Abstract
As national HIV programs across the world mature and continue to scale up towards UNAIDS’ 90-90-90 targets, it is increasingly important to accurately estimate HIV treatment needs in pediatric patient populations to prepare for anticipated increases in demand. This is particularly vital in sub-Saharan Africa, where the bulk of the global pediatric HIV burden remains concentrated, and for treatment-experienced populations, for which data are severely limited. This article discusses the conceptual framework behind and application of a five-year country-level quantification and decision-making tool aimed at providing national HIV programs and their partners with a better understanding of their evolving national HIV treatment and programming needs for second-and third-line pediatric populations. The conceptual framework of the algorithm which undergirds the tool is the patient pathway, along which key influencing factors that determine whether pediatric HIV patients are linked to care, remain in treatment, and are appropriately switched to later lines of treatment are accounted for quantitatively. Excel-based and arithmetic, the algorithm is designed to use available national, regional, and global data for factors impacting patient estimates including treatment coverage; routine viral load testing; viral load non-suppression; confirmed treatment failure; and patient loss to follow up—outcomes for which data are generally very limited in this patient population. The ultimate output of the tool is an estimate of the aggregate annual number of patients by treatment line. Given the limitations in available data for pediatric HIV, particularly for patients on second- and third-line treatments, this tool may help fill a data gap by providing a mechanism for policymakers to scenario plan, thus aiding resource allocation decisions for pediatric HIV program scale-up. The tool may be used to streamline national antiretroviral procurement of later lines of treatment, especially in resource-limited settings, and may also be used to add value to broader HIV policy and planning processes at the national level.
Introduction
The state of the global HIV epidemic among children under the age of 14 has changed considerably over the past decade. The scale-up of prevention of mother-to-child transmission programs has contributed to a significant reduction in the number of new HIV infections among children–from 270,000 in 2010 to 160,000 in 2018 [1]–as well as to a notable reduction in acquired immunodeficiency syndrome (AIDS)-related deaths–from 200,000 in 2010 to 100,000 in 2018 [1]. Although the global scale-up of antiretroviral therapy (ART) has also contributed to these reductions, treatment coverage for children living with HIV continues to lag significantly behind adult treatment: in 2016, 62% [1] of adults living with HIV had access to treatment whereas only 52% [1] of children were on treatment. Access to treatment is particularly challenging for children who have failed first- or second-line therapies in resource-limited countries, as viral load and genotypic resistance tests often are not routinely available and third-line treatment may not be provided free of charge in the public sector. [2,3]
As national HIV programs across the world mature and continue to scale up towards UNAIDS’ 90-90-90 targets, it is increasingly important to accurately estimate HIV treatment needs in pediatric patient populations to prepare for anticipated increases in demand. This is particularly vital in sub-Saharan Africa, where the bulk of the global pediatric HIV burden remains concentrated [4] and for treatment-experienced populations, for which data are severely limited. [5,6] For example, a recent World Health Organization (WHO) scoping review of all available evidence on children failing first- and second-line HIV medicines identified only 18 studies total in this population, including one randomized control trial, seven phase II trials, five prospective cohort studies and five retrospective cohort studies. [7] Moreover, neither UNAIDS nor WHO regularly report country-level data or estimates by line of treatment for pediatric patients in their annual global reports or other data and statistics resources. [8,9]
The New Horizons: Advancing Pediatric HIV Collaborative (“New Horizons”), formed by Janssen Pharmaceutical Companies of Johnson & Johnson with expert input from many stakeholders involved in pediatric HIV care, treatment, and prevention, aims to strengthen the public health approach to care and treatment for children living with HIV. Recognizing that a lack of data continues to impair efforts to improve treatment access for children living with HIV failing first- and second-line treatment, New Horizons developed a quantification tool aimed at providing national governments and other relevant partners with a resource to facilitate discussions, decision making, and short-term pediatric HIV program planning targeting this very underserved patient population. The tool is grounded in the primary factors that determine whether pediatric HIV patients (ages 0–14) are linked to care, remain in treatment, and are switched to later lines of treatment.
Methodology
Overview and conceptual framework of the tool
Our Excel-based tool was developed using an iterative approach, integrating publicly-available quantitative data with perspectives gained through stakeholder interviews. The arithmetic algorithm underpinning the tool was constructed to generate an annual estimate of pediatric patient need for second- and third-line HIV treatment in a national program over a five-year period (2018–2021) based on manually changeable parameter data across a variety of domains (described in detail below). In the next section, we describe the results generated by application of the tool when using publicly-available data from the Kenyan context.
