Abstract
Background:
Adaptive balance control is often compromised in children with developmental coordination disorder (DCD). Neuromuscular training (NMT) is commonly used in clinical settings to improve neuromuscular control and hence balance performance in these children. However, its effectiveness has not been proven scientifically. This randomized controlled study aimed to explore the effectiveness of NMT for improving adaptive balance performance and the associated leg muscle activation times in children with DCD.
Methods:
Eighty-eight children with DCD were randomly assigned to the NMT or control group (44 per group). The NMT group received two 40-minute NMT sessions/week for 3 months, whereas the control group received no intervention. The outcomes were measured at baseline and 3 and 6 months. The primary outcome was the sway energy score (SES) in both the toes-up and toes-down conditions as derived using the Adaptation Test (ADT). Secondary outcomes included the medial gastrocnemius, medial hamstring, tibialis anterior and rectus femoris muscle activation onset latencies during ADT, measured using surface electromyography and accelerometry. Data were analyzed using a repeated measures analysis of covariance based on the intention-to-treat principle.
Results:
At 3 months, no significant within-group or between-group differences were noted in the SESs for either group. At 6 months, the toes-down SES decreased by 6.8% compared to the baseline value in exclusively the NMT group (P = .004). No significant time, group or group-by-time interaction effects were observed in any leg muscle activation outcomes.
Conclusions:
Short-term NMT failed to improve adaptive balance performance and leg muscle activation times in children with DCD. Further studies should explore the clinical applications of longer-term task-specific interventions intended to improve the adaptive balance performance of these children.
Keywords: adaptation, dyspraxia, neuromuscular reaction time, postural control, rehabilitation exercises
1. Introduction
Developmental coordination disorder (DCD), a condition characterized by clumsiness in motor skills, affects approximately 6% of primary school-aged children.[1] This neurodevelopmental movement disorder not only affects activities of daily living, but also limits children's participation in activities and adversely affects their motor skills and psychosocial development.[2–4]
Approximately 73% to 87% of children with DCD demonstrate difficulties with postural control (balance).[5] Although most studies have focused on deficits in static balance,[2,6–9] recent neuroimaging studies of children with DCD have suggested that adaptive balance performance could also be compromised due to cerebellar pathology, which affects the grading or scaling of muscle force output in response to postural perturbations.[10,11] In fact, previous studies have suggested that children with DCD demonstrate atypical visuomotor adaptation associated with cerebellar dysfunction.[12,13] Aside from atypical or slowed central adaptation, poorer balance adaptation in children with DCD may also be caused by neuromuscular factors that increase mechanical stiffness at the ankle joints.[14] Therefore, rehabilitation interventions for children with DCD should be designed to treat both neuromuscular deficits and improve adaptive (and reactive) balance performance.
Perturbation-enhanced neuromuscular training (NMT) on a moving platform has been commonly used to treat neuromuscular deficits and improve reactive balance performance in children with postural control disorders. This type of impairment-based balance training (10 sessions over 3 to 4 weeks) could improve the timing (i.e., onset latency) of postural muscle activation and the balance responses of children.[15,16] The physiological mechanisms underlying this technique include increased excitation of the afferent pathways and increased sensitivity of the muscle spindles, resulting in the postural muscles having a heightened state of readiness to respond to postural perturbations.[17] Therefore, NMT may be suitable for improving the leg muscle activation times and adaptive and reactive balance performance of children with DCD. This study was the first to test the effectiveness of a modified short-term NMT regimen as a potential means of improving adaptive balance performance and leg muscle activation times in children with DCD. It was hypothesized that a short-term NMT may improve adaptive balance performance and the associated leg muscle activation times in these children.
2. Methods
2.1. Study design
This was a single-blinded and randomized controlled intervention trial (ClinicalTrials.gov NCT02397161). Ethical approval was obtained from the Human Research Ethics Committee of the University of Hong Kong. Informed written consent was obtained from all participants and parents before their enrollment in the study. All of the procedures were conducted in accordance with the Declaration of Helsinki.
2.2. Participants
Children with DCD were recruited from primary schools and the Heep Hong Society in Hong Kong through social media, poster advertisements and referrals. Relevant information, such as demographic and medical history data, was obtained from medical records and by interviewing the participants and their parents.
