Abstract
Nonsense-mediated mRNA decay (NMD) is a cellular mRNA degradation mechanism that inhibits the expression of aberrant mRNAs harboring premature termination codons (PTCs). Recent progress in transcriptome-wide sequencing techniques has revealed that NMD also degrades approximately 5–30% of non-mutated cellular mRNAs in a way that can be regulated in response to various cellular signals. In mammals, NMD is governed by the central NMD factor UPF1, which is activated by phosphorylation after translation terminates at a nonsense codon that triggers NMD. We have found that immunoprecipitation using an antibody that is specific for phosphorylated UPF1 is a useful tool to define not only cellular NMD targets but also the nature of NMD decay intermediates and, thus, the process of NMD. To this end, we describe here a detailed protocol for what we call “NMD degradome sequencing” using high-throughput technology.
Keywords: mRNP, RNA helicase, Phosphorylated UPF1, Co-translational mRNA decay, Immunoprecipitation, Next-generation transcriptome sequencing, RIP–seq
1. Introduction
Nonsense-mediated mRNA decay (NMD) is a fundamental mechanism of cellular mRNA degradation that targets not only aberrant mRNAs harboring a premature termination codon (PTC) but also normal physiologic mRNAs dependent on several NMD activating features. These features include an exon-junction complex (EJC) situated sufficiently downstream of a termination codon, an upstream open reading frame (uORF), a UGA selenocysteine codon in certain selenoprotein-encoding mRNAs, or a long 3′-untranslated region (3′UTR) [1–3]. Recent progress in the technology of transcriptome-wide high-throughput sequencing estimates that NMD targets ~5–30% of physiologic transcripts for the purpose of tuning gene expression in response to various environmental conditions [4–7].
Regardless of the eukaryote studied, NMD requires the ATP-dependent RNA helicase upframeshift 1 (UPF1). NMD is initiated in human cells by translation termination of the type that triggers the phosphorylation of UPF1 by the serine/threonine protein kinase SMG1 [1–3]. Although phosphorylated UPF1 (p-UPF1) is known to recruit endonucleolytic activity as well as decapping and 5′-to-3′ exonuclease activities, and deadenylating and 3′-to-5′ exonuclease activities, the molecular specifics of the decay mechanism remained unclear. To elucidate the decay steps of NMD, we deemed it critical to selectively isolate NMD-specific mRNA decay intermediates. Notably, since NMD utilizes many decay enzymes in common to other mRNA decay pathways, including the DCP2 decapping enzyme, the XRN1 5′-to-3′ exonuclease, the CCR4-NOT deadenylation complex, and exosomal 3′-to-5′ exonucleases [8–11], simply downregulating these enzymes and deep-sequencing RNAs that accumulate are insufficient to allow the identification of decay intermediates that arise specifically during the process of NMD.
We have reported that immunoprecipitation (IP) of p-UPF1, but not of total-cell hypo-phosphorylated (i.e. steady-state UPF1), effectively differentiates cellular mRNAs that are NMD targets from those that are not NMD targets [11–13]. We have also found that vast majority (> 90%) of RNA species that co-immunoprecipitate with p-UPF1 are short (< 120 nt), lack a full-length poly(A) tail, can harbor non-templated nucleotides at their 3′end, and constitute NMD decay intermediates [13]. Here we describe NMD-degradome sequencing (NMD-DegSeq), which is a novel approach we developed that utilizes p-UFP1 IP and high-throughput RNA sequencing to isolate and sequence direct mRNA decay intermediates [13].
2. NMD-degradome sequencing overview
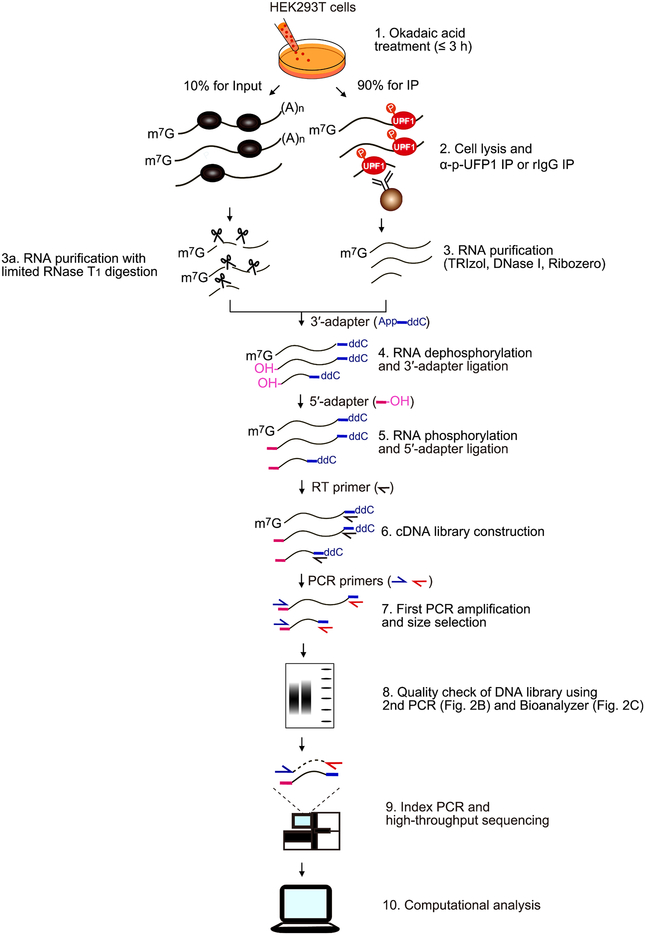
The purpose of the protocol described here is to characterize direct NMD decay intermediates at single- nucleotide resolution. All samples should be prepared in triplicate. Since this protocol uses human embryonic kidney (HEK) 293T cells, which only inefficiently engage in the p-UPF1-dependent pathway Staufen-mediated mRNA decay [11], very few if any decay intermediates obtained derived from this alternative pathway. As shown in schematic overview (Fig. 1), NMD-DegSeq can be divided into 10 steps as follows:
Fig. 1.
