Abstract
Purpose
To determine whether change in retinal sensitivity in areas with subretinal or intraretinal fluid secondary to age-related macular degeneration (AMD) precedes visual symptoms. If confirmed, retinal sensitivity testing could be used for home monitoring in AMD.
Methods
Individuals with intermediate AMD enrolled in a longitudinal study were seen every 6 months and underwent best-corrected visual acuity testing (BCVA), spectral domain–optical coherence tomography (SD-OCT), and microperimetry. Asymptomatic individuals who developed incidental, reading center–determined retinal fluid detected on SD-OCT were identified. The point-wise sensitivity (PWS) at the time of fluid detection was compared with 6 and 12 months prior.
Results
Fourteen of 161 individuals developed fluid without symptoms. PWS over fluid areas at detection was reduced compared with 6 (difference −2.04 dB, P < 0.001) and 12 months (−2.27 dB, P < 0.001) prior. PWS over fluid areas was reduced compared with perifluid areas (difference −1.02 dB, P = 0.03), peripheral areas (−1.51 dB, P < 0.001), nonprogressed fellow eyes (−1.49 dB, P = 0.006), and nonprogressed age-matched intermediate AMD eyes (−2.29 dB, P = 0.001). No difference in BCVA was observed in eyes developing fluid compared to eyes that do not develop fluid (P = 0.76).
Conclusions
Retinal areas with fluid on SD-OCT had a corresponding reduction in retinal sensitivity at the time of fluid detection compared with 6 and 12 months prior, in asymptomatic intermediate AMD without change in BCVA.
Translational Relevance
Development of self-monitoring tools to detect changes in retinal sensitivity may be helpful for early detection of retinal fluid suggestive of progression to neovascular AMD before acuity is affected.
Keywords: age-related macular degeneration, microperimetry, retinal sensitivity, SD-OCT, retinal fluid
Introduction
Neovascular age-related macular degeneration (nAMD) is a devastating disease that, if left untreated, will usually result in severe, permanent vision loss. The introduction of intravitreal injections of vascular endothelial growth factor inhibitors (anti-VEGF), has dramatically reduced the rate of irreversible vision loss, with rates of legal blindness decreasing by more than half in the past decade.1,2 However, many people do not notice the earliest changes in their vision, delaying presentation. Reports from the United States suggest that 78.5% to 88.3% of nAMD cases present with vision worse than 20/40, where there is often irreversible retinal damage, thereby limiting the ability of treatment to restore vision.3,4 Delay between symptom onset and initiation of treatment as well as poor presenting visual acuity (VA) have been identified as important predictors of final visual outcome after anti-VEGF treatment.5–9 Recently, the Fight Retinal Blindness real world audit reported that second eyes developing nAMD (mean VA of 61.2 letters; 20/63) present with a mean VA of approximately 12 letters better than the first eye that developed nAMD (mean VA of 49.7 letters; 20/100).10 It is likely this improved vision on presentation of the second eye is due to earlier detection, either as a result of more frequent review, or earlier recognition of vision loss by the individual. It is vitally important that we develop better ways to identify and monitor people with the earliest signs suggestive of nAMD as earlier identification and appropriate treatment with anti-VEGF therapy will impact greatly on the overall long-term visual outcomes. Because clinical follow-up of all patients with intermediate AMD (iAMD) at time intervals of less than 6 monthly is not practical; more effective home-monitoring tools need to be developed.
People with iAMD are at risk of developing nAMD, and are usually advised to self-monitor at home on a weekly basis using an Amsler grid to detect the onset of distortion or blur.11–13 While being used for many decades, the Amsler chart has been demonstrated to lack sensitivity and compliance.14,15 Newer home monitoring tools using hyperacuity measurements (e.g., ForeseeHome device and MyVisionTrack application), have shown some success with earlier detection of nAMD in clinical trials.16,17 However, there has not yet been widespread uptake of these acuity based home monitoring tools and they still rely on a detectable change in vision. Intraretinal fluid (IRF) and subretinal fluid (SRF) have been identified as being early and prominent signs of exudation associated with nAMD, and surrogate markers for the presence of excess VEGF, which drives neovascular tissue development.18–20 Potentially, there may be an early alteration in retinal sensitivity, as IRF and SRF accumulate, that might be able to be detected in a self-monitoring tool, perhaps even before a detectable change in acuity. If so, then closer monitoring and earlier intervention might be possible, assuring a better long-term visual outcome.12,21,22
Clinic-based microperimetry is a more sensitive measure of functional deficit than VA in iAMD.23–25 Longitudinal observation of individuals with iAMD who had changed clinical features showed corresponding changes in retinal sensitivity without changes in VA.26 Furthermore, microperimetric functional deficits are known to correspond to multimodal imaging (MMI) biomarkers of AMD, including increased fundus autofluorescence,27 outer segment thinning on spectral-domain optical coherence tomography (SD-OCT),28 pigment epithelial thickening and elevation on SD-OCT, and disruption of the second hyperreflective band on SD-OCT.29–31 One report suggests that a functional decline can precede progression to macular neovascularization (MNV) and geographic atrophy (GA) by several months,32 and it is known that retinal sensitivity change is associated with SRF and IRF identified with SD-OCT.33 However, it is currently unknown whether microperimetry functional deficits associated with SRF or IRF can be detected before the person develops any visual symptoms or loss of VA. It is also unknown if any change in sensitivity precedes the first signs of fluid detected with SD-OCT.
The aim of the study was to determine whether there is a reduction in retinal sensitivity in areas that develop SRF or IRF, implying a high likelihood of exudative MNV, before there are any visual symptoms or drop in VA. We also wished to determine if any change in sensitivity could be detected at visits preceding detection of the fluid. To do this, we prospectively followed a cohort of iAMD patients, all having MMI, microperimetry testing, and reading center evaluations every 6 months.
Methods
Study Cohort
Participants with iAMD, who were part of a 36-month longitudinal cohort study in the Macular Research Unit at the Centre for Eye Research Australia enrolled between 2012 and 2015, were eligible for this substudy. The study was approved by the Human Research Ethics Committee of the Royal Victorian Eye and Ear Hospital and was conducted according to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants after explanation of the nature and possible consequences of the study.
Inclusion criteria consisted of individuals aged 50 years or older with bilateral large drusen (>125 μm); consistent with the Beckman classification of iAMD.34 Best-corrected visual acuity (BCVA) read on the standard Early Treatment Diabetic Retinopathy Study (ETDRS) chart was required to be 70 letters (20/40) or better in both eyes.
Exclusion criteria included late AMD (GA or MNV) or any anatomic features recognized on MMI as signs that portend the development of late atrophic AMD.35–37 These included SD-OCT evidence of nascent atrophy,35,36 or fundus autofluorescence (FAF)-defined atrophy.37 Additional ocular exclusions included cataracts (≥2 in the World Health Organization Cataract Grading Scheme),38 glaucoma, amblyopia, or any corneal pathology. Individuals with neurologic or systemic conditions and those taking medications known to affect vision or retinal evaluation were excluded as were participants with cognitive deficits. Individuals who repeatedly scored a false-positive rate of more than 25% on microperimetry testing of retinal sensitivity during screening were excluded from the study.
