Abstract
Purpose of review
This review discusses recent advances in the rehabilitation of motor deficits after traumatic brain injury (TBI) and spinal cord injury (SCI) using neuromodulatory techniques.
Recent findings
Neurorehabilitation is currently the only treatment option for long-term improvement of motor functions that can be offered to patients with TBI or SCI. Major advances have been made in recent years in both preclinical and clinical rehabilitation. Activity-dependent plasticity of neuronal connections and circuits is considered key for successful recovery of motor functions, and great therapeutic potential is attributed to the combination of high-intensity training with electrical neuromodulation. First clinical case reports have demonstrated that repetitive training enabled or enhanced by electrical spinal cord stimulation can yield substantial improvements in motor function. Described achievements include regaining of overground walking capacity, independent standing and stepping, and improved pinch strength that recovered even years after injury.
Summary
Promising treatment options have emerged from research in recent years using neurostimulation to enable or enhance intense training. However, characterizing long-term benefits and side-effects in clinical trials and identifying patient subsets who can benefit are crucial. Regaining lost motor function remains challenging.
Keywords: electrical neuromodulation, motor recovery, rehabilitative training, spinal cord injury, traumatic brain injury
INTRODUCTION
A trauma to the central nervous system (CNS), that is, spinal cord injury (SCI) and traumatic brain injury (TBI), is a devastating event and an important global cause of morbidity and mortality exhibiting an upward trend in frequency [1,2]. Directed interventions during the acute injury period are designed to limit secondary damage [3,4], but effective therapeutic strategies to manage the neurological sequelae and to promote axon regeneration are yet beyond reach [5,6]. Rehabilitative training is currently the only treatment option for injured patients that bears the potential to improve short and long-term recovery of motor function [6,7]. The large number of patients who are dependent on a wheelchair or suffer from lifelong disabilities and impairments implies that reparative effects are highly limited. In recent years, the combination of rehabilitative training with neuromodulation of the brain or the spinal cord has been investigated as means to enhance the excitability of motor circuits and to increase training efficacy promoting motor recovery [8,9]. Latest findings are promising and might open up possibilities even for patients with severe spinal cord or traumatic brain injury.
The article mainly focuses on the recovery of motor function after CNS injury. It addresses the growing field of neurorehabilitation augmented by electrical neuromodulation and highlights some of the recent advances in both basic and clinical science. The fast-growing field of robotic and exoskeleton assisted training [10–12] is of great interest but lies beyond the scope of the present review.
Box 1.

no caption available
Injury-induced neuronal plasticity promotes motor recovery
Contrary to previous assumptions, the central nervous system has a substantial potential for structural and functional adaptations after injury. In the spinal cord, for example, various descending systems have been shown to exhibit pronounced spontaneous circuit reorganization of partially spared tracts after an SCI. A correlation and temporal overlap between recovery of function and injury-induced anatomical plasticity has been observed, and these plastic processes may be an important element and basis for spontaneous and training-enhanced recovery of motor function after neurotrauma.
Spinal cord injury
After sustaining an injury to the spinal cord, most patients experience some degree of spontaneous functional recovery within the first year, but improvement of motor function greatly decreases thereafter [13]. In the last few years, both projections descending from the motor cortex [14,15] or the brainstem [16,17▪▪] and the intraspinal circuits [18,19] (central pattern generators, CPGs) have been shown to reorganize following an injury. Using a dual viral silencing approach in rodents, Hilton et al.[14] demonstrated that spared corticospinal fibers play a pivotal role in spontaneous recovery after cervical SCI. Transient silencing of uninjured corticospinal neurons temporarily eliminated motor function that had recovered after injury. In another study in rodents with severe incomplete SCI (iSCI), Asboth et al.[17▪▪] showed that the cortex mediates recovery of hindlimb function via the brainstem by activating spared reticulospinal axons. However, spontaneous cortico-reticulospinal plasticity alone is insufficient to form sufficient relay connections between cortex and brainstem and to warrant substantial recovery. Changes in the excitability of motor neuron and interneuron circuits between acute and chronic SCI have been reported by Bellardita et al.[19]. Such changes may also play a crucial role for the development of spasms in SCI patients. Züchner et al.[20] demonstrated rewiring of spared serotonergic axons in the neonatal, injured rodent spinal cord paralleled by functional recovery and thus suggest modulatory changes within the CPG after SCI.
Taken together, these recent studies, among many others, suggest that a number of reorganizational processes are initiated by an SCI, leading to sprouting of surviving sensory and motor tract fibers as an adaptive mechanism that facilitates motor output. However, the CNS's innate repair mechanisms and growth capacity are insufficient for higher levels of recovery of motor function after large lesions.