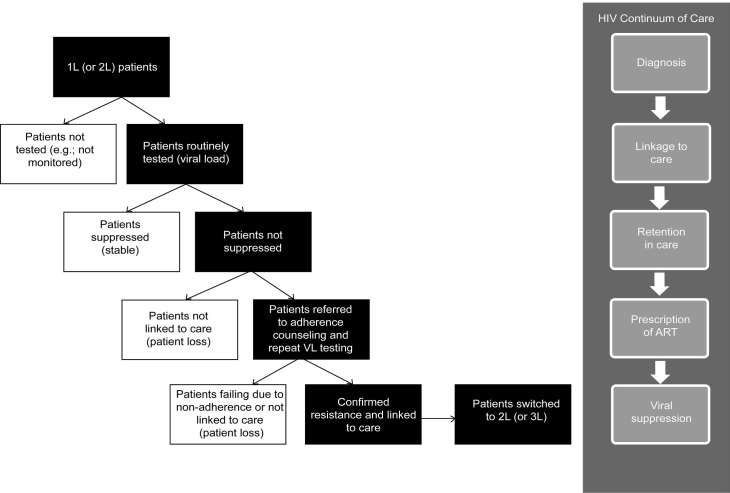
The conceptual basis for the tool’s central algorithm is a patient pathway depicting the connections between various key elements that determine whether an HIV-positive child is linked to appropriate care and treatment (Fig 1). The pathway was designed to reflect current evidence [10] and commonly shared understandings among practitioners regarding the pediatric patient journey [11,12], the clinical dimension of treatment failure and switching [13,14], and the on-the-ground realities of HIV treatment in resource-constrained settings [15,16,17,18].
Fig 1. Patient pathway.
Grounded in treatment guidelines [19,20], the patient pathway served to depict the generalized “steps” that pediatric HIV patients will take when moving to more advanced treatment lines. The sequencing of these steps and patient pool attrition–due to both positive (suppression) or negative (patient loss) factors–at each stage resulted in a pathway in which only a subset of patients progressed. A core assumption that resulted from this approach was that “lost” patients did not re-enter the pathway at a later stage nor did they progress to a later line of treatment.
Tool parameters
Key parameters of the tool include pediatric HIV treatment coverage; routine viral load testing rate; viral load non-suppression rate; confirmed treatment failure rate; and patient loss. Where appropriate, the tool allows for differences in these rates for first- and second-line treatment to be reflected. Additional foundational parameters, such as mortality, new cases, and rate of aging out, were included in the algorithm as well. Table 1 provides an overview of the parameters, the definitions applied, and methodological notes regarding sources and calculations.
Table 1. Overview of model parameters.
| Parameter | Description | Notes on Data Sources | Potential Data Sources |
|---|---|---|---|
| Key Parameters | |||
| Pediatric HIV treatment coverage | Percentage of eligible children (0–14 ages) in need of ART reached | National-level coverage rates are generally publicly reported. | Data reported by Ministries of Health, UNAIDS, United Nations Children's Fund (UNICEF), WHO |
| Routine viral load testing (different rates for first- and second-line treatment) | Percentage of ART patients who undergo viral load testing (assumes 1 routine test per patient per year) | Limited data exists regarding routine viral load testing among pediatric patients. In the absence of appropriate national-level data, values should reflect clinical study data, available testing data (adult populations), and qualitative data on testing infrastructure and routine testing practices in resource-limited settings. | Clinical study data, qualitative data from peer-reviewed articles, UNAIDS data (adults only) |
| Viral load non-suppression rate (different rates for first- and second-line treatment) | Percentage of patients receiving viral load tests who are not virologically suppressed | Limited data exists regarding pediatric viral load suppression rates. In the absence of appropriate national-level data, values should reflect relevant clinical study data, available viral load suppression rate data (adult populations), and qualitative data on factors influencing suppression rates and trends. | Clinical study data, qualitative data from peer-reviewed articles, UNAIDS data (adults only) |
