Abstract
Background
Drug-drug interactions (DDIs) are one of the most common drug-related problems. Recently, electronic databases have drug interaction tools to search for potential DDIs, for example, Micromedex and Drugs.com. However, Micromedex and Drugs.com have different abilities in detecting potential DDIs, and this might cause misinformation to occur between patients and health care providers.
Methods and findings
The aim of this study was to compare the ability of Micromedex and Drugs.com to detect potential DDIs with metabolic syndrome medications using the drug list from the U-central database, King Chulalongkorn Memorial Hospital in April 2019. There were 90 available drugs for the treatment of the metabolic syndrome and its associated complications, but six were not found in the Micromedex and Drugs.com databases; therefore, only 84 items were used in the present study. There were 1,285 potential DDI pairs found by the two databases. Micromedex reported DDIs of 724 pairs, while, Drugs.com reported 1,122 pairs. For the severity of the potential DDI reports, the same severity occurred between the two databases of 481 pairs (37.43%) and a different severity for 804 pairs (62.57%).
Conclusion
Drugs.com had a higher sensitivity to detect potential DDIs by approximately 1.5-fold, but Micromedex supplied more informative documentation for the severity classification. Therefore, pharmacists should use at least two databases to evaluate potential DDIs and determine the appropriate drug regimens for physician communications and patient consultations.
Introduction
Non-communicable diseases (NCDs) are a major health problem worldwide [1–3]. One of the major NCDs is metabolic syndrome, according to the NCEP ATP III definition, metabolic syndrome is present if three or more of the following five criteria are met: waist circumference over 40 inches (men) or 35 inches (women), blood pressure over 130/85 mmHg, fasting triglyceride level over 150 mg/dL, fasting high-density lipoprotein cholesterol level less than 40 mg/dL (men) or 50 mg/dL (women) and fasting blood sugar over 100 mg/dL [4, 5]. Metabolic syndrome is considered as a risk factor for various complications such as type 2 diabetes [6, 7]. The treatment of metabolic syndrome and its complications are usually related to multiple drug use, which might cause drug-drug interactions (DDIs) [8, 9]. DDIs can cause treatment failure, morbidity, and mortality to the affected patients [10, 11]. The severity of potential DDIs can be classified into contraindicated, major, moderate, minor, and none [12–13]. The severity levels of contraindicated and major seem to be a serious concern in drug dispensing in patients. In recent years, numerous tools have been developed to detect potential DDIs, and one of the most popular tools is online DDI databases; however, there are two major types of DDI databases, free online and copyrighted databases [14–16]. In the case of patient access for potential DDI determination, they usually use a free online database, e.g., Drugs.com. Meanwhile, health care providers usually detect potential DDIs using a copyrighted database, e.g., Micromedex. Ramos et al. reported that these two databases have different sensitivity and specificity in detecting potential DDIs between the prescriptions of HIV/AIDs patients in critical care [17]. Also, Bossaer et al. found that Drugs.com is the most sensitive DDI database for the detection of potential DDIs in oral antineoplastic combinations [18]. However, there are no studies regarding the ability of databases in detecting potential DDIs for the treatment of metabolic syndrome, which usually requires multiple drug use. The aim of this study was therefore to determine the different abilities of the two electronic databases in detecting potential DDIs with metabolic syndrome medications. The results of this study could increase the awareness of information obtained from different databases and lead to proper communication between metabolic syndrome patients and health care providers.
