Supplemental Digital Content is available in the text
Keywords: ambulatory, amlodipine, aortic pressure, arterial stiffness, blood pressure monitoring, central blood pressure, combination drug therapy, hypertension, losartan, smoothness index, thiazides, trough-peak ratio
Objectives:
The aim of this study was to identify associations between the smoothness index of central SBP (CSBP) and changes of ambulatory carotid femoral pulse wave velocity in response to 20-week treatments with losartan and amlodipine vs. losartan and hydrochlorthiazide combinations.
Methods:
For 142 (losartan and hydrochlorthiazide: 72, losartan and hydrochlorthiazide: 70) patients examined with ambulatory central blood pressure (BP) monitoring device, we calculated smoothness indices and trough-to-peak ratios of brachial SBP, CSBP, ambulatory pulse pressure amplification (APPA), ambulatory augmentation index at heart rate 75 beats per minute (AAIx75) and ambulatory carotid femoral pulse wave velocity (AcfPWV).
Results:
Mean age was 58.9 ± 12.3 years, and women accounted for 25.9%. Changes in office SBP/DBP were not different between groups (losartan and hydrochlorthiazide: −15.2 ± 15.0/−7.8 ± 8.0 vs. losartan and amlodipine: −14.9 ± 13.7/−9.2 ± 7.5 mmHg). Reduction of 24-h CSBP was not significantly different (losartan and hydrochlorthiazide: 6.4 ± 1.1 vs. losartan and amlodipine: 9.2 ± 1.1 mmHg, P = 0.074). Reduction in nocturnal AcfPWV was greater in the losartan and amlodipine group (losartan and hydrochlorthiazide: 0.09 ± 0.05 vs. losartan and amlodipine: 0.26 ± 0.05 m/s, P = 0.0216). Intraindividual SIs for CSBP were higher in the losartan and amlodipine group (0.40 ± 0.57 vs. 0.65 ± 0.74, P = 0.022). In multivariable regression analysis, smoothness index of CSBP was independently associated with the losartan and amlodipine group. In model additionally considering the changes in arterial stiffness, decrease in AcfPWV instead of the treatment group was independently associated with smoothness indices. In mediation analysis, smoothness index was fully mediated by reduction in night-time AcfPWV.
Conclusion:
Losartan and amlodipine combination was superior to the losartan and hydrochlorthiazide combination in terms of achieving higher smoothness index for CSBP after 20-week treatments. The effect of losartan and amlodipine on smoothness index was fully mediated by reduction of night-time AcfPWV.
INTRODUCTION
Hypertension is the key risk factor for cardiovascular death, the leading cause of death worldwide [1]. Control of blood pressure (BP) using antihypertensive medications (AHMs) has been proven to prevent hypertension-related cardiovascular complications [1]. In most hypertension patients, combination therapy is frequently needed as a stepwise escalation or as the first regimen, except in the fragile elderly or when SBP is less than 150 mmHg [2]. The preference for the combination including specific class of AHM is documented in regional hypertension guidelines [3]. Nevertheless, the rationale behind the preference is usually beyond the scope of BP measurement, as shown in some outcome trials; clinical outcomes for the preferred combination appeared to be better than the expectation conceived by BP difference [4,5].
With respect to the efficacy of BP reduction by AHM, central BP was suggested to be more important for clinical outcome. For example, compared with brachial BP, central BP showed better correlation with target organ damage and BP-lowering efficacy [6]. In the BP GUIDE study, it was demonstrated that central BP-guided practice could make the practice be more cost-effective [7].
Renin-angiotensin system (RAS) inhibitors are preferred for combination therapy with calcium antagonist or with diuretics. However, there is a preference for calcium channel blocker (CCB) over diuretics for combination therapy because of the superiorities of the BP-lowering efficacy and clinical outcome [8]. In addition to pharmacologic profiles, patient characteristics such as salt sensitivity, volume status, age, obesity, vascular stiffness and racial background appeared to be associated with efficacy [9]. As shown in the J-CORE study, superiority of CCB to hydrochlorothiazide on top of olmesartan-based treatment could be demonstrated only using aortic pulse wave velocity.
In terms of the limitations of use of clinical BP measurement, diurnal BP variability may be differently affected by drug class or some combination regimens [10]. For example, 24-h BP control or action duration of AHM represented by parameters such as smoothness index or trough–peak ratio may be different among some combination regimens [11]. Nevertheless, there are few studies comparing the efficacy of smoothness index and trough–peak ratio of combination therapy using RAS inhibitor and CCB vs. RAS inhibitor and diuretics.
The Mobil-O-Graph PWA ABPM device, with the multiple measurement during 24-h ambulatory monitoring, provides both brachial and central BP using the ARC Solver algorithm [12]. The generalized transfer function was assumed to be comparable to the individualized transfer function in resting status [13]. Currently used vascular parameters such as central BP and pulse pressure amplification (PPA), carotid femoral pulse wave velocity (cfPWV), augmentation index (AIx) are recommended to be measured in the fully resting state for hypertensive patients; however, the clinical value of central BP and other vascular parameters measured in the ambulatory state are unknown [14,15]. Moreover, whether the concept of smoothness index could be applied to those parameters is also unknown.
Recently, we reported that ambulatory central SBP (CSBP) was significantly more reduced by losartan and amlodipine than by losartan and hydrochlorothiazide after 20-week treatment, despite the fact that BP reduction was not significantly different [16].
Therefore, as a substudy, we analysed the association between SI of brachial or central BPs and ambulatory parameters of arterial stiffness in response to 20-week treatment with losartan and amlodipine vs. losartan and hydrochlorothiazide.