Visit Schedule
At intervals of every 6 months (±1 month) over a follow-up period of 36 months the following testing was performed: history regarding symptomatic changes in vision (including self-monitored Amsler grid), BCVA, Amsler grid testing, clinical examination, functional assessment with microperimetry, and MMI, including SD-OCT. Baseline assessment additionally included review of systemic, ocular, and family history. As part of the longitudinal study, participants were asked to home monitor for symptoms of visual changes using the Amsler grid weekly. In the event of new ocular symptoms reported to the study team, participants were reviewed on an ad-hoc basis to exclude new ocular pathology.
Multimodal Imaging
Near-infrared reflectance (NIR), SD-OCT, and short-wavelength FAF were performed using a Spectralis HRA+OCT (Heidelberg Engineering, Heidelberg, Germany) over a 30° × 30° area centered on the macula. SD-OCT volume scans were taken using 49 horizontal B-scans, 120 μm apart over 20° × 20° area centered on the macula, with automatic real time (ART) equal to 25 frames averaged for each B scan. The automatic scan alignment feature was used for follow-up scans. Nonstereoscopic posterior pole (macula and disc centered) digital color fundus photography was performed using the CR6-5NM nonmydriatic retinal camera (Canon Inc., Saitama, Japan) with a minimum resolution of 2000 × 2000 pixels.
Microperimetry
Microperimetry was performed using the Macular Assessment Integrity Analyzer (MAIA; CenterVue, Padova, Italy) microperimeter with our customized AMD 6° grid39 (Fig. 1). The AMD 6° grid consists of 37 test points at 0°, 1°, 2.33°, 4°, and 6° from fixation allowing regular measurement within the macula with a slight increased density proximal to the fovea. A red circular fixation target of 1° diameter and Goldman III stimuli were presented against a background of 1.27 cd/m2 with a 4-2 threshold strategy. The maximum stimulus luminance was 318 cd/m2, which give a dynamic range of 36 dB.39 Continuous fundus tracking allowed presentation of stimuli at the same retinal location throughout the test, and hence enabled precise measurement of individual retinal regions. A false-positive rate of less than 25% was classified as reliable (i.e., no participant had a false-positive rate >25%), with the second of two back-to-back reliable tests being used for the threshold values. The second test was conducted a few minutes after the first test at each visit to minimize effects of fatigue. The outcome parameter was point-wise sensitivity (PWS), which is the threshold of each individual test point. Room illumination was switched off immediately to ensure equivalent levels of light exposure prior to testing. Functional testing was performed prior to any investigations capable of significantly bleaching photoreceptors.
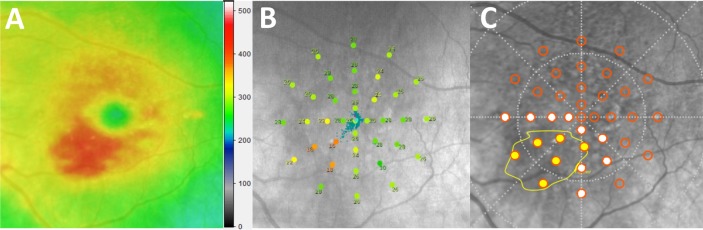
Figure 1.
(A) Retinal thickness heat map generated by the Eye Explorer software (Heidelberg Engineering) demonstrating an area of elevation (red) secondary to retinal fluid at the inferior nasal retina of a left eye. False-color scale bar shows retinal thickness (μm). (B) NIR scout image with overlaid retinal sensitivity results from the MAIA. The AMD 6° grid is colored to denote varying threshold measurement values (dB) showing the area of low sensitivity is associated with retinal fluid. (C) Relationship between the fluid location (yellow boundary) and the location of the sensitivity testing (orange circles). Points falling within or on the area demarcated by the yellow boundary were classified as co-located with fluid (yellow-filled test points), those immediately surrounding were classified as perifluid (white-filled test points) and those further removed were classified as peripheral to fluid.
Grading
All MMI images were graded in the reading center for AMD status according to the Beckman criteria. Any participant noted to have changes suggestive of progression to advanced AMD was flagged and images reviewed by clinicians within 1 week. Participants identified at any review as having developed incidental SRF or IRF on SD-OCT, were reviewed by senior retinal specialists and fluorescein and indocyanine green angiography was performed to determine if there was exudative MNV present.
Analysis
Eyes analyzed in the substudy were allocated into one of three groups after MMI grading; ‘progressor eyes' that developed asymptomatic SRF or IRF on SD-OCT, ‘fellow eyes' that did not develop any signs of late-stage disease at any time, and ‘nonprogressor eyes', matched for age, sex, and smoking status with the progressor group, that also did not develop signs of late-stage disease at any time. Only the first eye of an individual developing fluid detected with SD-OCT was included. Participants that reported symptoms (distortion, central blur, difficulty reading), either proactively or upon direct questioning, suggestive of exudative complications were excluded, so as to only examine asymptomatic cases of fluid that were picked up incidentally by the reading center.
Data at the time of fluid detection (“time of detection”), and also at the preceding 6- and 12-month time points (if available), were included in the analyses. In the nonprogressor group, an arbitrary “time of detection” was chosen as the visit where the participants' age most closely matched the age of the corresponding progressor at the time of fluid detection.
Identification of MAIA Test Points Affected by Pathology
Areas of SRF or IRF were mapped objectively on the Spectralis NIR scout image using inbuilt software (Fig. 1). The corresponding MAIA NIR image with the AMD 6° grid was manually overlaid on the Spectralis NIR image using the anatomic landmarks and retinal vessels to ensure correct positioning. As both the Spectralis and the MAIA use fundus tracking, the colocalization of NIR maps can be performed with confidence. In progressor eyes, the 37 test points making up the grid were classified as either falling (1) within, (2) adjacent (perifluid), or (3) peripheral to areas of fluid. If any part of a test point overlapped an area of fluid, they were classified as falling within an area of pathology. Points were classified as perifluid when they formed part of a circumferential perimeter around an area of fluid with the tightest fit possible (Fig. 1C). Points further peripheral to those considered perifluid were designated as peripheral. The PWS was determined for each region (fluid, perifluid, peripheral to fluid) of progressor eyes, as well as for all test locations in the grid in fellow eyes and nonprogressor eyes.
Statistics and Analysis
Baseline demographic characteristics between progressor and nonprogressor groups was performed using either two sample t-test (two-tailed) for continuous data or Fisher's exact test (two-tailed) for categoric data. Examination of longitudinal changes in microperimetry PWS were performed using the linear mixed-effects model with the fluid regions as the fixed effect and test points nested within an eye as a random effect, and age and smoking status as a covariate. Longitudinal changes in BCVA were performed using a mixed-effects piecewise linear-regression model with random intercepts and slope, accounting for correlation within eye and participant and adjusted for age and smoking status. Statistical significance was set at 0.05 for all tests. Statistical analyses were performed using Stata/MP version 14.0 (StataCorp LLP, College Station, TX).
