Abstract
The serine/threonine protein phosphatase 1 (PP1) inhibitors PPP1R2, PPP1R7, and PPP1R11 are evolutionarily ancient and highly conserved proteins. Four PP1 isoforms, PP1α, PP1β, PP1γ1, and PP1γ2, exist; three of them except PP1γ2 are ubiquitous. The fact that PP1γ2 isoform is present only in mammalian testis and sperm led to the notion that isoform-specific regulators for PP1γ2 in sperm may be responsible for its function. In this report, we studied these inhibitors, PPP1R2, R7, and R11, to determine their spatial and temporal expression in testis and their regulatory functions in sperm. We show that, similar to PP1γ2, the three inhibitors are expressed at high levels in developing spermatogenic cells. However, the transcripts for the regulators are expressed as unique sizes in testis compared with somatic tissues. The three regulators share localization with PP1γ2 in the head and the principal piece of sperm. We show that the association of inhibitors to PP1γ2 changes during epididymal sperm maturation. In immotile caput epididymal sperm, PPP1R2 and PPP1R7 are not bound to PP1γ2, whereas in motile caudal sperm, all three inhibitors are bound as heterodimers or heterotrimers. In caudal sperm from male mice lacking sAC and glycogen synthase kinase 3, where motility and fertility are impaired, the association of PP1γ2 to the inhibitors resembles immature caput sperm. Changes in the association of the regulators with PP1γ2, due to their phosphorylation, are part of biochemical mechanisms responsible for the development of motility and fertilizing ability of sperm during their passage through the epididymis.
Keywords: inhibitor-2 (I2), inhibitor-3 (I3), PP1γ2, PPP1R11, PPP1R17, PPP1R2, sds22, sperm, spermatogenesis, testis
1 |. INTRODUCTION
The serine/threonine protein phosphatase 1 (PP1) is a highly conserved enzyme involved in a wide range of cellular processes. It exists in the cell as an oligomer and comprised of the catalytic subunit bound to a variety of interacting proteins (>200) that modulate its intracellular localization, substrate specificity, and activity (Bollen, Peti, Ragusa, & Beullens, 2010; Cohen, 2002; Fardilha, Esteves, Korrodi-Gregorio, da Cruz e Silva, & da Cruz e Silva, 2010; Heroes et al., 2013; Korrodi-Gregório, Esteves, & Fardilha, 2014). In mammals there are four PP1 isoforms, PP1α, PP1β, PP1γ1, and PP1γ2, encoded by three separate genes. The PP1γ1 and PP1γ2 isoforms, arise from alternative spliced transcripts of a single gene, Ppp1cc (stands for PP1 catalytic subunit c). These two isoforms differ only in their extreme C-terminus end with PP1γ2 having a unique 22 amino acid C-terminus replacing a 6-amino acid counterpart in PPγ1. The PP1γ2 message arises due to removal of the terminal intron 7, which is retained in PP1γ1 transcript as an extended exon. PP1α, PP1β, and PP1γ1 are ubiquitously expressed whereas PP1γ2 is highly enriched in testis. PP1γ2 is the major PP1 isoform present in mature sperm (Kitagawa et al., 1990; Shima et al., 1993; Smith et al., 1996; Vijayaraghavan et al., 1996), although three isolated studies also state the presence in low amounts of PP1α, PP1β, and PP1γ1 in hamster, human, and monkey, respectively (Fardilha et al., 2013; Smith et al., 1996; Suzuki, Fujinoki, Shibahara, & Suzuki, 2010). Testis contains both Ppp1cc gene isoforms, with PP1γ1 expressed only in the sertoli and spermatogonial cells (Sinha, Puri, Nairn, & Vijayaraghavan, 2013) and PP1γ2 expressed in developing germ cells and dramatically increasing post-meiotically around Day 15 (Chakrabarti, Cheng, Puri, Soler, & Vijayaraghavan, 2007; Kitagawa et al., 1990; Shima et al., 1993; Sinha et al., 2013). Past studies from our group have shown that catalytic activity of PP1γ2 is higher in immotile caput epididymal spermatozoa than in motile caudal epididymal spermatozoa suggesting a role for PP1γ2 in the initiation and regulation of sperm motility (Smith et al., 1996; Vijayaraghavan, Chakrabarti, & Myers, 2007; Vijayaraghavan et al., 1996). The Ppp1cc knockout studies have shown that animals are healthy, however, males are infertile due to impaired spermatogenesis (Chakrabarti, Cheng, et al., 2007; Chakrabarti, Kline, Lu, Orth, Pilder, & Vijayaraghavan, 2007; Varmuza et al., 1999). The lack of a phenotype in females and other tissues in the male is due to isoform replacement, whereas in testis during spermatogenesis there is no compensation for the loss of PP1γ2. In fact, our recent studies have shown that temporal and adequate levels of PP1γ2 are essential for spermatogenesis and male fertility (Sinha et al., 2013; Sinha, Pilder, & Vijayaraghavan, 2012). Interestingly, the knockout of Lmtk2 (lemur tyrosine kinase 2) that is also a PP1-binding partner, causes male infertility azoospermia with severe impairment of spermiogenesis beginning at the round spermatid stage similar to PP1γ knockouts (Kawa et al., 2006).
Similar to PP1 in general, PP1γ2 activity is also regulated by its binding partners (Fardilha et al., 2011; Korrodi-Gregorio et al., 2014; Silva, Freitas, & Fardilha, 2014; Vijayaraghavan et al., 2007). The regulators of sperm PP1γ2 identified so far are, PPP1R2 (inhibitor-2, I2) (Vijayaraghavan et al., 1996; Vijayaraghavan, Mohan, Gray, Khatra, & Carr, 2000), PPP1R11 (inhibitor-3, I3, tctex5) (Cheng, Pilder, Nairn, Ramdas, & Vijayaraghavan, 2009) and PPP1R7 (sds22) (Huang, Khatra, Bollen, Carr, & Vijayaraghavan, 2002; Mishra, Somanath, Huang, & Vijayaraghavan, 2003) which are ubiquitously expressed and evolutionarily ancient PP1 regulatory proteins (R) (Ceulemans, Stalmans, & Bollen, 2002).
The heat stable inhibitors PPP1R1 (inhibitor-1, I1) and PPP1R2 were the first PP1 inhibitors identified (Huang & Glinsmann, 1976). Unlike PPP1R1, PPP1R2 inhibits PP1 in its dephosphorylated form (Huang & Glinsmann, 1976). The inactive form of PP1 bound to PPP1R2 could be activated by PPP1R2 phosphorylation through glycogen synthase kinase 3 (GSK3) (Aitken et al., 1984; DePaoli-Roach, 1984; Hemmings, Resink, & Cohen, 1982; Smith, Wolf, Trautman, & Vijayaraghavan, 1999). The presence of PPP1R2-like activity was shown in heat stable sperm extracts (Smith et al., 1996; Vijayaraghavan et al., 1996; Vijayaraghavan et al., 2000). This activity was thought to be PPP1R2 because inhibition of PP1 could be reversed by GSK3 in the presence of ATP. More recently, mass spectrometry evidence showed the presence of PPP1R2 and two related pseudogenes (P), PPP1R2P3 and PPP1R2P9, in human sperm (Korrodi-Gregorio, Abrantes et al., 2013; Korrodi-Gregorio, Ferreira et al., 2013). There was no conclusive immunoblot or protein sequence evidence for the presence of PPP1R2 in spermatozoa (Lin et al., 2003) largely because of the lack of reliable antibodies and the apparent lability of the protein during extract preparation.
The inhibitor PPP1R11 is another ubiquitous heat-stable inhibitor of PP1 (Zhang, Zhang, Zhao, & Lee, 1998). PPP1R11 exhibits one of the characteristics of PPP1R2, where it is inhibitory in its dephosphorylated form (Zhang et al., 1998). It was discovered as a PP1-binding protein by yeast two-hybrid screening (Zhang et al., 1998). The gene for Ppp1r11 is within the t-complex (Pilder et al., 2007), a region associated with sperm motility defects and infertility. Northern blot analyses showed a transcript, highly expressed in testis, smaller in size compared with the ubiquitous message (Cheng et al., 2009; Han et al., 2007).
PPP1R7 was originally identified as a PP1-binding protein in Schizosaccharomyces pombe for its role in mitosis (Ohkura & Yanagida, 1991; Stone, Yamano, Kinoshita, & Yanagida, 1993). Later, an ortholog of PPP1R7 was identified in Caenorhabditis elegans genome (Wilson et al., 1994) and its homolog discovered in Saccharomyces cerevisiae (MacKelvie, Andrews, & Stark, 1995). Thereafter, orthologs of this yeast protein have been found in several organisms including mammals where it is ubiquitously expressed (Ceulemans et al., 1999; Chun et al., 2000; MacKelvie et al., 1995; Renouf et al., 1995). It appears that PPP1R7 could either activate (Ohkura & Yanagida, 1991) or inhibit PP1 catalytic activity depending on the phosphoprotein substrate used in the assay (MacKelvie et al., 1995; Ohkura & Yanagida, 1991). Similar to PPP1R11, a smaller mRNA message for PPP1R7 was detected in testis (Chun et al., 2000). The proteins PP1γ2, PPP1R7, and PPP1R11 exist as a trimeric complex in extracts of bovine testis and caudal epididymal spermatozoa (Cheng et al., 2009).