MATERIALS AND METHODS
Study design
The original trial had a multicentre, double-blinded, active controlled, randomized design to compare the efficacy of a fixed-dose combination of losartan and amlodipine with that of losartan and hydrochlorothiazide after 20 weeks of treatment [17]. Clinical BP was the primary endpoint. Secondary endpoints were central BP and other vascular haemodynamic parameters such as ambulatory cfPWV (AcfPWV), AIx (AAIx) and ambulatory PPA (APPA). Microalbuminuria was expressed as albumin creatinine ratio (ACR) was also included.
Briefly, the study was performed as follows. After screening, eligible patients went through an open-labelled run-in period with losartan 50 mg daily for 4 weeks. Then, only patients with clinic SBP at least 140 mmHg were randomized into blinded treatment assignments, either losartan/amlodipine 50/5 mg daily or losartan/dihydrochlorothiazide 50/12.5 mg daily with a placebo drug of the other group. After 4-week treatment, uptitration was performed by increasing the dose to losartan/amlodipine 100/5 mg daily or losartan/dihydrochlorothiazide 100/25 mg daily when clinic SBP remained at least 140 mmHg.
Study population
Among 220 participants, 45 individuals refused follow-up ambulatory BP monitoring (ABPM); therefore, 185 individual data were analysed. Finally, 143 individual data were acquired for the final analyses with exclusion criteria (available data points less than 80% after applying popular editing criteria) [18].
Ambulatory monitoring of blood pressures, pulse pressure amplification, augmentation index and ambulatory carotid femoral pulse wave velocity
Clinical BP was obtained in the sitting position with the pressure cuff placed at either the right or left brachial area, using a semi-automated sphygmomanometer (HEM-7080IC; Omron Healthcare Co, Kyoto, Japan). After 5 min of rest, BP was measured three times with an interval of 2 min, and mean pressure was used for analysis. Measurements of BPs, AAIx, APPA and AcfPWV were performed for 24 h with 30-min intervals using the previously validated, automated oscillometric device (Mobil-O-graph 24 h PWA monitor; IEM Gmbh, Stolberg, Germany) [12]. Daytime and night-time were defined by narrow fixed interval method. BPs during the period between 0800 and 2100 h were regarded as daytime BP and BPs during the period between midnight and 0500 h was regarded as night-time BP. Twenty-four hour BP was calculated by daytime BP x [1–(sleep duration in hour/24)] + night-time BP x (sleep duration in hour/24). Nocturnal dipping was defined as night-time brachial SBP divided by daytime brachial SBP x 100 (%).
All patients took the study drug at the time of starting the ambulatory monitoring. PPA was defined as the ratio of brachial SBP to CSBP [19]. AAIx75 was calculated by calibrating the AAIx at the heart rate of 75 beats per minute.
The smoothness index was calculated using hourly changes of the study parameters from baseline to 20-week treatment. Smoothness index was defined as average of hourly changes divided by standard deviation of those hourly changes during a 24-h time period. All hourly data for each hour interval from the time when monitoring started and the study drug was taken were averaged to generate a single hourly change [18]. Intraindividual smoothness index was calculated by using hourly changes for each individual to test statistical comparison between groups [20].
Trough–peak ratio
Global trough–peak ratio was calculated using BP reduction in the trough period, defined as average BP over 22–24 h from drug intake, divided by peak BP reduction, defined as average BP reduction of peak reduction from second to eighth hours from drug intake and adjacent BPs. Intraindividual trough–peak ratio was calculated with 2-h average windows; the corresponding number of trough–peak ratio readings according to the groups were statistically compared [21,22].
Ethics
Patients were recruited from 20 medical centres nationwide in Korea. The study was approved by each institutional review board. Written consent was obtained for all study patients.
Statistical analysis
Data were expressed as mean ± standard deviation for normal distribution and median with 95% confidence interval (95% CI) for nonnormal distribution. Baseline characteristics were compared using the Student's t-test for continuous variables and the chi-square or Fisher's exact test for categorical variables.
Because the present study is a substudy for the randomized study, the baseline characteristics may differ for some variables. Efficacies according to treatment groups were compared using adjusted means and standard errors for baseline data using a generalized linear model. For the statistical testing for the difference in the reduction of AAIx75 and AcfPWV during treatment were adjusted for clinic brachial SBP at baseline and at week 20 because of the SBP dependencies.
For intergroup comparison of smoothness index, the intraindividual smoothness indices were compared using two-sample t-tests. For intergroup differences of nonnormally distributed trough–peak ratios, point estimates and 95% Cls of the net treatment difference between LH vs. LA groups were computed according to the method of calculating CI for nonparametric analyses [23,24]. For intraindividual trough–peak ratios, the Kruskal–Wallis nonparametric test was performed.
To examine the associated factors for smoothness indices of brachial SBP and CSBP, stepwise multivariate regression analysis was performed and the entry was decided by the P value less than 0.1 and the stay was decided by the P value less than 0.05. Model 1 includes independent variables such as baseline age, sex, smoking or drinking status, height and waist circumference, and treatment group. Model 2 additionally includes clinic brachial SBP at baseline and week 20, changes in ACR, fasting blood glucose, uric acid, mean ambulatory heart rate, mean APPA, mean AAIx75, mean AcfPWV, daytime AcfPWV and night-time AcfPWV. For significant factors associated with both smoothness index and treatment group, mediation analysis was performed using the ‘process’ macro using mediation model 4 [25]. Covariates adjusted for mediation analysis were identical to independent variables in model 1 except treatment group. The statistical software package used was SAS (version 9.4, SAS Institute Inc., Cary, North Carolina, USA). P values less than 0.05 were regarded as statistically significant.