Results
Demographic, Clinical, and Review Parameters of Groups
One-hundred sixty-one participants (322 eyes) were enrolled in the longitudinal AMD study and followed for 36 months. Of these, 16 eyes of 16 participants developed fluid detected with SD-OCT, but two participants were excluded from further analysis; one because the individual alerted the study staff to a sudden drop in vision in the preceding 2 weeks and the other case because the individual's fellow eye had already developed nAMD. The remaining 14 eyes developed fluid (progressors) over the follow-up period and were analyzed along with 14 eyes of 14 participants matched for age, sex, and smoking status, who had not progressed (nonprogressors). The SRF or IRF was detected at routine reading center review in all 14 individuals with no one describing symptoms at time of fluid detection (distortion, blur, reduced reading ability), even upon direct questioning. None were able to identify a defect on Amsler grid when tested in the clinic. Nine fellow nonprogressing eyes of the progressors were included for comparison, the five remaining fellow eyes were excluded for having been found to have progressed to later-stage disease (1 with geographic atrophy, 1 with nascent geographic atrophy, 3 with retinal fluid) during the follow-up period. None of the participants received anti-VEGF treatment during the course of the study.
Three participants in the progressor group had fluid detected at their first 6-month review after their baseline examination so data were not available 12 months prior to fluid detection for analysis, therefore just the “time of detection” and 6-month prior data were analyzed for these subjects. Demographic, clinical, and details relating to follow-up periods are summarized in Table 1. There was no significant difference between the progressor and nonprogressor group for age (P = 0.98), smoking status (P =0.46), and review intervals prior to detection (6 month to time of detection interval, P = 0.42; 12- to 6-month interval, P = 0.30).
Table 1.
Demographic and Clinical Characteristics of the Study Participants
| Study Group |
P Value* |
||
| Progressors (N = 14) |
Nonprogressors (N = 14) |
||
| Eyes, n (%) | |||
| Study eye | 14 (100) | 14 (100) | |
| Fellow eye | 9 (64) | 0 | |
| Data Available, n (%) | |||
| At time of fluid detection | 14 (100) | 14 (100) | |
| 6 months prior to fluid detection | 14 (100) | 14 (100) | |
| 12 months prior to fluid detection | 11 (79) | 14 (100) | |
| Age (mean ± SD) | 66.9 ± 7.0 | 66.9 ± 7.0 | 0.98 |
| Female sex, n (%) | 14 (100) | 14 (100) | 1.00 |
| Smoking status, n (%) | |||
| Never smoked | 8 (57) | 8 (57) | 0.46 |
| Exsmoker | 6 (43) | 4 (29) | |
| Current smoker | 0 (0) | 2 (14) | |
| Time in months between reviews, mean ± SD | |||
| −6 month to time of detection | 6.5 ± 0.8 | 6.2 ± 0.7 | 0.42 |
| −12- to −6-month period | 6.3 ± 0.6 | 6.0 ± 0.5 | 0.30 |
| Subretinal fluid detection at routine follow-up, n (%) | 14 (100) | 0 (0) | |
| Symptomatic at time fluid detection, n (%) | 0 (0) | NA | |
P values derived via two-sample t-tests for continuous data and Fisher's exact test for categoric data.
Longitudinal Changes in Best-Corrected Visual Acuity
No significant difference in BCVA was observed between the progressor study eyes and the nonprogressor eyes (P = 0.76, see Table 2) or the progressor fellow eyes and the nonprogressor eyes (P = 0.31) at fluid detection or at any other time. There was no significant change in BCVA within the progressor study eyes at the time of fluid detection in comparison to the review 6 months prior (P = 0.85, see Table 2 and Fig. 2).
Table 2.
Mean BCVA (Number of Letters) of the Nonprogressor Group and Study Eyes and Fellow Eyes of the Progressor Groupa
| Study Group |
||||||||
| Nonprogressors | Progressors |
|||||||
| (n = 14) |
Fellow Eye (n = 9) |
Study Eye (n = 14) |
||||||
| Mean |
[95%CI] |
Mean |
[95%CI] |
ΔPb |
Mean |
[95%CI] |
ΔPb |
|
| At fluid detection | 88.8 | 86.6–91.0 | 90.5 | 87.7–93.3 | 0.31 | 88.3 | 86.1–90.5 | 0.76 |
| 6-months predetection | 90.3 | 88.1–92.5 | 90.8 | 88.0–93.6 | 0.75 | 88.6 | 86.4–90.8 | 0.24 |
| 12-months predetectionc | 89.5 | 87.3–91.7 | 88.4 | 85.3–91.6 | 0.56 | 87.6 | 85.3–90.0 | 0.23 |
Estimated using a mixed-effects piecewise linear regression model with random intercepts and slope, accounting for correlation within eye and participant and adjusted for age and smoking status.
ΔP represents the P value for the mean difference in progressor groups compared with the study eye of nonprogressors.
No data available at this visit for 3 fellow eyes and 3 study eyes of progressors.
Figure 2.
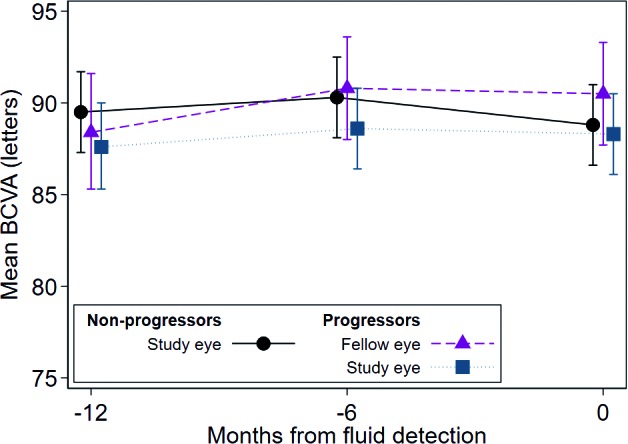
Longitudinal changes in mean BCVA (letters) of nonprogressor and progressor groups for three visits shows no change in mean BCVA over time for any group. Estimated using mixed-effects piecewise linear regression model with random intercepts, accounting for correlation within eye and participant and adjusted for age and smoking status. Error bars indicate 95%CI. See Table 2 for statistical analysis. Note that individual data points have a slight spread at the three longitudinal time points so that each data point is visible. All data points were obtained at the time of fluid detection and at 6 and 12 months prior.