| Confirmed treatment failure (different rates for first- and second-line treatment) | Percentage of patients whose treatment failure is confirmed by a confirmatory viral load test (or, where available and appropriate, a resistance test) prior to switching to second-line | Limited data exists regarding pediatric HIV treatment failure. In the absence of appropriate national-level data, values should reflect a survey of clinical studies and observations from practicing clinicians. Recent WHO data revealed a broad range of estimated treatment failure rates (from over 30% to 0.5%). | Clinical study data, qualitative data from peer-reviewed articles, WHO data |
| Patient loss (different rates for first- and second-line treatment) | Percentage of patients lost following viral load testing and not linked to additional care (drivers include lack of access, loss to follow up, and mortality) | At various stages over the course of treatment, patients will be lost due to lack of access (to treatment, services, and/or care), loss to follow up, and mortality. In the absence of appropriate national-level data, values should reflect clinical study data. A review of recent studies suggests that adherence is a larger problem for pediatric patients than for adult patients and that there are greater barriers to access for pediatric HIV services and care as compared to adult services. | Clinical study data, qualitative data from peer-reviewed articles |
| Foundational Parameters | |||
| Mortality | Annual number of pediatric HIV patient deaths expressed as a percentage of the total pediatric HIV patient pool | National-level AIDS-related mortality rates for the pediatric HIV+ population are generally publicly reported. | UNAIDS data (children) |
| New cases | Decrease in number of new cases of HIV infections among children (0–14 years) expressed as a percentage change | National-level data on new cases of pediatric HIV are generally publicly reported. | UNAIDS data (children) |
| Rate of aging out | Percentage of children who age out of the 0–14 age cohort (on an annual basis) | In the absence of national-level data for the pediatric HIV+ population, demographic data for the general population within the 0–14 age cohort can be used as a proxy. | UN World Population Prospects |
Results
Sample country application–Kenya
In Kenya, recent initiatives such as PEPFAR’s Accelerating Children’s HIV/AIDS Treatment Initiative [21,22] have helped to reduce the gap in treatment coverage between adults and children and expand access to treatment for children in need. As the Ministry of Health seeks to capitalize on these gains and works to continue scaling pediatric HIV treatment programs, forecasting demand for treatment, particularly for later lines of treatment, will become more important to ensure appropriate planning and resource allocation. (Table 2 provides an overview of the state of the epidemic today.)
Table 2. Snapshot of epidemic.
| Pediatric HIV Burden in Kenya: 2018 (UNAIDS) [1,22,23,24] | |
|---|---|
| Number of children living with HIV (0–14 years) | 120,000 |
| New cases | 7,600 |
| Treatment coverage (%)* | 61% |
| Number of children receiving treatment | 74,344 |
| Deaths due to AIDS | 5,200 |
*Reported treatment coverage rates vary by reporting source. For example, the treatment coverage rate for 2018 was reported as 61% by the UNAIDS AIDSinfo database, whereas the PEPFAR 2017 Kenya Country Operational Plan reported that pediatric treatment coverage was 82% as of April 2017, and the Kenya National AIDS & STI Control Programme (NASCOP) National ACT Dashboard reported that coverage was 54% in May 2016. While the different reporting periods likely play a role in these differences, comparing different sources in the same period also yields similar inconsistencies. For example, while the PEPFAR 2017 Kenya Country Operational Plan reports 82% treatment coverage of April 2017, UNAIDS reports that pediatric treatment coverage in 2017 was 66%.