The inclusion criteria were as follows:
-
1.
age of 6 to 9 years;
-
2.
formal diagnosis of DCD based on the Diagnostic and Statistical Manual of Mental Disorders V (DSM-V) [1];
-
3.
total impairment score of ≤15th percentile on the Movement Assessment Battery for Children (MABC) test (version 1)[18];
-
4.
total score of <46 (for ages 5–7.9 years) or <55 (ages 8–9.9 years) on the DCD questionnaire 2007[19];
-
5.
T score of ≥54.5 on the Child Behavior Checklist attention problem subscale[20];
-
6.
attendance at a local mainstream school; and
-
7.
an intelligence level within the normal range (Raven's Progressive Matrices - Color Progressive Matrices).
The exclusion criteria were as follows:
-
1.
diagnosis of a neurological or other movement disorder;
-
2.
any cognitive, psychiatric (other than comorbid attention deficit hyperactivity disorder), congenital, musculoskeletal or cardiopulmonary disorder that could affect motor performance;
-
3.
receipt of active treatments, including complementary, and alternative medicine;
-
4.
excessively disruptive behavior; or
-
5.
the inability to follow instructions.
2.3. Screening and randomization
A physiotherapist screened the volunteers by phone, and those who appeared to meet the study criteria underwent an in-person evaluation and baseline assessment. All eligible participants were randomly assigned to the NMT, attention training (AT), AT-NMT or control group, although only the NMT and control groups were included in this paper. The randomization procedure involved the use of a random number table to generate the allocation sequence. To ensure that the allocations were concealed, sealed opaque envelopes were prepared by an independent research assistant who was not involved in the subject recruitment process. Another independent research assistant conducted the allocation, which included opening the envelopes.
2.4. Intervention
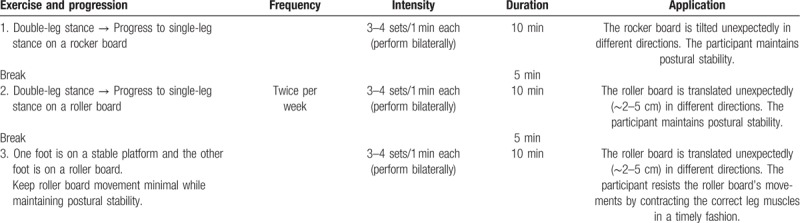
The NMT regime was modified from an NMT protocol used commonly to improve the neuromuscular and postural control of young people in clinical situations.[17] The detailed training protocol is described in Table 1. During exercises 1 and 2, the trainer disturbed the participant's balance unexpectedly by tilting or translating the supporting surface (a movable platform) in a random direction. The participant reacted by remaining upright without falling over. During exercise 3, the participant was required to resist the movement of the supporting surface (a roller board) by contracting the correct lower limb muscles in a timely fashion. The participant was instructed to neither contract the leg muscles in anticipation of the perturbation nor respond by co-contracting both the agonists and antagonists.[17] Children in the intervention group practiced these neuromuscular balance exercises for 40 minutes in each session. Each child received two face-to-face training sessions per week for 12 weeks at a university laboratory. All sessions were conducted by a physiotherapist and two research assistants with sports or rehabilitation background. Children in the control group continued their normal daily activities and usual medical care but did not receive any NMT within the study period.
Table 1.
Neuromuscular training protocol.
2.5. Outcome measurements
The participants were assessed by a blinded physiotherapist and two blinded research assistants at baseline and at 3 (i.e., after the NMT) and 6 months (i.e., 3 months after the end of the NMT) (Fig. 1). All of the participants underwent the following outcome assessments in the same order at a university laboratory.
Figure 1.