Topographic Variances in Microperimetric Sensitivity
The PWS (±95%CI) for the different locations within the progressor eyes (fluid, perifluid, and peripheral-to-fluid) along with the PWS for the fellow eyes and the nonprogressor eyes are shown in Table 3 and Figure 3. At the time of incidental SD-OCT fluid detection, all retinal locations with fluid (n = 51 points on the MAIA grid across 14 eyes) showed significantly worse PWS than those test locations, which had not developed fluid (including regions within the same eye adjacent [peri-] or peripheral to the fluid, as well as fellow eyes and nonprogressor eyes) as shown in Table 3 and Figure 3. The nonprogressor eyes had the highest PWS at the time of fluid detection in comparison to the fluid region and differed by 2.29 dB (P = 0.001). The PWS of the fellow eyes and the peripheral-to-fluid region differed from the fluid region by similar extents; 1.49 dB (P = 0.006) and 1.51 dB (P < 0.001), respectively. Sensitivity in the perifluid region differed from the fluid region by 1.02 dB (P = 0.03), the least of any nonfluid region. At both 6 and 12 months prior to detection of fluid, there is no significant difference in retinal sensitivity between fluid and nonfluid locations (Table 3). Examples of localized decreases in retinal sensitivity associated with fluid detected on SD-OCT compared with 6 months prior are shown in Figures 4 and 5.
Table 3.
Mean Pointwise Sensitivity (dB) Across Groups and Time Pointsa
| Study Group | Nonprogressors |
Progressors |
||||||
| Eye | Study Eye (n = 14) |
Fellow Eye (n = 9) |
||||||
| Area of Retina | No Fluid |
No Fluid |
||||||
| Mean |
[95%CI] |
P |
Mean |
[95%CI] |
P |
|||
| Fluid detection visit | 26.23 | 25.29 | 27.18 | 25.44 | 24.37 | 26.51 | ||
| Difference from previous visit | 0.18 | −0.07 | 0.43 | 0.16 | −0.43 | −0.75 | −0.12 | 0.007 |
| Difference from 12 months predetectionb | −0.26 | −0.51 | −0.00 | 0.048 | −1.51 | −1.89 | −1.14 | <0.001 |
| Difference from points with fluid (progressor eye) | 2.29 | 0.91 | 3.66 | 0.001 | 1.49 | 0.42 | 2.56 | 0.006 |
| 6 months predetection | 26.05 | 25.11 | 27.0 | 25.88 | 24.81 | 26.95 | ||
| Difference from previous visit | −0.44 | −0.69 | −0.19 | 0.001 | −1.08 | −1.45 | −0.71 | <0.001 |
| Difference from points with fluid (progressor eye) | 0.06 | −1.31 | 1.43 | 0.93 | −0.11 | −1.18 | 0.96 | 0.84 |
| 12 months predetectionb | 26.49 | 25.55 | 27.43 | 26.96 | 25.87 | 28.04 | ||
| Difference from points with fluid (progressor eye) | 0.28 | −1.18 | 1.73 | 0.71 | 0.74 | −0.46 | 1.93 | 0.23 |
Estimated using a mixed-effects piecewise linear regression model with random intercepts, accounting for correlation within eye, participant and stimulus location, and adjusted for age and smoking status.
No data at this visit for 3 fellow eyes and 3 study eyes of progressors.
Bolded numbers indicate P < 0.05.
Figure 3.
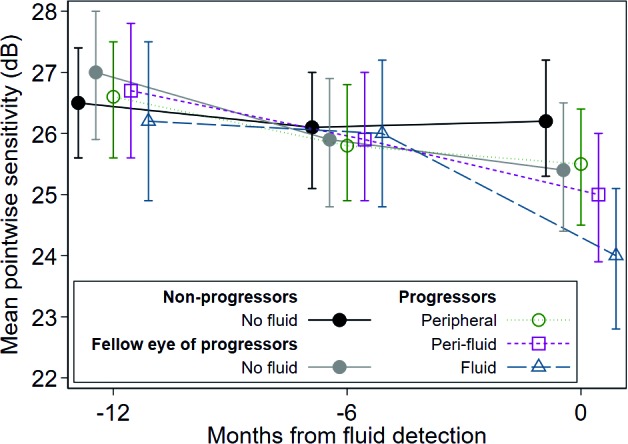
Mean pointwise sensitivity estimated using a mixed-effects piecewise linear regression model with random intercepts, accounting for correlation within eye, participant and stimulus location, and adjusted for age and smoking status. Note the greatest decrease in sensitivity demonstrated by areas overlying fluid at time of fluid detection. Error bars indicate 95%CI. See Table 3 for statistical analysis. Note that individual data points have a slight spread at the three longitudinal time points so that each data point is visible. All data points were obtained at the time of fluid detection and at 6 and 12 months prior.
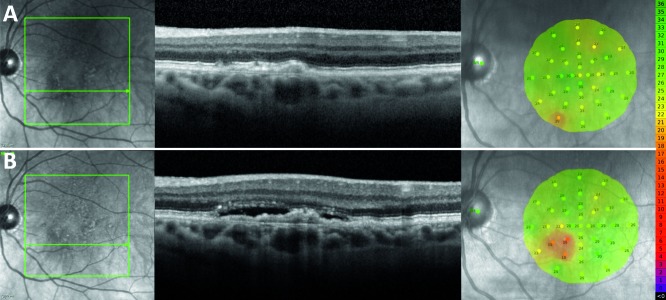
Figure 4.
Representative finding from a 70-year-old female study participant who developed SRF inferonasally to the fovea in their left eye. (A) NIR scout image, SD-OCT B-scan, and MAIA findings 6 months prior to development of SRF. (B) NIR, SD-OCT, and MAIA at the time of SRF detection. Microperimetry scale of decibel values and corresponding colors is shown on the right.
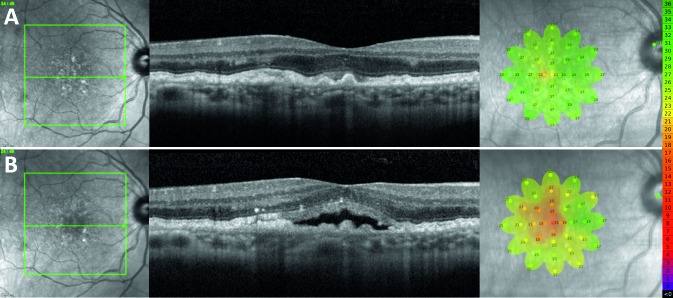
Figure 5.
Representative finding from a 64-year-old female study participant who developed SRF centrally in their right eye. (A) NIR scout image, SD-OCT B-scan, and MAIA findings 6 months prior to development of SRF. (B) NIR, SD-OCT, and MAIA at the time of SRF detection. Microperimetry scale of decibel values and corresponding colors is shown on the right.
Table 3.