Materials and methods
Drug selection
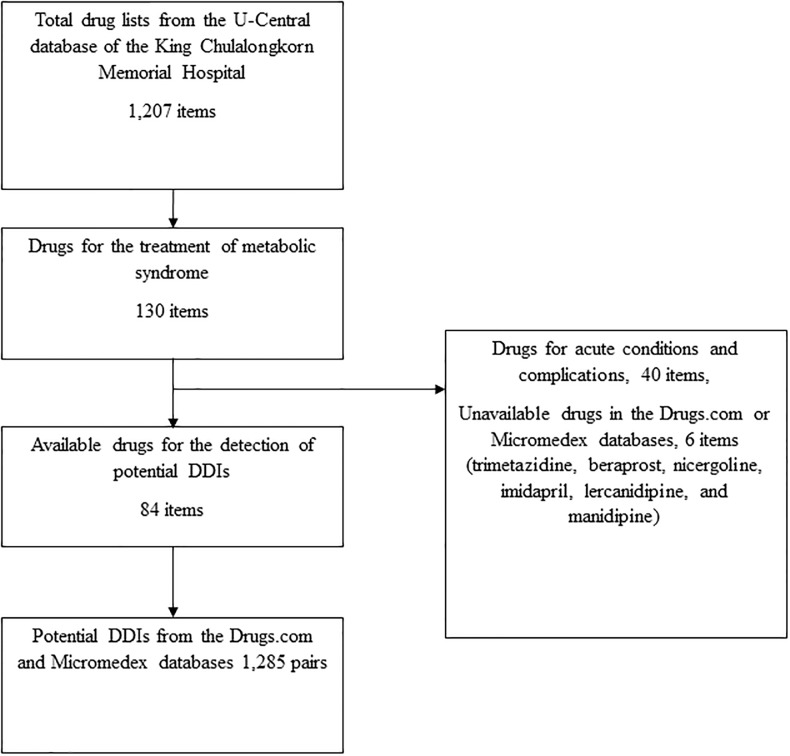
This descriptive study included a list of medicines for metabolic syndrome from the U-central database of the King Chulalongkorn Memorial Hospital that was taken on the 12th April 2019 [19]. Of the 1,207 items in total, only 90 drugs were used for the treatment of the syndrome. Surprisingly, six drugs were not found in the Micromedex and Drugs.com databases; therefore, only 84 items were included in the study (Fig 1 and Table 1).
Fig 1. Flowchart of the study.
Table 1. Drug lists for the detection of potential DDIs.
| Drug class | Drug groups | Drug lists |
|---|---|---|
| Cardiac drugs | Beta blockers | 1. Atenolol 2. Bisoprolol 3. Carvedilol 4. Esmolol 5. Metoprolol 6. Nebivolol 7. Propranolol |
| Antianginal agents | 8. Ranolazine 9. Trimetazidine |
|
| Vasodilating agents | Phosphodiesterase inhibitors | 10. Dipyridamole |
| Arteriolar dilators | 11. Hydralazine 12. Minoxidil |
|
| Prostacyclin analogues | 13. Iloprost 14. Beraprost |
|
| Nitrates | 15. Isosorbide dinitrate 16. Isosorbide mononitrate 17. Nitroglycerin |
|
| Phosphodiesterase-5 inhibitors | 18. Sildenafil | |
| Peripheral vasodilators and related agents | Peripheral vasodilator agents | 19. Nicergoline |
| Xanthine derivatives | 20. Pentoxifylline | |
| Antiplatelets | Cyclooxygenase inhibitors | 21. Acetylsalicylic acid |
| Phospholipase-3 inhibitors | 22. Cilostazol 23. Omega-3-acid ethyl ester |
|
| Antilipemic agents | Chelating agents | 24. Cholestyramine |
| Fibrates | 25. Fenofibrate 26. Gemfibrozil |
|
| HMG-CoA reductase inhibitors | 27. Atorvastatin 28. Pitavastatin 29. Pravastatin 30. Rosuvastatin 31. Simvastatin |
|
| Nicotinic acid | 32. Nicotinic acid | |
| Selective cholesterol absorption inhibitors | 33. Ezetimibe | |
| PCSK9 inhibitors | 34. Evolocumab | |
| Antihypertensive drugs | Angiotensin converting enzyme inhibitors | 35. Captopril 36. Enalapril 37. Imidapril 38. Perindopril 39. Quinapril 40. Ramipril |
| Angiotensin receptor blockers | 41. Azilsartan 42. Candesartan 43. Irbesartan 44. Losartan 45. Olmesartan 46. Telmisartan 47. Valsartan |
|
| Neprilysin inhibitors | 48. Sacubitril valsartan sodium salt complex | |
| Thiazide and related diuretics | 49. Hydrochlorothiazide 50. Indapamide 51. Chlorthalodine |
|
| Alpha-2 adrenergic receptors | 52. Methyldopa 53. Clonidine |
|
| Alpha adrenergic antagonists | 54. Doxazosin 55. Prazosin |
|
| Calcium channel blockers | 56. Amlodipine 57. Diltiazem 58. Felodipine 59. Lercanidipine 60. Manidipine 61. Nifedipine 62. Nimodipine 63. Verapamil |
|
| Direct renin inhibitors | 64. Aliskiren | |
| Endothelin-1 receptor antagonists | 65. Bosentan 66. Macitentan |
|
| Diuretics | Diuretics loop diuretics | 67. Furosemide |
| Carbonic anhydrase inhibitors | 68. Acetazolamide | |
| Osmotic diuretics | 69. Mannitol 70. Glycerin |
|
| Potassium-sparing diuretics | 71. Amiloride 72. Spironolactone |
|
| Antidiabetic drugs | Alpha-glucosidase inhibitors | 73. Acarbose |
| Biguanides | 74. Metformin | |
| Dipeptidyl peptidase-4 inhibitors | 75. Linagliptin | |
| Glucagon-like peptide-1 receptor agonists | 76. Liraglutide 77. Dulaglutide |
|
| Meglitinide analogs | 78. Repaglinide | |
| Sodium glucose co-transporter type 2 inhibitors | 79. Dapagliflozin 80. Empagliflozin |