RESULTS
Baseline characteristics
Mean age was 58.9 ± 12.3 years and women accounted for 25.9%. Prevalence of drinking and current smoking were 51.5 and 21.7%, respectively. Mean BMI was 25.9 ± 2.9 kg/m2 and diabetes mellitus was noted in 14.7%. Clinic SBP and DBP were 153.8 ± 10.2 and 92.4 ± 8.5 mmHg, respectively. Prevalence of women was marginally higher in the losartan and hydrochlorthiazide group and uric acid levels were significantly higher in the losartan and amlodipine group (Table 1). Baseline BPs were not different, but nocturnal brachial SBP was marginally higher and nocturnal CSBP was significantly higher in the losartan and amlodipine group than in the losartan and hydrochlorthiazide group. Nocturnal dipping was more prominent in the losartan and hydrochlorthiazide group than in the losartan and amlodipine group. APPA, AAIx75 and AcfPWV were similar between groups.
TABLE 1.
Baseline characteristics of losartan and hydrochlorothiazide group vs. losartan and amlodipine group
| Losartan and HCTZ (n = 72) | Losartan and amlodipine (n = 70) | P | |
| Age (years) | 59.3 ± 11.7 | 58.4 ± 12.9 | 0.6633 |
| Female (%) | 36.9% | 22.9% | 0.0655 |
| BMI (kg/m2) | 25.9 ± 3.0 | 25.9 ± 2.9 | 0.9758 |
| Drinking | 56.2% | 45.7% | 0.2142 |
| Smoking | 24.6% | 18.5% | 0.3808 |
| Waist circumference (cm) | 89.9 ± 9.0 | 89.5 ± 8.5 | 0.7849 |
| Clinic SBP (mmHg) | 153.7 ± 9.5 | 153.9 ± 11.0 | 0.5713 |
| Clinic DBP (mmHg) | 92.5 ± 8.4 | 92.9 ± 8.8 | 0.8778 |
| Heart rate (bpm) | 71.2 ± 10.5 | 69.4 ± 9.6 | 0.2530 |
| Fasting blood glucose (mg/dl) | 109.7 ± 17.0 | 105.4 ± 18.3 | 0.1576 |
| Creatinine (mg/dl) | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.1482 |
| Uric acid (mg/dl) | 5.4 ± 1.3 | 5.8 ± 1.2 | 0.0349 |
| Total cholesterol (mg/dl) | 187.2 ± 38.6 | 182.1 ± 36.5 | 0.4123 |
| Haemoglobin A1c (%) | 5.8 ± 0.7 | 5.8 ± 0.7 | 0.581 |
| Sodium (mEq/l) | 141.0 ± 2.2 | 140.8 ± 2.0 | 0.5204 |
| Potassium (mEq/l) | 4.4 ± 0.3 | 4.4 ± 0.3 | 0.4793 |
| Albumin creatinine ratio (mg/g) | 33.3 ± 91.3 | 24.9 ± 47.0 | 0.4986 |
| Brachial SBP, 24 h (mmHg) | 135.7 ± 10.4 | 137.2 ± 13.0 | 0.4619 |
| Brachial SBP, daytime (mmHg) | 141.0 ± 11.0 | 141.2 ± 13.9 | 0.9289 |
| Brachial SBP, night-time (mmHg) | 125.3 ± 13.7 | 129.7 ± 14.1 | 0.0631 |
| Brachial DBP, 24 h (mmHg) | 86.8 ± 9.4 | 87.0 ± 10.1 | 0.8943 |
| Brachial DBP, daytime (mmHg) | 91.2 ± 9.7 | 90.2 ± 10.3 | 0.5276 |
| Brachial DBP, night-time (mmHg) | 78.1 ± 11.6 | 81.0 ± 11.5 | 0.1355 |
| Heart rate, 24 h (bpm) | 71.6 ± 9.7 | 68.8 ± 8.5 | 0.0697 |
| Heart rate, daytime (bpm) | 75.7 ± 10.8 | 72.5 ± 8.9 | 0.0535 |
| Heart rate, night-time (bpm) | 63.8 ± 9.2 | 62.1 ± 9.8 | 0.2851 |
| Nocturnal dipping (mmHg) | 10.9 ± 9.2 | 7.9 ± 7.5 | 0.0364 |
| Dipper (%) | 58.9% | 40.0% | 0.0238 |
| Central SBP, 24 h (mmHg) | 125.4 ± 9.6 | 127.4 ± 12.4 | 0.2790 |
| Central SBP, daytime (mmHg) | 129.9 ± 10.1 | 130.4 ± 13.5 | 0.7727 |
| Central SBP, night-time (mmHg) | 116.7 ± 12.7 | 121.8 ± 13.6 | 0.022 |
| Ambulatory PPA, 24-h | 1.27 ± 0.08 | 1.25 ± 0.06 | 0.1032 |
| Ambulatory PPA, daytime | 1.29 ± 0.08 | 1.27 ± 0.07 | 0.1088 |
| Ambulatory PPA, night-time | 1.23 ± 0.11 | 1.22 ± 0.11 | 0.6368 |
| Ambulatory AIx75, 24-h (%) | 23.9 ± 9.5 | 22.3 ± 9.4 | 0.3173 |
| Ambulatory AIx75, daytime (%) | 24.4 ± 9.3 | 22.0 ± 8.7 | 0.123 |
| Ambulatory AIx75, night-time (%) | 23.2 ± 12.2 | 22.8 ± 12.6 | 0.8457 |
| Ambulatory cfPWV, 24-h (m/s) | 8.8 ± 1.6 | 9.0 ± 1.8 | 0.7202 |
| Ambulatory cfPWV, daytime (m/s) | 9.0 ± 1.6 | 9.1 ± 1.8 | 0.8345 |
| Ambulatory cfPWV, night-time (m/s) | 8.5 ± 1.7 | 8.7 ± 1.8 | 0.5051 |
AIx75, augmentation index calibrated for the assumed ambulatory heart rate of 75 beats per minute; cfPWV, carotid femoral pulse wave velocity; HCTZ, dihydrochlorothiazide; PPA, pulse pressure amplification.