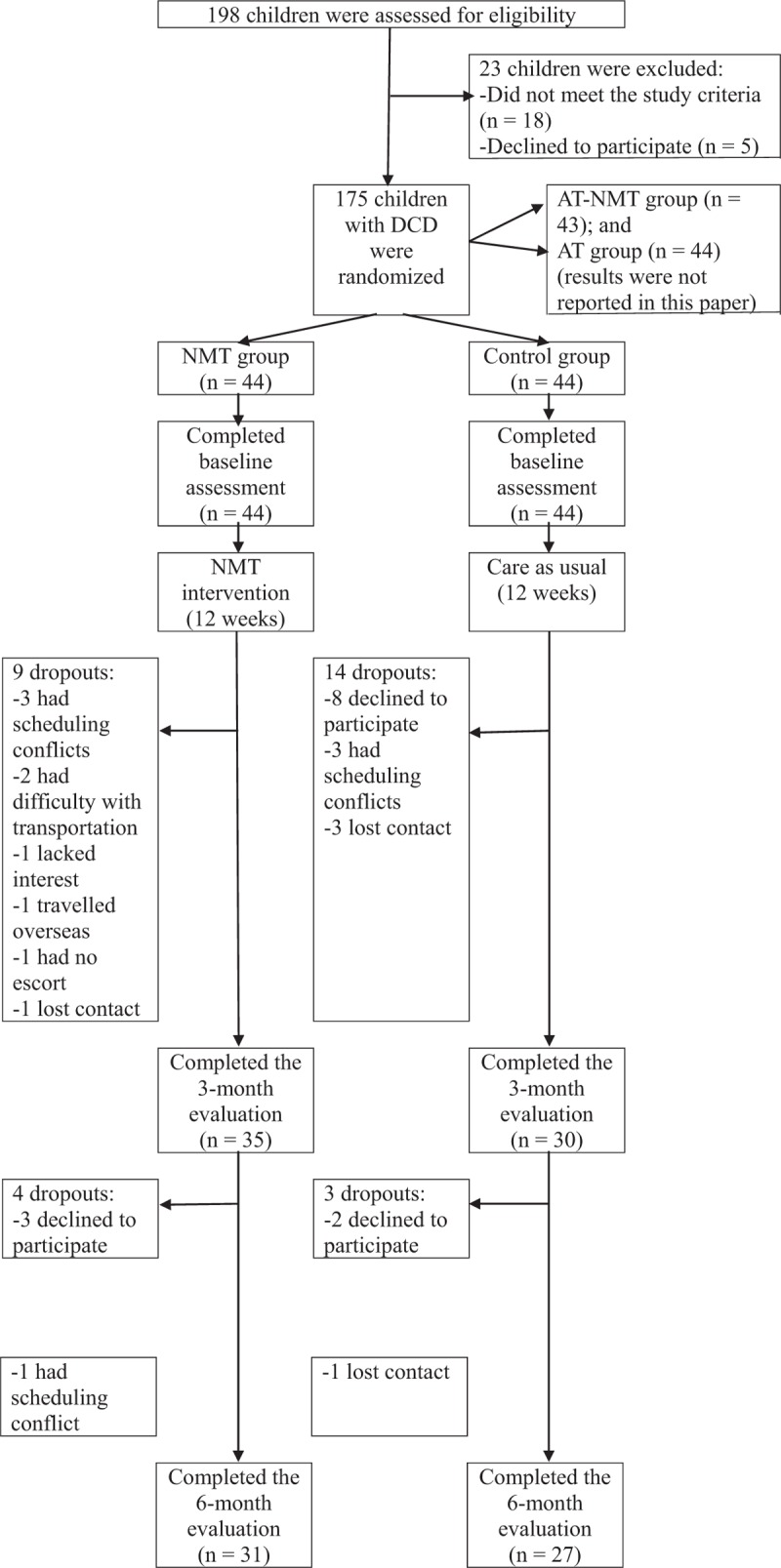
Study flow diagram. AT = attention training, DCD = developmental coordination disorder, NMT = neuromuscular training.
2.6. Primary outcomes
The adaptive balance performance (i.e., adaptive changes in postural control) of each participant while standing was measured using an Adaptation Test (ADT) administered via a computerized dynamic posturography (CDP) machine (Smart Equitest; NeuroCom International Inc, Clackamas, OR). This test assesses the motor system's ability to adapt to repeated support surface rotation (i.e., platform tilting) that causes the ankles to dorsiflex or plantar flex and thus trigger the toes to move up or down, without significantly displacing the participant's center of gravity (COG). During the test, the participant stood and looked straight forward while maintaining postural stability. She or he was then exposed to a series of five sudden platform rotations for each toes-up or toes-down cycle. Physiologically, the automatic postural control system is initially prepared to actively resist ankle joint dorsiflexion/plantar flexion. However, resisting ankle joint rotations in the ADT is destabilizing when the support surface rotates. Therefore, by the fourth or fifth platform rotation of the series, the automatic postural control system attenuates the ankle joint resistance and reduces COG sway during the recovery period.[14]
A non-dimensional sway energy score (SES) was generated based on the velocity and acceleration of the COG (i.e., body sway) of the participant during each testing trial of both toes-up and toes-down platform rotations. The SES is an accurate measure of the overall functional effect of changes in adaptive balance. Mean SESs were calculated for both the toes-up and toes-down conditions and used to quantify the amount of body sway and define the overall adaptation of the motor system. A smaller mean SES represents lesser body sway and better adaptive automatic postural responses to repeated platform rotations.[14,21] The mean SESs of both the toes-up and toes-down conditions were used for the analysis.