|
| Sulfonylureas | 81. Glimepiride 82. Glipizide |
|
| Thiazolidinediones | 83. Pioglitazone | |
| Insulins | 84. Insulin |
Databases
IBM Micromedex Web Application Access was used in this study; it is a copyrighted database of IBM Corp., USA. The official registration and operations were conducted for academic purposes under the license from Chulalongkorn University. The DDI reports of the Micromedex database consisted of severity levels, documentation, onset, probable mechanism, management, literature, and references. The Drug Interactions Checker is a free online database provided by Drugs.com. This database is powered by four independent leading medical-information suppliers: Wolters Kluwer Health, American Society of Health-System Pharmacists, Cerner Multum, and Micromedex from Truven Health. The DDI reports of Drugs.com consisted of severity levels, management, probable mechanism, literature, and references.
Identification of DDIs
The generic names of all the selected drugs were inputted into the database for potential DDI detection. All potential DDIs were recorded to determine the sensitivity and specificity of the reports. The severity of the potential DDIs from Micromedex was classified into five groups: contraindicated, major, moderate, minor, and none. Meanwhile, Drugs.com classified the potential DDIs into four groups: major, moderate, minor, and none. Micromedex classified the documentation of the outcomes as excellent, good, fair, and unknown, even though Drugs.com did not document these outcomes. Excellent documentation is defined as controlled studies have clearly established the existence of the interaction, good strongly suggests the interaction exists, but well-controlled studies are lacking, fair is defined as available documentation is poor, but pharmacologic considerations lead clinicians to suspect the interaction exists; or, documentation is good for a pharmacologically similar drug. Similarly, Micromedex also reported the onset of the potential DDIs as one topic; meanwhile, Drugs.com included the onset in the DDI monograph. All potential DDI reports were collected from the databases in April 2019.
Data analysis
The data analysis was conducted using SPSS version 16 (SPSS Inc., US). The assessment of the agreement between the DDIs identified by the two databases was performed using the kappa index. A kappa value of 0.81–1.00 indicated an almost perfect agreement, 0.61–0.80 indicated a substantial agreement, 0.41–0.60 indicated a moderate agreement, 0.21–0.40 indicated a fair agreement, 0.00–0.20 indicated a slight agreement, and below 0.00 indicated a poor agreement [20].
Results
From the 84 items analyzed, we found 1,285 pairs of potential DDIs from the two databases. Drugs.com reported DDIs of 1,122 pairs and Micromedex reported DDIs of 724 pairs. Of the 724 reported by Micromedex, the classification of severity was contraindicated in 23 pairs, major in 132 pairs, moderate in 566 pairs, and minor in 3 pairs. Drugs.com reported major DDIs of 130 pairs, moderate of 931 pairs, and minor of 61 pairs (Table 2). Among the 724 DDIs identified by the Micromedex, DDIs with excellent or good scientific documentation rating (47.79%) and stratifying the severity of DDIs according to documentation ratings are reported in Table 3.