Changes at 20 weeks
Reductions in clinic BPs were not different between groups. However, ambulatory brachial SBP decreased more significantly in the losartan and amlodipine group than in the losartan and hydrochlorthiazide group during the 24-h period and during daytime. Reduction of nocturnal CSBP unadjusted for baseline difference was significantly greater in the losartan and amlodipine group than in the losartan and hydrochlorthiazide group (4.1 ± 12.2 vs. 9.4 ± 12.2 mmHg, P = 0.0106); however, when adjusted for baseline level or regression to mean, it was not significantly different, as summarized in Table 2. APPA changed very little (less than 0.01). The changes in AcfPWV and AAIx75-adjusted baseline difference and clinic SBPs were not significantly different between groups except that the reduction in nocturnal AcfPWV was greater in the losartan and amlodipine group (losartan and hydrochlorthiazide: 0.09 ± 0.05 vs. losartan and amlodipine: 0.26 ± 0.05 m/s, P = 0.0235) even with adjustment for clinic brachial SBP at baseline and at week 20 because of the SBP dependencies.
TABLE 2.
Comparison between losartan and hydrochlorothiazide vs. losartan and amlodipine groups in the reduction of the study parameters at week 20
| Losartan and HCTZ (n = 72) | Losartan and amlodipine (n = 70) | Pa | |
| Clinic SBP (mmHg) | 15.2 ± 15.0 | 14.9 ± 13.7 | 0.9003 |
| Clinic DBP (mmHg) | 7.8 ± 8.0 | 9.2 ± 7.5 | 0.2833 |
| Brachial SBP, 24 h (mmHg) | 6.5 ± 1.2 | 9.9 ± 1.2 | 0.0449 |
| Brachial SBP, daytime (mmHg) | 7.0 ± 1.3 | 10.8 ± 1.3 | 0.0429 |
| Brachial SBP, night-time (mmHg) | 5.5 ± 1.3 | 8.4 ± 1.3 | 0.1264 |
| Central SBP, 24 h (mmHg) | 6.4 ± 1.1 | 9.2 ± 1.1 | 0.0738 |
| Central SBP, daytime (mmHg) | 6.8 ± 1.2 | 9.6 ± 1.1 | 0.0836 |
| Central SBP, night-time (mmHg) | 4.9 ± 1.3 | 8.3 ± 1.3 | 0.0613 |
| Ambulatory AIx75, 24-h (%) | −0.4 ± 0.6 | 1.1 ± 0.6 | 0.0955 |
| Ambulatory AIx75, daytime (%) | 0.1 ± 0.7 | 1.6 ± 0.7 | 0.1296 |
| Ambulatory AIx75, night-time (%) | −1.2 ± 1.0 | 0.4 ± 1.0 | 0.2705 |
| Ambulatory cfPWV, 24-h (m/s) | 0.15 ± 0.05 | 0.27 ± 0.05 | 0.0507 |
| Ambulatory cfPWV, daytime (m/s) | 0.16 ± 0.05 | 0.28 ± 0.05 | 0.0808 |
| Ambulatory cfPWV, night-time (m/s) | 0.09 ± 0.05 | 0.26 ± 0.05 | 0.0235 |
| Albumin creatinine ratio (mg/g) | 8.27 ± 2.43 | 10.56 ± 2.45 | 0.5100 |
| Fasting blood glucose (mg/dl) | −7.04 ± 1.99 | −2.63 ± 1.97 | 0.1487 |
| Uric acid (mg/dl) | −0.38 ± 0.11 | 0.19 ± 0.11 | 0.0002 |
AIx75, augmentation index calibrated for the assumed ambulatory heart rate of 75 beats per minute; cfPWV, carotid femoral pulse wave velocity; HCTZ, dihydrochlorothiazide.
aAdjusted for baseline difference and additionally adjusted for SBPs at baseline and at week 20 in case of AIx and cfPWV. Mean ± standard error.
Difference in smoothness index
As summarized in Table 3, intraindividual smoothness indices in both brachial SBP and CSBP in losartan and amlodipine group were higher than those in the losartan and hydrochlorthiazide group. This was attributable both to greater hourly reduction in mean BPs and less variability in hourly BP reduction.
TABLE 3.
Comparison between losartan and hydrochlorothiazide vs. losartan and amlodipine group in intraindividual smoothness index of brachial and central SBP and vascular parameters
| Losartan and HCTZ (n = 72) | Losartan and amlodipine (n = 70) | P | |
| Smoothness index for brachial SBP | 0.39 ± 0.57 | 0.66 ± 0.78 | 0.0151 |
| Mean hourly reduction (mmHg) | 6.33 ± 10.02 | 10.4 ± 12.5 | 0.0311 |
| SD of hourly reduction (mmHg) | 20.2 ± 6.7 | 17.0 ± 5.2 | 0.002 |
| Smoothness index for central SBP | 0.40 ± 0.57 | 0.65 ± 0.74 | 0.022 |
| Mean hourly reduction (mmHg) | 6.15 ± 9.03 | 9.79 ± 11.45 | 0.0362 |
| SD of hourly reduction (mmHg) | 18.59 ± 5.67 | 16.0 ± 4.8 | 0.0036 |
AIx, augmentation index; cfPWV, carotid femoral pulse wave velocity; HCTZ, dihydrochlorothiazide; PPA, pulse pressure amplification; SD, standard deviation.