Traumatic brain injury
A traumatic brain injury initiates a cascade of insufficiently studied pathological processes that can ultimately result in substantial sensori-motor as well as cognitive dysfunction, depending on the severity and location of the trauma. Even though motor dysfunction including gait disturbances or limb paralysis and spasticity is less frequent compared with neurocognitive and behavioral impairments [21] after a TBI, 30% of TBI survivors exhibit disabling motor deficits [22]. Motor recovery is largely restricted to a short-time window of approximately 3 months following the primary injury and starts to stagnate thereafter [23]. Even though the age-standardized incidence of TBI is 30 times higher than that of SCI [2], fundamental knowledge about neuroanatomical correlates of the observed behavioral changes and the dynamic circuit changes that follow a traumatic impact to the brain is scarce. It has been hypothesized that serotonergic axons bear potential for regrowth after TBI [24,25]. Kajstura et al.[24] demonstrated that a significant acute loss of serotonergic fibers was followed by substantial axonal outgrowth between 1 and 3 months postinjury in the neocortex of adult mice. However, no causal link or temporal correlation to functional recovery has been established. Interestingly, neuroplastic responses (c-Fos, Tgfb1) to a distant trauma have been pointed out by Kononenko et al.[26▪], suggesting a systemic upregulation of the regenerative capacity in the CNS. The reported findings indicate that a focal TBI can initiate plastic processes in distant spinal circuits and highlight that injury-induced plasticity could be a synergistic process taking place throughout the CNS. Whether the suggested interactions between TBI and spinal circuitry contribute to motor recovery remains to be seen.
Activity-dependent plasticity – the basis for rehabilitation
The vast majority of spinal cord injuries is anatomically incomplete [27,28] and thus do not entirely disconnect the sublesional spinal cord from the brain and brainstem [29]. In patients with a clinically complete injury (ASIA A) as well as in ASIA B and C patients, spared fibers at the lesion site are insufficient to transmit functionally meaningful signals for volitional motor control to the lower spinal cord [30]. Despite this deprivation of supraspinal input, locomotor circuits (CPGs) located below the injury remain functional and able to process information [31]. Furthermore, propriospinal circuits, which interconnect spinal segments over short or long distances, have been shown to be crucial for motor recovery after partial SCI [32,33]. A certain number of spared descending fibers, propriospinal fibers, and local interneuron and motoneuron circuits are the basis for use-dependent recovery of functions after an incomplete injury to the spinal cord [34]. Importantly, although by themselves insufficient for a functionally relevant recovery, they can be modulated and reintegrated into a functional state by intense activation, for example, during repetitive training of defined functional tasks [35▪▪,36]. The current concept of rehabilitation thus suggests that repetitive use leads to strengthening of spared projections as well as stabilization and strengthening of newly sprouted fibers and connections both between cortex and brainstem, between brainstem and spinal cord, and within the spinal cord [37]. Literature on activity-induced plasticity and circuit reorganization following TBI is scarce. However, it is hypothesized that compensatory anatomical plasticity occurs in large parts of the CNS. Spared and new fibers and connections are then integrated into functional circuits by intense rehabilitative training, in this way restoring a certain degree of both structural connectivity and motor function [38–40].
Electrical neuromodulation to enhance the efficacy of rehabilitative training
Rehabilitative training alone often does not yield sufficient recovery of motor functions, especially in patients with severe lesions and impairments. Over the last few years, translation of stimulation enhanced activity-based rehabilitation from the preclinical to a clinical setting has been carried out successfully, yielding substantial improvements in motor functionality [30,34]. The data published so far point out that the combination of intense rehabilitative training with neuromodulation by electrical stimulation might be a very promising treatment option for the recovery of motor function after SCI and TBI, at least in a subpopulation of patients [30,34]. Based on their anatomical target, current approaches of electrical neuromodulation can be roughly subdivided into cortical, deep brain, and spinal cord stimulation.
Cortical neuromodulation
Current electrical neuromodulation techniques after brain injury include epidural electrical cortical stimulation (eECS) and transcranial direct current stimulation (tDCS) [21,41]. eECS is a minimally invasive technique that involves the insertion of small electrodes into the epidural space and allows the selective stimulation of specific cortical areas. tDCS is a noninvasive method for brain stimulation, which uses directed current flow to activate restricted cortical areas. Yu et al.[42] recently compared the effects of eECS and tDCS on motor and cognitive recovery in rats with acute, focal TBI. After 4 weeks of either subthreshold eECS or tDCS during rehabilitation, rats outperformed their unstimulated controls in a motor cortex dependent skilled reaching movement task (single-pellet grasping) and locomotor task (the rotarod test), with a slight superiority of tDCS effects. A reduction of motor impulsivity with tDCS after bilateral frontal TBI was reported by Martens et al.[43] However, the only study that has tried to correlate both motor recovery and structural reorganization with motor training augmented by cortical stimulation after TBI is, to our knowledge, a study by Jefferson et al.[44] published in 2016. In this study, rats with an impact lesion to the caudal forelimb area underwent 9 weeks of rehabilitative training with or without subthreshold eECS of the injured motor cortex. Neuromodulation assisted rehabilitation led to significantly larger improvements over time, and intracortical microstimulation mapping revealed a structural reorganization of the wrist representation in the injured cortex upon long-term eECS. These results encourage further research on neuromodulation-assisted training for recovery of deficient motor function after TBI. Schönfeld et al.[45], who demonstrated that standalone cortical stimulation is insufficient for significant motor improvements in rats with severe TBI, outlined the importance of combining stimulation with training.