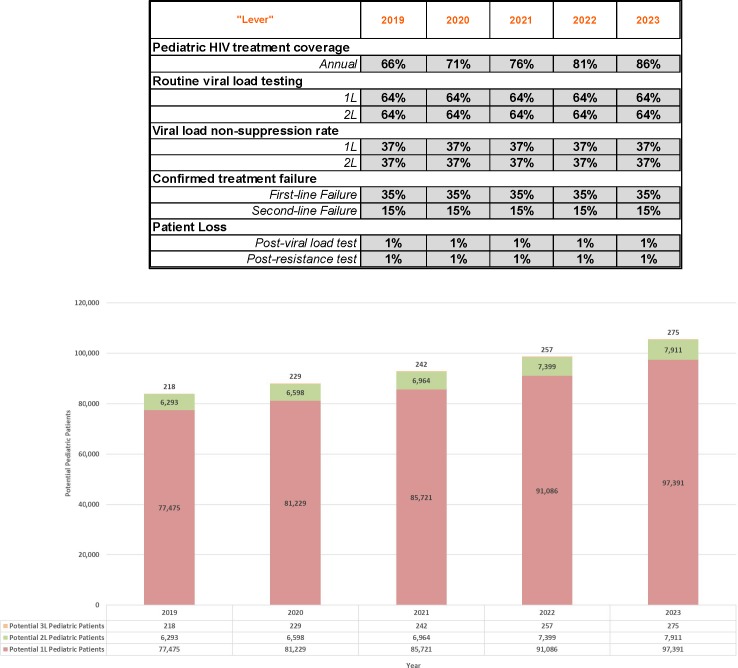
To generate an estimate of patient need over a five-year period, currently available UNAIDS [1], PEPFAR [22] and NASCOP [23,24] data were used to populate–and calculate or derive as necessary–baseline values for all the model parameters (described above) for 2019–2023. In the absence of reported, country-level data, values were derived based on the following available data points: relevant regional data or estimates; relevant clinical studies; and WHO [25] calculations of the global distribution of patients on ART (see S1 Dataset for additional information on data and calculations). The resulting annual patient estimates (Fig 2) represent a reasonable if somewhat conservative estimate based on publicly available data and latest findings and trends from current country-level programs and initiatives.
Fig 2. Baseline 2019–2023 forecast.
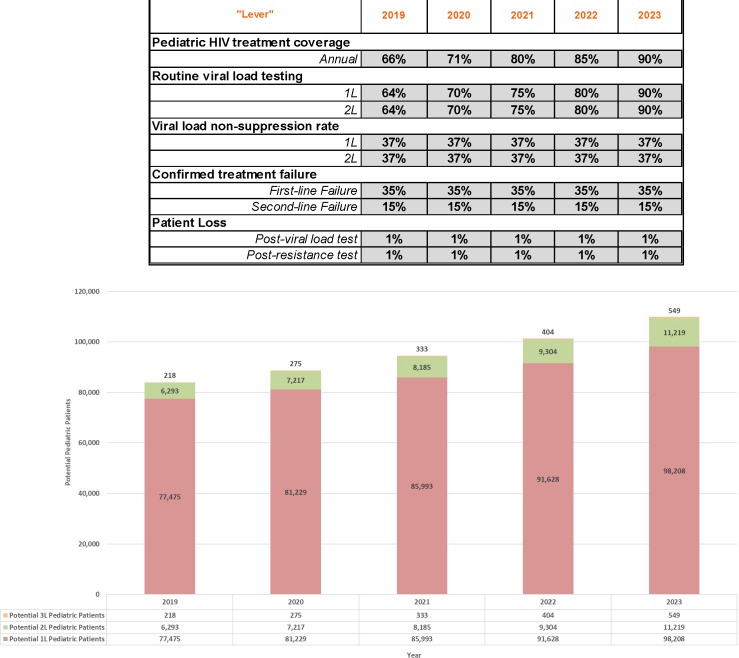
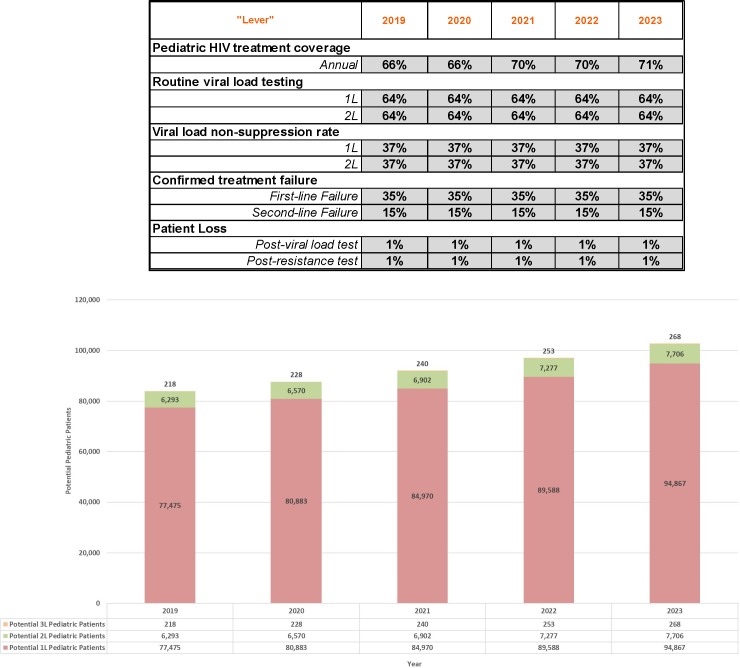
Using the adjustable levers in the forecasting tool, model parameters can be adjusted to reflect any number of potential scenarios. For example, Fig 3 below depicts an aggressive growth scenario in which 90-90-90 treatment coverage and routine viral load testing goals are achieved by 2023. By contrast, Fig 4 demonstrates what pediatric patient pool growth could look like where treatment coverage growth were slower and if viral load testing rates were to plateau at current estimated levels.