Extended
| Study Group | Progressors |
|||||||
| Eye | Study Eye (n = 14) |
|||||||
| Area of Retina | Peripheral to Fluid |
Perifluid |
||||||
| Mean |
[95%CI] |
P |
Mean |
[95%CI] |
P |
|||
| Fluid detection visit | 25.46 | 24.52 | 26.42 | 24.97 | 23.93 | 26.01 | ||
| Difference from previous visit | −0.35 | −0.65 | −0.04 | 0.03 | −0.96 | −1.51 | −0.42 | 0.001 |
| Difference from 12 months predetectionb | −1.11 | −1.44 | −0.78 | <0.001 | −1.71 | −2.33 | −1.11 | <0.001 |
| Difference from points with fluid (progressor eye) | 1.51 | 0.71 | 2.32 | <0.001 | 1.02 | 0.12 | 1.91 | 0.03 |
| 6 months predetection | 25.81 | 24.86 | 26.76 | 25.93 | 24.89 | 26.97 | ||
| Difference from previous visit | −0.77 | −1.10 | −0.44 | <0.001 | −0.75 | −1.36 | −0.14 | 0.02 |
| Difference from points with fluid (progressor eye) | −0.17 | −0.98 | 0.63 | 0.67 | −0.06 | −0.95 | 0.84 | 0.90 |
| 12 months predetectionb | 26.58 | 25.62 | 27.54 | 26.70 | 25.62 | 27.76 | ||
| Difference from points with fluid (progressor eye) | 0.36 | −0.59 | 1.31 | 0.46 | 0.47 | −0.59 | 1.53 | 0.39 |
Table 3.
Extended
| Study Group | Progressors |
|||
| Eye | Study Eye (n = 14) |
|||
| Area of Retina | Fluid |
|||
| Mean |
[95%CI] |
P |
||
| Fluid detection visit | 23.95 | 22.77 | 25.13 | |
| Difference from previous visit | −2.04 | −2.85 | −1.23 | <0.001 |
| Difference from 12 months predetectionb | −2.27 | −3.22 | −1.31 | <0.001 |
| Difference from points with fluid (progressor eye) | ||||
| 6 months predetection | 25.99 | 24.81 | 27.17 | |
| Difference from previous visit | −0.23 | −1.12 | 0.73 | 0.64 |
| Difference from points with fluid (progressor eye) | ||||
| 12 months predetectionb | 26.21 | 24.94 | 27.50 | |
| Difference from points with fluid (progressor eye) | ||||
Microperimetric Sensitivity Prior to Onset of SD-OCT Fluid
The difference in retinal sensitivity between the time of fluid detection and 6 and 12 months prior was calculated to determine if retinal sensitivity changes can be detected at the onset of retinal fluid, or even potentially prior to the detection of fluid with SD-OCT. At the time of fluid detection relative to 6 months prior, there was a reduction in retinal sensitivity at the fluid, perifluid, and peripheral-to-fluid regions, as well as in the fellow eyes (Table 3). Notably, the reduction in PWS at the fluid region at the time of fluid detection relative to 6 months prior (−2.04 dB, P < 0.001; Table 3) was larger than the reduction in PWS at the perifluid region (−0.96 dB, P = 0.001), peripheral region (−0.35 dB, P = 0.03), or in the progressor fellow eyes (−0.43 dB, P = 0.007). The nonprogressor eyes did not show change in sensitivity (0.18 dB, P = 0.16) in the 6 months prior to fluid detection. All groups demonstrated a significant decline in sensitivity at the time of fluid detection relative to 12 months prior, although nonprogressor eyes showed the least change (PWS decline of −0.26 dB, P = 0.048) and fluid regions showed the most change (−2.27 dB, P < 0.001; Table 3). The change in nonprogressor eyes indicates a slow reduction in retinal sensitivity over 12 months even in participants who do not develop fluid in either eye during the study period.
At 6 months prior to the detection of fluid, no change in PWS (−0.23 dB, P = 0.64) relative to 12 months prior was detected in the regions destined to develop fluid in another 6 months' time. However, all other groups showed a reduction in retinal sensitivity at 6 relative to 12 months prior to fluid detection (Table 3). The smallest change was in the nonprogressor eyes (−0.44 dB, P = 0.001), while the largest changes were in the remaining progressor groups; the fellow eyes (−1.08 dB, P < 0.001), the peripheral-to-fluid region (−0.77 dB, P < 0.001), and the perifluid region (−0.75 dB, P = 0.02).
Discussion
The aim of this study was to determine if there were changes in retinal sensitivity in areas overlying incident fluid in iAMD, prior to any visual symptoms suggestive of active exudative MNV. The key new finding from this study was that in asymptomatic iAMD eyes, the retinal sensitivity of areas with SRF or IRF detected by SD-OCT was significantly lower than the retinal sensitivity of (1) areas within the same eyes not associated with the fluid, (2) their nonprogressed fellow eyes, and (3) eyes matched for age, sex, and smoking status with iAMD, which did not develop fluid (nonprogressors). Critically, we have shown that microperimetry can detect a regional functional change in retinal sensitivity in participants with fluid in a presymptomatic stage, prior to both any change in BCVA or Amsler grid testing. Previous investigation by ourselves and others into intersession variability of the MAIA microperimeter has demonstrated a high degree of repeatability and consistency of fixation between tests.26,39,40 The reduced regional retinal function was measured by microperimetry prior to any symptoms of visual change that were either self-reported or noted upon direct questioning at visits every 6 months. Previous studies41,42 have reported reduced areas of sensitivity in clinically apparent nAMD, and improved function after anti-VEGF treatment, but our study was looking only at cases that did not have typical symptoms of nAMD.
The findings are important as they imply that it may be possible to use a measure of retinal sensitivity to detect the onset of retinal fluid (which is suggestive of nAMD) before patients report visual symptoms or lose BCVA. It has been assumed that in many cases of nAMD the neovascular leak occurs acutely and is an emergency event needing urgent intervention. This is because clinicians often see people present with “sudden” distortion, blur, or loss of vision and it has been assumed to be a recent, profound development. Our results indicate, that at least in some people, or perhaps the majority (15/16 in our study) with iAMD, that there is an opportunity to detect the first signs of likely exudation before there are any new visual symptoms or vision loss. This then also suggests that with regard to self-monitoring, the ability to detect a drop in retinal sensitivity may be another sensitive parameter to test. The average reduction in sensitivity in fluid regions at the time of detection (PWS differences of −2.04 and −2.27 dB compared with 6 and 12 months prior, respectively) was observed as statistically significant despite falling within the coefficient of repeatability (±4.12 dB) reported previously.39 It should be noted the coefficient of repeatability shown previously and the regional and longitudinal differences in retinal sensitivity shown in the current study represent different parameters. The coefficient of repeatability shows the variation in sensitivity when doing the test twice on 1 day, comparing all areas within a test, in participants who have never performed MAIA previously. The current study shows a reduction in sensitivity within a specific retinal region over time periods of 6 to 12 months in participants already very familiar with performing MAIA.
As part of this study, all participants were reminded to observe an Amsler grid weekly, but none of the 14 included, reported a defect at the time of detecting fluid. The findings also suggest there may be a period of time following the first detection of a sensitivity drop and accompanying fluid detection with SD-OCT, that a more frequent review could be the most appropriate course of management rather immediate treatment. Indeed, close monitoring of these people for signs of disease progression will quickly identify those where treatment becomes necessary.