Table 2. Comparison of the potential DDIs characterized by Micromedex and Drugs.com.
| Severity | Micromedex n (%) |
Drugs.com n (%) |
|---|---|---|
| Contraindicated | 23 (3.18) | N/A |
| Major | 132 (18.23) | 130 (11.59) |
| Moderate | 566 (78.18) | 931 (82.98) |
| Minor | 3 (0.41) | 61 (5.43) |
| Total | 724 (100.00) | 1,122 (100.00) |
N/A, not available
Table 3. Documentation of potential DDIs classified by Micromedex.
| Severity | Excellent n (%) |
Good n (%) |
Fair n (%) |
|---|---|---|---|
| Contraindicated | 5 (21.74) | 13 (56.52) | 5 (21.74) |
| Major | 52 (39.39) | 47 (35.61) | 33 (25.00) |
| Moderate | 8 (1.45) | 219 (38.69) | 339 (59.89) |
| Minor | 0 (0.00) | 2 (66.67) | 1 (33.33) |
| Total | 65 (8.98) | 281 (38.81) | 378 (52.21) |
For the severity of potential the DDI reports, the same severity between the two databases of 481 pairs was 37.43%. The number of reports with a different severity were 804 pairs (62.57%) when comparing the two databases. Of these differing severity reports, major DDIs of 9 pairs reported by Drugs.com were not detected by Micromedex. On the contrary, Micromedex reported major DDIs for 21 pairs, although Drugs.com determined only minor to none DDIs as shown in Tables 4 and 5. The agreement between the severity reports of the two databases, as determined by the kappa value, was -0.055 (95% CI, -0.07068 to -0.03932, p < 0.001), which was considered to be a poor agreement between the two databases.
Table 4. The severity of the potential DDIs detected by Micromedex and Drugs.com.
| Drug.com | Major n (%) |
Moderate n (%) |
Minor n (%) |
None n (%) |
Total n (%) |
|---|---|---|---|---|---|
| Micromedex | |||||
| Contraindicated | 22 (1.71) | 0 (0.00) | 0 (0.00) | 1 (0.08) | 23 (1.79) |
| Major | 83 (6.46) | 29 (2.26) | 3 (0.23) | 17 (1.32) | 132 (10.27) |
| Moderate | 16 (1.25) | 397 (30.89) | 9 (0.70) | 144 (11.21) | 566 (44.05) |
| Minor | 0 (0.00) | 1 (0.08) | 1 (0.08) | 1 (0.08) | 3 (0.24) |
| None | 9 (0.70) | 504 (39.22) | 48 (3.73) | 0 (0.00) | 561 (43.65) |
| Total | 130 (10.12) | 931 (72.45) | 61 (4.74) | 163 (12.69) | 1,285 (100.00) |
Table 5. The significant difference of the severity in potential DDIs analyzed by Micromedex and Drugs.com.
| Micromedex | Drugs.com | DDIs paired list with different severity |
|---|---|---|
| Contraindication | None | 1. Aliskiren—Sacubitril |
| Major | Minor | 1. Aspirin—Furosemide 2. Aspirin—Spironolactone 3. Nifedipine—Pioglitazone |
| Major | None | 1. Acarbose—Aspirin 2. Acarbose—Glipizide 3. Acarbose—Pioglitazone 4. Aspirin—Amiloride 5. Aspirin—Chlorthalidone 6. Aspirin—Hydrochlorothiazide 7. Aspirin—Indapamide 8. Aspirin—Metformin 9. Clonidine—Metoprolol succinate 10. Clonidine—Metoprolol tartrate 11. Glimepiride—Pioglitazone 12. Glipizide—Pioglitazone 13. Metformin—Pioglitazone 14. Metoprolol succinate—Diltiazem 15. Metoprolol succinate—Verapamil 16. Metoprolol tartrate—Diltiazem 17. Metoprolol tartrate—Verapamil |
| None | Major | 1. Amiloride—Azilsartan 2. Amiloride—Olmesartan 3. Amiloride—Spironolactone 4. Bosentan—Ranolazine 5. Clonidine—Metoprolol 6. Diltiazem—Metoprolol 7. Spironolactone—Azilsartan 8. Spironolactone—Olmesartan 9. Verapamil—Metoprolol |
Discussion
Drugs.com reported more potential DDIs than Micromedex by approximately 1.5-fold. These results were consistent with Ramos et al., who found that potential DDIs in HIV/AIDs patients detected by Drugs.com were more numerous than the Micromedex database indicated [17]. Bossaer et al. also mentioned that Drugs.com was more sensitive to detect the potential DDIs in cancer treatment than Micromedex [18]. This phenomenon may be explained by the fact that Drugs.com has a larger database which is contributed to by four suppliers, including Micromedex. Micromedex utilizes peer-review process to screen published medical studies as an evidence-based, pre-appraisal approach to assess the quality of documentation. The higher number of potential DDIs reported in Drugs.com might not always be fruitful in clinical practice. Some reports of potential DDIs are the combination of drugs used in the routine treatment of metabolic syndrome; for example, a combination of pioglitazone and metformin could generate benefits in controlling the symptoms of diabetes mellitus. The potential DDIs of these two antidiabetics seem to have positive clinical outcomes rather than negative adverse events. A large number of potential DDI alerts with limited documentation might lead to data alert fatigue to both health care providers and patients. Increased awareness regarding the discrepancy between the two databases should be made to both patients and providers who have access to these databases. Shared-decision making between providers and patients should be used for any significant potential DDIs in order to avoid alert fatigue and minimize liability.