In this work, we show that all these regulators are expressed in testis at high levels along with PP1γ2. We have also shown that changes in the association of PP1γ2 with its regulators occur during sperm maturation in the epididymis. These changes were shown to occur in both bovine and mouse epididymal sperm. The alteration in binding of the regulators is due to changes in their phosphorylation. This study lays the basis for understanding the biochemical mechanisms underlying sperm maturation in the epididymis.
2 |. MATERIALS AND METHODS
2.1 |. Ethics statement
Balb/c mice used in this study were acquired from Jackson Laboratory and were housed and used at the Kent State University animal facility. The housing and handling was in accordance with the Kent State Institutional Animal Care and Use Committee under Protocol 268DK 09–09 approved by the National Institute of Environmental Health Sciences Animal Care and Use Committee.
2.2 |. RNA extraction and cDNA preparation
Approximately 100 mg of different tissues were homogenized in 1 ml of TRI reagent (Sigma Aldrich, Darmstadt, Germany), and total RNA was extracted using manufacturer instructions. For complementary DNA (cDNA), 1μg of total testis RNA was prepared using the QuantiTect Reverse Transcription Kit (Qiagen, Germantown, MD). Spermatid cDNA library was purchased from ATCC. The transcripts for testis-specific isoforms were isolated using reverse transcription polymearse chain reaction (PCR). The PCR products for Ppp1r2, Ppp1r11, and Ppp1r7 were subcloned into a pGEM-T Easy cloning vector (Promega, USA) and was sent to the Genomics Core at Lerner Research Institute for sequencing. For developmental studies, the total RNA was extracted from mouse testis at different days postpartum (dpp). Testis at 3–6 dpp mainly contains sertoli cell and undifferentiated spermatogonial stem cells. During 7–10 dpp, the spermatogonial stem cells undergo mitosis in testis and during 10–14 dpp type B spermatogonia differentiate to form primary spermatocytes and meiosis I starts. At 15–19 dpp, meiosis II begins and secondary spermatocytes are formed. Haploid postmeiotic round spermatids are formed at 18–22 dpp, and mature spermatozoa are present in the testis around 25–30 dpp.
2.3 |. Probes and primers
Ppp1r2 probe was generated as an ~750 bp restriction digested (XhoI and BamHI) fragment from the plasmid harboring the entire human I2 coding sequence in pBluescript SK II backbone (in house). Probe for Ppp1r11 was restriction digested as an ~1.6 kb fragment from the pGEX-4 T-2 vector harboring the full-length Ppp1r11 cDNA (Cheng et al., 2009). A probe of length 1091 bp spanning the entire coding sequence Ppp1r7 was amplified from the cDNA clone (ATCC# MG C-19201) using the primer pair 5′-CTCGAGGCCAATATGGCGGC AGAG-3′ and 5′-CTCAAGCTTAGGGCTCAGAACCTGACGTA-3′.
2.4 |. Northern blot analysis
Total RNA (20 or 25 μg) of each sample was mixed with three volumes of Northern Max Formaldehyde loading dye (Ambion, USA) and then was incubated at 65°C water bath for 15min. The samples are then centrifuged briefly and of ethidium bromide (10–50 μg/ml) was added and loaded in the denaturing formaldehyde agarose gel. The samples were electrophoretically separated on a 1.5% agarose/0.66 M formaldehyde gel in 1× MOPS buffer at 70 V for 4–5 hr. Following separation, RNA was immobilized on a positively charged Hybond-XL nylon membrane (GE Healthcare) by capillary transfer in 10× saline sodium citrate buffer (SSC). The transfer was allowed for 16–18 hr overnight, followed by baking at 85°C for 2 hr in vacuum oven. The membrane was prehybridized in 10 ml of Ultrahyb ultrasensitive hybridization buffer (Ambion, USA) in a hybridization bottle at 42°C for one hour. Probes were labeled by random labeling using 32P-dCTP (MP Biomed, Solon, OH) and the Rediprime nick translation kit (GE Healthcare) and finally purified using Illustra NICKTM columns (GE Healthcare), following the manufacturer’s protocol. The purified radiolabeled probes were diluted in 10 ml of Ultrahyb ultrasensitive hybridization buffer and added to the blot in a hybridization bottle. The blot was then incubated overnight at 42°C. After hybridization, the blot was washed twice in wash buffer I (1% SSC and 0.1% SDS), twice in wash buffer II (0.5% SSC and 1% SDS), and twice in wash buffer III (0.1% SSC and 1% SDS). All the washes were carried out at 42°C for 5 min. After washing, the membrane was wrapped in saran wrap and exposed to a phosphor-imager screen (Molecular Dynamics) and developed in a Typhoon scanner (GE Healthcare). For tissue northern blots, premade mouse tissue blots were bought from Zyagen. The integrity of the blotted RNA (20 μg per lane) is tested by a beta actin-specific probe, and it is also standardized by the presence of 28 s and 18 s RNA. Full-length cDNA of the inhibitors were used as probes.
2.5 |. Protein extract preparation
Mice tissues were homogenized in 2× homogenization buffer (20 mM Tris-HCl pH 7.0, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM ethylene glycol-bis(β-aminoethyl ether)-N.N.N′N′-tetraacetic acid (EGTA), 10 mM Benzamidine, 1 mM phenylmethylsulfonyl fluoride (PMSF), 0.1 mM of tosyl phenylalanyl chloromethyl ketone (TPCK), and 0.1% 2-mercaptoethanol) for 15 s with a 1min interval on ice using a homogenizer and spun-down at 16,000 × g for 15 min at 4°C. The supernatant was collected for further analysis. Mouse sperm was collected from the caudal epididymis and vas deferens. The contents of the vas deferens were gently squeezed out using forceps into a petri dish containing 1× phosphate buffer saline (PBS) and transferred to a 1.5 ml microcentrifuge tube using a cut pipette tip. The caudal epididymis was punctured using a needle, and the contents were gently squeezed into 1× PBS. The epididymis was kept in 1× PBS for 20 min to allow the sperm to swim out. The sperm suspension was collected using a cut pipette tip and pooled, if required, with the sperm from the vas deferens. The sperm suspension was washed twice in 1× PBS, centrifuged 400 × g at 4°C and resuspended in an appropriate volume of homogenization buffer and sonicated. After sonication, it was spun-down at 16,000 × g for 15 min at 4°C. The supernatant was collected for further analysis. For developmental studies, protein was extracted from mouse testis at different dpp. When needed, heat stable protein extracts were also prepared by heating at 95°C for 5 min, followed by centrifugation at 16,000 × g and posterior collection of the supernatant. Mature bull testis was obtained from a local commercial slaughterhouse. Spermatozoa were isolated from caput and caudal epididymis as previously described. Sperm isolates were washed twice in 1× PBS, pH 7.0. Sperm pellets were adjusted to a volume of 1.25 × 108 by resuspension in homogenization buffer with protease inhibitors (HB+; 10 mM Tris [pH 7.2] containing 1 mM EDTA, 1 mM EGTA, 10mM benzamidine-HCl, 1 mM PMSF, 0.1 mM TPCK, 0.1% 2-mercaptoethanol, and 1 mM sodium orthovanadate). The sperm suspension was sonicated on ice with three 10-s bursts (level three) of a Microson ultrasonic cell disruptor (Misonix Inc.). The suspension was then centrifuged at 16,000 × g for 20 min at 4°C. The supernatants were used for the immunoprecipitation experiments.
2.6 |. Antibodies
The primary antibody used for PPP1R2 was raised against the C-terminal region (STTSDHLQHKSQSS, see Supporting Information Figure S2 for validation). The PPP1R11 antibody (1:1,000 dilution) was raised against the synthetic peptide (EPENQSLTMKLRKR) and was previously validated (Cheng et al., 2009). The PPP1R7 antibody (Affinity Bio Reagents, 1:2,000 dilution) was raised against the synthetic peptide containing the 347–360, amino acid residues (LPSVRQIDATYVS) and was previously validated (Cheng et al., 2009). This antibody will recognize both isoforms. PPP1R2 and PPP1R11 antibodies were made by Yenzym Antibodies, LLC.
2.7 |. Western blot analysis
Protein extracts were quantified using a DC Assay Kit (Bio-Rad) after 10% trichloroacetic acid precipitation and the pellet resuspended in 0.1 N NaOH. Bovine serum albumin standards were subjected to the same treatment (Sigma Aldrich). The assay was performed according to the manufacturer instructions. Protein extracts were boiled in Laemmli sample buffer, electrophoretically separated on 12% gels and transferred on to a polyvinylidene difluoride membrane (Millipore, Burlington, MA) and blocked in 5% nonfat dry milk in 1× TTBS (Tris buffer saline with 0.1% Tween-20) for 1hr. Following blocking, the membrane was incubated with the appropriate primary antibody diluted in 5% nonfat dry milk in 1× TTBS overnight at 4°C on an orbital shaker. The following day, the membrane was rinsed in 1× TTBS and incubated with the appropriate secondary antibody conjugated with horseradish-peroxidase in 5% nonfat dry milk in 1× TTBS for 1 hr at room temperature. The membrane was rinsed twice with 1× TTBS for 10 min and developed using a “homemade” enhanced chemiluminescence reagent. The images were captured using the Fujifilm Darkbox LAS-3000 (Fuji).