2.7. Secondary outcomes
During the ADT, lower limb postural muscle responses were measured concurrently using surface electromyography (EMG) (Biometrics, Newport, UK). An accelerometer (Biometrics, Newport, UK) was attached to the CDP machine's movable platform to register the onset of platform rotation. Both anterior (tibialis anterior and rectus femoris) and posterior (medial hamstrings and gastrocnemius) leg muscles were measured during both toes-up and toes-down platform rotations. The former condition stretches the gastrocnemius muscle, while a reflexive contraction of this muscle exaggerates rather than compensates for the associated postural disturbance (i.e., backward COG displacement). The tibialis anterior and quadriceps muscles must contract to maintain body balance. In contrast, the toes-down platform rotation stretches and provokes a reflexive contraction of the tibialis anterior muscle. It thus exaggerates the postural disturbance (i.e., forward COG displacement) and requires the gastrocnemius and hamstrings muscles to contract to maintain postural stability.[14] EMG and accelerometry signals were recorded during the first ADT toes-up and toes-down trials.
Circular Ag/AgCl bipolar surface EMG electrodes (center-to-center inter-electrode distance: 2 cm) were used to record the activities of the 4 lower limb muscles in the dominant leg during ADT. Active EMG electrodes were attached to the skin over the center of each muscle belly according to the recommendations of Barbero et al.[22] The reference electrode was attached to the lateral malleolus of the ipsilateral leg. Raw EMG signals were filtered at a bandwidth of 20 to 460 Hz, sampled at 1000 Hz, and amplified by a gain factor of 1000 using a single differential amplifier (input impedance >1015 and a common mode rejection ratio >96 dB). All electrodes were connected to a DataLOG device (Biometrics, Newport, UK) that was attached to the participant's waist to minimize artifacts. The DataLOG uses both a high-pass filter (20 Hz) and a low-pass filter (450 Hz) and stores filtered EMG data for later offline analysis.[23,24]
After data collection, the EMG signals of each muscle and the accelerometer signal were post-processed using Biometrics EMG analysis software for DataLOG, version 8.51 (Newport, UK). First, the means and standard deviations (SDs) of the resting EMG signals of each muscle were calculated. Second, the onset time of muscle activation, defined as the starting point of the EMG activity with a duration of >25 ms and 2 SD from the mean resting EMG value, was marked for each muscle. Third, the onset time of the accelerometer signal, defined as the time point at which the signal amplitude was 0.2 ms−2 from the resting value, was identified and used to represent the onset of ADT platform rotation. Finally, the muscle activation onset latency, defined as the time interval (ms) between the onset of the accelerometer signal and the first discernible EMG activity of each muscle, was calculated and used for analysis.[23–25] The synchronizing contraction times of the different lower limb muscles are thought to attenuate ankle joint resistance during ADT platform rotations and hence reduce body sway.[14]
2.8. Statistical analysis
The sample size was calculated using G∗Power, version 3.1.0 (Franz Faul, University of Kiel, Germany). In our previous studies,[6,8] children with DCD achieved significant improvements in standing balance performance (primary outcome) after 3 months of gross motor training when compared with controls (effect sizes = 0.404–1.248). Therefore, a moderate-to-large effect size of 0.7 was estimated for the between-group comparison of the primary outcome in this study. A total of 34 children per group would be needed to achieve a statistical power of 0.8 with a 2-tailed alpha level set at 0.05. Anticipating a 25% attrition rate,[8] a minimum of 43 children with DCD per group were required.
All of the data were analyzed based on the intention-to-treat principle (last observation carried forward) to address drop-out cases. The demographic and outcome variables of the two groups at baseline were compared using the independent t test (for continuous data) and chi-square test (for categorical data). Any significant between-group differences in the demographic and outcome variables at baseline were included as covariates in the subsequent analyses. A repeated measures analysis of covariance (ANCOVA, group × time) was used to compare the primary and secondary outcomes of the two groups across the three time points. Further within-group comparisons were made using the paired t test. The 2-tailed significance level was set at 5%. Bonferroni adjustment was applied to multiple comparisons. IBM SPSS software version 25 (IBM, Armonk, NY) was used for all the statistical analysis.