Interestingly, we found that the two databases reported different severities in some potential DDI pairs. This major finding may cause drug-related problems in pharmacotherapy and may generate conflict between patients and health care providers. For example, Micromedex, which is preferred by health care providers, reported potential DDIs between aspirin and furosemide as being a major severity with excellent documentation. Meanwhile, Drugs.com, which is generally used by the patients, determined this interaction as being of minor severity. In contrast, Micromedex reported no potential DDI between amiloride and olmesartan; meanwhile, Drugs.com determined this potential DDI as a major event. In a case where patients develop severe cardiovascular complications while using these two agents together, health care professionals may be held liable for the harms a patient experiences. This is because Micromedex, which is usually utilized by health care providers, reports no concern for this DDI, but Drugs.com generally used by patients reported this as a major potential DDI. The evaluation of agreement between the more serious DDI reports of the two databases should be conducted and prioritized in order to prevent patient complications and also medical complaints.
We found that more than 80% of the potential DDIs of metabolic syndrome medications are pharmacodynamic interactions rather than those of pharmacokinetics. This phenomenon can be explained by the fact that metabolic syndrome is a complex pathological condition of the cardiovascular and endocrine systems. Combinations of several drugs for the treatment of metabolic syndrome are expected for the drug synergism of action, especially for pharmacodynamics rather than pharmacokinetic purposes. Interestingly, Drugs.com has a special feature to detect therapeutic duplications. This feature could reduce medication errors or the overdose of two drugs with an identical mechanism of action, e.g., atenolol and metoprolol. These two beta blockers have a similar mechanism of action by inhibiting the beta-1 receptors in the tissues of the heart. Even though this phenomenon seems prone to be caused by medication errors rather than true potential DDIs, it could be useful in some instances. In developing countries, patients can easily access dangerous medicines with a similar mechanism of action from drug stores or convenience stores. The determination of repetitive medicine with a similar mechanism of action by the free online database or mobile applications of Drugs.com might be useful in this circumstance.
The limitations of this study seem to be the dynamic changes of the drug list in the hospital, new drugs are entered and old drugs removed from time to time; therefore, this study made the cut off for the drug's list in early 2019. The current drug list in late 2019 might have had some changes from the time when we cut off the drug list. In addition, the two databases have frequently updated their potential DDI reports, so we collected all the potential DDI reports during early 2019. The current version of Micromedex and Drugs.com in late 2019 might report potential DDIs that are different from our study in early 2019. This study used only drugs from the electronic health record, thus not expanding the search to all drugs available in each of the respective classes. Therefore, there may be a greater (or lesser) discrepancy impact between the databases if all drugs in each class were included.
Conclusion
The Drugs.com database had a higher sensitivity to detect potential DDIs, but Micromedex could provide more informative documentation for the severity classification. Both databases could be used to screen for potential DDIs, and the final justification should be conducted in accordance with the agreement and communication between patients and health care providers.
Acknowledgments
The authors express their gratitude to the Faculty of Pharmaceutical Sciences, Chulalongkorn University for providing research funding to Bovornpat Suriyapakorn (Phar2563-RG001). We are sincerely thankful to Dr. Suthira Taychakhoonavudh for her kind assistance in data analysis.
Abbreviations
- AIDS
Acquired Immunodeficiency Syndrome
- DDIs
Drug-Drug Interactions
- HIV
Human Immunodeficiency Virus
- NCDs
Non-Communicable Diseases
- NCEP ATP
the National Cholesterol Education Program of Adult Treatment Panel
Data Availability
All relevant data are within the paper.