Schematic for NMD-DegSeq. Details are provided in the text.
Incubate cells with the serine/threonine protein phosphatase inhibitor okadaic acid to increase the level of cellular p-UPF1.
Generate cell lysates, saving 10% as “Input” and immunoprecipitating half of the rest with anti-p-UFP1 S1116 antibody, and the other half as a negative control with rabbit (r)IgG.
Purify the RNA from all three fractions using TRIzol, DNase I and Ribozero. For Input RNA samples only, perform limited RNase T1 digestion, and subsequently repeat the RNA purification.
Dephosphorylate RNA using recombinant shrimp alkaline phosphatase (rSAP), and ligate the resulting 3′-ends to a 34-nucleotide DNA adapter.
Phosphorylate RNA 5′-ends using T4 polynucleotide kinase (T4 PNK), and ligate the resulting 5′-ends to an RNA adapter.
Construct a cDNA library using RT and a 34-nucleotide DNA primer that anneals to the 3′-end DNA adapter.
Perform the PCR amplification using a 16-nucleotide DNA primer that anneals to the 5′-end adapter and a 16-nucleotide DNA primer that anneals to the 3′-end adapter, and size select after electrophoresis in acrylamide.
Check the quality of the PCR products by assaying (i) size using a second PCR and quality-control DNA primers and also (ii) amount using an Agilent Bioanalyzer, which also provides information on product size.
When quality is assured, perform the index PCR using Illumina Index PCR primers in the Nextera Index Kit followed by high-throughput sequencing.
Undertake computational and comparative analyses of the three libraries.
This protocol is generally applicable to the any cultured cell type from a variety mammalian species. Notably, since p-UPF1 is generally formed as a consequence of NMD activation and UPF1 functions in pathways in addition to mRNA decay, the level of p-UPF1 constitutes only a small fraction of total-cell UPF1 [11,14]. In the case of HEK 293T-cell lysates, the level of cellular p-UFP1 is barely detectable by western blotting using antibodies that recognize either UPF1 phosphorylation at S1078 and S1096, S1089, or S1116 unless the cells are incubated with okadaic acid [11]. Likewise, to obtain sufficient levels of NMD decay intermediates as identified by bound p-UPF1, it is critical that cells are incubated for 3 h with 200 nM okadaic acid, which is a potent and specific inhibitor of type 1 and type 2A protein phosphatases [15]. Okadaic acid increases the cellular abundance of p-UFP1 by blocking p-UPF1 recycling [11,14,16], which in turn increases p-UPF1 binding to cellular NMD targets.
3. Methods
3.1. Augmenting the abundance of cellular p-UFP1
-
3.1.1.
HEK293T cells (3 × 108 cells/4 × 150 mm dish) are cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS).
-
3.1.2.
Culture cells with 200 nM okadaic acid for 3h immediately prior to lysis.
-
3.1.3.
Collect cells using a cell lifter, and pellet cells in a 1.5 ml microcentrifuge tube at 1000×g and 4°C for 5 min. Wash cell pellets two times with ice-cold PBS.
-
3.1.4.
Cell pellets can be stored at −80°C.
3.2. Cell lysis and immunoprecipitation (IP)
-
3.2.1.
Resuspend cell pellets (~100 mg/ml) using a vortex mixer in ice-cold Hypotonic Gentle Lysis Buffer, and keep on ice for 10 min.
-
3.2.2.
Add 5 M NaCl to a final concentration of 150 mM, and vortex vigorously for 15 s.
-
3.2.3.
Pellet cell debris at 15,000×g and 4°C for 10 min, and transfer the supernatant to a new ice-cold 1.5 ml microcentrifuge tube.
-
3.2.4.
Measure protein concentration using a Protein Assay Kit.
-
3.2.5.
Pre-clear each cell lysate by adding supernatant to Dynabeads Protein A, and mix using end-over-end tube rotation at 4°C for 30–60 min.
-
3.2.6.
Centrifuge pre-cleared lysates at 15,000×g at 4°C for 10 min, and carefully transfer supernatants to a clean microfuge tube.
-
3.2.7.
While pre-clearing each lysate, prepare antibody-bound Dynabeads Protein A by adding 5–10 μg of anti-p-UPF1 (Ser1116) antibody or, as a negative control, rabbit IgG to 50 μl of Dynabeads Protein A in 300 μl of Hypotonic Gentle Lysis Buffer containing 150 mM NaCl (use 5–10 μg of antibody for a lysate that contains 1–3 mg of total HEK293T-cell protein). Mix using end-over-end tube rotation at 25°C for 30 min. Then, carefully remove the buffer using aspiration after concentrating the beads to the sides of the tubes using a Magnetic Tube Rack.
-
3.2.8.
Add the supernatant from 3.2.6. to the antibody-Dynabeads Protein A mixture from 3.2.7., and mix using end-over-end tube rotation at 4°C for 2 h.
-
3.2.9.
Wash the antibody–Dynabeads Protein A mixture with 1 ml of ice-cold NET-2 Buffer (0.1% NP-40). Repeat this step two more times.
-
3.2.10.
Wash antibody–Dynabeads Protein A mixture with 1 ml of ice-cold NET-2 Buffer (0.5% NP-40). Repeat this step two more times.
-
3.2.11.
Elute RNA and proteins using 50 μl of 2× SDS-PAGE Sample Elution Buffer by incubating at 95°C for 5 min.
-
3.2.12.