Fig 3. Aggressive growth 2019–2023 forecast.
Fig 4. Plateauing growth 2019–2023 forecast.
Additionally, as Kenya’s national pediatric HIV treatment program matures and additional resources are dedicated to achieving 90-90-90 targets, more pediatric patients are likely to be identified and put on treatment; these higher volumes may potentially lead to more pediatric patients advancing to later lines of treatment. Model parameters can be adjusted to reflect 90% treatment coverage and 90% viral load testing coverage (assuming a standard of 1 test per year as indicated in treatment guidelines in many resource-limited settings) so as to forecast anticipated patient need for later lines of treatment under these conditions, particularly third-line treatment. The difference between anticipated third-line patient estimates using those parameters and the third-line patient estimates using parameters that reflect the current national treatment landscape represents a way to conceptualize and quantify future pediatric patients in need of such treatment (Table 3). It also offers an approach for conceptualizing potential “unmet need” for third-line treatment by offering a method for estimating the range of potential patients in need of treatment under different scenarios. Comparing these scenarios to anticipated and/or planned national treatment program resource levels could help governments and other relevant partners identify potential shortfalls.
Table 3. Anticipated third-line patients under different scenarios.
| 2019 | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
| Anticipated total third-line patients with 90% treatment coverage and 90% routing viral load testing | 442 | 471 | 501 | 534 | 572 |
| Patients on third-line treatment currently forecasted | 218 | 229 | 242 | 257 | 275 |
| Difference between current forecast and anticipated total with partial 90-90-90 achievement | 224 | 242 | 259 | 277 | 297 |
Discussion
Strengths and limitations of the tool
Designed to be used for quantification and decision making at the national level for a population of patients for which little data—historic or current—exists, the tool’s strength lies in its changeable levers and ability to be used for scenario planning. Notably, the patient pathway algorithm which undergirds the tool was designed to reflect the on-the-ground conditions and patient journey in countries with the highest prevalence of pediatric HIV today where scenario planning would be of greatest use. Additionally, the baseline values used to populate the model parameters–and assumptions used to derive proxies as needed–in our Kenya example were calculated based on recent trends in the pediatric HIV treatment data as well as a synthesis of findings from available literature.
Limited availability of real-world data, however, posed a challenge to the development of the tool and is a limitation of its future application. Age-disaggregated data is not widely available; in some cases, different data sources (e.g., the U.S. President’s Emergency Plan for AIDS Relief [PEPFAR] vs. UNAIDS vs. Ministry of Health) may be reporting different statistics for comparable time periods. The use of adult data as a proxy, which was necessary in our Kenya example, can lead to distortions as adherence, retention, and loss to follow up rates are generally higher among adult patient populations as compared to pediatric patients. The accuracy of available data, in turn, impacts the accuracy of the tool’s output and the resulting patient estimate.
Additionally, as the tool’s output is limited to aggregate annual patient numbers, ART nuances that could impact patient numbers within a given year are not captured (e.g., length of time on treatment prior to failure, age at treatment initiation, etc.). Moreover, the tool’s design does not factor in the possibility of “re-entry” for patients lost at an earlier stage. Although re-entry may happen in practice, current available data is neither robust nor generalizable enough to derive a proxy that could be applied to the tool with a reasonable degree of confidence. Lastly, the algorithm is arithmetic-based, and, as a result, the forecast is limited to linear patient pool growth (within annual intervals as parameters can be adjusted by year included in the time horizon).
