2 | 2.6 ± 1.2 | 2.6 ± 1.3 | 0.979 |
| Resistant hypertension | 14 (23) | 6 (20) | 8 (25) | 0.638 |
| Haemoglobin (g/l) | 140.8 ± 12.8 | 140.2 ± 12.0 | 141.3 ± 13.7 | 0.736 |
| Creatinine (mmol/l) | 71.2 ± 13.5 | 73.0 ± 10.9 | 69.6 ± 15.5 | 0.315 |
| Glucose (mmol/l) | 5.6 ± 0.9 | 5.4 ± 0.8 | 5.8 ± 1.0 | 0.70 |
| Urate (μmol/l) | 362.2 ± 96.7 | 367.3 ± 81.5 | 357.3 ± 110.1 | 0.690 |
| HsCRP (mg/l) | 2.4 ± 3.3 | 2.6 ± 3.7 | 2.3 ± 3.0 | 0.770 |
| TBARs (μmol/l) | 2.8 ± 0.9 | 2.9 ± 1.0 | 2.7 ± 0.8 | 0.342 |
| NTproBNP (ρg/ml) | 792.0 ± 891.5 | 617.8 ± 583.3 | 960.5 ± 1095.9 | 0.133 |
| PICP (ng/l) | 1.6 ± 0.9 | 1.7 ± 1.0 | 1.5 ± 0.7 | 0.269 |
| Soluble ST2 (ng/ml) | 19.9 ± 9.9 | 19.7 ± 8.1 | 20.2 ± 11.6 | 0.834 |
| Echo LVMI (g/m2) | 125.9 ± 18.7 | 127.1 ± 21.0 | 124.8 ± 16.5 | 0.625 |
| MRI LVM (g) | 131.3 ± 36.7 | 132.5 ± 35.2 | 130.3 ± 38.5 | 0.812 |
| MRI LVM Height1.7 (g/m1.7) | 53.2 ± 11.8 | 54.3 ± 11.9 | 52.2 ± 11.7 | 0.489 |
| MRI EDV (ml) | 143.5 ± 34.4 | 142.5 ± 38.0 | 144.6 ± 31.2 | 0.815 |
| MRI ESV (ml) | 36.9 ± 16.1 | 36.6 ± 18.9 | 37.3 ± 13.3 | 0.862 |
| MRI SV (ml) | 106.6 ± 21.6 | 105.9 ± 22.6 | 107.3 ± 20.9 | 0.807 |
| MRI ejection Fraction (%) | 75.1 ± 6.1 | 75.5 ± 7.2 | 74.7 ± 4.9 | 0.604 |
| FMD (%) | 5.4 ± 3.5 | 4.9 ± 3.2 | 5.8 ± 3.8 | 0.330 |
| AIx (%) | 22.3 ± 13.5 | 21.2 ± 12.7 | 23.3 ± 14.4 | 0.534 |
| PWV (m/s) | 8.4 ± 1.2 | 8.2 ± 1.1 | 8.5 ± 1.4 | 0.359 |
Values are n, mean ± SD, or n (%). ABPM, ambulatory blood pressure monitoring; ACEI, angiotensin converting enzyme inhibitor; AIx, augmentation index; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; CVA, cerebrovascular accident; DM, diabetes mellitus; EDV, end-diastolic volume; ESV, end-systolic volume; FMD, flow-mediated dilation; HsCRP, high-sensitivity C-reactive protein; IHD, ischaemic heart disease; LVM, left ventricular mass; MRA, magnetic resonance angiogram; NTproBNP, N-terminal pro B natriuretic peptide; PICP, N-terminal pro b-type natriuretic peptide; PVD, peripheral vascular disease; PWV, pulse wave velocity; SV, stroke volume; TBARs, thiobarbituric acid reactive substances; TIA, transient ischemic attack.
Primary endpoint
We found that treating patients with controlled essential hypertension and LVH with allopurinol resulted in potential harm because of significantly reduced LVM regression compared with placebo, individual changes in LVM are illustrated in Fig. 3a and b. The cohort taking allopurinol were found to have a significantly higher final absolute LVM than those taking placebo, after correction for sex, baseline LVM and baseline SBP (Table 2). Forty-five percent of the participants were women. When sex differences were analysed, women on allopurinol had significantly reduced LVM regression compared with men. We did not find a difference between those with high baseline urate versus those with low baseline urate.
FIGURE 3.