2.8 |. Immunocytochemistry
Caudal epididymal spermatozoa were isolated in 1× PBS. The cells were fixed in 4% paraformaldehyde, EM grade (Electron Microscopy Sciences) in 1× PBS at 4°C for 20 min. The sperm suspension was then treated with 0.2% Triton-X (5 min) for permeabilization. After the fixation step, sperm were attached to poly-l-lysine-coated slides. To remove excess paraformaldehyde, the samples were washed three times with 1× TTBS. 1× TTBS supplemented with 5% normal goat serum was used as blocking solution and samples were incubated at room temperature for 3 hr followed by incubation with first primary antibody (1:200) diluted in 5% blocking buffer overnight at 4°C in a moist chamber. Anti-PPP1R2 (1:200), anti-PPP1R7 (1:200), and anti-PPP1R11 (1:200) were used as the first primary antibodies. Following day, the samples were washed with 1× TTBS and then incubated with Alexa Fluor 488 conjugated anti-rabbit secondary antibody (Jackson Immunoresearch) for 1 hr and 30 min at room temperature in the dark. All the samples were washed twice with 1× TTBS. The slides were then incubated with rabbit serum for 30 min to saturate open-binding sites on the first secondary antibody with IgG so that they cannot capture the second primary antibody. The slides were then washed again with 1× TTBS and incubated with unconjugated Fab antibody (Jackson Immunoresearch) for 45 min. This step is done to cover the rabbit IgG so that it does not bind to the second secondary antibody. The wash step with 1× TTBS is repeated. All the slides were then incubated with second primary antibody Anti-PP1γ2 (1:200) and kept in a dark, moist chamber overnight. Next day, the slides were washed in 1× TTBS and incubated with Cyanine 3 conjugated anti-rabbit second secondary antibody (Jackson Immunoresearch) for 2 hr. Slides were then washed and incubated with Hoechst dye for 10 min. Finally, the samples were washed three times with 1× TTBS and mounted in slides using the mounting media Prolong Diamond Antifade (Thermo Fisher Scientific). All the slides were examined under a fluorescence microscope (Olympus 81).
2.9 |. Immunohistochemistry
Testes from wild type (WT) mice were collected and fixed in 4% paraformaldehyde in PBS at 4°C for 6 hr. The fixed testes were transferred to 75% ethanol and dehydrated. They were permeabilized and embedded in paraffin using a Shandon Tissue Processor (Thermo Electron Corp., Waltham, MA). Multiple 5-μm-thick sections of the whole testis were attached to poly-l-lysine-coated slides, deparaffinized, and rehydrated using a standard procedure. Antigen retrieval was performed using 1× Antigen Retrieval Citra Solution (BioGenex, San Ramon, CA). Sections immersed in Citra solution were microwaved three times for 2 min, with a cooling period of 1 min between each heating cycle. Slides were incubated for 1 hr at room temperature in a blocking solution containing 5% normal goat serum (Jackson Immunoresearch Laboratory, West Grove, PA) in PBS. The slides were then incubated with primary antibodies for PP1γ2, PPP1R11, PPP1R2, and PPP1R7 (1:200) overnight at 4°C. Slides were washed three times with 1× PBS, and incubated with the appropriate secondary antibody (1:250) conjugated with Cy3 or Alexa fluor (Jackson Immunoresearch Laboratory) for 2 hr at room temperature. The slides were washed five times with PBS. Nuclei were labeled with Hoechst dye (Thermo Fisher Scientific Pierce, OR). The slides were mounted with Prolong Diamond Antifade Mountant (Thermo Fisher Scientific Pierce) mounting media, and examined using a Fluo View 500 Confocal Fluorescence Microscope (Olympus, Melville, NY).
2.10 |. Immunoprecipitation
Crude lysates of testis and sperm from mouse or bull were incubated for 2 hr at 4°C with Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) which were washed once with distilled water and twice with homogenization buffer (20 mM Tris-HCl pH 7.0, 1 mM EDTA, 1 mM EGTA, 10 mM Benzamidine, 1 mM PMSF, 0.1 mM of TPCK, and 0.1% 2-mercaptoethanol). It was then spun-down at 10,000 × g for 1 min and the supernatant was incubated with 5 μg of the appropriate antibody or diluted rabbit preimmune serum as a negative control, overnight with gentle rocking at 4°C. Following day, Protein G-Sepharose 4 Fast Flow beads (GE Healthcare) were washed once with distilled water and twice with 1× TTBS. Each extract/antibody solution was incubated with the beads by rocking for 2 hr at 4°C. After incubation, the beads were washed five times with 1× TTBS. After washing, the beads were resuspended in 2× SDS reducing sample buffer (6% SDS, 25 mM Tris-HCl pH 6.5, 50 mM dithiothreitol (DTT), 10% glycerol, and bromophenol blue), boiled for 10 min and centrifuged at 10,000 × g for 10 min. The supernatants were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), followed by western blot analysis.
2.11 |. Phosphoprotein enrichment from bull caput and caudal sperm
Phosphoprotein enrichment was carried out as per the manufacturer’s protocol using Pro-Q Diamond Phospho-enrichment Kit (Invitrogen, cat no. P33361). Bovine caput and caudal sperm were collected in TBS. It was then washed three times with TBS for 10 min at 700 g. Sperm pellet was resuspended in lysis buffer supplemented with endonuclease, protease inhibitors, and sodium orthovanadate. The samples were sonicated three times at 30% amplitude for 10 s. Protein estimation was carried out using modified BCA method as mentioned earlier. Enrichment columns were prepared by adding 200 μl of ethanol to wet the column. One milliliter of resin was added to each column. Resin was washed twice with 1 ml of dH2O and in the meanwhile 1 mg of samples were diluted with wash buffer to get the final concentration of 0.1 mg/ml. Columns were equilibrated by passing 1 ml of wash buffer twice. Diluted samples were passed through the column and flow-through was collected. Columns were washed with 1 ml of wash buffer three times, and phosphoproteins were eluted by passing 250 μl elution buffer five times. Eluted phosphoproteins and flow-through were concentrated using 3 kDa amicon ultra centrifugal filter units and buffer exchange was performed to 25 mm Tris and 0.25% CHAPS. Methanol-chloroform precipitation was carried out and air-dried pellet was reconstituted in 6× gel loading buffer and was subjected to western blot analysis.
3 |. RESULTS
3.1 |. Expression of PPP1R2, PPP1R11, and PPP1R7 in testis
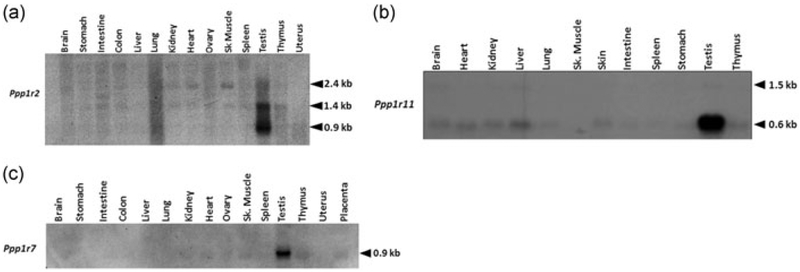
It is known that PP1γ2, expressed at high levels in testis, is present as the sole, if not predominant, isoform in sperm (Chakrabarti, Cheng, et al., 2007; Chakrabarti, Kline, et al., 2007). The three inhibitors of the protein phosphatase PP1, PPP1R2, PPP1R11, and PPP1R7 (also known as inhibitors I2, I3, and sds22) are found in a wide range of organisms and tissues. The levels of proteins regulators are expected to match levels of the phosphatase to prevent unregulated catalytic activity of the enzyme. We, therefore, expected that these three proteins should be expressed in testis at significant levels compared with other tissues. This expression should also be synchronized with the expression of PP1γ2 in testis. We determined levels of transcripts for these proteins in testis compared with other tissues. We used a northern blot of RNA from different mouse tissues probed with cDNA corresponding to the coding sequence of PPP1R2, PPP1R11, or PPP1R7. A transcript for Ppp1r2, around 0.9 kb, is present almost exclusively in testis (Figure 1a). In addition, a 1.4 kb mRNA also predominant in testis is present in lower amounts in other tissues. A band at 2.4 kb is present in relatively low levels in all tissues. For Ppp1r11 northern blot analysis showed that mRNA species at 0.6 kb is present at significantly higher levels in testis compared with other tissues. A band at 1.5 kb was present at relatively low levels in all tissues. Tissue blot probed with Ppp1r7 cDNA showed a unique message size of ~0.9 kb abundant in the testis compared with other tissues (Figure 1c). Thus, overall, unique message sizes for all the three proteins were present in significantly high levels in testis compared with other tissues.
FIGURE 1.
mRNA analysis of PP1γ2 binding partners in tissues. (a) Northern blot analysis of total RNA (25 μg) from mouse tissues shows a~0.9 kb unique Ppp1r2 (I2) message in testis which is different from the ubiquitous 1.4 kb message. Equal amounts were loaded in each lane. (b) Northern blot analysis of total RNA (25 μg) from mouse tissues shows abundant expression of 0.6 kb message of Ppp1r11 (I3) in testis apart from the ubiquitously expressed Ppp1r11 message (1.5 kb). Equal amounts were loaded in each lane. (c) Northern blot analysis of total RNA (25 μg) demonstrating the specific expression of a unique message for Ppp1r7 (sds22) in testis. Equal amounts were loaded in each lane
3.2 |. Expression of the inhibitors in postnatal developing testis
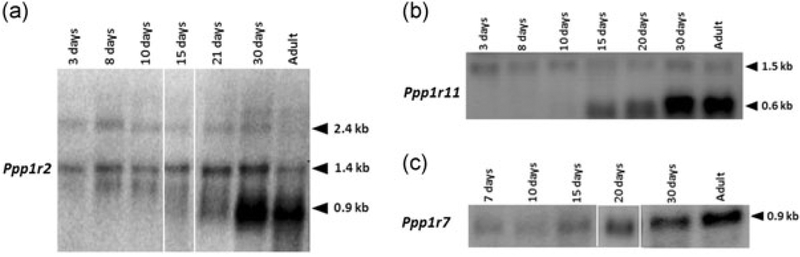
To determine the stage of spermatogenesis at which the message for the Ppp1r2 was expressed, total RNA was prepared from postnatal developing mouse testis. Northern blot analysis showed that the 0.9 kb Ppp1r2 message starts increasing from Day 21 coinciding with the appearance of round spermatids and reaching a maximum in adult testis (Figure 2a). The 1.4 and 2.4 kb messages remain relatively constant at these developing stages. We next determined the expression levels of the other two PP1 inhibitors. Northern blot shows that the 0.6 kb mRNA for Ppp1r11 is first detected between Days 15–20 of postnatal testis which corresponds with an emergence of early/mid pachytene spermatocytes and round spermatids (Figure 2b). The message level further increases in Day 30 postnatal testis, when elongating spermatids are formed and remains elevated in adult testis. For Ppp1r7, the 0.9 kb mRNA was detected starting from Day 7 and with the progressive development of the testis, the levels of this mRNA significantly increased particularly after Days 15–20, reaching a maximum in the adult mice (Figure 2c).