In a small clinical study, Middleton et al.[46] reported an improved upper extremity Fugl–Meyer score with upper-extremity physiotherapy augmented by bihemispheric tDCS in two TBI patients. However, most clinical studies focus on the effect of cortical stimulation on the nonmotor impairments in patients with TBI [21], and reports on motor recovery are scarce.
Deep brain stimulation
The application of deep brain stimulation (DBS) is routine in the treatment of pharmacotherapy-resistant movement disorders. DBS of the subthalamic nucleus and the internal globus pallidus is a highly effective treatment for drug-resistant Parkinson's disease, especially for patients with marked dyskinesia or motor fluctuation [47]. However, literature on the use of DBS to improve motor function in the context of neurotrauma is scarce. Chan et al.[48] showed that DBS of the lateral cerebellar nucleus contralateral to a unilateral fluid percussion injury of the motor cortex promotes motor recovery in rats. Additionally, DBS of the midbrain locomotor center (mesencephalic locomotor region [MLR]) has been proposed as a treatment strategy for locomotor recovery after SCI and stroke [49,50]. Highly promising results were achieved in a rodent model with more than 80% spinal cord transection where MLR-DBS acutely led to functional hindlimb walking and swimming movements [50]. A clinical study to investigate DBS of the MLR for its potential to enhance training and improve gait in nonambulatory patients with chronic iSCI (DBS-SCI, ClinicalTrials.gov identifier: NCT03053791) is currently recruiting patients.
Spinal cord stimulation
Spinal cord stimulation (SCS) is currently the most frequently investigated type of electrical circuit modulation and comprises intraspinal, transcutaneous, and epidural stimulation. In the past years, both preclinical and clinical literature have focused primarily on epidural SCS (eSCS), whose combination with rehabilitative training was suggested as a promising treatment strategy for deficient motor function after severe SCI [17▪▪,29,51▪▪,52▪▪,53,54,55▪]. Preclinically, a recent study by Gerasimenko et al.[55▪] highlighted the capability of eSCS to initiate hindlimb stepping in rats with complete SCI. The authors additionally observed that the more caudal spinal networks are insufficient to control locomotion in the absence of more rostral, upper lumbar and lower thoracic segments, a criterion that should be considered when recruiting patients for clinical testing. The therapeutic potential of eSCS was also emphasized by Asboth et al.[17▪▪], who additionally demonstrated that stimulated rats were capable of engaging context-specific locomotor behavior. Further, Capogrosso et al.[56] showed that antigravitational strength could be improved with eSCS during overground locomotion in the nonhuman primate with acute, incomplete SCI.
eSCS is currently the clinically most studied neuromodulatory technique in the context of neurotrauma [35▪▪,51▪▪,52▪▪,57,58]. Gill et al.[35▪▪] published the first report on a chronic, clinically motor complete SCI patient that regained independent stepping ability with task-specific training supported by eSCS 3 years after injury. In contrast to bilateral stepping on the treadmill, walker and trainer assistance was required during overground stepping. Angeli et al.[51▪▪] tested the effects of intense locomotor treadmill training with weight support accompanied by eSCS in four patients that had failed to improve with training alone. Although all four patients recovered independent standing and trunk stability, two patients even regained overground walking capability. Wagner et al.[57] and Calvert et al.[58] demonstrated improved voluntary control during walking or cycling and rhythmic motor activity, respectively. The ‘Epidural Stimulation After Neurologic Damage clinical trial’ (E-STAND, Trial Number: NCT03026816) is currently ongoing and has been designed to investigate the generalizability of eSCS in a greater population with, for example, differences in age, sex, time postinjury, and lesion size. Darrow et al.[52▪▪] published preliminary findings proposing that eSCS might be beneficial for a greater variety of patients than previously thought, without requiring preimplantation training in contrast to previous studies. Their preliminary data further indicate beneficial effects of eSCS beyond motor function. Inanici et al.[59▪▪] reported improved long-term recovery of upper extremity function with noninvasive transcutaneous electrical stimulation (tSCS) and physical therapy in a patient with chronic iSCI. In all these patients who developed certain degrees of volitional motor control after combined eSCS and rehabilitation therapy, spared fibers must have been present in their spinal cords in spite of an initial clinical complete ASIA A diagnosis. Additionally, all these patients were younger patients, often former athletes, in very good physical condition and able to go through a physically very demanding training over many weeks and months. Overall, there is great variability in stimulation parameters used in both preclinical and clinical studies. At a given frequency and pulse width, each individual has a certain threshold intensity eliciting, for example, rhythmic muscle activity. As the SCI population is highly heterogeneous, future research should focus on the establishment of stimulation parameters that are effective and safe according to patient subgroup, for example, depending on lesion level, lesion extent, or time that has passed since injury, and specific stimulation sites, for example, with electrode arrays targeting different segments of the lumbar spinal cord. This would increase comparability among individuals and between studies, which is required to ultimately draw conclusions on the effectiveness of neuromodulation. The E-STAND trial (Trial Number: NCT03026816) has taken the first step in this direction.