Comparison with other quantification tools
As national HIV programs continue focusing on the challenge of reaching HIV-positive children in need of treatment, patient quantification tools can add value to broader HIV policy and planning processes at the country level. A number of estimation and projection tools are currently available for country-level use, including the SPECTRUM AIDS Impact Module (SPECTRUM AIM) [26] and the Clinton Health Access Initiative Simple Tool [27]. Used by UNAIDS, SPECTRUM AIM is a mathematical model that uses country-level demographic data and HIV surveillance, survey, and program data to generate estimates of HIV indicators (historical and short-term projections). These indicators include key statistics such as the number of people living with HIV, HIV incidence, number of new infections, etc. As country reporting has evolved to meet 90-90-90 requirements, UNAIDS SPECTRUM AIM estimates have expanded to capture progress towards these goals and include indicators such as viral suppression. Many of these indicators are not yet age-disaggregated and forecasts are limited to the immediate year, posing a challenge for pediatric HIV planning. SPECTRUM AIM also does not report statistics broken down by line of treatment.
While the Clinton Health Access Initiative (CHAI) Simple Tool [27] provides a three-year forecast, this tool focuses on quantifying antiretroviral (ARV) needs instead of estimating patients and other key HIV indicators. A morbidity-based commodity forecast, the Simple Tool uses the number of patients per ART regimen treated in the past 12 month period (actual or estimated) and estimated incidence or prevalence rates to generate an estimate of the number of patients that can be expected to be treated and the number of ARVs these patients will need. The pediatric portion of the Simple Tool also incorporates the weight distribution of the pediatric cohort in question as well as weight distribution by ARV formulation. As a quantification tool, the Simple Tool focuses on estimating the supply needs for ARVs and does not incorporate dimensions of patient treatment access that also serve to determine the size of potential pediatric patient pools in practice.
Conclusion
Among our analytical tool’s potential applications, we envision it being used to complement ongoing commodity forecasting to strengthen and streamline national ARV procurement, particularly for second- and third-line ARVs, as the tool accounts for clinical and patient-specific challenges associated with treatment switching in resource-limited settings. Additionally, the tool could be used to compare a country’s current patient forecast to an anticipated (or desired) scenario–e.g., partial achievement of the 90-90-90 targets. This comparison data would help policymakers and partners think critically and creatively about potential patients in need of treatment that they have either not yet identified or that they will need to account for as they scale up their national programs. Given ongoing pediatric HIV data limitations, particularly for later lines of treatment, a patient estimate of this type can provide a national baseline to ground planning discussions and support strategy development and decision making around resource allocation for effective pediatric HIV programs scale up.
Supporting information
(XLS)
Acknowledgments
The authors gratefully acknowledge the contributions of their J&J Global Public Health colleagues, as well as all New Horizons Collaborative (NHC) implementing partners. The development of this forecasting model would not have been possible without their input, insights, and sustained feedback over the course of several years of iterative piloting.
Data Availability
Primary data that informed model development is available from the UNAIDS AIDSInfo database (http://aidsinfo.unaids.org/), the UN World Population Prospects database (https://population.un.org/wpp/), Kenya's NASCOP National ACT Dashboard and Viral Load System Dashboard (https://www.nascop.or.ke/), and various study and articles listed in the references. Specific information concerning which data sets and variables were utilized from each of these sources is available in the Supporting Information. The authors of this study confirm that they had no special access privileges in accessing any of these data.