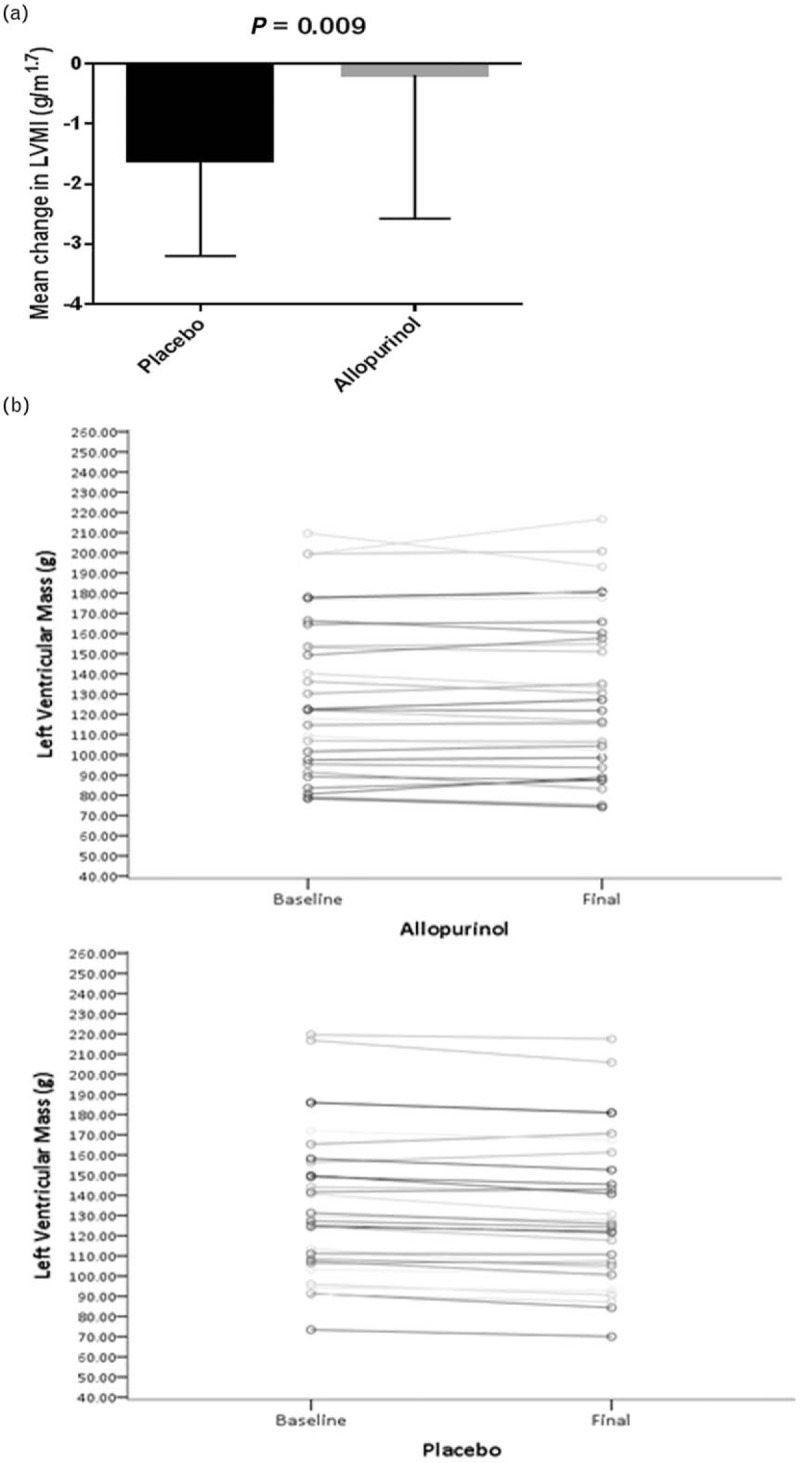
(a) The effect of allopurinol on left ventricular mass index (height1.7). Data expressed as mean ± SD. (b) The effect of allopurinol on left ventricular mass.
TABLE 2.
Multiple regression: adjusted for sex, baseline SBP, baseline left ventricular mass
| Dependent variable | B (difference in change) | 95% confidence interval | P | R2 | |
| Lower bound | Upper bound | ||||
| Absolute LVM (g) | 3.43 | 0.91 | 5.95 | 0.008 | 0.983 |
LVM, left ventricular mass.