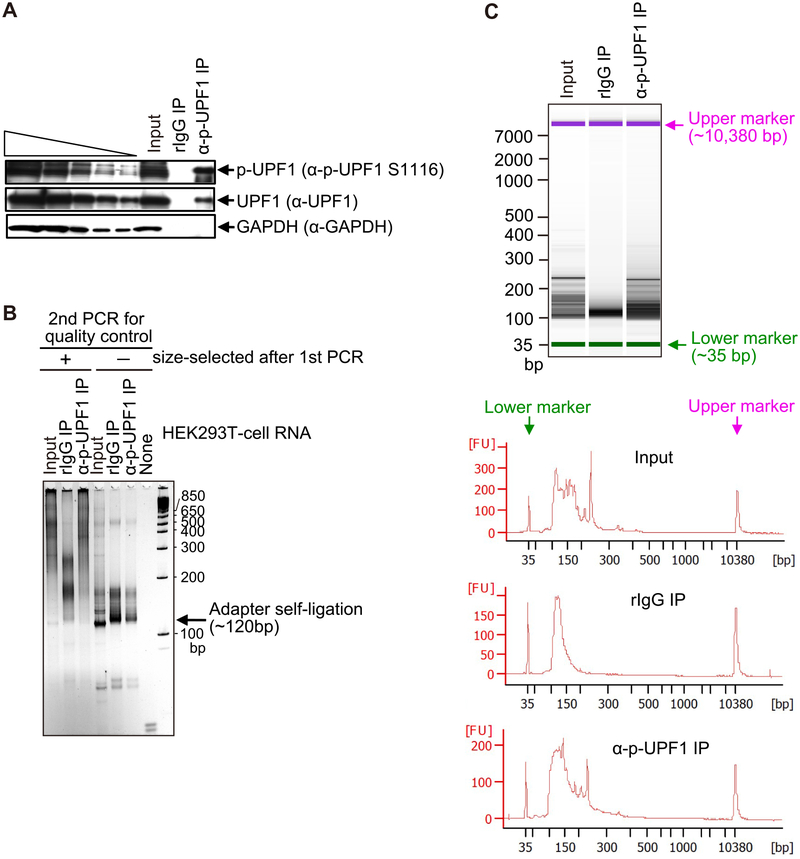
Check the IP quality by western blotting (Fig. 2A).
-
3.2.13.
Sample eluate can be stored at −20°C.
-
3.2.14.
Note: Serine 1,116 in the short (1,118-amino acid) isoform of human UPF1 is equivalent to serine 1,127 in the long (1,129-amino acid) isoform of human UPF1. Although the shorter isoform is more abundant than the longer isoform in most of tissues and cell types, the functional difference of these isoforms in NMD remains unclear [17,18].
Fig. 2.
Quality check of IP specificity and first and second PCR-sample sizes and amounts used for NMD-DegSeq. (A) Western blot of lysates of HEK293T cells using the specified antibodies prior to (Input) or after IP using anti(α)-p-UFP1 S1116 antibody (i.e. (α)-p-UFPl) or, as a negative control, rabbit (r)IgG. The left-most five lanes are 3-fold dilutions of Input sample (i.e. before IP). (B) SYBR Gold staining after the second PCR-amplification for the purpose of quality control. Analyses are of RNA samples prior to (Input); after (+) IP using anti(α)-p-UFP1 S1116 antibody or, as a negative control, rabbit (r)IgG; or no RNA (None). These RNAs were subjected to the first PCR and, subsequently, as second PCR, after which PCR products were (+) or were not (−) size-selected (the latter has Adapter self-ligation products that should be eliminated prior to library construction). (C) Agilent Bioanalyzer 2100 analyses using capillary electrophoresis (upper) and tracing (lower) of fluorescent samples after the first PCR. Results confirm adequate quality and quantity of samples before (Input) and after IP so that index PCR can be performed to generate samples for Illumina sequencing. FU, fluorescence units; bp, base pairs.
3.3. RNA purification
-
3.3.1.
Transfer eluted IPs to a microcentrifuge tube and adjust the volume to 100 μl with water.
-
3.3.2.
Add 500 μl of TRIzol Reagent and 100 μl of Chloroform. Mix well by manual inversions.
-
3.3.3.
Centrifuge samples at 15,000×g at 4°C for 5 min to separate aqueous and organic phases.
-
3.3.4.
Transfer upper aqueous phase to a new microcentrifuge tube, add an equal volume (~500 μl) of 2-propanol and 20 μg of Glycogen Solution, and incubate at −20°C for at least 1 h.
-
3.3.5.
Centrifuge samples at 15,000×g at 4°C for 10 min to pellet precipitated RNA.
-
3.3.6.
Wash RNA pellets with 1 ml of 75% ethanol, and centrifuge at 15,000×g at 4°C for 5 min. Repeat this wash step once more.
-
3.3.7.
After completely pipetting away the 75% ethanol, open tube lids and air-dry RNA pellets at room temperature for 5 min.
-
3.3.8.
Dissolve RNA pellets in 15 μl of water.
-
3.3.9.
Determine RNA concentration using a NanoDrop 1000 Spectrophotometer. Typical RNA yields are ~1–2 μg/1 × 108 cells.
-
3.3.10.
Digest any remaining DNA with 2.5 U RQ1 DNase in 1× RQ1 Reaction Buffer containing 10 mM DTT and 6 U RNaseOUT RNase Inhibitor at 37°C for 15 min.
-
3.3.11.
Repeat steps 3.3.1. to 3.3.9.
-
3.3.12.
Using ~5 μg of each RNA sample and remove rRNA according to the instruction manual of Ribo Zero Gold rRNA Removal Kit.
-
3.3.13.
Determine RNA concentration using a NanoDrop 1000 Spectrophotometer. Typical RNA yields are ~0.5–1 μg.
-
3.3.14.
Partially digest 1 μg of the Input RNA sample with 0.1 U of RNase T1 in 1× RNA Sequencing Buffer at 4°C for 30 min. Note that this step applies only to control Input RNA samples. See 4.4 below for additional information.
-
3.3.15.
Repeat steps 3.3.1. to 3.3.9 for only the Input RNA sample.
-
3.3.16.
RNA samples can be stored at −80°C.
3.4. RNA dephosphorylation and 3′-end DNA adapter ligation
-
3.4.1.
Treat ~1 μg of each RNA sample with 2 U of recombinant shrimp alkaline phosphatase (rSAP) in 1× CutSmart Buffer containing 20 U of RNaseOUT RNase inhibitor at 37°C for 1 h.
-
3.4.2.
Inactivate enzymes at 65°C for 15 min.
-
3.4.3.