3. Results
3.1. Study population
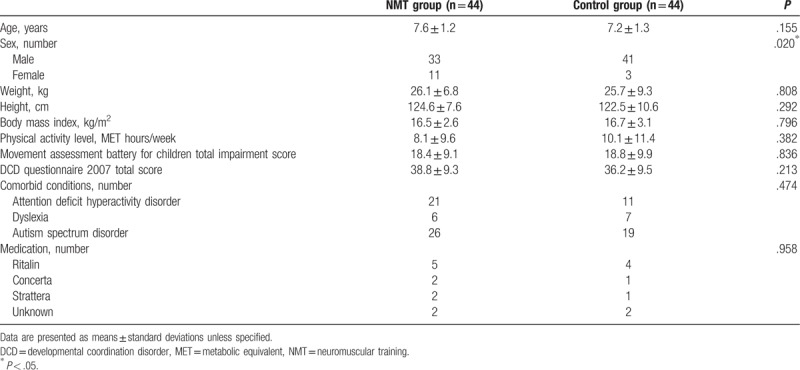
From July 2016 to March 2017, 198 children were screened; 175 were eligible and underwent randomization: 44 to the NMT group, 44 to the control group, 44 to the AT group, and 43 to the AT-NMT group (both AT groups are not discussed in this paper). Figure 1 presents the reasons for exclusion from the study and the flow of the study. No statistically significant between-group differences were observed in any baseline characteristics except that the control group had a higher boy-to-girl ratio (P = .020) (Table 2). Therefore, sex was treated as a covariate in the subsequent ANCOVA.
Table 2.
Demographic characteristics of children with DCD at baseline.
Thirty-five children in the NMT group and 30 children in the control group completed the intervention and 3-month evaluation. Only 31 children in the NMT group and 27 children in the control group completed the 6-month evaluation (Fig. 1). No significant differences were observed in the baseline demographic data between the children who completed the study and those who did not. The attendance rate (i.e., participation in the NMT sessions) was 72%. No changes in the participants’ medications, treatments and physical activity levels were noted during the study period. No adverse events were reported.
3.2. Primary outcomes
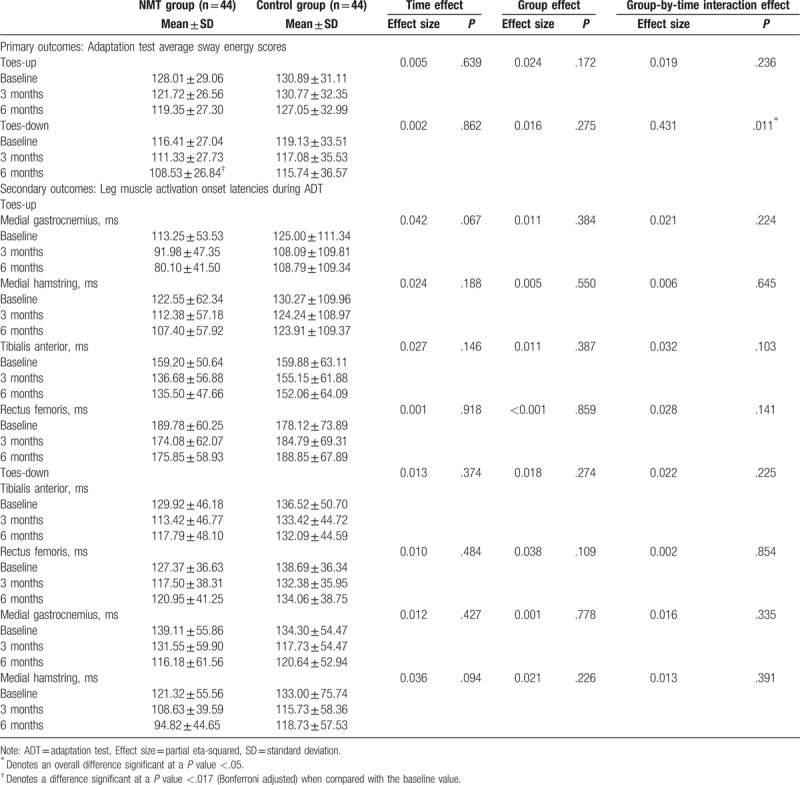
At 3 months, no significant within-group changes or between-group differences were observed in either the toes-up or toes-down condition (P > .05). At 6 months, however, the toes-down SES decreased by 6.8% relative to the baseline only in the NMT group (P = .004). No such change was observed in the toes-up condition (Table 3).