For other parameters measured on cardiac MRI, there were no significant differences in LV ejection fraction, end-diastolic volume, end-systolic volume, stroke volume or left atrial volumes. Sub-group analysis by baseline LV mass, baseline oxidative stress (TBARs) or BP changes all yielded consistent results with the primary analysis.
Secondary endpoints
There was no statistically significant difference seen in FMD, PWV and PWA between the two treatment arms (Table 3).
TABLE 3.
Effect of allopurinol on haemodynamics, endothelial function and vascular stiffness
| Change in measured parameter | Placebo | Allopurinol | P value |
| 24 h systolic (mmHg) | 1.2 ± 8.0 | 0.61 ± 8.0 | 0.799 |
| 24 h diastolic (mmHg) | −0.04 ± 5.40 | 0.67 ± 4.76 | 0.634 |
| Daytime systolic (mmHg) | 1.57 ± 7.30 | −0.94 ± 8.05 | 0.205 |
| Daytime diastolic (mmHg) | 0.07 ± 5.41 | 0.34 ± 5.72 | 0.846 |
| FMD (%) | −0.23 ± 3.65 | 0.14 ± 4.12 | 0.718 |
| AIx | −0.30 ± 13.46 | 0.06 ± 12.41 | 0.913 |
| PWV (m/s) | −0.09 ± 1.12 | −0.25 ± 1.07 | 0.581 |
AIx, augmentation index; FMD, flow-mediated dilatation; PWV, pulse wave velocity.
Blood pressure control
Baseline blood pressure, number of antihypertensives (2.5 in placebo arm versus 2.7 in allopurinol arm) and prescribed major classes of antihypertensives was similar between both groups at baseline and remained so throughout the trial. Both cohorts had consistently controlled blood pressure at baseline (125.6 ± 7.4 mmHg placebo versus 124.3 ± 8.8 mmHg allopurinol) and at the end of the trial period (Table 3).
Biomarkers
The expected significant reduction in uric acid was demonstrated in the allopurinol group (Table 4). There was a significant increase in thiobarbituric acid reactive substances (TBARs), a biomarker for oxidative stress and a nonsignificant increase in high-sensitivity CRP, a marker of inflammation in the allopurinol cohort. There was no significant change between the cohorts for NTProBNP, PICP and soluble ST2 levels (Table 4).
TABLE 4.
Effect of allopurinol on biomarkers
| Change in measured parameter | Placebo | Allopurinol | P value |
| Uric acid (umol/l) | −1.33 ± 37.04 | −189.56 ± 91.95 | <0.001 |
| HsCRP (mg/l) | −0.55 ± 2.10 | 0.22 ± 1.71 | 0.122 |
| TBARS (μmol/l) | −0.34 ± 0.83 | 0.26 ± 0.85 | 0.007 |
| NTProBNP (pg/ml) | 109.08 ± 491.03 | −109.03 ± 612.84 | 0.131 |
| PICP (ng/l) | −0.18 ± 0.60 | −0.05 ± 0.43 | 0.322 |
| Soluble ST2 (ng/ml) | −1.02 ± 3.39 | −0.61 ± 8.63 | 0.573 |
HsCRP, high-sensitivity C-reactive protein; NTproBNP, N-terminal pro B natriuretic peptide; PICP, N-terminal pro b-type natriuretic peptide; TBARs, thiobarbituric acid reactive substances.
Adverse events
There were no suspected unexpected serious adverse reactions (SUSARs). Overall, there were three serious adverse events that required hospital admission; however, all were unrelated to the study medication. Of the three participants who withdrew because of side effects in the allopurinol arm, two developed nausea and one had a rash.
DISCUSSION
The main finding from this study is that over a 12-month period, allopurinol treatment adversely impacts on the LV mass regression expected with good blood pressure control in patients with hypertension and LVH. Indeed, the expected LVM regression with time because of good blood pressure control was actually reduced in the allopurinol cohort compared with the placebo cohort. We also found that, unlike cohorts with preexisting cardiovascular disease, CKD or diabetes, endothelial function and vascular stiffness did not improve with high-dose allopurinol in this cohort.
Allopurinol has been shown to regress LVM in different cohorts along the cardiovascular spectrum [4–6] with significant preexisting disease, oxidative stress and inflammation. However, the present study has identified that LVM regression may not be seen universally in all populations and that cohort selection of hyperuricemic patients is needed for defining populations that benefit from uric acid lowering. The cohort in this present study was normouricemic. There is a possibility that such patients rely on urate, the most abundant naturally occurring aqueous antioxidant for redox balance.