Trough–peak ratio
The individual trough–peak ratios for brachial SBP and CSBP were higher in the losartan and amlodipine group than in the losartan and hydrochlorthiazide group, but the difference was not statistically significant. The global trough–peak ratio of brachial SBP and CSBP were higher in the losartan and amlodipine group than in the losartan and hydrochlorthiazide group as shown in Fig. 1 (Table 4).
FIGURE 1.
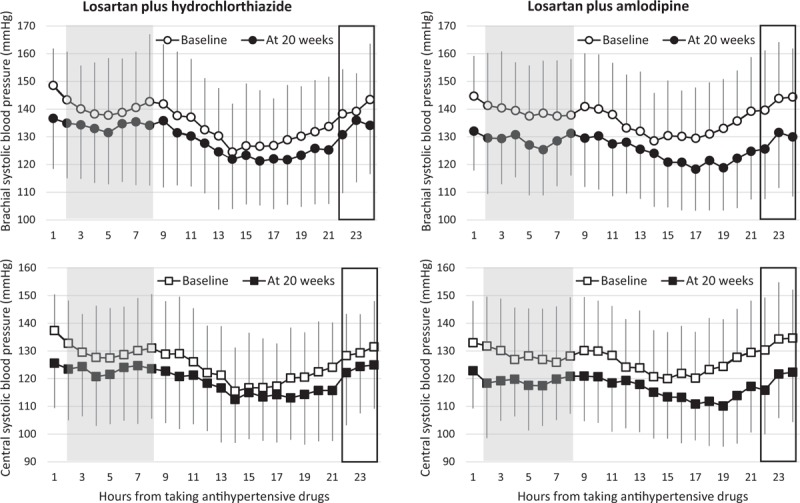
Brachial SBP profiles (upper) and central systolic blood pressure profiles (lower) at baseline and at week 20. Shaded area is the period to find peak efficacy of treatment and period with rectangle is to find trough efficacy of treatment.
TABLE 4.
Comparison between losartan and hydrochlorothiazide vs. losartan and amlodipine group in intraindividual and global trough-peak ratio of brachial and central SBPs
| Intraindividual TP ratio | |||
| Group | Median | Difference | P |
| Brachial SBP | |||
| Losartan and HCTZ (n = 72) | 0.26 [−0.68 to 0.80] | 0.30 [−0.07 to 1.30] | 0.7202 |
| Losartan and amlodipine (n = 70) | 0.45 [−0.45 to 1.08] | ||
| Central SBP | |||
| Losartan and HCTZ (n = 72) | 0.34 [−0.67 to 0.75] | 0.24 [−0.51 to 3.80] | 0.0885 |
| Losartan and amlodipine (n = 70) | 0.48 [−0.25 to 1.18] | ||
| Global TP ratio | |||
| Group | Peak | Trough | TP ratio |
| Brachial SBP | |||
| Losartan and HCTZ (n = 72) | −9.52 ± 2.02 | −4.90 ± 0.82 | 0.51 |
| Losartan and amlodipine (n = 70) | −11.83 ± 1.18 | −12.16 ± 0.24 | 1.03 |
| Central SBP | |||
| Losartan and HCTZ (n = 72) | −8.44 ± 3.09 | −5.16 ± 0.55 | 0.62 |
| Losartan and amlodipine (n = 70) | −11.71 ± 1.79 | −12.70 ± 0.66 | 1.08 |
[], 95% confidence interval; HCTZ, dihydrochlorothiazide; TP ratio, trough to peak ratio.
Factors related to smoothness index of brachial SBP and central SBP
As summarized in Table 5, in multivariable regression analysis model 1 to explore the factors related to smoothness index of brachial SBP and CSBP, treatment group and age were independent factors associated with smoothness index in brachial SBP and the effect of treatment regimen was the only factor associated with smoothness index in CSBP. In model 2 for smoothness index of brachial SBP, improvement of AcfPWV was the only independently associated factor. In model 2 for smoothness index of CSBP, the improvement of AcfPWV and increase in APPA were independently associated factors (Table 5). In mediation analysis for reduction in night-time AcfPWV, which was the only covariate showing statistically significant correlations with both treatment groups and smoothness index of CSBP, the total effect between treatment group and smoothness index of CSBP was fully mediated by reduction in night-time AcfPWV, showing a significant indirect effect. Therefore, the direct effect became no longer significant considering the mediation or indirect effect, which was completely attributable to the reduction in night-time AcfPWV (Fig. 2, supplementary Table).
TABLE 5.