Table 3.
Comparison of primary and secondary outcome variables between groups from baseline to 6 months.
3.3. Secondary outcomes
No significant time, group or group-by-time interaction effects were observed in any EMG outcomes. In other words, NMT did not induce any changes in leg muscle activation times in response to a sudden tilting of the support surface (Table 3).
4. Discussion
This trial was the first to explore the effectiveness of an NMT program on adaptive balance performance and leg muscle activation time in children with DCD. In partial contrast to our hypothesis, however, the results revealed that 3 months of NMT did not improve adaptive balance performance and the associated leg muscle activation times in these children. Accordingly, NMT could not hasten central adaptation or optimize the peripheral neuromuscular reaction time (which attenuates ankle joint resistance and reduces COG sway during– ADT)[14] in these children.
We initially asked why NMT did not effectively improve the adaptive balance performance of children with DCD. First, the balance exercises included in the NMT program focus on remediating the underlying neuromuscular impairments, with the expectation of subsequent improvements in functional adaptive balance performance.[15–17] This type of bottom-up treatment approach is considered less effective than the top-down treatment approach because it may not be able to induce neuroplastic changes in the brain or hasten central adaptation through the learning process.[26] A previous study demonstrated that during a task-specific intervention (i.e., learning functional balance tasks directly), a top-down treatment approach could effectively improve the functional dynamic balance performance of children with DCD by improving sensory organization in the central nervous system.[27] Second, we postulated that 3 months of NMT may not be sufficient to improve the adaptive balance performance and leg muscle activation times in children with DCD. A 6-month training period may be more appropriate. The observed improvement in adaptive balance performance in the ADT toes-down condition at 6 months may suggest that the participants continued to practice NMT exercises after the intervention period but did not report this to our research personnel. Further studies may increase the training duration to at least 6 months and closely monitor the participants, possibly by sending regular reminders during the follow-up period.
We also asked why NMT did not effectively improve the leg muscle activation times, even though it had been designed to remediate neuromuscular impairments in children with DCD. Again, this may have been a consequence of the inadequate training duration (3 months), as explained above. Moreover, the NMT exercises may have been too static, as they comprised primarily balance-challenging activities while standing. Further studies should refine the NMT protocol to include a variety of exercises such as stretching, muscle strengthening and functional power training (e.g., lateral hops)[28] intended to stimulate neuromuscular changes in children with DCD.
Although our study design was robust, this randomized controlled trial had some additional limitations. First, the participants were not blinded to their group assignment because of the nature of the exercise intervention. The participants’ optimism about the benefits of the exercises might have introduced some bias to the results.[29] Second, as this was a laboratory-based experimental study, the generalizability of results to a natural environment remains uncertain. Third, only the lower limb muscle activation times were measured during ADT in the present study. Further studies could measure the ankle joint kinematics simultaneously to quantify the magnitudes of adaptive postural control changes over time. Further studies could also explore the optimum exercise training duration for improving the adaptive balance performance and other kinetic and kinematic parameters in children with DCD.
5. Conclusions
A 3-month course of NMT did not improve adaptive balance performance and the associated leg muscle activation times in children with DCD. Further studies are needed to explore other clinical interventions that may improve the adaptive balance performance of these children and treat their neuromuscular deficits.
Acknowledgments
The authors thank Ms. Lily Yuen (Heep Hong Society, Hong Kong) and Dr. C.Y. Cheung (Department of Social Work and Social Administration, University of Hong Kong) for providing support and advice.
Author contributions
Conceptualization: Thomas K.S. Wong, William W.N. Tsang, Catherine Mary Schooling, Shirley S.M. Fong, Daniel Y.T. Fong, Yang Gao, Joanne W.Y. Chung.
Data curation: Yoyo T.Y. Cheng, William W.N. Tsang.
Formal analysis: Yoyo T.Y. Cheng, Catherine Mary Schooling, Shirley S.M. Fong.