The results of this present study is consistent with the findings of the Oxypurinol Therapy for Congestive Heart Failure (OPT-CHF) trial where those with uric acid levels of less than 565 μmol/l (9.5 mg/dl) showed a trend towards worsening compared with those with uric acid levels greater than 565 μmol/l [12]. The data relating to urate lowering is also consistent with two recently reported trials, the Febuxostat for Cerebral and Cardiorenovascular Events Prevention Study (FREED) study [13], which suggested the presence of a J-shaped curve with regards to uric acid and clinical events [13] and the Cardiovascular safety of Febuxostat or Allopurinol in Patients with Gout (CARES) study [14], which showed increased all-cause and cardiovascular mortality in the Febuxostat cohort who also had significantly higher proportion of patients achieving uric acid levels less than 0.35 mmol/l, although the latter trial did not prove conclusively that the increase in mortality was related to urate-lowering efficacy.
It is widely accepted that LVH regression occurs with time in patients whose blood pressure is well controlled and this has been confirmed in meta-analysis and systematic reviews of the available trial data [15,16] and incorporated into various national and international guidelines [17]. This is consistent with what we observed in the placebo cohort over a period of 12 months, which showed a significantly greater reduction in LV mass compared with the allopurinol cohort. This, despite the fact that blood pressure in both cohorts remained similar throughout the duration of the study suggesting that any improvement in LVM regression that would have happened in the blood pressure-controlled allopurinol cohort over 12 months was negated by the effect of increased burden of oxidative stress because of reduction in the naturally occurring antioxidant, uric acid, in the allopurinol cohort.
Myocardial hypertrophy is known to be triggered by reactive oxygen species (ROS) and oxidative stress [7,8,18,19]. Therefore, an adverse redox imbalance would be expected to result in an attenuation of the positive impact of good blood pressure control on LVM regression. Data from our group and others have previously suggested that the mechanism by which allopurinol improves LVM regression was mediated by xanthine oxidase inhibition and the consequent reduction in oxidative stress in cohorts with background ischemic heart disease, CKD and type-2 diabetes mellitus [4–6]. The LVH regression in these previous cohorts was also associated with an improvement in vascular endothelial function suggesting potential impact on vascular stiffness and cardiac afterload, independent of blood pressure.
The lack of LVM regression in this present cohort, unlike other cohorts we have previously studied, suggests that reducing uric acid in normouricemic patients but without established vascular disease, and therefore low-background oxidative stress or inflammation could tip the anti-oxidant pro-oxidant balance negatively and cause detriment.
This might be an explanation for our findings as uric acid is the most abundant naturally occurring aqueous antioxidant in humans and contributes as much as two-thirds of all free radical scavenging capacity in plasma [20,21]. Uric acid can, however, switch from an antioxidant to pro-oxidant under certain conditions, such as ischemia and inflammation, termed the uric acid paradox [22] or the uric acid redox shuttle [23–25]. Whether lower doses of allopurinol may be less detrimental in this specific cohort requires further investigation. We selected this high dose (600 mg/day) as previous trials using this dose had shown LVM regression in cohorts with established disease [4–6].
We stress that the results of this study is a preliminary indication of a possible mechanism. Further in-depth research is required to evaluate if indeed a redox imbalance directly results in a reduction in LV mass regression. This study suggests that reducing uric acid had a detrimental impact on redox balance and of ROS levels and myocardial structure, consistent with findings of previous trials, such as FREED [13], CARES [14] and OPT-CHF [12] as well as the redox shuttle uric acid paradox previously described [22–25]. This is further suggested, but not definitively confirmed, by our finding that TBARs were significantly increased in the allopurinol cohort. A multiple regression analysis with treatment, BP changes and oxidative stress changes revealed that increasing TBARs impacted final LVM. We found an increased but not statistically significant, level of hs-CRP in the allopurinol cohort suggesting the possibility of downstream inflammatory impact of increased ROS. It is reassuring that when the small number of patients with pre-existing vascular disease was excluded, the results were consistent with the primary analysis for all measures (LV mass index, augmentation index and change in FMD).