FIGURE 2.
mRNA analysis of PP1γ2 testis enriched binding partners in developmental testis. (a) Total RNA (25 μg) extracted from developing testis was also subjected to northern blot analysis and showed a steady expression of both 2.4 and 1.4 kb ubiquitous Ppp1r2 messages but showed a gradual increase of the ~0.9 kb testis-specific isoform from Day 20 to adult testis. The 1.4 kb message levels also increase markedly post-meiotically in elongating spermatids and remains elevated in adult testis. Equal amounts were loaded in each lane. (b) Northern blot analysis of testis total RNA (25 μg) demonstrating the developmental expression of Ppp1r11 isoforms when probed with the whole cDNA of Ppp1r11. The testis-specific isoform of Ppp1r11 is highly expressed post-meiotically. Equal amounts were loaded in each lane (c) northern blot analysis of testis total RNA (25 μg) demonstrating the developmental expression of Ppp1r7 alternate isoform when probed with whole cDNA of Ppp1r7. The alternate isoform of Ppp1r7 is highly expressed post-meiotically. Equal amounts were loaded in each lane
We also examined the levels of Ppp1r2, Ppp1r11, and Ppp1r7 by northern blot analyses of total RNA from Ppp1cc−/− testis mice which lack PP1γ2. Comparison of the message levels of Ppp1r2 revealed that the 0.9 kb transcript was decreased in Ppp1cc−/−testis when compared with wild type whereas the levels of the other transcripts for Ppp1r2 were unaltered. Similarly, the messages for Ppp1r11 and Ppp1r7 (Supporting Information Figure S2) decreased in Ppp1cc−/− mouse testis. Thus, the unique testis expressed messages for the three inhibitors were reduced or absent in testis of Ppp1cc−/− compared with wild-type mice.
3.3 |. Protein expression of PP1 inhibitors in testis
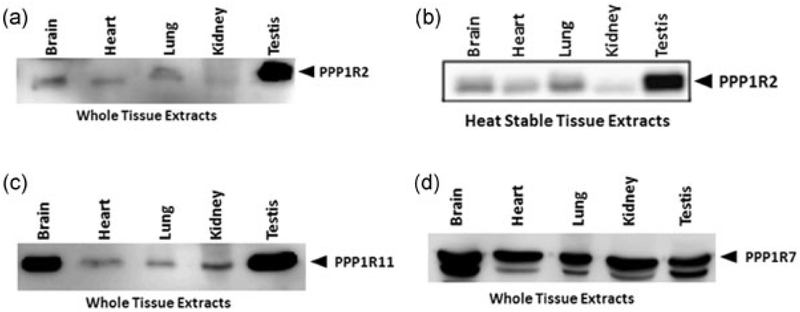
The presence of PPP1R7 and PPP1R11 have been shown in bovine sperm (Cheng et al., 2009). Well-characterized antibodies for these proteins exist. The antibodies were generated against conserved amino acid sequence segments and thus should be able to recognize the proteins in other species including mice. A western blot of extracts from various tissues shows that PPP1R11 is highly expressed in testis with two bands seen at molecular weights around 26 kDa (Figure 3c). PPP1R11, which is hydrophilic and rich in amino acid residues, migrates anomalously at 26 kDa while its calculated molecular weight is 15 kDa. Similar to PPP1R11, the western blot of whole tissue extracts probed for PPP1R7 shows two bands around 43 kDa with higher amounts in testis (Figure 3d). The two bands for PPP1R7 were also seen in bovine testis and sperm extracts.
FIGURE 3.
Protein analysis of PP1γ2 testis enriched binding partners in different mouse tissues. Western blots using the new C-terminus PPP1R2 antibody in (a) whole protein extracts and in (b) heat-stable extracts of brain, heart, lung, kidney, and testis. Both blots show the PPP1R2 (I2) protein at 32 kDa position and that it is heat stable. (c) Western blots developed with PPP1R11 (I3) and (d) PPP1R7 (sds22) antibodies in brain, heart, lung, kidney, and testis both with whole protein extracts show bands at 26 and 43 kDa, respectively
Definitive identification of PPP1R2 protein has been hampered by the lack of an appropriate antibody. An antibody raised against internal amino acids of PPP1R2 (135REKKRQFEMKRKLH148) while reacting against recombinant PPP1R2 due to low affinity was unable to detect heat stable PPP1R2 in testis and sperm extracts (data not shown). We generated a C-terminus antibody for PPP1R2 (STTSDHLQHKSQSS). This antibody recognized recombinant PPP1R2 (Supporting Information Figure S3) and PPP1R2 in a panel of tissues, the protein being most abundant in testis compared with other tissues (Figure 3a). It is known that PPP1R2 migrates anomalously in SDS gel electrophoresis at 32 kDa compared with its calculated molecular weight of 23 kDa, due to its hydrophilicity and acidity (Korrodi-Gregorio, Abrantes et al., 2013; Korrodi-Gregorio, Ferreira et al., 2013). More important, being a heat-stable protein, the antibodies were able to recognize the protein in heat stable tissue extracts (Figure 3b) at a position identical to whole tissue extracts.
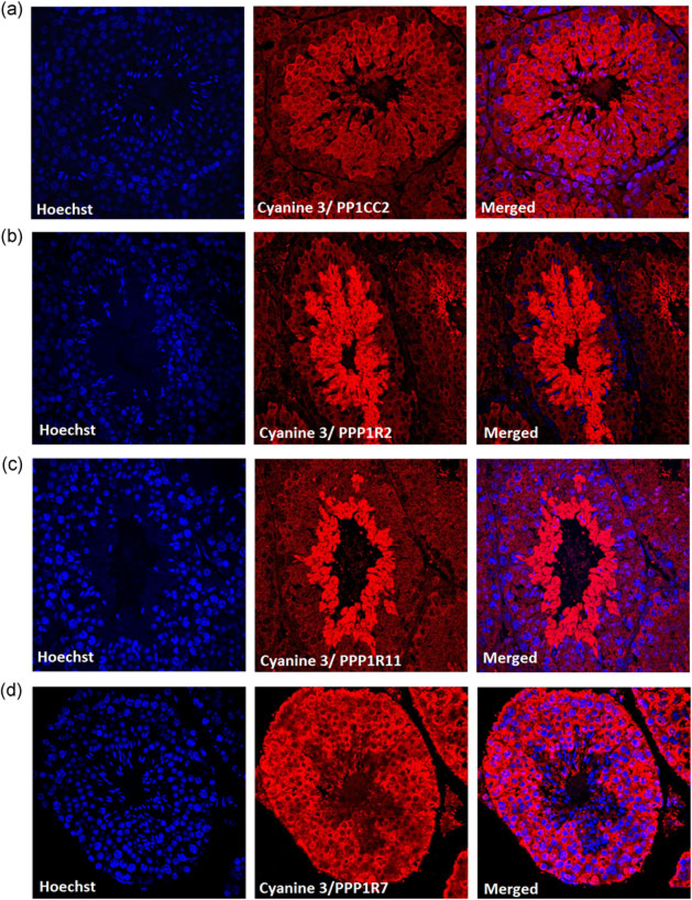
3.4 |. Localization of PP1γ2 and its inhibitors in testis section
The immunohistological analysis of testis section show presence of PP1γ2 (Figure 4a) from secondary spermatocytes onward. PPP1R11 (Figure 4c), and PPP1R2 (Figure 4b) are predominant in starting from spermatids to mature spermatozoa. Whereas PPP1R7 (Figure 4d) is seen from spermatogonia to mature spermatozoa. Expression of PPP1R11 and PPP1R7 has been previously reported, here, we show that PPP1R2 is also expressed in a similar way. Thus, all the three inhibitors and PP1γ2 share expression from round spermatids to mature spermatozoa.
FIGURE 4.