These findings re-emphasize that a change in BCVA and patient symptomology are not necessarily very early indicators of the onset of exudation.10,18,43 It has been established that initiation of treatment when BCVA remains good leads to the best long-term visual outcome.9,44 Yet, it still remains that vision is often lost before presentation.10 If it was possible to self-monitor retinal sensitivity routinely it could lead to the earlier detection of disease progression to exudative complications. Our preliminary work with the Psypad application, presenting a sensitivity test on a tablet device, used in the home has shown some promise in this regard.45,46 Nevertheless, the sensitivity and specificity of the self-testing tool of retinal sensitivity in detecting the development of retinal fluid needs to be further evaluated.
Of further interest was the pattern of sensitivity change displayed by different regions over time. Within the progressor group, all tested regions that did not develop fluid, including the fellow eye, showed a decline in PWS over the 12-months period before exudation was detected. Such a longitudinal decrease in sensitivity in individuals with iAMD, of similar magnitude, has been reported by our group previously.26 This incremental longitudinal decline in sensitivity was not seen in the actual regions, which went on to develop fluid; these regions only exhibited a decrease in PWS over the 6-month period immediately prior to the fluid detection, with no drop off in sensitivity in the preceding 6 months (6- to 12-month interval prior to fluid detection). Given that areas, which develop exudation likely constitute regions of worst disease (from hypoxia due to upregulation of angiogenic mediators such as VEGF),47–50 this lack of longitudinal sensitivity decline was unexpected. It is possible that initially, the angiogenic drive delivers nonexudative abnormal choroidal vessels to the area providing a level of nutrients and oxygen that is not seen in surrounding less hypoxic areas. This nutritional supply may then keep retinal function preserved, which we observed as maintained sensitivity, prior to development of exudation. Our findings of no difference in retinal sensitivity between 6 and 12 months prior to fluid detection in the fluid region is consistent with Querques et al.51 who reported no reduction in microperimetric retinal sensitivity in the region of a nonexudative “quiescent” MNV. The recent availability of noninvasive OCT angiography (OCTA) to closely monitor iAMD cases at high risk of nAMD will enable the time course of MNV development and exudation to be determined. Indeed, Rosenfeld et al.18 have demonstrated with OCTA that nonexudative MNV can be detected in cases that would otherwise be considered as typical iAMD. Further investigation will be required to determine whether new onset of loss of regional sensitivity, in the absence of any SD-OCT signs of exudative disease, may also portend the imminent development of nAMD.
Over the 12-month review period all progressor regions, including the fellow eye, displayed a greater magnitude of decline in sensitivity in comparison to the nonprogressor eyes, suggestive that collectively the progressors possessed factors, which lead to accelerated impairment of function. The development and progression of AMD is a multifactorial process, but understanding which variables contribute the greatest predilection toward developing late disease has not been definitively established.52–54 Further investigation of the subset of individuals noted to display hastened impairment of function using microperimetry, may allow for greater insight into factors involved with AMD pathogenesis and progression.
Strengths of this study include the large homogeneous cohort with a unique dataset. This cohort, all with bilateral large drusen, had regular review every 6 months with extensive MMI and functional testing at each visit. Grading center review of all images at each visit ensured a high degree of confidence that cases of progression of disease at each time point were detected. As a consequence, we have a unique cohort where retinal function has been determined at the time of first detection of asymptomatic retinal fluid and also at the preceding 6- and 12-month visits. The prospective, longitudinal nature of this study made it possible to measure changes in sensitivity over worsening stages of disease where previously this has only been done in cases that remained phenotypically stable,26 or after overt MNV had developed and been treated.55,56 Fundus tracking MAIA microperimetry allowed the same retinal areas to be remeasured accurately, and also allowed differentiation between areas over fluid to those adjacent and peripheral to the fluid with great accuracy.
Limitations of this study included the 6-month intervals between reviews such that the asymptomatic fluid may have been present for days or months prior to its routine detection, making it impossible to determine the exact time line between fluid accumulation and sensitivity decreases. Furthermore, from 166 participants in the total cohort, only 16 (∼10%) developed incidental fluid detected on SD-OCT. A larger sample size of cases progressing to develop fluid would allow some subanalysis of fluid location and effect on sensitivity. A further limitation is the size Goldmann III stimuli, which is the only size stimulus available in the MAIA at this time. Recently, it has been found that test sizes within spatial summation improve functional sensitivity57 (Phu J, et al. IOVS. 2016;57:ARVO E-Abstract). Hence, it is possible that larger deficits in sensitivity associated with fluid may have been found with a smaller stimulus. However, most of the test points were well within the area of fluid (except for a small number at the border), thus a decrease in stimulus size may not improve the sensitivity of the detection in this study. In conclusion, localized retinal functional deficits associated with the development of asymptomatic, SD-OCT–defined retinal fluid secondary to AMD have been observed through use of microperimetry. These changes preceded changes in BCVA and symptomology suggesting that microperimetry could be used as a parameter to self-monitor and detect the first evidence of potential nAMD. This in turn could initiate an earlier and more regular review, with time to develop a monitoring and treatment plan, which would begin before there was any loss of vision, thus leading to better long-term visual outcomes than currently seen with nAMD.
Acknowledgments
The authors thank Elizabeth Baglin, Pyrawy Sivarajah, Maria Kolic, and Galina Makeyeva for technical assistance collecting the clinical and microperimetry data, and Khin Zaw Aung and Lauren Hodgson for grading of retinal images and Nicole Tindill for data extraction assistance.
Supported by National Health and Medical Research Council (NHMRC) Fellowship (#1103013, RHG) and NHMRC Project Grant (1084081). The Centre for Eye Research Australia (CERA) receives operational infrastructure support from the Victorian State Government.