Multivariable regression model for the factors associated with smoothness index
| Variable | Beta | Model Rsq | Pa |
| Model 1 | |||
| Smoothness index of brachial SBP | |||
| LA group (reference: LH group) | 0.2698 | 0.0274 | 0.0148 |
| Age | 0.0097 | 0.0365 | 0.0327 |
| Smoothness index of central SBP | |||
| LA group (reference: LH group) | 0.2526 | 0.0363 | 0.0200 |
| Model 2 | |||
| Smoothness index of brachial SBP | |||
| Reduction of 24-h ambulatory cfPWV | 1.5904 | 0.7878 | <0.0001 |
| Smoothness index of central SBP | |||
| Reduction of 24-h ambulatory cfPWV | 1.5476 | 0.8253 | <0.0001 |
| Increase in mean pulse pressure amplification | 0.8510 | 0.8323 | 0.0243 |
Model 1 includes covariates of baseline age, sex, smoking or drinking status, height, waist circumference and treatment group. Model 2 additionally includes clinic brachial SBP at baseline and week 20, the changes in ACR, fasting blood glucose, uric acid, mean ambulatory heart rate, mean APPA, mean AAIx75, mean AcfPWV, daytime AcfPWV and night-time AcfPWV in addition to the model 1.
LA, losartan and amlodipine combination; LH, losartan and dihydrochlorothiazide combination.
aStepwise multivariate regression analysis.
FIGURE 2.
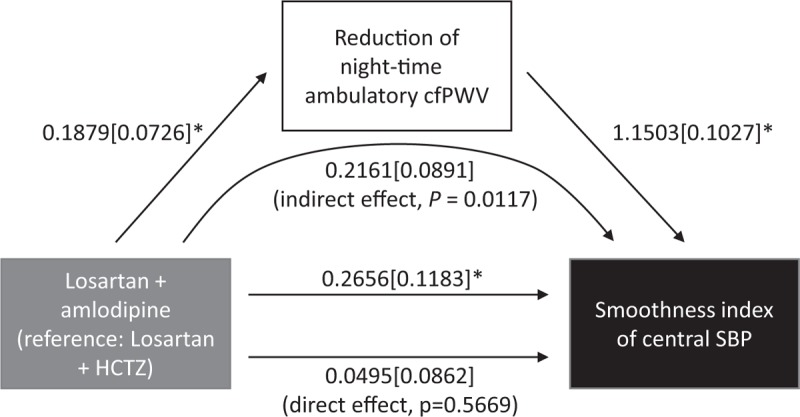
Path diagram showing full mediation of reduction of night-time carotid femoral pulse wave velocity regarding the relationship between different treatment groups and smoothness index of central SBP. Data were expressed as estimate [standard error]. Adjusted covariates: baseline age, sex, brachial SBP, smoking or drinking status, height and waist circumference. AcfPWV, ambulatory carotid femoral pulse wave velocity; HCTZ, dihydrochlorothiazide; SI, smoothness index. ∗P < 0.05; P for indirect effect was calculated using Sobel test.
DISCUSSION
The main finding of the present study was that, compared with the losartan and hydrochlorthiazide group, the losartan and amlodipine group was independently associated with intraindividual smoothness indices of brachial SBP and CSBP, even though the reduction of 24-h CSBP was not different between groups. The finding that reduction in CSBP was not significantly different between groups contradicted to findings of the original study can be explained by the limited sample size because of exclusion of more patients to calculate smoothness indices.
In contrast to the trough–peak ratio, which failed to show a difference between the groups, intraindividual smoothness indices successfully showed differences between losartan and amlodipine and losartan and hydrochlorthiazide combinations. These findings appeared to be consistent with the previous report suggesting the superiority of smoothness index to trough–peak ratio in assessing the 24-h BP-lowering effect of AHM [20]. In our study, global trough–peak ratio was so high to be about 1 in losartan and amlodipine combination group as shown in Fig. 1. This unusually high value can be attributable to the relatively weak peak effect of losartan and amlodipine regimen, which could be attributable to occupational stress which could blunt the effect of losartan and amlodipine combination [26,27]. The fact that such an apparent difference does not mean statistical difference might be the limitation of trough–peak ratio as the parameter indicating 24-h BP control efficacy of AHM. Taken together, smoothness index seems to be better to reflect the 24-h BP-lowering efficacy of AHM in the study population who are actively engaged with physical or social activities.
Smoothness indices were first reported to be independently associated with the improvement of AcfPWV in our study. These findings are in line with those of previous studies showing the correlation between smoothness index with changes in left ventricular mass or carotid intima media thickness in terms of hypertension-mediated organ damage [28,29]. By contrast to the finding in model 1 that the treatment group was independently associated with smoothness indices, according to the model 2, including parameters for arterial stiffness, it could be suggested that individual reduction of AcfPWV was more important than the treatment regimen itself. Further study with larger sample size is needed to demonstrate the association of treatment regimen independently of the reduction in AcfPWV.
In our original study, the losartan and amlodipine group showed a higher reduction in 24-h AcfPWV than the losartan and hydrochlorthiazide group [16]. In the present study, the reduction in AcfPWV was significantly different only during the night-time between groups. This appears to be attributable to reduced sample size for calculating the smoothness index. However, it also suggests that the measurement of cfPWV in the resting state may have more differential value than the ambulatory nonresting state to show the differences between treatment groups. Even though CSBP and cfPWV are recommended to be measured at resting status, our multivariable regression model 2 showed that reduction in 24-h AcfPWV instead of night-time AcfPWV was independently associated with smoothness indices. This finding suggests that further study to define clinical usefulness of nonresting or AcfPWV is needed.
Mediation analysis using the reduction in night-time AcfPWV strongly suggested that the smoothness index achieved by 20-week treatment by the two combination regimens can be fully mediated by the reduction of night-time AcfPWV (suppl. Table 1).
Therefore, during antihypertensive therapy for an individual patient, reduction of AcfPWV might be important to predict smoothness index during AHM regardless of the antihypertensive regimen. Whether this hypothesis can be applied to resting change in cfPWV regarding the association with smoothness index or clinical outcome needs further study [30].