CONCLUSION
Despite major advances in the field of neurorehabilitation, the management of severe motor impairments resulting from TBI and SCI continues to challenge both basic scientists and clinicians. Figure 1 schematically illustrates electrical neuromodulation approaches and main clinical implications of the literature discussed in this review. Preclinical and clinical literature on electrical neuromodulatory approaches to regain motor function after TBI is still scarce and has focused on different postinjury phases. This is different for the field of SCI where neuromodulatory interventions to enable or enhance intense locomotor training are currently well studied in animal models and the first clinical trials. First case reports of patients with chronic SCI have shown that electrical neuromodulation of the spinal cord bears promising therapeutic potential to enable a different form and intensity of training which can lead to a significantly higher degree of recovery of lost motor functions. However, considering the heterogeneity of the TBI and SCI patient population, well-controlled clinical trials with larger numbers of participants are required to define the specific effects of the treatment and to identify the subsets of patients that can benefit. Identifying potential long-term adverse effects of electrical stimulation and the physical consequences of high-intensity training on the organism is also key. Furthermore, the optimal temporal relationship between neuromodulation and rehabilitative training needs to be identified in both preclinical and clinical studies to maximize therapeutic efficacy. The majority of current and recent SCI studies hypothesize that electrical stimulation restores the excitability of sublesional neurons, which can then be reintegrated into functional circuits by repetitive use, and these studies, thus, focus on neuromodulation applied during training (stimulation-enabled training). However, subthreshold stimulation over prolonged time periods has been shown to induce neuronal growth [60], and it has been suggested previously that the effects of a sequential application of a growth-promoting treatment followed by training might be superior [61,62]. Therefore, both preclinical and clinical studies investigating the implementation of neuromodulation prior to training to promote the expression of plasticity genes are required, as not only the absolute time point of treatment start (acute versus chronic SCI state) but also the relative timing of treatment options (sequential versus parallel) are essential for an optimal therapeutic schedule. The many ways by which electrical stimulation can affect neurons and how electrical stimulation positively influences functional recovery remain to be analyzed in detail. Figure 2 illustrates putative mechanisms of action at the example of epidural spinal cord stimulation after incomplete spinal cord injury. Preclinical and clinical studies applying high-precision stimulation are required to determine which subsets of neuronal populations and structures (soma versus axon) respond most strongly to stimulation and whether acute or long-term responses are more crucial. The combination of multiple approaches, including multilevel neuromodulation [60,63], should be pursued in the long run to meet the wide range of needs that arise from a trauma to the CNS and go far beyond motor dysfunction.
FIGURE 1.
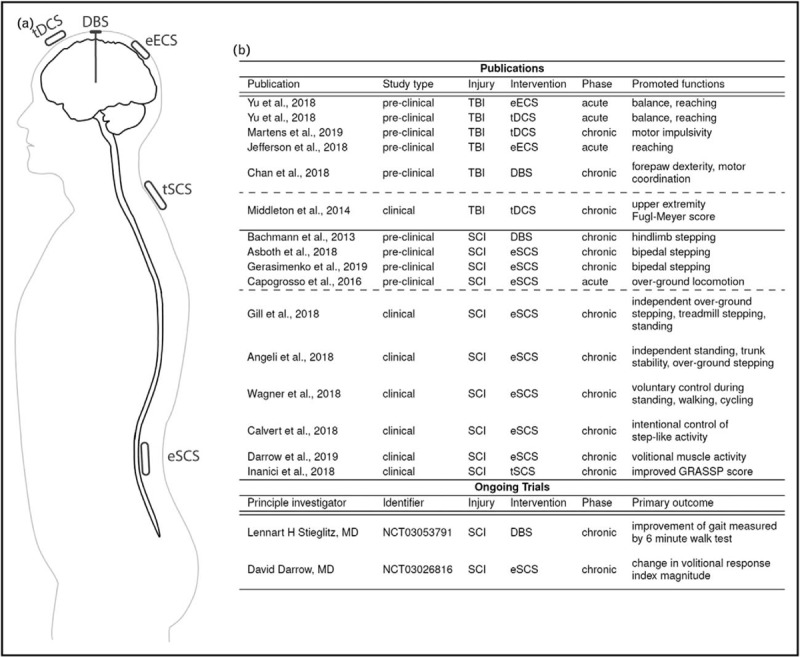
Summary of electrical neuromodulatory approaches, publications, and ongoing clinical trials discussed in this review. (a) Schematic illustration of different neuromodulatory approaches. (b) List of publications and ongoing trials by study type, injury type, intervention, and postinjury phase with the observed facilitated or enhanced functions. eECS, Epidural electrical cortical stimulation; tDCS, transcranial direct current stimulation; DBS, deep brain stimulation; eSCS, epidural spinal cord stimulation, tSCS, transcutaneous spinal cord stimulation; TBI, traumatic brain injury; SCI, spinal cord injury; GRASSP, Graded and Redefined Assessment of Strength, Sensibility and Prehension. Phase refers to the postinjury phase. Identifier refers to ongoing studies’ ClinicalTrials.gov identifier.