Distribution of PP1γ2 and its binding partners in testis section. Immunohistochemistry of wild-type testis section show (a) PP1γ2 expression from secondary spermatocytes cytoplasm, spermatids, and mature spermatozoa. (b) PPP1R2 and (c) PPP1R11 is expressed in round spermatids and mature spermatozoa whereas (d) PPP1R7 is expressed throughout the cytoplasm of primary spermatocyte secondary spermatocyte round spermatid and mature spermatozoa. Cyanine 3 was used as a secondary antibody to visualize the proteins. Hoechst dye was used to stain the nucleus
3.5 |. The presence and distribution of PPP1R2, PPP1R7, and PPP1R11 in mouse sperm
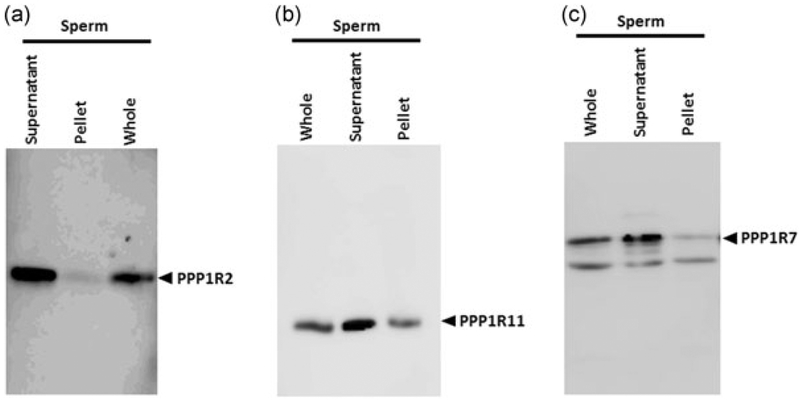
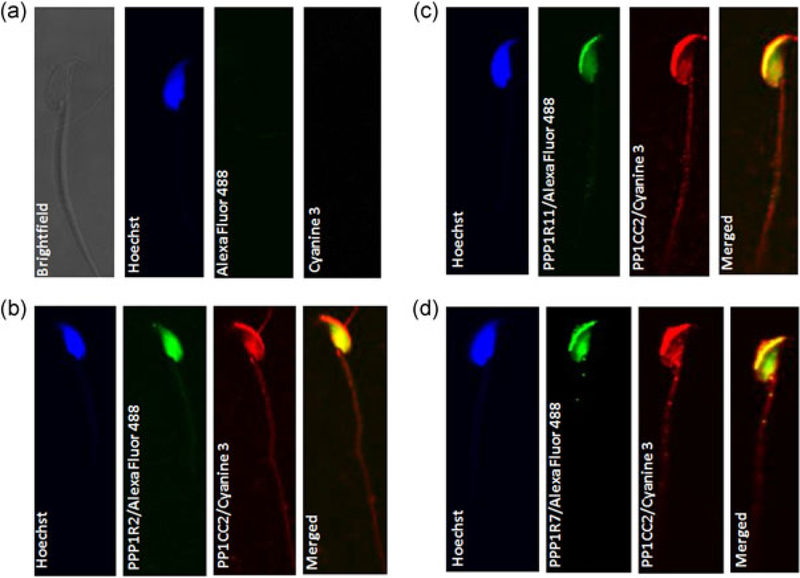
We have previously documented the presence of PPP1R11 and PPP1R7 in bovine sperm (Cheng et al., 2009). The presence of the inhibitor PPP1R2 was inferred based on the effect of GSK3 in increasing sperm PP1 catalytic activity. Our next objective was to determine the presence of the three regulators in mouse sperm. Figure 5 shows that PPP1R2, R7, and R11 are present in caudal epididymal sperm. The proteins are predominantly present in the soluble fraction of sperm extracts. Immunofluorescence was used to determine where PP1γ2 and its regulators PPP1R2, PPP1R11, and PPP1R7 are present within sperm. We used monovalent Fab fragment for blocking and for double labeling primary antibodies from the same host species. As seen in Figure 6b–d, PP1γ2 (Cyanine 3) is present along the entire length of the flagellum, in the mid- and principal pieces, and neck. In the head, staining is intense in the acrosome region and in the equatorial segment. PPP1R11 and PPP1R7 colocalize with PP1γ2 in acrosome region, but PPP1R2 is present in the entire head region except the acrosome. The inhibitors are present along the entire flagellum, although PPP1R2 and PPP1R11 seems to be more prominent in the mid- and principal pieces, whereas PPP1R7 is mostly present in the principal piece (Figure 6b–d). The inhibitors are also present in the head of spermatozoa and colocalize with PP1γ2 (merged figure shown in yellow).
FIGURE 5.
Distribution of PP1γ2 testis enriched binding partners in soluble and insoluble fraction of sperm lysate. Western blot analysis was done to determine the supernatant versus pellet distribution of PPP1R2, PPP1R11, and PPP1R7 in sperm was done to determine the relative distribution of PPP1R2, PPP1R11, and PPP1R7, probed with the respective antibodies. (a) After sonication, PPP1R2 comes out mostly in the supernatant and is heat stable although there is some present in the pellet. (b,c) The proteins PPP1R11 and PPP1R7 are present in both sperm supernatant and pellet, although in higher amounts in the first
FIGURE 6.
Colocalization of γ and PPP1R2, PPP1R7 and PPP1R11 in morphologically normal mouse spermatozoa. Mouse spermatozoa were labeled with fluorescence conjugated secondary antibody as (a) negative control, (b) rabbit anti-PPlγ2 rabbit anti-PPP1R2, (c) rabbit anti-PPlγ2 rabbit anti-Ppp1r11, and (d) rabbit anti-PPly2 rabbit anti-PPP1R7 antibodies. Specific secondary antibodies conjugated with Cyanine 3 fluorophores, and Alexa Fluor 488 were used. To identify the nucleus, a Hoechst dye (blue) was used. Merged pictures show that PPP1R2, PPP1R11, and PPP1R7 are localized in mouse spermatozoa head region as well as in the mid-piece and principle piece just like PP1γ2. (To see the flagellum staining for the inhibitors refer to Supporting Information Figure S6.)
3.6 |. Binding of PP1γ2 to its inhibitors changes during epididymal sperm maturation
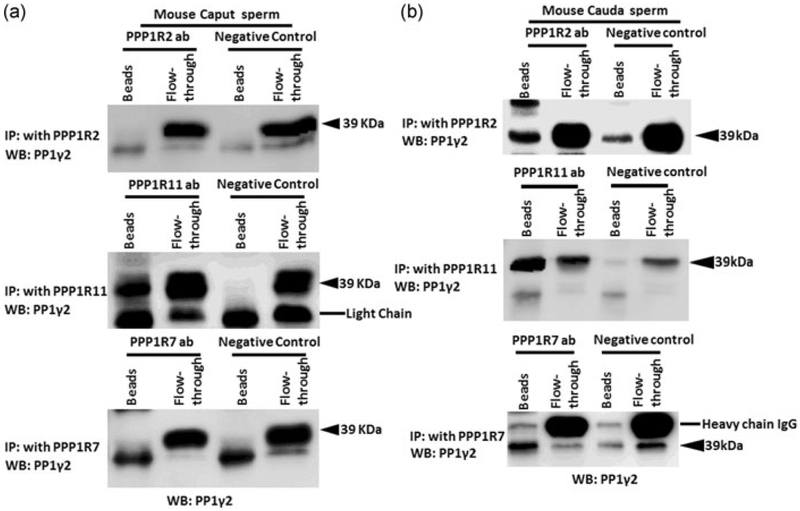
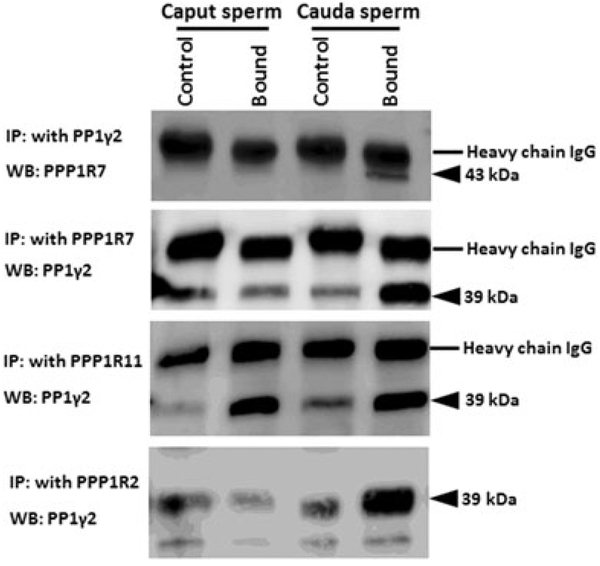
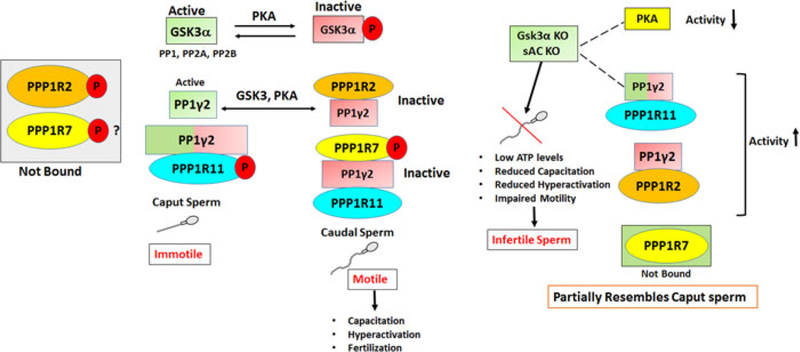
The inhibitors and PP1γ2 shared localization within sperm. We next determined whether the inhibitors were bound to PP1γ2 in sperm. Binding of PPP1R7 and PPP1R11 to PP1γ2 in bovine caudal epididymal sperm is reported (Cheng et al., 2009). We next determined whether the inhibitors were bound to PP1γ2 and whether their binding is altered during passage of sperm through the epididymis. We performed immunoprecipitation experiments with sperm extracts to determine whether antibodies to the inhibitors co-precipitated PP1γ2. Immunoprecipitation was performed with mouse caput and caudal epididymal sperm extracts. Antibodies to PPP1R2 and PPP1R7 precipitate PP1γ2 in caudal but not caput sperm extracts (Figure 7a,b). That is, PP1γ2 is bound to PPP1R2 and PPP1R7 in caudal but not in caput sperm. Figure 6 also shows that PPPR11 antibody precipitates PP1γ2 from both caput and caudal sperm extracts. That is, PP1γ2 is bound to PPP1R11 in both caput and caudal sperm. Table 1 summarizes the binding data determined in Figure 7. These binding data were also confirmed by reciprocal IP with PP1γ2 antibody and probed with antibodies to each of the inhibitor proteins (data not shown). We also determined whether inhibitor binding also exists in bovine epididymal as in mouse epididymal sperm. Data in Figure 8 show that same differences in the binding of PP1γ2 to its inhibitors are also found in bovine caput and caudal epididymal sperm as in mouse epididymal sperm. That is, PP1γ2 is associated with all three regulators in caudal sperm but only with PPP1R11 in caput epididymal sperm.