Funding Statement
This work was supported exclusively by Johnson & Johnson Global Public Health. The authors did not receive separate funding for this work. Johnson & Johnson and Rabin Martin provided support in the form of salaries for authors PM and AL and MR, respectively, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Joint United Nations Programme on HIV/AIDS (UNAIDS). AIDSinfo Database [Internet]. Geneva: UNAIDS; 2019. [cited 2019 Sep 30]. Available from: http://aidsinfo.unaids.org/#. [Google Scholar]
- 2.Lecher S, Williams J, Fonjungo PN, Kim AA, Ellenberger D, Zhang G, et al. Progress with scale-up of HIV viral load monitoring—Seven Sub-Saharan African countries, January 2015–June 2016. MMWR Morb Mortal Wkly Rep. 2016. December 2;65(47): 1332–1335. 10.15585/mmwr.mm6547a2 [DOI] [PubMed] [Google Scholar]
- 3.Moorhouse M, Maartens G, Venter WDF, Moosa M-Y, Steegen K, Jamaloodien K, et al. Third-line antiretroviral therapy program in the South African public sector: Cohort description and virological outcomes. J Acquir Immune Defic Syndr. 2019. January 1;80(1): 73–78. 10.1097/QAI.0000000000001883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations International Children’s Fund (UNICEF). Children and AIDS: Statistical update [Internet]. New York (NY): UNICEF; 2017. December [cited 2019 April 4]. Available from: https://data.unicef.org/wp-content/uploads/2017/11/HIVAIDS-Statistical-Update-2017.pdf. [Google Scholar]
- 5.Meintjes G, Dunn L, Coetsee M, Hislop M, Leisegang R, Regensberg L, et al. Third-line antiretroviral therapy in Africa: effectiveness in a Southern African retrospective cohort study. AIDS Res Ther. 2015;12: 39 10.1186/s12981-015-0081-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corrigan B, Mukui I, Mulenga L, Mthethwa N, Letsie M, Bruno S, et al. Characteristics of treatment-experienced HIV-infected African children and adolescents initiating darunavir and/or etravirine-based antiretroviral treatment. Pediatr Infect Dis J. 2018. July;37(7): 669–672. 10.1097/INF.0000000000001843 [DOI] [PubMed] [Google Scholar]
- 7.Lazarus E, Nicol S, Frigati L, Penazzato M, Cotton MF, Centeno-Tablante E, et al. Second- and third-line antiretroviral therapy for children and adolescents: A scoping review. Pediatr Infect Dis J. 2017. May;36(5): 492–499. 10.1097/INF.0000000000001481 [DOI] [PubMed] [Google Scholar]
- 8.Joint United Nations Programme on HIV/AIDS (UNAIDS). Communities at the centre: Defending rights, breaking barriers, reaching people with HIV services [Internet]. Geneva: UNAIDS; 2019. [cited 2019 Sep 30]. Available from: https://www.unaids.org/sites/default/files/media_asset/2019-global-AIDS-update_en.pdf. [Google Scholar]
- 9.World Health Organization (WHO). HIV/AIDS data and statistics [Internet]. Geneva: WHO; 2019. [cited 2019 Sep 30]. Available from: https://www.who.int/hiv/data/en/. [Google Scholar]
- 10.Penazzato M, Bendaud V, Nelson L, Stover J, Mahy M. Estimating future trends in paediatric HIV. AIDS. 2014. [cited 2019 Apr 4];28: S445–S451. 10.1097/QAD.0000000000000481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bastard M, Poulet E, Nicolay N, Szumilin E, Balkan S, Pujades-Rodriguez M. Pediatric access and continuity of HIV care before the start of antiretroviral therapy in sub-Saharan Africa. Pediatr Infect Dis J. 2016;35(9): 981–86. 10.1097/INF.0000000000001213 [DOI] [PubMed] [Google Scholar]
- 12.United States Centers for Disease Control and Prevention (CDC). Strategies for identifying and linking HIV-infected infants, children, and adolescents to HIV care and treatment [Internet]. Washington, DC: United States President’s Emergency Plan for AIDS Relief (PEPFAR); 2015. June 23 [cited 2019 Apr 4]. Available from: https://www.pepfar.gov/documents/organization/244347.pdf. [Google Scholar]
- 13.Wynberg E, Williams E, Tudor-Williams G, Lyall H, Foster C. Discontinuation of efavirenz in paediatric patients: Why do children switch?. Clin Drug Investig. 2017;38(3): 231–238. 10.1007/s40261-017-0605-1 [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization (WHO), Center for Disease Control and Prevention (CDC), The Global Fund. HIV drug resistance report 2017 [Internet]. Geneva: World Health Organization; 2017. [cited 2019 Apr 4]. Available from: http://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf?sequence=1. [Google Scholar]
- 15.African Society for Laboratory Medicine (ASLM). Laboratory challenges to viral load implementation in resource-limited settings [Internet]. Lusaka (Zambia): International AIDS Society; 2014. May 5 [cited 2019 Apr 4]. Available from: https://www.iasociety.org/Web/WebContent/File/IAS-ILF__3_Lab_Challenges__Peter.pdf. [Google Scholar]