FIGURE 2.
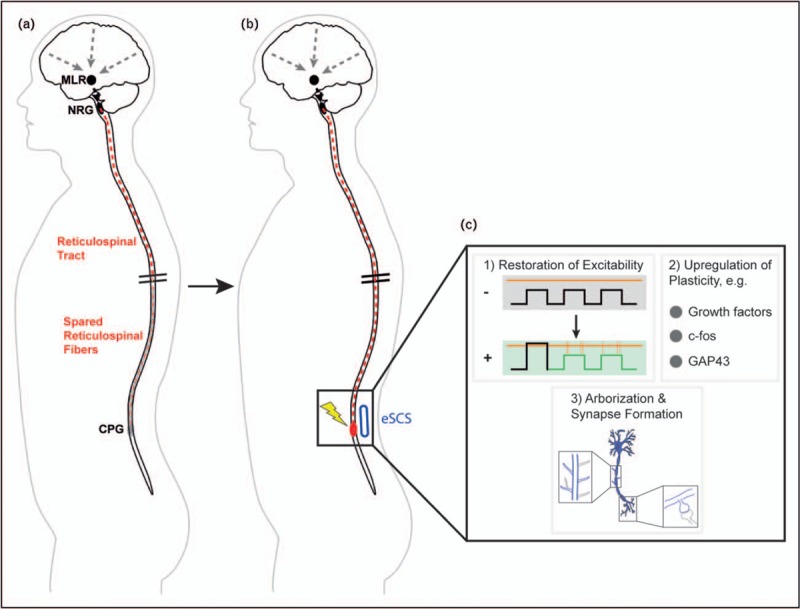
Putative biological effects of epidural spinal cord stimulation on neuronal structures. (a) After large, incomplete spinal cord injury, spared reticulospinal fibers are incapable to sufficiently activate the sublesional CPGs to generate rhythmic muscle activity and locomotion. (b) With epidural stimulation of the lumbar spinal cord, the local neurons including the CPGs regain a certain level of background activity, which makes them excitable by spared reticulospinal fibers. (c) Inset summarizing putative mechanisms. (1) Stimulation changes the resting membrane potential of CPGs, either directly or by enhancing input from propriospinal sensory fibers, thereby restoring excitability (− = no stimulation; + = stimulation; orange horizontal line = threshold potential; black and green squares = membrane potential; orange vertical lines = spikes of muscle activity). (2) Plasticity markers are upregulated by electrical activity, including, for example, growth factors, c-fos, and the growth-associated protein GAP43. (3) Neurons start to sprout, to reorganize, and to adapt the local circuits to the decreased descending input of spared fibers. CPG, Central pattern generator; eSCS, epidural spinal cord stimulation; MLR, mesencephalic locomotor region; NRG, gigantocellular reticular nucleus; GAP, growth-associated protein.
Acknowledgements
None.