Ligate 100 pmol of the preadenylated NMD-DegSeq 3′-DNA adapter to the RNA 3′ ends by incubating with 600 U of T4 RNA Ligase 2, truncated KQ in 1× T4 RNA Ligase Buffer containing 7.5% of polyethylene glycol (PEG) 8000 and 20 U of RNaseOUT RNase inhibitor at 25°C overnight.
-
3.4.4.
Adjust total volume to 300 μl with water, add Phenol: Chloroform: Isoamyl Alcohol 25:24:1 (pH 8.0), and mix well by manual inversions.
-
3.4.5.
Centrifuge samples at 15,000×g at 4°C for 10 min to separate aqueous and organic phases.
-
3.4.6.
Transfer upper aqueous phase to a new microcentrifuge tube, add an equal volume (~300 μl) of 2-propanol, one-tenth volume (~30 μl) of 3 M Sodium Acetate and 1 μl of Glycogen Solution, and incubate at −20°C for least 1 h.
-
3.4.7.
Centrifuge samples at 15,000×g at 4°C for 10 min.
-
3.4.8.
Wash nucleic-acid pellets with 1 ml of 75% ethanol, and centrifuge at 15,000×g at 4°C for 5 min. Repeat this wash step two more times.
-
3.4.9.
After completely pipetting away the 75% ethanol, open tube lids and air-dry pellets at room temperature for 5 min.
-
3.4.10.
Dissolve pellets in 10 μl of water.
-
3.4.11.
Samples can be stored at −80°C.
3.5. 5′-end RNA phosphorylation, and 5′-end RNA adapter ligation
-
3.5.1.
Treat samples (10 μl) with 20 U of T4 polynucleotide kinase (T4 PNK) in 1× T4 PNK Buffer containing 20 U of RNaseOUT RNase inhibitor at 37°C for 30 min.
-
3.5.2.
Inactivate enzymes at 65°C for 15 min.
-
3.5.3.
Ligate 100 pmol of the NMD-DegSeq 5′-RNA adapter to RNA 5′ ends by incubating with 15 U of T4 RNA Ligase in 1× T4 RNA Ligase Buffer containing 20 U of RNaseOUT RNase inhibitor at 4°C overnight.
-
3.5.4.
Purify the resulting RNA–DNA hybrids by repeating steps 3.4.4. to 3.4.10.
-
3.5.5.
Samples can be stored at −80°C.
3.6. cDNA library construction
-
3.6.1
Denature purified RNA–DNA hybrids (~100 ng, which is the expected yield) at 65°C for 5 min.
-
3.6.2.
Generate cDNAs using the 34-nucleotide NMD-DegSeq 1st-strand RT primer, which is complementary to the 3′-adapter, by incubation with 50 U of Superscript III Reverse Transcriptase in First-Strand Buffer containing 10 mM DTT, 0.5 mM dNTP Mix and 10 U of RNaseOUT RNase inhibitor at 50°C for 2 h.
-
3.6.3.
cDNA samples can be stored at −20°C.
3.7. First PCR amplification and size selection
-
3.7.1.
PCR-amplify the resulting cDNAs by incubating with 0.5 U of Q5 High-Fidelity DNA Polymerase, 12.5 pmol of the 16-nucleotide NMD-DegSeq forward primer (i.e. the 1st PCR sense primer that anneals to cDNA deriving from the 5′-RNA adapter) and 12.5 pmol of the 16-nucleotide NMD-DegSeq reverse primer (1st PCR antisense primer that anneals to cDNA deriving from the 3′-DNA adapter) in 1× Q5 Reaction Buffer with 0.2 mM dNTP Mix, initially denaturing at 98°C for 30 s, followed first by 20 PCR cycles at 98°C for 10 s →68°C for 30 s → 72°C for 1 min and, subsequently, by a final incubation at 72°C for 2 min.
-
3.7.2.
Load PCR products onto a 6% polyacrylamide gel in parallel to a 1 Kb Plus DNA Ladder, and gently rock at room temperature for 30 min with SYBR Gold Nucleic Acid Gel Stain. Using a clean single-edge razor blade, excise the PCR products (> 85 bp; data not shown), avoiding shorter PCR products that result from adapter ligations without cellular RNA inserts or with short RNA inserts. Transfer the excised gel pieces to a clean microcentrifuge tube.
-
3.7.3.
Add 400 μl of RNA Extraction Buffer to each sample, and crush the gel slices by passage through a Monoject 1 ml Tuberculin Syringe.
-
3.7.3.
Load the resulting gel slurry onto a Corning Coastar Spin-X Centrifuge Tube Filter, and remove acrylamide-gel debris at 15,000×g and 25°C for 5 min.
-
3.7.4
Further purify the eluted PCR products by repeating steps 3.4.4. to 3.4.10.
-
3.7.5.
Samples (now in 10 μl) can be stored at −20°C.
3.8. Quality check of DNA library using two methods
3.8.1. Assess DNA library quality first using a second PCR amplification
-
3.8.1.1.
PCR-amplify 1 μl of each sample from step 3.7.5 by incubating with 0.5 U of Q5 High-Fidelity DNA Polymerase, 12.5 pmol of the 39-nucleotide Quality Check forward primer, i.e. sense primer for 2nd PCR, and 12.5 pmol of the 43-nucleotide Quality Check reverse primer, i.e. antisense primer for 2nd PCR, in 1× Q5 Reaction Buffer with 0.2 mM dNTP Mix, initially denaturing at 98°C for 30 sec, followed by 20 PCR cycles at 98°C for 10 sec → 66°C for 30 sec → 72°C for 1 min and, subsequently, by a final incubation at 72°C for 2 min.
-
3.8.1.2.
Load PCR products onto a 6% polyacrylamide gel in parallel with a 1 Kb Plus DNA Ladder, and gently rock at room temperature for 30 min with SYBR Gold Nucleic Acid Gel Stain (Fig. 2B). See 4.5 below for additional information.
3.8.2. Assess DNA library quality
-
3.8.2.1.
DNA libraries are analyzed in a second way using an Agilent Bioanalyzer and Qubit dsDNA HS Assay Reagent according to manufacturer’s instructions (Fig. 2C).