Funding acquisition: Shirley S.M. Fong, Daniel Y.T. Fong, Joanne W.Y. Chung.
Investigation: Yoyo T.Y. Cheng, William W.N. Tsang, Catherine Mary Schooling, Shirley S.M. Fong.
Methodology: Yoyo T.Y. Cheng, William W.N. Tsang, Catherine Mary Schooling, Shirley S.M. Fong.
Project administration: Shirley S.M. Fong.
Resources: Shirley S.M. Fong.
Software: Yang Gao.
Supervision: William W.N. Tsang, Catherine Mary Schooling, Shirley S.M. Fong.
Validation: William W.N. Tsang, Shirley S.M. Fong.
Visualization: William W.N. Tsang, Catherine Mary Schooling, Shirley S.M. Fong.
Writing – original draft: Yoyo T.Y. Cheng, Shirley S.M. Fong.
Writing – review & editing: Yoyo T.Y. Cheng, Thomas K.S. Wong, William W.N. Tsang, Catherine Mary Schooling, Shirley S.M. Fong, Daniel Y.T. Fong, Yang Gao, Joanne W.Y. Chung.
Shirley S.M. Fong orcid: 0000-0001-6410-7606.
Footnotes
Abbreviations: ADT = Adaptation Test, ANCOVA = analysis of covariance, AT = attention training, CDP = computerized dynamic posturography, COG = center of gravity, DCD = developmental coordination disorder, DSM-V = Diagnostic and Statistical Manual of Mental Disorders V, EMG = electromyography, MABC = Movement Assessment Battery for Children, NMT = neuromuscular training, SD = standard deviation, SES = sway energy score.
How to cite this article: Cheng YT, Wong TK, Tsang WW, Schooling CM, Fong SS, Fong DY, Gao Y, Chung JW. Neuromuscular training for children with developmental coordination disorder. Medicine. 2019;98:45(e17946).
The work described in this paper was supported by two General Research Funds (project numbers: 17658516 and 17112018) from the Research Grants Council of the Hong Kong Special Administrative Region, China.
The authors have no conflicts of interests to disclose.
References
- [1].American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th edArlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- [2].Fong SSM, Lee VYL, Pang MYC. Sensory organization of balance control in children with developmental coordination disorder. Res Dev Disabil 2011;32:2376–82. [DOI] [PubMed] [Google Scholar]
- [3].Fong SSM, Lee VYL, Chan NNC, et al. Motor ability and weight status are determinants of out-of-school activity participation for children with developmental coordination disorder. Res Dev Disabil 2011;32:2614–23. [DOI] [PubMed] [Google Scholar]
- [4].Piek JP, Baynam GB, Barrett NC. The relationship between fine and gross motor ability, self-perceptions and self-worth in children and adolescents. Hum Mov Sci 2006;25:65–75. [DOI] [PubMed] [Google Scholar]
- [5].Macnab JJ, Miller LT, Polatajko HJ. The search for subtypes of DCD: Is cluster analysis the answer? Hum Mov Sci 2001;20:49–72. [DOI] [PubMed] [Google Scholar]
- [6].Fong SSM, Chung JWY, Chow LPY, et al. Differential effect of taekwondo training on knee muscle strength and reactive and static balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2013;34:1446–55. [DOI] [PubMed] [Google Scholar]
- [7].Fong SSM, Tsang WWN, Ng GYF. Altered postural control strategies and sensory organization in children with developmental coordination disorder. Hum Mov Sci 2012;31:1317–27. [DOI] [PubMed] [Google Scholar]
- [8].Fong SSM, Tsang WWN, Ng GYF. Taekwondo training improves sensory organization and balance control in children with developmental coordination disorder: a randomized controlled trial. Res Dev Disabil 2012;33:85–95. [DOI] [PubMed] [Google Scholar]
- [9].Fong SSM, Chung LMY, Bae YH, et al. Neuromuscular processes in the control of posture in children with developmental coordination disorder: current evidence and future research directions. Curr Dev Disord Rep 2018;5:43–8. [Google Scholar]
- [10].Horak F, Diener HC. Cerebellar control of postural scaling and central set in stance. J Neurophysiol 1994;72:479–93. [DOI] [PubMed] [Google Scholar]
- [11].Marien P, Wackenier P, Surgeloose D, et al. Developmental coordination disorder: disruption of the cerebello-cerebral network evidenced by SPECT. Cerebellum 2010;9:405410. [DOI] [PubMed] [Google Scholar]