- 16.Clinton Health Access Initiative (CHAI). The state of antiretroviral drug market in low- and middle-income countries, 2014–2019: ARV market report [Internet]. New York (NY): CHAI; 2015. [cited 2019 Apr 4]. Available from: https://clintonhealthaccess.org/content/uploads/2015/11/CHAI-ARV-Market-Report-2015_FINAL.pdf. [Google Scholar]
- 17.TREAT Asia Pediatric HIV Observational Database (TApHOD) and the International Epidemiologic Databases to Evaluate AIDS (IeDEA) Southern Africa Paediatric Group. A bioregional survey and review of first-line treatment failure and second-line paediatric antiretroviral access and use in Asia and southern Africa. J Int AIDS Soc. 2011;14(17):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwinkels D. An update on the Coordinated Procurement Planning Initiative (CPP): A multi-partner approach to preventing ARV stockouts In: WHO/AMDS Partners Meeting [Internet]. Geneva: WHO; 2012. June 19 [cited 2019 Apr 4]. Available from: http://www.who.int/hiv/events/scms_cpp_dominique.pdf. [Google Scholar]
- 19.World Health Organization (WHO). Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy [Internet]. Geneva: World Health Organization; 2017. July [cited 2019 Apr 4]. Available from: https://www.who.int/hiv/pub/guidelines/advanced-HIV-disease/en/. [PubMed] [Google Scholar]
- 20.World Health Organization (WHO). Guidelines on the public health response to pretreatment HIV drug resistance: Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Second edition Jun 2016 [Internet]. Geneva: World Health Organization; 2017. July [cited 2019 Apr 4]. Available from: https://www.who.int/hiv/pub/guidelines/hivdr-guidelines-2017/en/. [Google Scholar]
- 21.United States President’s Emergency Plan for AIDS Relief (PEPFAR) and Children’s Investment Fund Foundation (CIFF). Promising practices and lessons learned from the implementation of the ACT Initiative Accelerating Children’s HIV/AIDS Treatment [Internet]. Washington, DC.: PEPFAR and CIFF; 2017. April [cited 2019 Apr 4]. Available from: http://www.pedaids.org/wp-content/uploads/2017/11/ACT_Report_04_2017_FINAL-digital.pdf. [Google Scholar]
- 22.United States President’s Emergency Plan for AIDS Relief (PEPFAR). Kenya country operational plan (COP) 2018 strategic direction summary [Internet]. Washington, DC: United States President’s Emergency Plan for AIDS Relief (PEPFAR); 2018. April 10 [cited 2019 Sep 30]. Available from: http://www.pepfar.gov/documents/organization/285861.pdf. [Google Scholar]
- 23.National AIDS & STI Control Programme (NASCOP). National ACT dashboard [Internet]. Nairobi, Kenya: NASCOP; 2016; [cited 2019 Apr 4]. Available from: http://www.nascop.or.ke/wp-content/uploads/2016/07/National-ACT-Dashboard-1.xlsx. [Google Scholar]
- 24.National AIDS & STI Control Programme (NASCOP). Viral load system [Internet]. Nairobi, Kenya: NASCOP; 2019; [cited 2019 Apr 4]. Available from: https://viralload.nascop.org/. [Google Scholar]
- 25.Habiyambere V. WHO global ARVs and diagnostic use survey in 2015 in low- and middle-income countries [Internet]. Geneva: WHO HIV Department, AIDS Medicines and Diagnostic Service (AMDS), Strategic Information and Planning Unit; 2016. March [cited 2019 Apr 4]. Available from: https://www.who.int/hiv/amds/amds2016-ppt-WHO-2015ARVUseSurvey.pdf?ua=1. [Google Scholar]
- 26.Mahy M, Penazzato M, Ciaranello A, Mofenson L, Yianoutsos C, Davies M, et al. Improving estimates of children living with HIV from the Spectrum AIDS impact model. AIDS. 2017. [cited 2019 Apr 4];31:S13–S22. 10.1097/QAD.0000000000001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clinton Health Access Initiative (CHAI). Simple Pediatric ARV Forecasting Tool [Internet]. Boston (MA): CHAI; 2017; [cited 2019 Apr 4]. Available from: https://childrenandaids.org/sites/default/files/2017-05/PAEDS%203-year%20Simple%20ARV%20forecasting%20tool%20v2016_BLANK.xlsx. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
Primary data that informed model development is available from the UNAIDS AIDSInfo database (http://aidsinfo.unaids.org/), the UN World Population Prospects database (https://population.un.org/wpp/), Kenya's NASCOP National ACT Dashboard and Viral Load System Dashboard (https://www.nascop.or.ke/), and various study and articles listed in the references. Specific information concerning which data sets and variables were utilized from each of these sources is available in the Supporting Information. The authors of this study confirm that they had no special access privileges in accessing any of these data.