Financial support and sponsorship
The authors received financial support from Wings for Life and the University of Zurich.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Hale AC, Bohnert KM, Grekin R, Sripada RK. Traumatic brain injury in the general population: Incidence, mental health comorbidity, and functional impact. J Nerv Ment Dis 2019; 207:38–42. [DOI] [PubMed] [Google Scholar]
- 2.Badhiwala JH, Wilson JR, Fehlings MG. Global burden of traumatic brain and spinal cord injury. Lancet Neurol 2019; 18:24–25. [DOI] [PubMed] [Google Scholar]
- 3.Hachem LD, Ahuja CS, Fehlings MG. Assessment and management of acute spinal cord injury: from point of injury to rehabilitation. J Spinal Cord Med 2017; 40:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vella MA, Crandall M, Patel MB. Acute management of traumatic brain injury. Surg Clin 2017; 97:1015–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loy K, Bareyre FM. Rehabilitation following spinal cord injury: how animal models can help our understanding of exercise-induced neuroplasticity. Neural Regen Res 2019; 14:405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zaninotto ALC, Costa BT, Ferreira IS. Fregni F, et al. Traumatic Brain Injury. Clinical Trials in Neurology. Neuromethods vol 138New York, NY: Humana Press; 2018. 105–138. [Google Scholar]
- 7.Côté MP, Murray M, Lemay MA. Rehabilitation strategies after spinal cord injury: inquiry into the mechanisms of success and failure. J Neurotrauma 2017; 34:1841–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rejc E, Angeli CA. Spinal cord epidural stimulation for lower limb motor function recovery in individuals with motor complete spinal cord injury. Phys Med Rehabil Clin N Am 2019; 30:337–354. [DOI] [PubMed] [Google Scholar]
- 9.Clayton E, Kinley-Cooper SK, Weber RA, Adkins DL. Brain stimulation: neuromodulation as a potential treatment for motor recovery following traumatic brain injury. Brain Res 2016; 1640:130–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esquenazi A, Lee S, Packel AT, Braitman L. A randomized comparative study of manually assisted versus robotic-assisted body weight supported treadmill training in persons with a traumatic brain injury. PM R 2013; 5:280–290. [DOI] [PubMed] [Google Scholar]
- 11.Fisahn C, Aach M, Jansen O, et al. The effectiveness and safety of exoskeletons as assistive and rehabilitation devices in the treatment of neurologic gait disorders in patients with spinal cord injury: a systematic review. Glob Spine J 2016; 6:822–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrholz J, Harvey LA, Thomas S, Elsner B. Is body-weight-supported treadmill training or robotic-assisted gait training superior to overground gait training and other forms of physiotherapy in people with spinal cord injury? A systematic review. Spinal Cord 2017; 55:722. [DOI] [PubMed] [Google Scholar]
- 13.Khorasanizadeh M, Yousefifard M, Eskian M, et al. Neurological recovery following traumatic spinal cord injury: a systematic review and meta-analysis. J Neurosurg Spine 2019; 1:1–17. [DOI] [PubMed] [Google Scholar]
- 14.Hilton BJ, Anenberg E, Harrison TC, et al. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci 2016; 36:4080–4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosberger AC, Miehlbradt JC, Bjelopoljak N, et al. Axotomized corticospinal neurons increase supra-lesional innervation and remain crucial for skilled reaching after bilateral pyramidotomy. Cereb Cortex 2018; 28:625–643. [DOI] [PubMed] [Google Scholar]
- 16.Baker SN, Perez MA. Reticulospinal contributions to gross hand function after human spinal cord injury. J Neurosci 2017; 37:9778–9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17▪▪.Asboth L, Friedli L, Beauparlant J, et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat Neurosci 2018; 21:576–588. [DOI] [PubMed] [Google Scholar]; This study illustrates the complexity of reorganizational processes underlying functional recovery after incomplete SCI. It highlights the significance of the interplay between cortex, brainstem, and spinal cord for functional plasticity and the crucial role of spared reticulospinal fibers.
- 18.Takeoka A, Vollenweider I, Courtine G, Arber S. Muscle spindle feedback directs locomotor recovery and circuit reorganization after spinal cord injury. Cell 2014; 159:1626–1639. [DOI] [PubMed] [Google Scholar]
- 19.Bellardita C, Caggiano V, Leiras R, et al. Spatiotemporal correlation of spinal network dynamics underlying spasms in chronic spinalized mice. eLife 2017; 6:e23011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Züchner M, Kondratskaya E, Sylte CB, et al. Rapid recovery and altered neurochemical dependence of locomotor central pattern generation following lumbar neonatal spinal cord injury. J Physiol 2018; 596:281–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim WS, Lee K, Kim S, et al. Transcranial direct current stimulation for the treatment of motor impairment following traumatic brain injury. J Neuroeng Rehabil 2019; 16:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker WC. Motor impairment after severe traumatic brain injury: a longitudinal multicenter study. J Rehabil Res Dev 2009; 44:975–982. [DOI] [PubMed] [Google Scholar]
- 23.Zarshenas S, Colantonio A, Horn SD, et al. Cognitive and motor recovery and predictors of long-term outcome in patients with traumatic brain injury. Arch Phys Med Rehabil 2019; 100:1274–1282. [DOI] [PubMed] [Google Scholar]
- 24.Kajstura TJ, Dougherty SE, Linden DJ. Serotonin axons in the neocortex of the adult female mouse regrow after traumatic brain injury. J Neurosci Res 2018; 96:512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jin Y, Dougherty SE, Wood K, et al. Regrowth of serotonin axons in the adult mouse brain following injury. Neuron 2016; 91:748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Kononenko O, Watanabe H, Stålhandske L, et al. Focal traumatic brain injury induces neuroplastic molecular responses in lumbar spinal cord. Restor Neurol Neurosci 2019; 37:87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study indicates that injury-induced plasticity is a systemic process involving both local and distant sites of the CNS. The authors implicate a sustained activation and enhanced growth capacity of lumbar spinal circuits after TBI.