Study limitations
This is a single-centre study. Screening for LVH was done by echocardiogram by a qualified echocardiographer. Due to the cost of CMR, it was not used as a screening tool. However, LV mass regression was determined by cardiac MRI, the current gold standard modality for this purpose. Reassuringly, the baseline CMR-determined LV mass was comparable with previously published studies on LV Mass regression on other cohorts [4–6].
As the results of this study contrast with the beneficial effect of allopurinol in LVH regression seen in patients with IHD, type 2 diabetes and CKD, we acknowledge that there is always a possibility that the results of this study may have occurred by chance. However, the lack of LVM regression is consistent with results in all other CMR, vascular stiffness and endothelial function measures we studied. Furthermore, the worsening of oxidative stress burden seen with the TBAR levels, although a secondary outcome, and therefore not powered, lends support to the findings.
In conclusion, this study, in conjunction with previously published data, including the OPT-CHF trial [12], CARES [14] and FREED [13] suggests that the cardiovascular benefits of allopurinol are mainly seen in hyperuricemic, high oxidative stress states driven by pre-existing ischemia and/or inflammation. Therefore, cohorts for future large cardiovascular outcomes trials with allopurinol should be carefully selected based on only populations that have demonstrated benefit.
ACKNOWLEDGEMENTS
Author contributions: A.D.S. and J.G. conceived the idea for the study. A.D.S., J.G., S.J.G., T.M.M., C.C.L. and J.G.H. designed the study. C.R.G., R.S. and S.J.G. performed research. S.J.G., P.T.D. and C.R.G. analyzed the data. All authors contributed to the writing of the manuscript.
Sources of funding: This study was funded by a grant from the British Heart Foundation (PG/13/67/30444). This trial was supported by the Tayside Clinical Trials Unit.
Clinical Trial Registration: ClinicalTrials.gov Identifier: NCT02237339;
ISRCTN number: ISRCTN40476871.
EUDRACT: https://www.clinicaltrialsregister.eu/. Unique identifier: 2014-002083-33.
Conflicts of interest
A.D.S. and the University of Dundee have applied for a patent on the use of xanthine oxidase inhibitors to treat angina pectoris. All other authors have no conflicts of interest.
Footnotes
Abbreviations: ABPM, ambulatory blood pressure monitoring; ACE, angiotensin-converting enzyme; ACEI, angiotensin converting enzyme inhibitor; AIx, augmentation index; ARB, angiotensin receptor blocker; BP, blood pressure; CCB, calcium channel blocker; CMRI, cardiac magnetic resonance imaging; GCP, good clinical practice; HsCRP, high sensitivity C-reactive protein; IHD, ischaemic heart disease; ISRCTN, International Standard Randomised Controlled Trial Number; LDL, low-density lipoprotein; LFTs, liver function tests; LV, left ventricle; LVH, left ventricular hypertrophy; LVIDd, left ventricular internal dimension diastole; LVM, left ventricular mass; LVMI, left ventricular mass index; PWA, pulse wave analysis; PWTd, posterior wall thickness diastole; PWV, pulse wave velocity; ROS, reactive oxygen species; SUSAR, serious unexpected adverse reaction; TBARS, thiobarbituric acid reactive substances; TCTU, Tayside Clinical Trials Unit; XOR, xanthine oxidoreductase
REFERENCES
- 1.Cuspidi C, Meani S, Sala C, Valerio C, Negri F, Mancia G. Age related prevalence of severe left ventricular hypertrophy in essential hypertension: echocardiographic findings from the ETODH study. Blood Press 2012; 21:139–145. [DOI] [PubMed] [Google Scholar]
- 2.Gosse P. Left ventricular hypertrophy--the problem and possible solutions. J Int Med Res 2005; 33 Suppl 1:3A–11A. [DOI] [PubMed] [Google Scholar]
- 3.George J, Carr E, Davies J, Belch JJF, Struthers A. High-dose allopurinol improves endothelial function by profoundly reducing vascular oxidative stress and not by lowering uric acid. Circulation 2006; 114:2508–2516. [DOI] [PubMed] [Google Scholar]
- 4.Rekhraj S, Gandy SJ, Szwejkowski BR, Nadir MA, Noman A, Houston JG, et al. High-dose allopurinol reduces left ventricular mass in patients with ischemic heart disease. J Am Coll Cardiol 2013; 61:926–932. [DOI] [PubMed] [Google Scholar]