3.9. Index PCR and high-throughput sequencing
-
3.9.1.
Prepare the final i5 and i7 indexed DNA libraries for Illumina sequencing using modified Nextera Indexing with Q5 High Fidelity 2× master mix, 3% DMSO, and Nextera DNA Library indices. PCR amplifications are performed after initially denaturing at 98°C for 30 sec, followed first by 20 PCR cycles at 98°C for 10 s → 66°C for 30 sec → 72°C for 1 min and, subsequently, by a final incubation at 72°C for 2 min.
-
3.9.2.
Indexed libraries are purified using Agencourt AMPure XP SPRI beads followed by quality assessment and quantification using an Agilent Bioanalyzer 2100 and Qubit 3.0 Flourometer, respectively.
-
3.9.3.
Libraries are diluted to 2 nM and sequenced using an Illumina HiSeq2500v4 system with a 2 × 150-bp configuration. Notably, two types of sequence information, single-end or paired-end reads, are provided in the analysis. Since NMD-DegSeq requires reliable sequence information at the ends of sequence reads, paired-end sequence reads are more useful to downstream analyses.
3.10. Computational sequence processing
-
3.10.1.
Raw reads generated from the Illumina HiSeq2500v4 sequencer are demultiplexed using configurebcl2fastq.pl version 2.19.0.
-
3.10.2.
CutAdapt 1.15 [19] is used to trim Nextera transposase sequences. An additional round of CutAdapt 1.15 removes reads with multiple transposase sequences or any other oligonucleotides used in preparing the library.
-
3.10.3.
Processed and cleaned reads are mapped to the human genome database (Fig. 3) using STAR_2.5.2b [20] and the following parameters: “–twopassMode Basic –runMode alignReads –genomeDir ${GENOME} –readFilesIn ${SAMPLE} –outSAMtype BAM SortedByCoordinate –outSAMstrandField intronMotif –out-FilterIntronMotifs RemoveNoncanonical”.
-
3.10.4.
Read counts per gene are derived in a strand-specific manner using featureCounts from the subread-1.5.0p3 package [21] with the following parameters: “-s 1 -t exon -g gene_name”.
-
3.10.5.
Samtools 1.5 [22] is used to isolate uniquely aligned reads: “view -q 255”. Duplicate reads are removed using picard-2.12.0 MarkDuplicates and the removeclipping.py. Python script from ngsutils-0.5.9 [23] can be modified to output only soft-clipped bases from either the 3′ or 5′ end of the inserts.
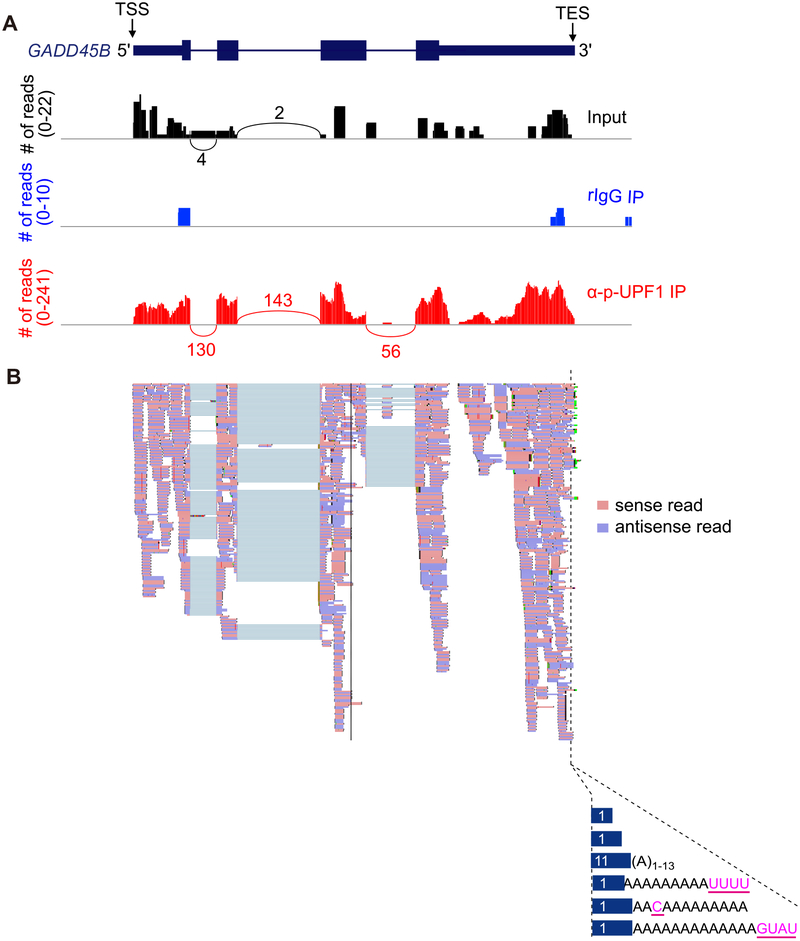
Fig. 3.
Results from NMD-DegSeq demonstrate the presence of nontemplated nucleotide additions at the 3′ends of p-UFP1-bound NMD decay intermediates. (A) Sashimi plots (black, Input; blue, rIgG IP; red, anti-p-UPF1 IP) developed using Integrative Genomics Viewer software [24] exemplifying NMD-DegSeq-read mapping to growth arrest and DNA-damage-inducible beta (GADD45B) mRNA, which is a bona fide NMD target, superimposed on the GADD45B gene as shown in the uppermost diagram [5,11]. TSS, transcript start site; TES, mature transcript end site, i.e. the site to which poly(A) is appended; numbers above or below arcs denote read numbers. (B) Using data shown in A for the p-UFP1 IP sample, individual sense reads (pink) and antisense reads (light purple) are shown (upper) and aligned with nontemplated nucleotide additions (pink and underlined; lower) observed within the poly(A) tail of GADD45B transcripts. Numbers in blue bars specify the number of paired reads having the corresponding 3′-end.