- 27.Barthélemy D, Willerslev-Olsen M, Lundell H, et al. Assessment of transmission in specific descending pathways in relation to gait and balance following spinal cord injury. In Progress in Brain Research 2015; 218:79–101. [DOI] [PubMed] [Google Scholar]
- 28.Kakulas BA, Kaelan C. The neuropathological foundations for the restorative neurology of spinal cord injury. Clin Neurol Neurosurg 2015; 129 (S1):S1–S7. [DOI] [PubMed] [Google Scholar]
- 29.Rejc E, Angeli CA, Atkinson D, Harkema SJ. Motor recovery after activity-based training with spinal cord epidural stimulation in a chronic motor complete paraplegic. Sci Rep 2017; 7:13476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taccola G, Sayenko D, Gad P, et al. And yet it moves: recovery of volitional control after spinal cord injury. Prog Neurobiol 2018; 160:64–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diaz-Ríos M, Guertin PA, Rivera-Oliver M. Neuromodulation of spinal locomotor networks in rodents. Curr Pharm Des 2017; 23:1741–1752. [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi T, Fujita Y, Yamashita T. Neuropilin-1-mediated pruning of corticospinal tract fibers is required for motor recovery after spinal cord injury. Cell Death Dis 2019; 10:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swieck K, Conta-Steencken A, Middleton FA, et al. Effect of lesion proximity on the regenerative response of long descending propriospinal neurons after spinal transection injury. BMC Neurosci 2019; 20:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Courtine G, Sofroniew MV. Spinal cord repair: advances in biology and technology. Nat Med 2019; 25:898–908. [DOI] [PubMed] [Google Scholar]
- 35▪▪.Gill ML, Grahn PJ, Calvert JS, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 2018; 24:1677–1682. [DOI] [PubMed] [Google Scholar]; This is the first case report on a chronic SCI patient that could regain independent stepping capacity because of task-specific training enabled by epidural spinal cord stimulation after 3 years of completely absent motor function. Intensive training was performed before implantation of electrodes and combined with stimulation thereafter.
- 36.Marques MR, Nicola FC, Sanches EF, et al. Locomotor training promotes time-dependent functional recovery after experimental spinal cord contusion. Neuroscience 2018; 392:258–269. [DOI] [PubMed] [Google Scholar]
- 37.Hilton BJ, Tetzlaff W. A brainstem bypass for spinal cord injury. Nat Neurosci 2018; 21:457–458. [DOI] [PubMed] [Google Scholar]
- 38.Turolla A, Venneri A, Farina D, et al. Rehabilitation induced neural plasticity after acquired brain injury. Neural Plast 2018; 2018:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahl AS, Erlebach E, Brattoli B, et al. Early reduced behavioral activity induced by large strokes affects the efficiency of enriched environment in rats. J Cereb Blood Flow Metab 2018; 17:271678X18777661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones TA, Liput DJ, Maresh EL, et al. Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J Neurotrauma 2012; 29:1455–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moisset X, Lefaucheur JP. Non pharmacological treatment for neuropathic pain: Invasive and noninvasive cortical stimulation. Rev Neurol (Paris) 2018; 175:51–58. [DOI] [PubMed] [Google Scholar]
- 42.Yu KP, Yoon YS, Lee JG, et al. Effects of electric cortical stimulation (ECS) and transcranial direct current stimulation (tDCS) on rats with a traumatic brain injury. Ann Rehabil Med 2018; 42:502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martens KM, Pechacek KM, Modrak CG, et al. Cathodal transcranial direct-current stimulation selectively decreases impulsivity after traumatic brain injury in rats. J Neurotrauma 2019; 10.1089/neu.2019.6470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jefferson SC, Clayton ER, Donlan NA, et al. Cortical stimulation concurrent with skilled motor training improves forelimb function and enhances motor cortical reorganization following controlled cortical impact. Neurorehabil Neural Repair 2016; 30:155–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schönfeld LM, Jahanshahi A, Lemmens E, et al. Motor cortex stimulation does not lead to functional recovery after experimental cortical injury in rats. Restor Neurol Neurosci 2017; 35:295–305. [DOI] [PubMed] [Google Scholar]
- 46.Middleton A, Fritz SL, Liuzzo DM, et al. Using clinical and robotic assessment tools to examine the feasibility of pairing tDCS with upper extremity physical therapy in patients with stroke and TBI: a consideration-of-concept pilot study. NeuroRehabilitation 2014; 35:741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen KS, Chen R. Invasive and noninvasive brain stimulation in Parkinson's disease: clinical effects and future perspectives. Clin Pharmacol Ther 2019; 106:763–775. [DOI] [PubMed] [Google Scholar]
- 48.Chan HH, Wathen CA, Mathews ND, et al. Lateral cerebellar nucleus stimulation promotes motor recovery and suppresses neuroinflammation in a fluid percussion injury rodent model. Brain Stimul 2018; 11:1356–1367. [DOI] [PubMed] [Google Scholar]
- 49.Fluri F, Malzahn U, Homola GA, et al. Stimulation of the mesencephalic locomotor region for gait recovery after stroke. Ann Neurol 2017; 82:828–840. [DOI] [PubMed] [Google Scholar]
- 50.Bachmann LC, Matis A, Lindau NT, et al. Deep brain stimulation of the midbrain locomotor region improves paretic hindlimb function after spinal cord injury in rats. Sci Transl Med 2013; 5:208ra146. [DOI] [PubMed] [Google Scholar]
- 51▪▪.Angeli CA, Boakye M, Morton RA, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 2018; 379:1244–1250. [DOI] [PubMed] [Google Scholar]; This study highlights the therapeutic potential of combining intensive gravity-assisted locomotor training with epidural spinal cord stimulation in motor complete SCI patients. Two of four patients were able to walk over the ground after extensive rehabilitation. Intense locomotor training with manual facilitation of stepping preceded locomotor training combined with stimulation.