The difference between the ambulatory brachial SBP and CSBP was about 10 mmHg and the resultant mean APPA appears to be consistent with findings of a previous study done for single measurements [31]. However, the change in APPA was negligible with 20-week treatment, even with the changes in BP and AcfPWV. Even though PPA has been known to be important as a therapeutic response, there are few studies of the therapeutic response of APPA [32].
The reduction of AAIx was not different between groups in the present study. Losartan and hydrochlorthiazide combination was reported to be inferior to maximal losartan dosage uptitration in reducing AAIx [33]. Because there was a tendency for more reduction in AAIx in the losartan and amlodipine group than in the losartan and hydrochlorthiazide group in which some increase in AAIx was noted, further study to compare losartan and amlodipine combination vs. maximal losartan dosage would be interesting.
Our study has some limitations. First, because it is a substudy of a randomized clinical trial, selection bias may be present and some of the baseline characteristics were significantly different between groups. Even though the changes of the study parameters were adjusted for baseline difference, the study result could be biased. Second, because of the smaller sample size, the marginal difference in CSBP can be suggested as being significant by referring to the original study result. Despite the marginal difference, demonstration of the stronger association of CSBP and AcfPWV than brachial SBP may be an interesting finding in the present study.
In conclusion, 20-week treatment using losartan and amlodipine combination is superior to losartan and dihydrochlorothiazide combination in achieving higher smoothness indices of both brachial SBP and CSBP. Its efficacy on higher smoothness index of CSBP was mediated fully by reduction in night-time AcfPWV.
ACKNOWLEDGEMENTS
Conflicts of interest
There are no conflicts of interest.
Supplementary Material
Footnotes
Abbreviations: AAIx, ambulatory augmentation index; AAIx75, augmentation index at heart rate 75 beats per minute; ABPM, ambulatory blood pressure monitoring; AcfPWV, ambulatory carotid femoral pulse wave velocity; ACR, albumin creatinine ratio; AHM, antihypertensive medication; AIx, augmentation index; APPA, ambulatory pulse pressure amplification; BP, blood pressure; CCB, calcium channel blocker; cfPWV, carotid femoral pulse wave velocity; CSBP, central SBP; HCTZ, hydrochlorthiazide; PPA, pulse pressure amplification; RAS, renin–angiotensin system
REFERENCES
- 1.A global brief on hypertension. Silent killer, global public health crisis. World Health Day 2013. Health Organization. Document number: WHO/DCO/WHD/2013.2; 2013. [Google Scholar]
- 2.Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. 2018 ESC/ESH Guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 2018; 36:1953–2041. [DOI] [PubMed] [Google Scholar]
- 3.James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2014; 311:507–520. [DOI] [PubMed] [Google Scholar]
- 4.Bloch MJ, Basile J. Analysis of recent papers in hypertension: differences in central aortic blood pressure explain outcome differences in ASCOT: the CAFE substudy; and rimonabant improves multiple cardiometabolic parameters in overweight and obese individuals. J Clin Hypertens (Greenwich) 2006; 8:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skoglund PH, Svensson P, Asp J, Dahlof B, Kjeldsen SE, Jamerson KA, et al. Amlodipine+benazepril is superior to hydrochlorothiazide+benazepril irrespective of baseline pulse pressure: subanalysis of the ACCOMPLISH trial. J Clin Hypertens (Greenwich) 2015; 17:141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roman MJ, Devereux RB. Association of central and peripheral blood pressures with intermediate cardiovascular phenotypes. Hypertension 2014; 63:1148–1153. [DOI] [PubMed] [Google Scholar]
- 7.Sharman JE, Marwick TH, Gilroy D, Otahal P, Abhayaratna WP, Stowasser M, et al. Randomized trial of guiding hypertension management using central aortic blood pressure compared with best-practice care: principal findings of the BP GUIDE study. Hypertension 2013; 62:1138–1145. [DOI] [PubMed] [Google Scholar]
- 8.Jamerson K, Weber MA, Bakris GL, Dahlof B, Pitt B, Shi V, et al. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428. [DOI] [PubMed] [Google Scholar]
- 9.Oshikawa J, Toya Y, Morita S, Taguri M, Hanaoka K, Hasegawa T, et al. Angiotensin receptor blocker (ARB)-diuretic versus ARB-calcium channel blocker combination therapy for hypertension uncontrolled by ARB monotherapy. Clin Exp Hypertens 2014; 36:244–250. [DOI] [PubMed] [Google Scholar]
- 10.Parati G, Schumacher H. Blood pressure variability over 24 h: prognostic implications and treatment perspectives. An assessment using the smoothness index with telmisartan-amlodipine monotherapy and combination. Hypertens Res 2014; 37:187–193. [DOI] [PubMed] [Google Scholar]
- 11.de la Sierra A, Gil-Extremera B, Calvo C, Campo C, Garcia-Puig J, Marquez E, et al. Comparison of the antihypertensive effects of the fixed dose combination enalapril 10 mg/nitrendipine 20 mg vs losartan 50 mg/hydrochlorothiazide 12.5 mg, assessed by 24-h ambulatory blood pressure monitoring, in essential hypertensive patients. J Hum Hypertens 2004; 18:215–222. [DOI] [PubMed] [Google Scholar]