FIGURE 7.
(a,b) Differential interaction of PP1γ2 with its inhibitors in mouse caput and caudal epididymal sperm. Immunoprecipitation with PPP1R7 (43 kDa) and PPP1R2 (32 kDa) in caput and caudal sperm extracts show that PP1γ2 do not bind with regulators PPP1R7 and PPP1R2 in (a) mouse caput sperm extracts but bind in mouse caudal sperm extracts (B). Immunoprecipitation with PPP1R11 antibody shows that it binds with PP1γ2 in (a) caput as well as in (b) caudal sperm extracts
TABLE 1.
The status of binding of the inhibitors with PP1y2 in caput and caudal epididymis is summarized in the table along with Gsk3a KO and sAC KO mice
| Wild type | GSK3α KO | sAC KO | ||||
|---|---|---|---|---|---|---|
| Inhibitors | Caput | Cauda | Caput | Cauda | Caput | Cauda |
| PPP1R2 | Not bound | Bound | Not bound | Bound | Not bound | Bound |
| PPP1R7 | Not bound | Bound | Not bound | Not bound | Not bound | Not bound |
| PPP1R11 | Bound | Bound | Bound | Bound | Bound | Bound |
Note. PPP1R2, PPP1R7, and PPP1R11 are bound to PP1y2 in caudal epididymis whereas PPP1R7, PPP1R2 remains unbound in caput epididymis. Caudal sperm from Gsk3a KO and sAC ko behave partially like wild-type caput sperm.
FIGURE 8.
Differential binding of PP1γ2 to PPP1R2, PPP1R7, and PPP1R11 in bull caput and caudal epididymal sperm. Immunoprecipitation s were done using PP1γ2, PPP1R7, PPP1R11, or PPP1R2 antibodies in bull caput and caudal sperm extracts and western blots were then probed with PPP1R7 or PP1γ2 antibodies. PP1γ2 antibody precipitates PPP1R2 and PPP1R7 from extracts of caudal but not from caput epididymal sperm. This is confirmed by reciprocal immunoprecipitation with a PPP1R7 antibody. Antibodies to PPP1R11 precipitate PP1γ2 from both caput and caudal sperm extracts. (Note: Western blots of PP1γ2 IP developed with PPP1R11 antibodies are not shown because the band for PPP1R11 runs close to the IgG light chain.)
3.7 |. Differential binding with PP1γ2 is due to a change in phosphorylation status
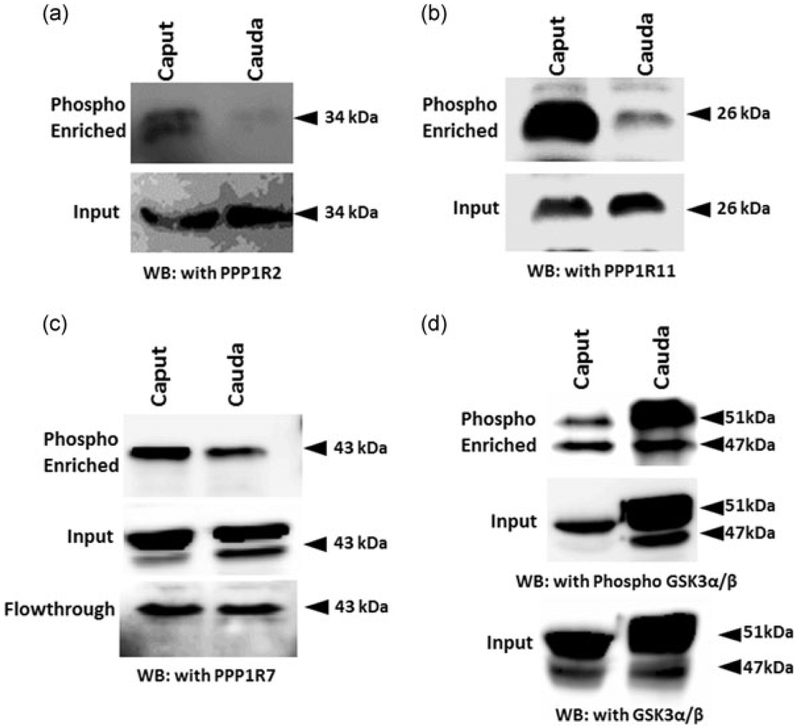
We suspected that the change in binding of the inhibitors to PP1γ2 is likely due to differences in their phosphorylation status. Because phospho-specific antibodies for these protein are not available, we determined the phosphorylation status of the inhibitors using Pro-Q diamond phosphoprotein enrichment columns. Extracts before and after phospho-enrichment were compared using extracts from equal numbers of caput and caudal epididymal sperm. Figure 9 shows that both PPP1R2 and PPP1R11 are significantly phosphorylated in caput sperm compared with caudal sperm. Their levels were identical in caput and caudal sperm before phosphoprotein enrichment. A portion of inhibitor PPP1R7 is almost equally phosphorylated in both caput and caudal sperm. A considerable proportion of PPP1R7 is not phosphorylated in both caput and caudal sperm as evidenced by its presence in the unbound flow-through fraction of the Pro-Q diamond columns. The ability of the procedure to enrich phospho-proteins was verified with GSK3 which is known to be more phosphorylated in caudal compared with caput sperm (Somanath, Jack, & Vijayaraghavan, 2004).
FIGURE 9.
The inhibitors are differentially phosphorylated in caput and caudal epididymis. Bull caput and caudal sperm lysate was phosphor enriched by using columns. Western blot analysis of the sample show that (a) PPP1R2 and (b) PPP1R11 is highly phosphorylated in caput sperm lysate whereas (c) PPP1R7 phosphorylation status is comparable in caput and caudal sperm lysate. Input samples were also ran to show equal loading. Phospho-enriched the samples were probed with phospho-GSK3α/β and GSK3α/β as controls as it is already reported that the GSK3 phosphorylation is high in caput. GSK3: glycogen synthase kinase 3
4 |. DISCUSSION
Protein phosphatases, in general, are regulated by one or more of several proteins (Aggen, Nairn, & Chamberlin, 2000). An important question relevant to PP1 regulation is how the amounts of the protein regulators compare with the levels of the enzyme in a cell.
The levels of the regulators, PPP1R2, PPP1R7, and PPP1R11 must match that of PP1γ2 which is expressed starting from initiation of spermatogenesis which occurs around Day 10 postnatal testis in sperm. We show that increased and overlapping expression of all three inhibitors with PP1γ2 occurs in developing testis starting with the onset of spermatogenesis (Figure 2). Their localization within testis by immunohistochemistry (IHC) show that PPP1R2 and PPP1R11 are predominant in developing haploid cells. The protein PPP1R7 has a more uniform localization within testis. However, the unique sized messenger RNA for PPP1R7 is more abundant coinciding with the onset of spermatogenesis. Remarkably all three inhibitors are expressed as unique mRNA forms in testis compared with other tissues (Figure 1). It is intriguing that the unique mRNA sizes for the inhibitors, found only in testis are expressed at high levels coinciding with onset of spermatogenesis. The mRNA forms found in somatic cells do not change in developing testis. Why the unique message forms exist and how their expression is coordinated with an expression of PP1γ2 during the onset of spermatogenesis are not known. Preliminary analysis with genomic and EST databases suggest that the unique sizes of the mRNA likely arise due to alternate splicing, alternate transcript start sites, and alternate polyadenylation (data not shown). It is likely that common regulatory elements for transcription exist in the genes for PP1γ2 and its three inhibitors enabling increased transcription during spermatogenesis. What these regulatory elements and the corresponding transcription factors are, remains to be determined.