- 52▪▪.Darrow D, Balser D, Netoff TI, et al. Epidural spinal cord stimulation facilitates immediate restoration of dormant motor and autonomic supraspinal pathways after chronic neurologically complete spinal cord injury. J Neurotrauma 2019; 36:2325–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]; Report on the ‘Epidural Stimulation After Neurologic Damage clinical trial’ (E-STAND, Trial Number: NCT03026816) that has been designed to investigate the potential of epidural stimulation in a broader SCI population with spared, but nonfunctional descending tracts. Preliminary findings are promising, even without intensive locomotor training prior to electrode implantation.
- 53.Formento E, Minassian K, Wagner F, et al. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat Neurosci 2018; 21:1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Goganau I, Sandner B, Weidner N, et al. Depolarization and electrical stimulation enhance in vitro and in vivo sensory axon growth after spinal cord injury. Exp Neurol 2018; 300:247–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55▪.Gerasimenko Y, Preston C, Zhong H, et al. Rostral lumbar segments are the key controllers of hindlimb locomotor rhythmicity in the adult spinal rat. J Neurophysiol 2019; 122:585–600. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study in rats illustrates the importance of rostral lumbar segments for the initiation and maintenance of locomotion with epidural stimulation after complete thoracic SCI. The results confirm previous observations in human patients showing that the lumbar GCP extends over several, closely interconnected segments of the lumbo-sacral spinal cord. The results have implications for patient recruitment in future clinical trials.
- 56.Capogrosso M, Milekovic T, Borton D, et al. A brain-spine interface alleviating gait deficits after spinal cord injury in primates. Nature 2016; 539:284–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wagner FB, Mignardot JB, Le Goff-Mignardot CG, et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018; 563:65–93. [DOI] [PubMed] [Google Scholar]
- 58.Calvert JS, Grahn PJ, Strommen JA, et al. Electrophysiological guidance of epidural electrode array implantation over the human lumbosacral spinal cord to enable motor function after chronic paralysis. J Neurotrauma 2019; 36:1451–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59▪▪.Inanici F, Samejima S, Gad P, et al. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans Neural Syst Rehabil Eng 2018; 26:1272–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first case report of a chronic cervical SCI patient treated with noninvasive transcutaneous electrical spinal cord stimulation combined with intense upper extremity training for 4 weeks. Hand function and sensation improved, and the improvements were stable over three successive months.
- 60.Yang Q, Ramamurthy A, Lall S, et al. Independent replication of motor cortex and cervical spinal cord electrical stimulation to promote forelimb motor function after spinal cord injury in rats. Exp Neurol 2019; 320:112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen K, Marsh BC, Cowan M, et al. Sequential therapy of anti-Nogo-A antibody treatment and treadmill training leads to cumulative improvements after spinal cord injury in rats. Exp Neurol 2017; 292:135–144. [DOI] [PubMed] [Google Scholar]
- 62.Wahl AS, Omlor W, Rubio JC, et al. Asynchronous therapy restores motor control by rewiring of the rat corticospinal tract after stroke. Science (80–) 2014; 344:1250–1255. [DOI] [PubMed] [Google Scholar]
- 63.Bonizzato M, Pidpruzhnykova G, DiGiovanna J, et al. Brain-controlled modulation of spinal circuits improves recovery from spinal cord injury. Nat Commun 2018; 9:3015. [DOI] [PMC free article] [PubMed] [Google Scholar]