4. Hints for troubleshooting
4.1. Accumulation of cellular p-UPF1
Okadaic acid induces apoptosis in many types of cells. Therefore, prior to performing p-UPF1 IPs, it is important to optimize the okadaic acid concentration and cell exposure time to be used by assaying cell viability concomitantly with the level of cellular p-UPF1. While the 100 μM DMSO stock of okadaic acid can be stored at −20°C, it is recommended that a fresh stock be prepared for each use.
4.2. Cell lysis
Hypotonic Gentle Lysis Buffer should be freshly prepared. cOmplete mini EDTA-free Protease Inhibitor cocktail tablet (Roche Diagnostics) and PhosSTOP Phosphatase Inhibitor cocktail tablet (Roche Diagnostics) can be used in place of 1× Halt Protease and Phosphatase Inhibitor Cocktail (100×).
4.3. p-UPF1 immunoprecipitation
Although Protein A Agarose or Protein A Sepharose can be used in the p-UPF1 and rabbit IgG IPs in place of Protein A Dynabeads, Protein A Dynabeads provide increased sensitivity and specificity relative to other Protein A beads.
4.4. p-UPF1 immunoprecipitation and RNA purification
Since RNAs captured by rabbit IgG-Protein A Dynabeads are barely detectable by RT-PCR, an additional negative control should include Input RNA treated with RNase T1. Conditions for the limited RNase digestion should be carefully optimized so that the bulk of RNA is < 100-nucleotides after digestion (data not shown).
4.5. Quality check of DNA library
The DNA library quality-check step is critical. If evidence of adapter self-ligation (~120 bp) is visible (see right lanes for size selection in Fig. 2B), the size-selection steps 3.7.2. to 3.7.4 should be repeated before proceeding.
5. Concluding remarks and perspective
All cellular mRNAs, including NMD targets, are eventually turned over through various cellular degradation mechanisms. The key point in the NMD-DegSeq protocol described here is that it isolates mRNA decay intermediates that specifically derive from the process of NMD by virtue of their co-IP with p-UPF1, which is the activated form of the key NMD factor and proven reliable marker of human-cell NMD targets [11,12,14]. NMD-DegSeq reveals that the dynamic nature of NMD decay, which occur co-translationally, involves the addition of non-templated nucleotides at decay-intermediate 3′-ends [13]. Notably, NMD-DegSeq provides information on the initial phase of NMD, during which decay intermediates are bound by p-UPF1. We have found that it is possible to determine the enzymatic activities that generate specific NMD decay intermediates by downregulating the cellular abundance of one or more NMD degradative activities, performing RNA-specific RT-PCR (sometimes simultaneously selecting for a specific 3′-end non-templated nucleotide), cloning the resulting products after excision from polyacrylamide gels, and performing Sanger sequencing [13].
Acknowledgements
We thank the University of Rochester Genomics Research Center for assistance in library design, construction, and next-generation sequencing support; and John Ashton for comments on this manuscript. Work on NMD in the Maquat lab is supported by NIH R01 GM059614 to L.E. M. T.K. was partially supported by a postdoctoral fellowship from the FRAXA Research Foundation.
Appendix A. Equipment and supply list
A.1. Reagent, chemicals and recombinant proteins
Dulbecco’s Modified Eagle’s Medium (DMEM) (Thermo Fisher Scientific Gibco, Catalog # 11995)
Fetal bovine serum (FBS) (VWR, Catalog # 97068–085)
Okadaic acid, free acid > 98% (LC Laboratories, Catalog # O-2220)
TRIzol Reagent (Thermo Fisher Scientific, Catalog # 15596018)
Chloroform (J.T. Baker, Catalog # JT9182–01)
Ethanol (100% and 75%) (Koptec, Catalog # QDEV1001TP)
2-Propanol (Fisher Scientific, Catalog # A4514)
Glycogen Solution (20 mg/ml) (Thermo Fisher Scienctific, Catalog # R0561)
SYBR Gold Nucleic Acid Gel Stain (Thermo Fisher Scientific, Catalog # S11494)
Phenol: Chloroform: Isoamyl Alcohol 25:24:1 Saturated with 10 mM Tris (pH 8.0), 1 mM EDTA (Sigma-Aldrich, Catalog # P2069)
Halt Protease and Phosphatase Inhibitor Cocktail, EDTA-free (100×) (Thermo Fisher Scientific, Catalog # 78443)
IgG from rabbit serum (Sigma-Aldrich, Catalog # I5006)
DNase I (RQ1 RNase-Free DNase) (Promega, Catalog # M6101)
RNaseOUT Recombinant Ribonuclease Inhibitor (Thermo Fisher Scientific Invitrogen, Catalog # 10777019)
Shrimp alkaline phosphatase (rSAP) (New England Biolabs, Catalog # M0371L)
T4 RNA Ligase 2, truncated KQ (New England Biolabs, Catalog # M0373L)
T4 polynucleotide kinase (T4 PNK) (New England Biolabs, Catalog # M0201L)
T4 RNA Ligase, cloned, 5 U/μl (Thermo Fisher Scientific, Ambion, Catalog # AM2141)
SuperScript III Reverse Transcriptase (Thermo Fisher Scientific, Invitrogen, Catalog # 18080085)
Q5 High-Fidelity DNA Polymerase (New England Biolabs, Catalog # M0491S)
Urea (EMD Millipore, Catalog # 9510)
Acrylamide/bis-acrylamide 19:1 (40%) (Fisher scientific, Catalog # BP1406–1)
Acrylamide/bis-acrylamide 29:1 (40%) (Fisher scientific, Catalog # BP1408–1)
Ammonium persulfate, crystal (Mallinckrodt chemicals, Catalog # 3460–04)