- 5.Kao MP, Ang DS, Gandy SJ, Nadir MA, Houston JG, Lang CC, et al. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J Am Soc Nephrol 2011; 22:1382–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szwejkowski BR, Gandy SJ, Rekhraj S, Houston JG, Lang CC, Morris AD, et al. Allopurinol reduces left ventricular mass in patients with type 2 diabetes and left ventricular hypertrophy. J Am Coll Cardiol 2013; 62:2284–2293. [DOI] [PubMed] [Google Scholar]
- 7.Takimoto E, Champion HC, Li M, Ren S, Rodriguez ER, Tavazzi B, et al. Oxidant stress from nitric oxide synthase-3 uncoupling stimulates cardiac pathologic remodeling from chronic pressure load. J Clin Invest 2005; 115:1221–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takimoto E, Kass DA. Role of oxidative stress in cardiac hypertrophy and remodeling. Hypertension 2007; 49:241–248. [DOI] [PubMed] [Google Scholar]
- 9.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015; 16:233–270. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Chamber Quantification Writing Group; American Society of Echocardiography's Guidelines and Standards Committee; European Association of Echocardiography. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 11.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, et al. International Brachial Artery Reactivity Task Force. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery - a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002; 39:257–265. [DOI] [PubMed] [Google Scholar]
- 12.Hare JM, Mangal B, Brown J, Fisher C, Jr, Freudenberger R, Colucci WS, et al. OPT-CHF Investigators. Impact of oxypurinol in patients with symptomatic heart failure. Results of the OPT-CHF study. J Am Coll Cardiol 2008; 51:2301–2309. [DOI] [PubMed] [Google Scholar]
- 13.Kojima S, Matsuii K, Hiramitsu S, Hisatome I, Waki M, Uchiyama K, et al. on behalf of the Febuxostat for Cerebral and CaRdiorenovascular Events PrEvEntion StuDy (FREED) investigators. Febuxostat for cerebral and cardiorenovascular events prevention study. Eur Heart J 2019; 40:1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378:1200–1210. [DOI] [PubMed] [Google Scholar]
- 15.Pokharel PBJ. Regression of left ventricular hypertrophy: lessons from clinical trials. OA Evidence-Based Med 2013; 1:13. [Google Scholar]
- 16.Schmieder RE, Martus P, Klingbeil A. Reversal of left ventricular hypertrophy in essential hypertension. A meta-analysis of randomized double-blind studies. JAMA 1996; 275:1507–1513. [PubMed] [Google Scholar]
- 17.Nerenberg KA, Zarnke KB, Leung AA, Dasgupta K, Butalia S, McBrien K, et al. Hypertension Canada. Hypertension Canada's 2018 Guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults and children. Can J Cardiol 2018; 34:506–525. [DOI] [PubMed] [Google Scholar]
- 18.Maulik SK, Kumar S. Oxidative stress and cardiac hypertrophy: a review. Toxicol Mech Methods 2012; 22:359–366. [DOI] [PubMed] [Google Scholar]
- 19.Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart 2007; 93:903–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ames BN, Cathcart R, Schwiers E, Hochstein P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc Natl Acad Sci USA 1981; 78:6858–6862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sautin YY, Johnson RJ, ACID: URIC. The oxidant–antioxidant paradox. Nucleosides Nucleotides Nucleic Acids 2008; 27:608–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lippi G, Montagnana M, Franchini M, Favaloro EJ, Targher G. The paradoxical relationship between serum uric acid and cardiovascular disease. Clin Chim Acta 2008; 392:1–7. [DOI] [PubMed] [Google Scholar]
- 23.Bagnati M, Perugini C, Cau C, Bordone R, Albano E, Bellomo G. When and why a water-soluble antioxidant becomes pro-oxidant during copper-induced low-density lipoprotein oxidation: a study using uric acid. Biochem J 1999; 340 (Pt 1):143–152. [PMC free article] [PubMed] [Google Scholar]
- 24.Radi R, Tan S, Prodanov E, Evans RA, Parks DA. Inhibition of xanthine oxidase by uric acid and its influence on superoxide radical production. Biochim Biophys Acta 1992; 1122:178–182. [DOI] [PubMed] [Google Scholar]
- 25.Hayden MR, Tyagi SC. Uric acid: a new look at an old risk marker for cardiovascular disease, metabolic syndrome, and type 2 diabetes mellitus: the urate redox shuttle. Nutr Metab (Lond) 2004; 1:10. [DOI] [PMC free article] [PubMed] [Google Scholar]