- [12].Kagerer FA, Bo J, Contreras-Vidal JL, et al. Visuomotor adaptation in children with developmental coordination disorder. Motor Control 2004;8:450–60. [DOI] [PubMed] [Google Scholar]
- [13].Kagerer FA, Contreras-Vidal JL, Bo J, et al. Abrupt, but not gradual visuomotor distortion facilitates adaptation in children with developmental coordination disorder. Hum Mov Sci 2006;25:622–33. [DOI] [PubMed] [Google Scholar]
- [14].Nashner LM. Jacobson GP, Newman CW, Kartush JM. Computerized dynamic posturography. Handbook of Balance Function Testing. San Diego, London: Singular Publishing Group Inc; 1997. 280–307. [Google Scholar]
- [15].Woollacott M, Shumway-Cook A, Hutchinson S, et al. Effect of balance training on muscle activity used in recovery of stability in children with cerebral palsy: a pilot study. Dev Med Child Neurol 2005;47:455–61. [DOI] [PubMed] [Google Scholar]
- [16].Shumway-Cook A, Hutchinson S, Kartin D, et al. Effect of balance training on recovery of stability in children with cerebral palsy. Dev Med Child Neurol 2003;45:591–602. [DOI] [PubMed] [Google Scholar]
- [17].Hurd WJ, Chmielewski TL, Snyder-Mackler L. Perturbation-enhanced neuromuscular training alters muscle activity in female athletes. Knee Surg Sports Traumatol Arthrosc 2006;14:60–9. [DOI] [PubMed] [Google Scholar]
- [18].Henderson SE, Sugden DA. Movement Assessment Battery for Children Manual. London: The Psychological Corporation Ltd; 1992. [Google Scholar]
- [19].Wilson BN, Crawford SG, Green D, et al. Psychometric properties of the revised developmental coordination disorder questionnaire. Phys Occup Ther Pediatr 2009;29:182–202. [DOI] [PubMed] [Google Scholar]
- [20].Dewey D, Kaplan BJ, Crawford SG, et al. Developmental coordination disorder: associated problems in attention, learning, and psychosocial adjustment. Hum Mov Sci 2002;21:905–18. [DOI] [PubMed] [Google Scholar]
- [21].NeuroCom.. Balance Manager Systems: Instructions for Use. Oregon: NeuroCom International Inc; 2008. [Google Scholar]
- [22].Barbero M, Merletti R, Rainoldi A. Atlas of Muscle Innervation Zones - Understanding Surface Electromyography and its Applications. Milan, Italy: Springer-Verlag Italia; 2012. [Google Scholar]
- [23].Fong SSM, Ng SSM, Guo X, et al. Deficits in lower limb muscle reflex contraction latency and peak force are associated with impairments in postural control and gross motor skills of children with developmental coordination disorder: a cross-sectional study. Med (Baltimore) 2015;94:e1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cheng YTY, Tsang WWN, Schooling CM, et al. Reactive balance performance and neuromuscular and cognitive responses to unpredictable balance perturbations in children with developmental coordination disorder. Gait Posture 2018;62:20–6. [DOI] [PubMed] [Google Scholar]
- [25].Fong SM, Ng GYF. The effects on sensorimotor performance and balance with Tai Chi training. Arch Phys Med Rehabil 2006;87:82–7. [DOI] [PubMed] [Google Scholar]
- [26].Mandich AD, Polatajko HJ, Macnab JJ, et al. Treatment of children with developmental coordination disorder: what is the evidence? Phys Occup Ther Pediatr 2001;20:51–68. [PubMed] [Google Scholar]
- [27].Fong SSM, Guo X, Liu KPY, et al. Task-specific balance training improves the sensory organization of balance control in children with developmental coordination disorder: a randomized controlled trial. Sci Rep 2016;6:20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Linford CW, Hopkins JT, Schulthies SS, et al. Effects of neuromuscular training on the reaction time and electromechanical delay of the peroneus longus muscle. Arch Phys Med Rehabil 2006;87:395–401. [DOI] [PubMed] [Google Scholar]
- [29].Crocetti MT, Amin DD, Scherer R. Assessment of risk of bias among pediatric randomized controlled trials. Pediatrics 2010;126:298–305. [DOI] [PubMed] [Google Scholar]