- 12.Luzardo L, Lujambio I, Sottolano M, da Rosa A, Thijs L, Noboa O, et al. 24-h ambulatory recording of aortic pulse wave velocity and central systolic augmentation: a feasibility study. Hypertens Res 2012; 35:980–987. [DOI] [PubMed] [Google Scholar]
- 13.Westerhof BE, Guelen I, Stok WJ, Lasance HA, Ascoop CA, Wesseling KH, et al. Individualization of transfer function in estimation of central aortic pressure from the peripheral pulse is not required in patients at rest. J Appl Physiol (1985) 2008; 105:1858–1863. [DOI] [PubMed] [Google Scholar]
- 14.McEniery CM, Cockcroft JR, Roman MJ, Franklin SS, Wilkinson IB. Central blood pressure: current evidence and clinical importance. Eur Heart J 2014; 35:1719–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 2012; 30:445–448. [DOI] [PubMed] [Google Scholar]
- 16.Cho EJ, Lee HY, Sung KC, Park S, Sohn IS, Park CG, et al. Comparison of 24 h ambulatory central blood pressure reduction efficacy between fixed amlodipine or up-titrated hydrochlorothiazide plus losartan: the K-Central study. Am J Hypertens 2019; doi: 10.1093/ajh/hpz050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oh GC, Lee HY, Chung WJ, Youn HJ, Cho EJ, Sung KC, et al. Comparison of effects between calcium channel blocker and diuretics in combination with angiotensin II receptor blocker on 24-h central blood pressure and vascular hemodynamic parameters in hypertensive patients: study design for a multicenter, double-blinded, active-controlled, phase 4, randomized trial. Clin Hypertens 2017; 23:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parati G, Omboni S, Palatini P, Rizzoni D, Bilo G, Valentini M, et al. Italian society of hypertension guidelines for conventional and automated blood pressure measurement in the office, at home and over 24 h. High Blood Press Cardiovasc Prev 2008; 15:283–310. [DOI] [PubMed] [Google Scholar]
- 19.Agnoletti D, Zhang Y, Salvi P, Borghi C, Topouchian J, Safar ME, et al. Pulse pressure amplification, pressure waveform calibration and clinical applications. Atherosclerosis 2012; 224:108–112. [DOI] [PubMed] [Google Scholar]
- 20.Palatini P, Malacco E, Di SS, Carretta R, Dorigatti F, Bertocchi F, et al. Trough:peak ratio and smoothness index in the evaluation of 24-h blood pressure control in hypertension: a comparative study between valsartan/hydrochlorothiazide combination and amlodipine. Eur J Clin Pharmacol 2002; 57:765–770. [DOI] [PubMed] [Google Scholar]
- 21.Parati G, Omboni S, Rizzoni D, Agabiti-Rosei E, Mancia G. The smoothness index: a new, reproducible and clinically relevant measure of the homogeneity of the blood pressure reduction with treatment for hypertension. J Hypertens 1998; 16:1685–1691. [DOI] [PubMed] [Google Scholar]
- 22.Staessen JA, Thijs L, Bijttebier G, Clement D, O’Brien ET, Palatini P, et al. Determining the trough-to-peak ratio in parallel-group trials. Systolic Hypertension in Europe (SYST-EUR) Trial Investigators. Hypertension 1997; 29:659–667. [DOI] [PubMed] [Google Scholar]
- 23.Campbell MJ, Gardner MJ. Calculating confidence intervals for some nonparametric analyses. Br Med J (Clin Res Ed) 1988; 296:1454–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omboni S, Parati G, Mancia G. The trough:peak ratio and the smoothness index in the evaluation of control of 24 h blood pressure by treatment in hypertension. Blood Press Monit 1998; 3:201–204. [PubMed] [Google Scholar]
- 25.Hayes AF. 2012. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. [White paper]. Retrieved from http://www.afhayes.com/. [Google Scholar]
- 26.Kim D, Ha JW. Hypertensive response to exercise: mechanisms and clinical implication. Clin Hypertens 2016; 22:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schultz MG, Sharman JE. Exercise hypertension. Pulse (Basel) 2014; 1:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Campo C, Saavedra J, Segura J, Roldan C, Ruilope LM, Parati G. Correlations of smoothness index and trough-to-peak ratio with left ventricular mass index changes induced by lercanidipine in hypertensive patients. A pilot trial. Minerva Med 2005; 96:365–371. [PubMed] [Google Scholar]
- 29.Rizzoni D, Muiesan ML, Salvetti M, Castellano M, Bettoni G, Monteduro C, et al. The smoothness index, but not the trough-to-peak ratio predicts changes in carotid artery wall thickness during antihypertensive treatment. J Hypertens 2001; 19:703–711. [DOI] [PubMed] [Google Scholar]
- 30.Williams B, Cockcroft JR, Kario K, Zappe DH, Cardenas P, Hester A, et al. Rationale and study design of the Prospective comparison of Angiotensin Receptor neprilysin inhibitor with Angiotensin receptor blocker MEasuring arterial sTiffness in the eldERly (PARAMETER) study. BMJ Open 2014; 4:e004254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pichler G, Martinez F, Vicente A, Solaz E, Calaforra O, Redon J. Pulse pressure amplification and its determinants. Blood Press 2016; 25:21–27. [DOI] [PubMed] [Google Scholar]
- 32.Protogerou AD, Papaioannou TG, Lekakis JP, Blacher J, Safar ME. The effect of antihypertensive drugs on central blood pressure beyond peripheral blood pressure. Part I: (Patho)-physiology, rationale and perspective on pulse pressure amplification. Curr Pharm Des 2009; 15:267–271. [DOI] [PubMed] [Google Scholar]
- 33.Metoki H, Obara T, Asayama K, Satoh M, Hosaka M, Elnagar N, et al. Differential effects of angiotensin II receptor blocker and losartan/hydrochlorothiazide combination on central blood pressure and augmentation index. Clin Exp Hypertens 2015; 37:294–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