Disclosure: A.J. Wightman, None; C.J. Abbott, None; M.B. McGuinness, None; E. Caruso, None; R.H. Guymer, None; C.D. Luu, None
References
- 1.Bloch SB, Larsen M, Munch IC. Incidence of legal blindness from age-related macular degeneration in Denmark: year 2000 to 2010. Am J Ophthalmol. 2012;153:209–213. doi: 10.1016/j.ajo.2011.10.016. e202. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P, Bressler N, Doan QV, et al. Estimated cases of blindness and visual impairment from neovascular age-related macular degeneration avoided in Australia by ranibizumab treatment. PLoS One. 2014;9:e101072. doi: 10.1371/journal.pone.0101072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fong DS, Custis P, Howes J, Hsu J-W. Intravitreal bevacizumab and ranibizumab for age-related macular degeneration: a multicenter, retrospective study. Ophthalmology. 2010;117:298–302. doi: 10.1016/j.ophtha.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Olsen TW, Feng X, Kasper TJ, Rath PP, Steuer ER. Fluorescein angiographic lesion type frequency in neovascular age-related macular degeneration. Ophthalmology. 2004;111:250–255. doi: 10.1016/j.ophtha.2003.05.030. [DOI] [PubMed] [Google Scholar]
- 5.Finger RP, Wickremasinghe SS, Baird PN, Guymer RH. Predictors of anti-VEGF treatment response in neovascular age-related macular degeneration. Surv Ophthalmol. 2014;59:1–18. doi: 10.1016/j.survophthal.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Ying G-S, Huang J, Maguire MG, et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology. 2013;120:122–129. doi: 10.1016/j.ophtha.2012.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim JH, Wickremasinghe SS, Xie J, et al. Delay to treatment and visual outcomes in patients treated with anti-vascular endothelial growth factor for age-related macular degeneration. Am J Ophthalmol. 2012;153:678–686. doi: 10.1016/j.ajo.2011.09.013. e672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyer DS, Antoszyk AN, Awh CC, et al. Subgroup analysis of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:246–252. doi: 10.1016/j.ophtha.2006.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Tufail A, Xing W, Johnston R, et al. The neovascular age-related macular degeneration database: multicenter study of 92 976 ranibizumab injections report 1: visual acuity. Ophthalmology. 2014;121:1092–1101. doi: 10.1016/j.ophtha.2013.11.031. [DOI] [PubMed] [Google Scholar]
- 10.Barthelmes D, Walton RJ, Arnold JJ, et al. Intravitreal therapy in bilateral neovascular age-related macular degeneration. Ophthalmology. 2014;121:2073–2074. doi: 10.1016/j.ophtha.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Marmor MF. A brief history of macular grids: from Thomas Reid to Edvard Munch and Marc Amsler. Surv Ophthalmol. 2000;44:343–353. doi: 10.1016/s0039-6257(99)00113-7. [DOI] [PubMed] [Google Scholar]
- 12.Trevino R, Kynn MG. Macular function surveillance revisited. Optometry. 2008;79:397–403. doi: 10.1016/j.optm.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 13.Amsler M. Earliest symptoms of diseases of the macula. Br J Ophthalmol. 1953;37:521. doi: 10.1136/bjo.37.9.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fine AM, Elman MJ, Ebert JE, Prestia PA, Starr JS, Fine SL. Earliest symptoms caused by neovascular membranes in the macula. Arch Ophthalmol. 1986;104:513–514. doi: 10.1001/archopht.1986.01050160069013. [DOI] [PubMed] [Google Scholar]
- 15.Razavi H, Baglin E, Sharangan P, et al. Gaming to improve vision: 21st century self-monitoring for patients with age-related macular degeneration. Clin Exp Ophthalmol. 2018;46:480–484. doi: 10.1111/ceo.13097. [DOI] [PubMed] [Google Scholar]
- 16.Chew EY, Clemons TE, Bressler SB, et al. Randomized trial of a home monitoring system for early detection of choroidal neovascularization home monitoring of the Eye (HOME) study. Ophthalmology. 2014;121:535–544. doi: 10.1016/j.ophtha.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser PK, Wang Y-Z, He Y-G, Weisberger A, Wolf S, Smith CH. Feasibility of a novel remote daily monitoring system for age-related macular degeneration using mobile handheld devices: results of a pilot study. Retina. 2013;33:1863–1870. doi: 10.1097/IAE.0b013e3182899258. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld PJ. Optical coherence tomography and the development of antiangiogenic therapies in neovascular age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2016;(57):OCT14–OCT26. doi: 10.1167/iovs.16-19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ting TD, Oh M, Cox TA, Meyer CH, Toth CA. Decreased visual acuity associated with cystoid macular edema in neovascular age-related macular degeneration. Arch. Ophthalmol. 2002;120:731–737. doi: 10.1001/archopht.120.6.731. [DOI] [PubMed] [Google Scholar]
- 20.Jung JJ, Chen CY, Mrejen S, et al. The incidence of neovascular subtypes in newly diagnosed neovascular age-related macular degeneration. Am. J. Ophthalmol. 2014;158:769–779. doi: 10.1016/j.ajo.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Schuchard RA. Validity and interpretation of Amsler grid reports. Arch Ophthalmol. 1993;111:776–780. doi: 10.1001/archopht.1993.01090060064024. [DOI] [PubMed] [Google Scholar]
- 22.Zaidi F, Cheong-Leen R, Gair E, et al. The Amsler chart is of doubtful value in retinal screening for early laser therapy of subretinal membranes. The West London Survey. Eye. 2004;18:503. doi: 10.1038/sj.eye.6700708. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Ayton LN, Guymer RH, Luu CD. Low-luminance visual acuity and microperimetry in age-related macular degeneration. Ophthalmology. 2014;121:1612–1619. doi: 10.1016/j.ophtha.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Cassels NK, Wild JM, Margrain TH, Chong V, Acton JH. The use of microperimetry in assessing visual function in age-related macular degeneration. Surv Ophthalmol. 2018;63:40–55. doi: 10.1016/j.survophthal.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 25.von der Emde L, Pfau M, Thiele S, et al. Mesopic and dark-adapted two-color fundus-controlled perimetry in choroidal neovascularization secondary to age-related macular degeneration. Transl Vis Sci Technol. 2019;8(1):7. doi: 10.1167/tvst.8.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z, Ayton LN, Luu CD, Guymer RH. Longitudinal changes in microperimetry and low luminance visual acuity in age-related macular degeneration. JAMA Ophthalmol. 2015;133:442–448. doi: 10.1001/jamaophthalmol.2014.5963. [DOI] [PubMed] [Google Scholar]
- 27.Midena E, Vujosevic S, Convento E, Cavarzeran F, Pilotto E. Microperimetry and fundus autofluorescence in patients with early age-related macular degeneration. Br J Ophthalmol. 2007;91:1499–1503. doi: 10.1136/bjo.2007.119685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Acton JH, Smith RT, Hood DC, Greenstein VC. Relationship between retinal layer thickness and the visual field in early age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:7618–7624. doi: 10.1167/iovs.12-10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landa G, Su E, Garcia PM, Seiple WH, Rosen RB. Inner segment–outer segment junctional layer integrity and corresponding retinal sensitivity in dry and wet forms of age-related macular degeneration. Retina. 2011;31:364–370. doi: 10.1097/IAE.0b013e3181e91132. [DOI] [PubMed] [Google Scholar]