Sperm maturation in the epididymis is likely to involve changes in protein phosphorylation. It was thought that elevation of cAMP levels and activation of PKA initiate motility in epididymal sperm (Vijayaraghavan et al., 1996; Vijayaraghavan et al., 2007). How cAMP action is altered in epididymal sperm is not known. However, it was later noted that cAMP and PKA action in sperm is limited by protein phosphatase activity (Vijayaraghavan et al., 1996; Vijayaraghavan et al., 2007). The activity of sperm PP1γ2 is high in immotile caput compared with motile caudal sperm. The PP1 inhibitors calyculin A and okadaic acid cause motility initiation and stimulation (Vijayaraghavan et al., 1996) suggesting that a decline in PP1γ2 activity may contribute to motility initiation in epididymal sperm. How this decline in PP1γ2 activity during sperm maturation occurs was not known. Here we show, for the first time, that association of PP1γ2 with its proteins regulators is altered in sperm during their passage through the epididymis. In immotile caput sperm, PP1γ2 is not bound to PPP1R2 or PPP1R7. It is known that PP1 when not bound to PPP1R2 is catalytically active (DePaoli-Roach, 1984; Vijayaraghavan et al., 1996). It is also known that PP1 found as a trimeric complex with PPP1R7 and R11 is catalytically inactive (Cheng et al., 2009; Lesage et al., 2007). Thus one of the likely reasons for the high catalytic activity of PP1γ2 in caput sperm is because it is not associated with the inhibitors PPPP1R2 and PPP1R7. In caudal sperm, all the three inhibitors are bound to PP1γ2. We hypothesized that differences in binding of the inhibitor proteins to PP1γ2 are due to differences in their phosphorylation. Indeed, phosphoprotein enrichment shows that PPP1R2 and PPP1R11 are phosphorylated in caput compared with caudal sperm. Phosphorylation of PPP1R7 is more or less comparable in caput and caudal sperm. A substantial portion of PPP1R7 is not phosphorylated in both caput and caudal sperm (Figure 9). Thus, lack of binding of PPP1R7 to PP1γ2 is most likely due to phosphorylation of PPP1R11 in caput sperm. That is, phosphorylated PPP1R11 bound to PP1γ2 in caput sperm may prevent binding of PPP1R7 thus preventing the formation of the trimer that is present in caudal sperm. It is already known that PPP1R2 remains phosphorylated in caput sperm by GSK3, so data here confirm this observation. Simultaneously we also found that PPP1R11 is highly phosphorylated in caput sperm, even though it remains bound to PP1γ2 in caput sperm. We can speculate from this that high phosphorylation of PPP1R11 prevents binding of PPP1R7 with PP1γ2. We have previously shown that in bovine caudal sperm PP1γ2 as a trimeric complex with PPP1R7, PPP1R11 is catalytically inactive (Cheng et al., 2009). Therefore epididymal sperm maturation involves dephosphorylation of PPP1R11 and PPP1R2. This is a surprising result because we anticipated that in general phosphorylation of protein increase in sperm during epididymal maturation. Thus decreased rather than increased phosphorylation may be responsible for sperm motility initiation in the epididymis. The protein kinase responsible for both PPP1R2 and PPP1R11 phosphorylation in caput epididymis could be GSK3. We have shown that GSK3 activity in caput sperm is significantly higher than caudal sperm (Somanath et al., 2004). It is well known that GSK3 is an enzyme that phosphorylates PPP1R2. Verification of whether the amino acid sequence domain in PPP1R11 corresponds to the consensus sequence for GSK3 phosphorylation remains to be determined. In testis binding of all three inhibitors resemble caudal sperm (Supporting Information Figure 4). Therefore phosphorylation of PPP1R11 and lack of binding of PPP1R7 to PP1γ2 occurs only in caput sperm. We have shown that in caudal sperm from GSK3α and soluble adenylyl cyclase knockout mice PPP1R7 is not bound to PP1γ2, suggesting impaired epididymal sperm maturation (Bhattacharjee et al., 2018) (Supporting Information Figure S5). Pull-down experiments with phosphorylated and unphosphorylated recombinant PPP1R11 along with recombinant PPP1R7 should provide further verification for our suggestion that phosphorylation of PPP1R11 prevents PPP1R7 from forming a trimeric complex with PP1g2. Direct examination of how these complexes form and change within sperm is not feasible. However, our data clearly show that phosphorylation changes in PPP1R2 and R11 occur during epididymal sperm maturation. Future studies will be directed towards determining how changes in phosphorylation of the regulators and thus epididymal sperm maturation can be prevented by pharmacological or by genetic approaches. It was recently suggested that a noncanonical Wnt signaling resulting in inactivation of GSK3 is involved in epididymal sperm maturation (Koch, Acebron, Herbst, Hatiboglu, & Niehrs, 2015). Thus Wnt signaling resulting in decreased phosphorylation of the regulatory proteins of PP1γ2 could be a key signaling event responsible for the acquisition of sperm motility in the epididymis.
In summary, we have shown that expression levels of the regulators of PP1γ2 match the expression of the enzyme during spermatogenesis. Appropriate levels of the inhibitors should be required for regulation of PP1γ2 in sperm. We also show for the first time that binding of PPP1R2, PPP1R11, and PPP1R7 to PP1γ2 changes during passage of sperm through the epididymis. The changes in binding to PP1γ2 occur due to decreased phosphorylation of the inhibitors PPP1R2 and PPP1R11. We suspect that GSK3 could be one of the protein kinases that is responsible for increased phosphorylation of PPP1R2 and PPP1R11. The possible roles of how changes in phosphorylation are involved in epididymal sperm maturation is shown in Figure 10. This study reveals, for the first time, the basis for understanding the biochemical mechanisms underlying sperm maturation in the epididymis.
FIGURE 10.
Schematic diagram of how PP1y2 is regulated by its inhibitors in sperm during its transition from caput to caudal epididymis
Supplementary Material
ACKNOWLEDGMENT
We sincerely thank Dr. Shandilya Ramdas for his contribution and his expertise.
Funding information
Eunice Kennedy Shriver National Institute of Child Health and Human Development, Grant/Award Numbers: R21HD086839, R15HD068971; Fundação para a Ciência e a Tecnologia, Grant/Award Numbers: SFRH/BPD/91766/2012, UID/BIM/04501/2013, POCI-01–0145-FEDER-007628
Footnotes
CONFLICTS OF INTEREST
The authors declare that there is no conflicts of interest that could be perceived as prejudicing the impartiality of the research reported.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- Aggen JB, Nairn AC, & Chamberlin R (2000). Regulation of protein phosphatase-1. Chemistry & Biology, 7(1), R13–R23. [DOI] [PubMed] [Google Scholar]
- Aitken A, Holmes CFB, Campbell DG, Resink TJ, Cohen P, Leung CTW, & Williams DH (1984). Amino acid sequence at the site on protein phosphatase inhibitor-2, phosphorylated by glycogen synthase kinase-3. Biochimica et Biophysica Acta, 790(3), 288–291. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee R, Goswami S, Dey S, Gangoda M, Brothag C, Eisa A, … Vijayaraghavan S (2018). Isoform specific requirement for GSK3α in sperm for male fertility. Biology of Reproduction, 99(2), 384–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen M, Peti W, Ragusa MJ, & Beullens M (2010). The extended PP1 toolkit: Designed to create specificity. Trends in Biochemical Sciences, 35(8), 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceulemans H, Stalmans W, & Bollen M (2002). Regulator-driven functional diversification of protein phosphatase-1 in eukaryotic evolution. BioEssays: News And Reviews In Molecular, Cellular And Developmental Biology, 24(4), 371–381. [DOI] [PubMed] [Google Scholar]
- Ceulemans H, Van Eynde A, Perez-Callejon E, Beullens M, Stalmans W, & Bollen M (1999). Structure and splice products of the human gene encoding sds22, a putative mitotic regulator of protein phosphatase-1. EuropeaN Journal Of Biochemistry/FEBS, 262(1), 36–42. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Cheng L, Puri P, Soler D, & Vijayaraghavan S (2007). Protein phosphatase PP1 gamma 2 in sperm morphogenesis and epididymal initiation of sperm motility. Asian Journal Of Andrology, 9(4), 445–452. [DOI] [PubMed] [Google Scholar]
- Chakrabarti R, Kline D, Lu J, Orth J, Pilder S, & Vijayaraghavan S (2007). Analysis of Ppp1cc-null mice suggests a role for PP1gamma2 in sperm morphogenesis. Biology of Reproduction, 76(6), 992–1001. [DOI] [PubMed] [Google Scholar]
- Cheng L, Pilder S, Nairn AC, Ramdas S, & Vijayaraghavan S (2009). PP1gamma2 and PPP1R11 are parts of a multimeric complex in developing testicular germ cells in which their steady state levels are reciprocally related. PLoS One, 4(3), e4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun YS, Park JW, Kim GT, Shima H, Nagao M, Kim MS, & Chung MH (2000). A sds22 homolog that is associated with the testis-specific serine/threonine protein phosphatase 1gamma2 in rat testis. Biochemical and Biophysical Research Communications, 273(3), 972–976. [DOI] [PubMed] [Google Scholar]
- Cohen PT (2002). Protein phosphatase 1--targeted in many directions. Journal of Cell Science, 115(Pt 2), 241–256. [DOI] [PubMed] [Google Scholar]