Funding Statement
The authors express their gratitude to the Faculty of Pharmaceutical Sciences, Chulalongkorn University for providing research funding to Bovornpat Suriyapakorn (Phar2563-RG001). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12 10.1007/s11906-018-0812-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. 10.1111/obr.12229 [DOI] [PubMed] [Google Scholar]
- 3.Bundhamcharoen K, Odton P, Phulkerd S, Tangcharoensathien V. Burden of disease in Thailand: changes in health gap between 1999 and 2004. BMC Public Health. 2011;11:53 10.1186/1471-2458-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Cholesterol Education Program Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–421. [PubMed] [Google Scholar]
- 5.Grundy SM, Brewer HB Jr., Cleeman JI, Smith SC Jr., Lenfant C, American Heart A, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109(3):433–8. 10.1161/01.CIR.0000111245.75752.C6 [DOI] [PubMed] [Google Scholar]
- 6.Grundy SM. Metabolic syndrome: connecting and reconciling cardiovascular and diabetes worlds. J Am Coll Cardiol. 2006;47(6):1093–100. 10.1016/j.jacc.2005.11.046 [DOI] [PubMed] [Google Scholar]
- 7.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. 10.1161/CIRCULATIONAHA.109.192644 [DOI] [PubMed] [Google Scholar]
- 8.Ersoy S, Engin VS. Risk factors for polypharmacy in older adults in a primary care setting: a cross-sectional study. Clin Interv Aging. 2018;13:2003–11. 10.2147/CIA.S176329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menditto E, Gimeno Miguel A, Moreno Juste A, Poblador Plou B, Aza Pascual-Salcedo M, Orlando V, et al. Patterns of multimorbidity and polypharmacy in young and adult population: Systematic associations among chronic diseases and drugs using factor analysis. PLoS One. 2019;14(2):e0210701 10.1371/journal.pone.0210701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dechanont S, Maphanta S, Butthum B, Kongkaew C. Hospital admissions/visits associated with drug-drug interactions: a systematic review and meta-analysis. Pharmacoepidemiol Drug Saf. 2014;23(5):489–97. 10.1002/pds.3592 [DOI] [PubMed] [Google Scholar]
- 11.Reimche L, Forster AJ, van Walraven C. Incidence and contributors to potential drug-drug interactions in hospitalized patients. J Clin Pharmacol. 2011;51(7):1043–50. 10.1177/0091270010378858 [DOI] [PubMed] [Google Scholar]
- 12.Micromedex.com. http://micromedex.com/about-micromedex. Accessed January 9, 2017.
- 13.Drugs.com. https://www.drugs.com/support/about.html. Accessed January 9, 2017.
- 14.Kheshti R, Aalipour M, Namazi S. A comparison of five common drug-drug interaction software programs regarding accuracy and comprehensiveness. J Res Pharm Pract. 2016;5(4):257–63. 10.4103/2279-042X.192461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Patel RI, Beckett RD. Evaluation of resources for analyzing drug interactions. J Med Libr Assoc. 2016;104(4):290–5. 10.3163/1536-5050.104.4.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clauson KA, Marsh WA, Polen HH, Seamon MJ, Ortiz BI. Clinical decision support tools: analysis of online drug information databases. BMC Med Inform Decis Mak. 2007;7:7 10.1186/1472-6947-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramos GV, Guaraldo L, Japiassu AM, Bozza FA. Comparison of two databases to detect potential drug-drug interactions between prescriptions of HIV/AIDS patients in critical care. J Clin Pharm Ther. 2015;40(1):63–7. 10.1111/jcpt.12222 [DOI] [PubMed] [Google Scholar]
- 18.Bossaer JB, Thomas CM. Drug Interaction Database Sensitivity With Oral Antineoplastics: An Exploratory Analysis. J Oncol Pract. 2017;13(3):e217–e22. 10.1200/JOP.2016.016212 [DOI] [PubMed] [Google Scholar]
- 19.Unbound Medicine, Inc. MDCU-KCMH Hospital Formulary [Mobile application software]. (Version 1.22) Charlottesville: Unbound Medicine, Inc. https://www.unboundmedicine.com/ucentral/index/KCMH%20-%20Formulary/Generic_TRADE_NAME_Index/A. Accessed January 9, 2017.
- 20.Marston L. Introductory statistics for health and nursing using SPSS: Sage Publications; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.