UltraPure TEMED (Thermo Fisher Scientific, Invitrogen, Catalog # 15524010)
Sodium dodecyl sulfate (Sigma-Aldrich, Catalog # 75746)
TWEEN 20 (Sigma-Aldrich, Catalog # P7949)
TRITON X-100 (Sigma-Aldrich, Catalog # T9284)
Nonidet P-40 (NP-40) (Sigma-Aldrich, Catalog # I3021)
2-Mercaptoethanol (Sigma-Aldrich, Catalog # M3148)
RNase T1 (5 U/μl) (Thermo Fisher Scientific Ambion, AM2283)
Halt Protease and Phosphatase Inhibitor Cocktail (100×) (Thermo Fisher Scientific, Catalog # PI78443)
Bromophenol blue (Fisher Scientific, Catalog # B-392)
1 Kb Plus DNA Ladder (Thermo Fisher Scientific Invitrogen, Catalog # 10787018)
Dynabeads Protein A (Thermo Fisher Scientific, Catalog # 10002D)
Anti-phospho-UPF1 Ser1116 antibody (anti-phospho-UPF1 (Ser1127), EMD Millipore, Catalog # 07–1016)
3 M Sodium Acetate (pH 5.5) (Thermo Fisher Scientific Ambion, Catalog # AM9740)
Trizma base (Sigma-Aldrich, Catalog # T6066)
Boric acid (Millipore Sigma, Catalog # 2720–5KG)
Ethylenediaminetetraacetic acid (EDTA), disodium salt (J.T. Baker, Catalog # 8993–01)
Acrylamide:Bis-Acrylamide (19:1) 40% solution (Fisher Scientific, Catalog # BP1406–1)
Ammonium persulfate (APS) (Acros Organics, Catalog # 401165000)
A.2. Buffers and formulations
-
Hypotonic Gentle Lysis Buffer
10 mM Tris-HCl (pH 7.4)
10 mM NaCl
10 mM EDTA
0.5% (w/w) Triton X-100
1× of Halt Protease and Phosphatase Inhibitor Cocktail
-
Phosphate Buffered Saline (PBS)
10 mM Na2HPO4
1.8 mM KH2PO4
137 mM NaCl
2.7 mM KCl
-
NET-2 Buffer (0.1 or 0.5% Nonidet P-40 (NP-40))
50 mM Tris-HCl (pH 7.4)
150 mM NaCl
0.1 or 0.5% NP-40
-
2× SDS-PAGE Sample Elution Buffer
125 mM Tris-HCl (pH 6.8)
4% SDS
20% Glycerol
% Bromophenol blue
10% 2-Mercaptoethanol
-
RNA Extraction Buffer
20 mM Tris-HCl (pH 7.5)
300 mM Sodium acetate
2 mM EDTA
0.2 % (v/v) SDS
-
6% polyacrylamide gel
6% Acrylamide: Bis-Acrylamide (19:1) solution
100 mM Tris-HCl (pH 8.3)
100 mM Boric acid
2 mM EDTA
0.07% APS
0.05% TEMED
A.3. Kits and supplies
Ribozero Gold rRNA Removal Kit (illumine, Catalog # MRZG12324)
Nextra Index Kit (Illumina, Catalog # FC-121–1012)
SPRIselect Reagent (Beckman Coulter, Catalog # B23319)
Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific Invitrogen, Catalog # Q32854,)
Protein Assay Kit (Bio-Rad, Catalog # 5000002)
Disposable Cell Lifter (Fisher Scientific, Catalog # 08-100-240)
Magnetic Tube Rack (Bio Rad Laboratories, Catalog # 1614916)
Corning Costar Spin-X Centrifuge Tube Filter (pore size: 0.45 μm) (Sigma-Aldrich, Catalog # 8162)
Monoject 1 ml Tuberculin syringe (Covidien, Catalog # 8881501400)
A.4. Equipment
CO2 incubator (Thermo Fisher Scientific, Model # 3110)
Refrigerated microcentrifuge (Beckman Coulter, Model # Microfuge 22R)
Labquake Shaker (Barnstead Thermolyne, Model # 415110)
NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Model # ND-1000)
Thermal Cycler (Bio-Rad Laboratories, Model # T100)
Molecular Imager Gel Doc XR + System with Image Lab Software (Bio-Rad Laboratories, Catalog # 1708195)
2100 Bioanalyzer (Agilent Technologies, Catalog # G2939BA)
Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Catalog # Q33226)
HiSeq 2500 System (Illumina)
Vortex mixer (VWR, Model # VM-3000)
Mini-PROTEAN II Cell Electrophoresis System (Bio-Rad Laboratories, Model # 125BR)
PowerPac1000 Basic Power Supply (Bio-Rad Laboratories, Model # 285BR)
A.5. Nucleotide sequences of adapters and primers
3′-DNA adapter for NMD-DegSeq: 5′-App-CTGTCTCTTATACACATC TCCGAGCCCACGAGAC-ddC-3′ (Integrated DNA Technologies)
5′-RNA adapter for NMD-DegSeq: 5′-UCGUCGGCAGCGUCAGAUGU GUAUAAGAGACAG-3′ (Integrated DNA Technologies)
1st strand RT primer for NMD-DegSeq: 5′-GTCTCGTGGGCTCGGAG ATGTGTATAAGAGACAG-3′ 3′ (Integrated DNA Technologies)
1st PCR sense primer for NMD-DegSeq: 5′-TCGTCGGCAGCGTCAG-3 3′ (Integrated DNA Technologies)
1st PCR antisense primer or NMD-DegSeq: 5′-GTCTCGTGGGCTC GGA-3′ (Integrated DNA Technologies)
2nd PCR sense primer for quality check: 5′-AATGATACGGCGACC ACCGAGATCTACACTCGTCGGCAGCGTC-3′ (Integrated DNA Technologies)
2nd PCR antisense primer for quality check: 5′- CAAGCAGAAGAC GGCATACGAGATGTCTCGTGGGCTCGG-3′ (Integrated DNA Technologies)
Nucleotide sequences were removed using CutAdapt 1.15: 5′-CTGT CTCTTATACACATCT-3′; CCGAGCCCACGAGAC-3′; 5′-GACGCTGCC GACGA-3′; 5′-AGATGTGTATAAGAGACAG-3′; 5′-TCGTCGGCAGCG TCAGATGTGTATAAGAGACAG-3′; 5′-GTCTCGTGGGCTCGGAGATG TGTATAAGAGACAG-3′
A.6. Cell line
Human embryonic kidney (HEK)293T cells (ATCC, CRL-11268)
A7. Software and algorithms
CutAdapt 1.15 (http://code.google.com/p/cutadapt/)
STAR_2.5.2b (https://code.google.com/archive/p/rna-star/)
Subread (http://subread.sourceforge.net/)
Samtools (http://www.htslib.org/)
NGSutils (https://github.com/ngsutils/ngsutils)
Integrative Genomics Viewer (IGV) 2.4 (http://software.broadinstitute.org/software/igv/)
References
- [1].Goetz AE, Wilkinson M, Stress and the nonsense-mediated RNA decay pathway, Cell. Mol. Life Sci. 74 (2017) 3509–3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Nasif S, Contu L, Mühlemann O, Beyond quality control: The role of nonsense-mediated mRNA decay (NMD) in regulating gene expression, Semin. Cell Dev. Biol 75 (2017) 78–87. [DOI] [PubMed] [Google Scholar]