- DePaoli-Roach AA (1984). Synergistic phosphorylation and activation of ATP-Mg-dependent phosphoprotein phosphatase by F A/GSK-3 and casein kinase II (PC0.7). The Journal of Biological Chemistry, 259(19), 12144–12152. [PubMed] [Google Scholar]
- Fardilha M, Esteves SL, Korrodi-Gregorio L, da Cruz e Silva OA, & da Cruz e Silva FF (2010). The physiological relevance of protein phosphatase 1 and its interacting proteins to health and disease. Current Medicinal Chemistry, 17(33), 3996–4017. [DOI] [PubMed] [Google Scholar]
- Fardilha M, Esteves SLC, Korrodi-Gregorio L, Pelech S, da Cruz e Silva OAB, & da Cruz e Silva E (2011). Protein phosphatase 1 complexes modulate sperm motility and present novel targets for male infertility. Molecular Human Reproduction, 17(8), 466–477. [DOI] [PubMed] [Google Scholar]
- Fardilha M, Ferreira M, Pelech S, Vieira S, Rebelo S, Korrodi-Gregorio L,… da Cruz e Silva EF (2013). “Omics” of human sperm: Profiling protein phosphatases. Omics: A Journal Of Integrative Biology, 17(9), 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YB, Feng HL, Cheung CK, Lam PM, Wang CC, & Haines CJ (2007). Expression of a novel T-complex testis expressed 5 (Tctex5) in mouse testis, epididymis, and spermatozoa. Molecular Reproduction and Development, 74(9), 1132–1140. [DOI] [PubMed] [Google Scholar]
- Hemmings BA, Resink TJ, & Cohen P (1982). Reconstitution of a Mg-ATP-dependent protein phosphatase and its activation through a phosphorylation mechanism. FEBS Letters, 150(2), 319–324. [DOI] [PubMed] [Google Scholar]
- Heroes E, Lesage B, Görnemann J, Beullens M, Van Meervelt L, & Bollen M (2013). The PP1 binding code: A molecular-lego strategy that governs specificity. The FEBS journal, 280(2), 584–595. [DOI] [PubMed] [Google Scholar]
- Huang FL, & Glinsmann WH (1976). Separation and characterization of two phosphorylase phosphatase inhibitors from rabbit skeletal muscle. EuropeaN Journal Of Biochemistry/FEBS, 70(2), 419–426. [DOI] [PubMed] [Google Scholar]
- Huang Z, Khatra B, Bollen M, Carr DW, & Vijayaraghavan S (2002). Sperm PP1gamma2 is regulated by a homologue of the yeast protein phosphatase binding protein sds22. Biology of Reproduction, 67(6), 1936–1942. [DOI] [PubMed] [Google Scholar]
- Kawa S, Ito C, Toyama Y, Maekawa M, Tezuka T, Nakamura T, … Yamamoto T (2006). Azoospermia in mice with targeted disruption of the Brek/Lmtk2 (brain-enriched kinase/lemur tyrosine kinase 2) gene. Proceedings of the National Academy of Sciences of the United States of America, 103(51), 19344–19349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa Y, Sasaki K, Shima H, Shibuya M, Sugimura T, & Nagao M (1990). Protein phosphatases possibly involved in rat spermatogenesis. Biochemical and Biophysical Research Communications, 171(1), 230–235. [DOI] [PubMed] [Google Scholar]
- Koch S, Acebron SP, Herbst J, Hatiboglu G, & Niehrs C (2015). Post-transcriptional Wnt signaling governs epididymal sperm maturation. Cell, 163(5), 1225–1236. [DOI] [PubMed] [Google Scholar]
- Korrodi-Gregório L, Esteves SLC, & Fardilha M (2014). Protein phosphatase 1 catalytic isoforms: Specificity toward interacting proteins. Translational Research: The Journal Of Laboratory And Clinical Medicine, 164(5), 366–391. [DOI] [PubMed] [Google Scholar]
- Korrodi-Gregório L, Abrantes J, Muller T, Melo-Ferreira J, Marcus K, da Cruz e Silva OA, … Esteves PJ (2013). Not so pseudo: The evolutionary history of protein phosphatase 1 regulatory subunit 2 and related pseudogenes. BMC Evolutionary Biology, 13, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korrodi-Gregório L, Ferreira M, Vintém AP, Wu W, Muller T, Marcus K, … Da cruz e silva EF (2013). Identification and characterization of two distinct PPP1R2 isoforms in human spermatozoa. BMC Cell Biology, 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage B, Beullens M, Pedelini L, Garcia-Gimeno MA, Waelkens E, Sanz P, & Bollen M (2007). A complex of catalytically inactive protein phosphatase-1 sandwiched between Sds22 and inhibitor-3. Biochemistry, 46(31), 8909–8919. [DOI] [PubMed] [Google Scholar]
- Lin TH, Chen YC, Chyan C, Tsay L, Tang TC, Jeng HH,… Huang H (2003). Phosphorylation by glycogen synthase kinase of inhibitor-2 does not change its structure in free state. FEBS Letters, 554(3), 253–256. [DOI] [PubMed] [Google Scholar]
- MacKelvie SH, Andrews PD, & Stark MJ (1995). The Saccharomyces cerevisiae gene SDS22 encodes a potential regulator of the mitotic function of yeast type 1 protein phosphatase. Molecular and Cellular Biology, 15(7), 3777–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra S, Somanath PR, Huang Z, & Vijayaraghavan S (2003). Binding and inactivation of the germ cell-specific protein phosphatase PP1gamma2 by sds22 during epididymal sperm maturation. Biology of Reproduction, 69(5), 1572–1579. [DOI] [PubMed] [Google Scholar]
- Ohkura H, & Yanagida M (1991). S. pombe gene sds22+ essential for a midmitotic transition encodes a leucine-rich repeat protein that positively modulates protein phosphatase-1. Cell, 64(1), 149–157. [DOI] [PubMed] [Google Scholar]
- Pilder SH, Lu J, Han Y, Hui L, Samant SA, Olugbemiga OO, … Vijayaraghavan S (2007). The molecular basis of “curlicue”: A sperm motility abnormality linked to the sterility of t haplotype homozygous male mice. Society of Reproduction and Fertility supplement, 63, 123–133. [PubMed] [Google Scholar]
- Renouf S, Beullens M, Wera S, Van Eynde A, Sikela J, Stalmans W, & Bollen M (1995). Molecular cloning of a human polypeptide related to yeast sds22, a regulator of protein phosphatase-1. FEBS Letters, 375(1–2), 75–78. [DOI] [PubMed] [Google Scholar]
- Shima H, Haneji T, Hatano Y, Kasugai I, Sugimura T, & Nagao M (1993). Protein phosphatase 1 gamma 2 is associated with nuclei of meiotic cells in rat testis. Biochemical and Biophysical Research Communications, 194(2), 930–937. [DOI] [PubMed] [Google Scholar]
- Silva J, Freitas M, & Fardilha M (2014). Phosphoprotein phosphatase 1 complexes in spermatogenesis. Current Molecular Pharmacology, 7(2), 136–146. [DOI] [PubMed] [Google Scholar]
- Sinha N, Pilder S, & Vijayaraghavan S (2012). Significant expression levels of transgenic PPP1CC2 in testis and sperm are required to overcome the male infertility phenotype of Ppp1cc null mice. PLoS One, 7(10), e47623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha N, Puri P, Nairn AC, & Vijayaraghavan S (2013). Selective ablation of Ppp1cc gene in testicular germ cells causes oligo-teratozoospermia and infertility in mice. Biology of Reproduction, 89(5), 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Wolf DP, Trautman KC, & Vijayaraghavan S (1999). Motility potential of macaque epididymal sperm: The role of protein phosphatase and glycogen synthase kinase-3 activities. Journal of Andrology, 20(1), 47–53. [PubMed] [Google Scholar]
- Smith GD, Wolf DP, Trautman KC, da Cruz e Silva EF, Greengard P, Vijayaraghavan S, Greengard P, & Vijayaraghavan S (1996). Primate sperm contain protein phosphatase 1, a biochemical mediator of motility. Biology of Reproduction, 54(3), 719–727. [DOI] [PubMed] [Google Scholar]
- Somanath PR, Jack SL, & Vijayaraghavan S (2004). Changes in sperm glycogen synthase kinase-3 serine phosphorylation and activity accompany motility initiation and stimulation. Journal of Andrology, 25(4), 605–617. [DOI] [PubMed] [Google Scholar]
- Stone EM, Yamano H, Kinoshita N, & Yanagida M (1993). Mitotic regulation of protein phosphatases by the fission yeast sds22 protein. Current Biology: CB, 3(1), 13–26. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Fujinoki M, Shibahara H, & Suzuki M (2010). Regulation of hyperactivation by PPP2 in hamster spermatozoa. Reproduction, 139(5), 847–856. [DOI] [PubMed] [Google Scholar]
- Varmuza S, Jurisicova A, Okano K, Hudson J, Boekelheide K, & Shipp EB (1999). Spermiogenesis is impaired in mice bearing a targeted mutation in the protein phosphatase 1cgamma gene. Developmental Biology, 205(1), 98–110. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Chakrabarti R, & Myers K (2007). Regulation of sperm function by protein phosphatase PP1gamma2. Society of Reproduction and Fertility supplement, 63, 111–121. [PubMed] [Google Scholar]
- Vijayaraghavan S, Mohan J, Gray H, Khatra B, & Carr DW (2000). A role for phosphorylation of glycogen synthase kinase-3alpha in bovine sperm motility regulation. Biology of Reproduction, 62(6), 1647–1654. [DOI] [PubMed] [Google Scholar]
- Vijayaraghavan S, Stephens DT, Trautman K, Smith GD, Khatra B, da Cruz e Silva EF, & Greengard P (1996). Sperm motility development in the epididymis is associated with decreased glycogen synthase kinase-3 and protein phosphatase 1 activity. Biology of Reproduction, 54(3), 709–718. [DOI] [PubMed] [Google Scholar]
- Wilson R, Ainscough R, Anderson K, Baynes C, Berks M, Bonfield J, … Wohldman P (1994). 2.2 Mb of contiguous nucleotide sequence from chromosome III of C. elegans. Nature, 368(6466), 32–38. [DOI] [PubMed] [Google Scholar]
- Zhang J, Zhang L, Zhao S, & Lee EYC (1998). Identification and characterization of the human HCG V gene product as a novel inhibitor of protein phosphatase-1. Biochemistry, 37(47), 16728–16734. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.