- 30.Wu Z, Ayton LN, Guymer RH, Luu CD. Comparison between multifocal electroretinography and microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2014;55:6431–6439. doi: 10.1167/iovs.14-14407. [DOI] [PubMed] [Google Scholar]
- 31.Querques L, Querques G, Forte R, Souied EH. Microperimetric correlations of autofluorescence and optical coherence tomography imaging in dry age-related macular degeneration. Am J Ophthalmol. 2012;153:1110–1115. doi: 10.1016/j.ajo.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 32.Luu CD, Dimitrov PN, Robman L, et al. Role of flicker perimetry in predicting onset of late-stage age-related macular degeneration. Arch Ophthalmol. 2012;130:690–699. doi: 10.1001/archophthalmol.2012.277. [DOI] [PubMed] [Google Scholar]
- 33.Sulzbacher F, Kiss C, Kaider A, et al. Correlation of SD-OCT features and retinal sensitivity in neovascular age-related macular degeneration. Invest Ophthalmol Vis Sci. 2012;53:6448–6455. doi: 10.1167/iovs.11-9162. [DOI] [PubMed] [Google Scholar]
- 34.Ferris FL, Wilkinson C, Bird A, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–851. doi: 10.1016/j.ophtha.2012.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouyang Y, Heussen FM, Hariri A, Keane PA, Sadda SR. Optical coherence tomography–based observation of the natural history of drusenoid lesion in eyes with dry age-related macular degeneration. Ophthalmology. 2013;120:2656–2665. doi: 10.1016/j.ophtha.2013.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Z, Luu CD, Ayton LN, et al. Optical coherence tomography–defined changes preceding the development of drusen-associated atrophy in age-related macular degeneration. Ophthalmology. 2014;121:2415–2422. doi: 10.1016/j.ophtha.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz-Valckenberg S, Sahel J-A, Danis R, et al. Natural history of geographic atrophy progression secondary to age-related macular degeneration (Geographic Atrophy Progression Study) Ophthalmology. 2016;123:361–368. doi: 10.1016/j.ophtha.2015.09.036. [DOI] [PubMed] [Google Scholar]
- 38.Thylefors B, Chylack L, Jr, Konyama K, et al. for the WHO Cataract Grading Group. A simplified cataract grading system. Ophthalmic Epidemiol. 2002;9:83–95. doi: 10.1076/opep.9.2.83.1523. [DOI] [PubMed] [Google Scholar]
- 39.Wu Z, Ayton LN, Guymer RH, Luu CD. Intrasession test–retest variability of microperimetry in age-related macular degeneration. Invest Ophthalmol Vis Sci. 2013;54:7378–7385. doi: 10.1167/iovs.13-12617. [DOI] [PubMed] [Google Scholar]
- 40.Molina-Martín A, Piñero DP, Pérez-Cambrodí RJ. Reliability and intersession agreement of microperimetric and fixation measurements obtained with a new microperimeter in normal eyes. Curr Eye Res. 2016;41:400–409. doi: 10.3109/02713683.2015.1020170. [DOI] [PubMed] [Google Scholar]
- 41.Munk MR, Kiss C, Huf W, et al. One year follow-up of functional recovery in neovascular AMD during monthly anti-VEGF treatment. Am J Ophthalmol. 2013;156:633–643. doi: 10.1016/j.ajo.2013.05.037. e632. [DOI] [PubMed] [Google Scholar]
- 42.Prager F, Michels S, Simader C, Geitzenauer W, Schmidt-Erfurth U. Changes in retinal sensitivity in patients with neovascular age-related macular degeneration after systemic bevacizumab (avastin) therapy. Retina. 2008;28:682–688. doi: 10.1097/IAE.0b013e318161dc70. [DOI] [PubMed] [Google Scholar]
- 43.de Oliveira Dias JR, Zhang Q, Garcia JM, et al. Natural history of subclinical neovascularization in nonexudative age-related macular degeneration using swept-source OCT angiography. Ophthalmology. 2018;125:255–266. doi: 10.1016/j.ophtha.2017.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee AY, Lee CS, Butt T, et al. UK AMD EMR Users Group Report V: benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br J Ophthalmol. 2015;99:1045–1050. doi: 10.1136/bjophthalmol-2014-306229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu Z, Guymer RH, Jung CJ, et al. Measurement of retinal sensitivity on tablet devices in age-related macular degeneration. Transl Vis Sci Technol. 2015;4(3):13. doi: 10.1167/tvst.4.3.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ho CYD, Wu Z, Turpin A, et al. A tablet-based retinal function test in neovascular age-related macular degeneration eyes and at-risk fellow eye. Transl Vis Sci Technol. 2018;7(2):2. doi: 10.1167/tvst.7.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelly BD, Hackett SF, Hirota K, et al. Cell type–specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 48.Ozaki H, Yu A, Della N, et al. Hypoxia inducible factor-1alpha is increased in ischemic retina: temporal and spatial correlation with VEGF expression. Invest Ophthalmol Vis Sci. 1999;40:182–189. [PubMed] [Google Scholar]
- 49.Stefánsson E, Geirsdóttir Á, Sigurdsson H. Metabolic physiology in age related macular degeneration. Prog Retin Eye Res. 2011;30:72–80. doi: 10.1016/j.preteyeres.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 50.Asahara T, Takahashi T, Masuda H, et al. VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 1999;18:3964–3972. doi: 10.1093/emboj/18.14.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Querques G, Srour M, Massamba N, et al. Functional characterization and multimodal imaging of treatment-naive “quiescent” choroidal neovascularization. Invest Ophthalmol Vis Sci. 2013;54:6886–6892. doi: 10.1167/iovs.13-11665. [DOI] [PubMed] [Google Scholar]
- 52.Wang W, Gawlik K, Lopez J, et al. Genetic and environmental factors strongly influence risk, severity and progression of age-related macular degeneration. Signal Transduct Target Ther. 2016;1:16016. doi: 10.1038/sigtrans.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sardell RJ, Persad PJ, Pan SS, et al. Progression rate from intermediate to advanced age-related macular degeneration is correlated with the number of risk alleles at the CFH locus. Invest Ophthalmol Vis Sci. 2016;57:6107–6115. doi: 10.1167/iovs.16-19519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clemons T, Milton R, Klein R, Seddon J, Ferris F., III Risk factors for the incidence of advanced age-related macular degeneration in the Age-Related Eye Disease Study (AREDS): AREDS report no. 19. Ophthalmology. 2005;112:533–539. doi: 10.1016/j.ophtha.2004.10.047. e531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Parravano M, Oddone F, Tedeschi M, et al. Retinal functional changes measured by microperimetry in neovascular age-related macular degeneration treated with ranibizumab: 24-month results. Retina. 2010;30:1017–1024. doi: 10.1097/IAE.0b013e3181cfd3c6. [DOI] [PubMed] [Google Scholar]
- 56.Squirrell DM, Mawer NP, Mody CH, Brand CS. Visual outcome after intravitreal ranibizumab for wet age-related macular degeneration: a comparison between best-corrected visual acuity and microperimetry. Retina. 2010;30:436–442. doi: 10.1097/IAE.0b013e3181bd2f29. [DOI] [PubMed] [Google Scholar]
- 57.Redmond T, Garway-Heath DF, Zlatkova MB, Anderson RS. Sensitivity loss in early glaucoma can be mapped to an enlargement of the area of complete spatial summation. Invest Ophthalmol Vis Sci. 2010;51:6540–6548. doi: 10.1167/iovs.10-5718. [DOI] [PubMed] [Google Scholar]