- [3].Kurosaki T, Maquat LE, Nonsense-mediated mRNA decay in humans at a glance, J. Cell Sci. 129 (2016) 461–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].He F, Li X, Spatrick P, Casillo R, Dong S, Jacobson A, Genome-wide analysis of mRNAs regulated by the nonsense-mediated and 5′ to 3′ mRNA decay pathways in yeast, Mol. Cell 12 (2003) 1439–1452. [DOI] [PubMed] [Google Scholar]
- [5].Mendell JT, Sharifi NA, Meyers JL, Martinez-Murillo F, Dietz HC, Nonsense surveillance regulates expression of diverse classes of mammalian transcripts and mutes genomic noise, Nat. Genet 36 (2004) 1073–1078. [DOI] [PubMed] [Google Scholar]
- [6].Ramani AK, Nelson AC, Kapranov P, Bell I, Gingeras TR, Fraser AG, High resolution transcriptome maps for wild-type and nonsense-mediated decay-defective Caenorhabditis elegans, Genome Biol. 10 (2009) R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Weischenfeldt J, Waage J, Tian G, Zhao J, Damgaard I, Jakobsen JS, Kristiansen K, Krogh A, Wang J, Porse BT, Mammalian tissues defective in nonsense-mediated mRNA decay display highly aberrant splicing patterns, Genome Biol. 13 (2012) R35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lejeune F, Li X, Maquat LE, Nonsense-mediated mRNA decay in mammalian cells involves decapping, deadenylating, and exonucleolytic activities, Mol. Cell 12 (2003) 675–687. [DOI] [PubMed] [Google Scholar]
- [9].van Dijk EL, Schilders G, Pruijn GJM, Human cell growth requires a functional cytoplasmic exosome, which is involved in various mRNA decay pathways, RNA 13 (2007) 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Hu W, Petzold C, Coller J, Baker KE, Nonsense-mediated mRNA decapping occurs on polyribosomes in Saccharomyces cerevisiae, Nat. Struct. Mol. Biol 17 (2010) 244–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kurosaki T, Li W, Hoque M, Popp MW-L, Ermolenko DN, Tian B, Maquat LE, A post-translational regulatory switch on UPF1 controls targeted mRNA degradation, Genes Dev. 28 (2014) 1900–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Imamachi N, Salam KA, Suzuki Y, Akimitsu N, A GC-rich sequence feature in the 3′ UTR directs UPF1-dependent mRNA decay in mammalian cells, Genome Res. 27 (2017) 407–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kurosaki T, Miyoshi K, Myers JR, Maquat LE, NMD-degradome sequencing reveals ribosome-bound intermediates with 3′-end non-templated nucleotides, Nat. Struct. Mol. Biol 25 (2018) 940–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kurosaki T, Hoque M, Maquat LE, Identifying cellular nonsense-mediated mRNA decay (NMD) targets: immunoprecipitation of phosphorylated UPF1 followed by RNA sequencing (p-UPF1 RIP-Seq), Methods Mol. Biol 1720 (2018) 175–186. [DOI] [PubMed] [Google Scholar]
- [15].Cohen P, Holmes CFB, Tsukitani Y, Okadaic acid: a new probe for the study of cellular regulation, Trends Biochem. Sci 15 (1990) 98–102. [DOI] [PubMed] [Google Scholar]
- [16].Yamashita A, Ohnishi T, Kashima I, et al. , Human SMG-1, a novel phosphatidylinositol 3-kinase-related protein kinase, associates with components of the mRNA surveillance complex and is involved in the regulation of nonsense-mediated mRNA decay, Genes Dev. 15 (2001) 2215–2228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nicholson P, Josi C, Kurosawa H, Yamashita A, Mühlemann O, A novel phosphorylation-independent interaction between SMG6 and UPF1 is essential for human NMD, Nucleic Acids Res. 42 (2014) 9217–9235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Gowravaram M, Bonneau F, Kanaan J, Maciej VD, Fiorini F, Raj S, Croquette V, Le Hir H, Chakrabarti S, A conserved structural element in the RNA helicase UPF1 regulates its catalytic activity in an isoform-specific manner, Nucleic Acids Res. 46 (2018) 2648–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Martin M, Cutadapt removes adapter sequences from high-throughput sequencing reads, EMBnet. J 17 (2011) 10–12. [Google Scholar]
- [20].Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR, STAR: ultrafast universal RNA-seq aligner, Bioinformatics 29 (2012) 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liao Y, Smyth GK, Shi W, featureCounts: an efficient general-purpose program for assigning sequence reads to genomic features, Bioinformatics 30 (2014) 923–930. [DOI] [PubMed] [Google Scholar]
- [22].Li H, Handsaker B, Wysoker A, Fennell T, et al. , The sequence alignment/map (SAM) format and SAMtools, Bioinformatics 25 (2009) 2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Breese MR, Liu Y, NGSUtils: a software suite for analyzing and manipulating next-generation sequencing datasets, Bioinformatics 29 (2013) 494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thorvaldsdóttir H, Robinson JT, Mesirov JP, Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration, Brief Bioinform. 14 (2013) 178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]